Abstract
Sleep and circadian rhythms are complex and inter-connected physiological processes. Relative to the remarkable progress made in identifying the genetic basis of circadian rhythms and some specific sleep disorders, efforts to identify genetic variants associated with normal variation in sleep have progressed more slowly. Two key issues concerning the design of such studies must be addressed in order to facilitate further progress. The first concerns the sleep related traits to be targeted. The second issue is the choice of the gene mapping method (linkage, candidate gene association or genome-wide association). This paper discusses these issues, reviews published studies of sleep phenotypes, and recommends cost-effective methods to advance knowledge of the genetic determinants of normal sleep patterns.
Keywords: Sleep, Polymorphisms, Circadian, Gene Mapping, Genome wide association studies
Introduction
The past two decades have witnessed major advances in genetics research. The availability of the complete human genome sequence, its ongoing annotation, the public availability of DNA polymorphism data, and the successful implementation of rapid, highly accurate and economical genotyping assays, have all contributed to an exponential increase in our understanding of the human genome, its variation, and the molecular pathology of a large number of Mendelian diseases. Remarkable success has already been attained in mapping genes for several multi-factorial polygenic diseases, included age related macular degeneration, Crohn’s disease, type 1 and type 2 diabetes mellitus, breast and prostate cancer, as well as coronary heart disease (1). It remains to be seen whether similar strategies can be successfully implemented for other genetically complex disorders and traits, including those related to the duration, patterning and quality of sleep.
In the following sections, we summarize the physiology of sleep and provide a rationale for gene mapping studies of sleep related variables. The range of sleep related traits (phenotypes) are surveyed and suggestions about selection of such traits are provided. The concepts of gene mapping and current techniques are explained next, with relevant reference to published studies in sleep genetics. We conclude with suggested designs for future studies.
The structure and physiology of sleep
Sleep and wakefulness are fundamental neurobiological conditions that are coordinated by homeostatic and circadian (24-hour rhythm) processes. Recent studies involving animal and drosophila models have established the evolutionary conservation of genetic regulation of sleep and circadian processes (2). The neuronal circuitry of the sleep-wake cycle has also been clearly delineated in animals as well as in humans (3), (4). Epidemiological studies indicate that approximately 30% to 40% of adults exhibit signs of insomnia and about 5% to 15% demonstrate excessive sleepiness (5). Several studies have also indicated that sleep related traits like sleep duration, quality, and daytime sleepiness are detrimental or risk promoting factors for common disorders such as obesity, hypertension, the metabolic syndrome, vulnerability to the common cold, and even all-cause mortality (6). Similarly, many other studies have demonstrated that sleeplessness is a risk factor for major depression and other psychiatric disorders including a wide variety of deficits related with sleep loss (7). As sleep plays an important role in health and disease, further knowledge about the genetic components regulating normal sleep and its disorders would have significant relevance for public health.
Since intimately related circadian and homeostatic regulatory processes govern sleep and wakefulness, these processes can be used to provide “starting points” for biological studies. While a number of circadian genes have been identified and studied in relation to sleep, the precise molecular mechanisms or components involved in homeostatic sleep regulation are less well understood.
The motivation for sleep gene mapping studies
Understanding genetic factors underpinning a trait or disorder provides a natural route to an understanding of its biological basis and manifestations. There are several persuasive reasons for considering genetic variants as plausible etiological factors, or moderators of, sleep related variables and/or sleep disorders. Animal studies have suggested that genetic variation contributes to normal sleep and sleep dysfunction (8). Several features of normal sleep are known to be heritable in humans. In a population based sample of 3,810 adult Australian twin pairs genetic differences accounted for at least 33% of the variance in sleep quality and sleep disturbance and 40% of the variance in sleep pattern, with no evidence for a decline in the importance of genetic predisposition with age (9). A recent twin study estimated broad sense heritability (the proportion of total phenotypic variation due to all genetic variance effects) for different self reported measures of sleep at 21% – 41% (10). In another independent twin study, Gregory et al observed significant genetic influence on sleep difficulties (11). Our studies among the Hutterites, a genetically isolated religious community has suggested significant narrow sense heritability estimates (i.e., the fraction of phenotypic variation explained by additive genetic variation) for sleep measures between 12.4% and 29.4%, even in the face of rigid environmental lifestyle restrictions (12). Familial aggregation of insomnia has been observed in independent samples (13). The number and identity of the genetic variants contributing to the heritability of normal and abnormal sleep variation remain largely unknown. The identification of such factors would undoubtedly help illuminate the neurobiology of human sleep and sleep disorders. Recognizing that many different non-genetic factors combine to determine human sleep patterns, the aim is to explain a portion of inter-individual variance, rather than to specifically identify, for example, a gene that causes insomnia (8).
Key issues related to the design of gene mapping studies
As with any gene mapping study, two key issues must be addressed in the study of human sleep: (i) the selection of appropriate sleep related traits; i.e., which normal variants or sleep disorders will be targeted; (ii) the choice of the gene mapping method (linkage, candidate gene association or genome-wide association).
Sleep related phenotypes for genetic studies
Two approaches have typically been utilized for gene mapping studies. The first involves the search for mutations that cause disease. Such an approach has obvious appeal from a public health perspective and can also yield valuable information about physiology (14). The second approach results from advances in quantitative genetics, which have enabled studies of normal variation in conjunction with the disease/disorder status (15). Moreover, sleep researchers need to evaluate the pros and cons of investigating particular sleep related traits in selecting appropriate phenotypic variables. Some sleep related traits can only be measured accurately in carefully controlled settings, such as sleep laboratories, or with relatively complex techniques, such as polysomnography (PSG). Even though PSG is now days being given to many thousands of people, it remains very expensive, and investigators may consider simple pen and paper behavioral measures as alternative phenotype variables, particularly in initial studies. More detailed analyses using sleep laboratory measures could then be later used for validation, once key genetic variants have been identified using the self-report variables. Such analyses could also exploit data gathered on pertinent individuals in the clinical setting.
Which sleep related phenotypes/traits should be selected for genetic analysis? There are numerous physiological and behavioral measures to choose from. Sleep-wake function can be conceptualized and measured along several orthogonal (uncorrelated) domains, each of which may be worth investigating. There are several common sleep electrophysiological measures that can be measured with electroencephalography (EEG) or with PSG. Such measures can be used to evaluate potential sub-domains such as sleep “continuity” measures (sleep latency, number and duration of awakenings, total wakefulness after sleep onset), sleep duration or sleep efficiency (ratio of sleep to time in bed). While these measures can be measured using EEG/PSG in the sleep lab, the individual’s perspective needs to be kept in mind. Quantitative sleep measures determined in an individual’s usual environment are also important, e.g. actigraphy measures or sleep diaries. Using these relatively simple techniques, it is possible to estimate sleep duration and continuity measures (analogous to PSG measures). Arguably, such estimates provide better measures of a person’s “usual” sleep. Indeed, current diagnoses of insomnia tend to rely more upon subjective measures than PSG ones (16).
Another important variable is sleep timing, which also provides a reference to a person’s circadian timing of sleep and is likely to be influenced not only by innate factors such as genetic variation, but also by social, occupational, and environmental demands. In the same vein, subjective sleep quality is an important measure that can only be obtained by sleep report. It reflects depth, satisfaction and restfulness that are important to individuals.
If several such traits can be estimated uniformly in a group of individuals who are also genotyped for the same set of genetic markers, significant economy of scale may be attained. Many such traits may be uncorrelated or there may be orthogonal sets of traits. Thus, a rich and informative dataset may be generated for gene mapping purposes. On the other hand, many available measures may be correlated. While such redundancy may arguably increase cost, it also provides more confidence for gene mapping results. The redundancy can also increase the ‘penalty’ for corrections due to multiple comparisons, but appropriate statistical methods are available. In summary, we advocate a combination of uncorrelated and overlapping measures that are likely to provide meaningful physiological insights and other variables that are meaningful to individuals. The key yardstick for selection of a variable for such analyses should be its heritability.
General approaches to gene mapping
The goal of gene mapping techniques is the identification of an ancestral mutation or genetic variant that is etiologically related to the trait of interest. As the relevant ancestor/s are usually unavailable, gene-mapping efforts had to rely on living individuals. Most current gene-mapping techniques rely on recombination, the phenomenon whereby chromosomal fragments are exchanged between homologous chromosomes during meiosis. Recombination ensures that chromosomal fragments are ‘shuffled’ when parental chromosomes are passed down to offspring, thus introducing variation between related individuals. Recombination typically occurs once or twice per chromosome in a cell undergoing meiosis, so sets of closely spaced chromosomal loci are inherited ‘en bloc’. Thus, selected polymorphisms can be used to ‘tag’ particular chromosomal fragments during the initial stages of a gene mapping study. Further ‘fine mapping’, or detailed analysis can be used to home in on a causative mutation once a chromosomal fragment is targeted for further analysis. This two-stage approach can be utilized among families or at the population level. Patently, the extent of chromosomal sharing is greater among members of a family compared with individuals from a population sample (where it is also likely to be obscured by other factors). The two stage approach has served gene mapping initiatives well, but it may well be surpassed once high throughput sequencing strategies enable facile and economic whole genome sequencing (17). Mapping the familial breast cancer causing gene BRCA1 described in the following section is a good example of this two stage approach.
Linkage Studies
Linkage studies investigate shared chromosomal fragments among members of a family who manifest the trait of interest. By analyzing the co-segregation of the trait and chromosomal markers, it may be possible to statistically identify chromosomal loci ‘linked’ to the trait, implying that those chromosomal regions harbor the variant or mutation of interest. These initial efforts are typically complimented by subsequent fine mapping studies to enable further localization. Identification of BRCA1, the gene harboring a mutation for familial breast cancer gene is an illustrative example. Linkage to early onset breast cancer was initially reported with polymorphisms on chromosome 17q21 (18). Soon thereafter, Miki et al identified the causal mutation in a gene located within this region, aptly named BRCA1 (19).
Relatively few linkage studies have targeted normal sleep related phenotypes, but the classical linkage approach has been exploited successfully to identify rare mutations and DNA markers, associated with specific sleep disorders, such as Delayed sleep phase syndrome (DSPS), Restless Legs syndrome (20) and narcolepsy (21). Mutations in the Period 2 (Per2) and the Casein Kinase Delta (CK1δ) gene that predispose to Familial advanced sleep phase syndrome (FASPS), a rare autosomal dominant disorder have also been identified (22), (23). The Per2 gene mutation also provides comparable phenotypes in a mouse model (24).
Association studies
Linkage studies have been very successful in mapping genes for rare Mendelian diseases, but they are limited by the availability of family based samples. They have also been less successful for diseases that are not inherited in a classical Mendelian fashion (25). For such traits, gene-mapping researchers have turned to association analyses. Association studies of genetically complex traits, which typically require larger samples, have also been facilitated by the availability of highly accurate, economical genotyping assays (1), (26). They involve comparisons between cases and controls with respect to selected polymorphisms. Like linkage studies, association studies also rely on correlation between closely spaced polymorphisms to help narrow chromosomal regions likely to harbor mutations. At the population level, the correlation is based on a phenomenon called Linkage Disequilibrium (LD). LD is influenced by recombination, as well as a number of phenomena impacted by population history, such as selection and genetic drift (Selection is the process by which certain traits, and the DNA variant encoding them are retained at greater than chance expectations. Genetic drift is the phenomenon whereby the frequency of a genetic variant gradually changes through succeeding generations, even in the absence of selection) (27). The researcher also has to be mindful of potential artifacts related to ethnic admixture. For example, if the ethnic background of the cases differs substantially from controls, then case-control differences noted for specific genetic polymorphisms may reflect such ethnic differences, rather than etiological relationships to case status. To control for such artifacts, family based association analyses have been advocated (28). Alternatively, corrections for ethnic admixture are possible among cases and unrelated controls using methods such as genomic control (29) or principal components approaches (30). Thus, the interpretation of a statistical association between a polymorphism and the trait of interest may be more complex and prone to artifacts than with linkage analyses. Nevertheless, recent advances summarized below in following sections have validated this approach.
Interpretation and follow up of association studies
Once association is initially detected, there are two important steps that need to be completed: replication of the initial result, and identification of the genetic variant primarily conferring risk. While replication is necessary in any biological research, it is particularly relevant in the gene-mapping context as the initial association is a probabilistic construct that requires independent verification. It is also important to note that the genetic variant initially found to be linked or associated with the trait in question might be in LD with an unanalyzed primary risk variant that should be more strongly linked or associated. Therefore, the follow up studies need to carefully evaluate associations with all other variants in the identified genomic region. In regions with high levels of LD, it may be difficult to identify the primary risk variant solely on the basis of the strength of the statistical association. It is therefore also necessary to identify a plausible biological function for the variant. Such functional analyses are also necessary before ascribing primacy for risk to a particular variant.
Candidate gene association studies
In the presence of random or near random mating, the extent of LD based correlations is typically limited to relatively small genomic regions (typically in the kilobase range, as opposed to LD across several megabases in some inbred populations) (12). Thus, several fold more polymorphisms are needed in order to survey the genome, in comparison with linkage studies. This not only increases cost, but also increases the likelihood of false positive results due to multiple comparisons. Therefore, many association studies focus on particular genes or sets of genes, based on prior evidence from other biological studies. This candidate gene approach is useful for disorders with known physiological bases. For example, the discovery of associations between Alzheimer’s disorder and the ApoE gene variants was motivated by prior physiological evidence, as well as linkage analyses (31).
Can association strategies be applied to identify genes for sleep related characteristics? Based on studies in other fields, there are grounds for optimism. However, several factors need to be kept in mind, though their individual impact is uncertain at the outset of any genetic study. They include genetic heterogeneity (the possibility that mutations in more than one gene may cause the same disease), and the impact of environmental factors.
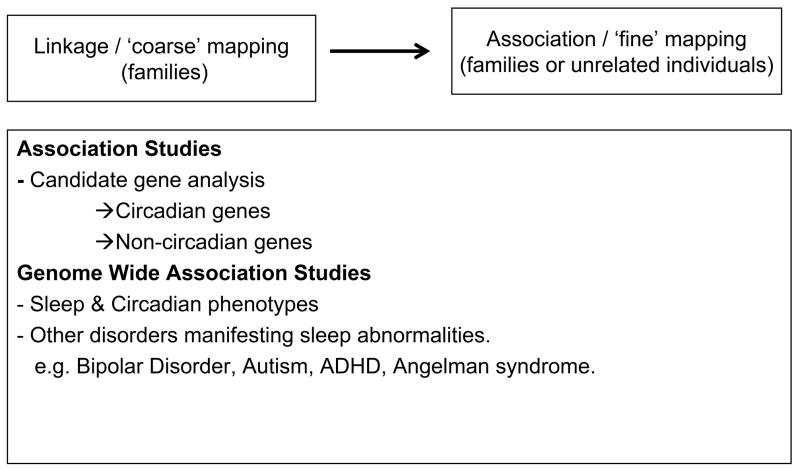
To date, a large number of studies in sleep genetics have utilized the candidate gene approach, with mixed results (Table 1, below). The majorities of the candidate gene studies have targeted circadian genes, based on well-known connections between circadian functions and sleep regulation in humans and model organisms (32). Among other factors, the success of this approach is based on a clear understanding of molecular pathways involved in sleep mechanism and explicit definition of phenotypes in human subjects. The studies based on circadian genes, as well as other association studies are reviewed below (see Figure 1).
Table 1.
Circadian gene variants investigated in sleep related traits and disorders.
| Gene | Polymorphism | Authors | Diurnal preference | DSPS | FASP |
|---|---|---|---|---|---|
| Clock | 3111C/T (3′UTR) | (66) | 3111C→E | ||
| (67) | + | ||||
| (68) | + | ||||
| (69) | − | − | |||
| (70) | − | ||||
| Per3 | Length repeat | (33) | (L →M; S →E) | + | |
| Haplotype composed of SNPs 5 | (40) | + | |||
| Timeless | * A2634G: Q831R | (71) | − | ||
| CKIepsilon binding region of hPer2 | * S662G | (22) | + | ||
| Per2 | C111G (5′UTR) (rs2304672) |
(72) | 111G → M | + | |
| Per1 | T2434C (exonic) | (73) | + | ||
| A2548G (exonic) | (74) | − | |||
| CKIepsilon | * S408N | (41) | Protective | ||
| Per3 | Exonic VNTR | (75) | + | ||
| Clock, Per2, Per3 & NPAS2 |
* NPAS2: L471 S * Period3: V647G |
(76) | + |
DSPS: Delayed Sleep Phase Syndrome; FASP: Familial Advanced Sleep-Phase; UTR: Untranslated Region. +: Nominally significant association detected; −: Association not detected; 3111C→E: Allele 3111C→ Preference for Eveningness; L→M: Longer allele→ Morningness; S→E: Shorter allele→ Eveningness; 111 G → M: Allele 111G→preference for Morningness.
causes amnio acid change
Figure 1.
Current and prospective gene mapping designs
Association studies involving circadian genes polymorphisms
The majority of published association studies involving sleep related measures have targeted circadian genes, presumably under the assumption that circadian variation may impact sleep. An exonic polymorphism of the circadian clock gene Per3 is instructive, as it has been investigated in relation to several physiological and pathological traits, including those related to circadian phase and sleep homeostasis. This polymorphism involves repetitive genomic sequences and is therefore called a “variable number of tandem repeat” (VNTR). It is likely to be important functionally, as the repeat motifs may lead to variations in the protein sequence of Per3. Archer and colleagues at the University of Surrey first noted that the Per3 VNTR is associated with diurnal preference, rated using the Horne-Ostberg scale for Morningness Eveningness (M/E) (33). Among 484 volunteers, they observed a nominally significant association between individuals at the extreme ends of the M/E score distribution (allelic associations, odds ratio, OR = 2.205, p = 0.047). Homozygosity for the 5-repeat allele (denoted as Per35/5) was associated with morning preference. These results were later reevaluated and corroborated by the same group using an enlarged sample. Among 80 individuals with extreme M/E scores selected from 1590 volunteers, they noted again that Per35/5 was associated with morningness (genotype-wise associations, OR = 0.53, 95% confidence intervals, CI, 0.33–0.85, p = 0.016) (34). The association was most evident among younger individuals. The investigators speculated that among younger individuals, Per3 genotypes might have a greater impact than exogenous environmental factors, in contrast to older individuals (34). Subsequently, the association between the Per35/5 and morningness was independently replicated in a smaller sample of Brazilian volunteers (n = 156, OR = 2.23, 95% CI 1.1–4.5) (35). In a series of subsequent studies, the group at the University of Surrey intensively investigated healthy participants selected on the basis of homozygosity for Per3 VNTR genotypes (Per35/5, n = 10; homozygous for the 4-repeat allele denoted as Per34/4, n = 14).
Homozygosity for the 5 repeat allele (Per35/5) was associated not only with circadian preference, but also with several markers of sleep structure and homeostasis: increased visually-scored slow-wave sleep (SWS, 22.7% in Per35/5 vs. 15.7% in Per34/4) and EEG slow wave activity in NREM sleep, and theta and alpha activity during wakefulness and REM sleep (36). In contrast, no significant differences in bed time, wake time or sleep duration were noted in this relatively small sample. Thus, genotype wise associations were noted on both sleep and waking EEG markers of sleep homeostasis. When the sleep homeostatic system was challenged by sleep deprivation, genotype-dependent differences in non-REM and REM sleep persisted (36). In addition, the decrement of cognitive performance in response to sleep loss was significantly greater among the Per35/5 individuals. On the other hand, the circadian rhythms of melatonin, cortisol and peripheral Per3 mRNA expression in lymphocytes were not affected. Later, the same group reported that the Per35/5 genotype significantly influenced the impact of sleep deprivation on measures of executive and non-executive function in the early morning hours (37). In addition, the Per35/5 individuals had less global heart rate variability (HRV) and genotypic variation significantly affected the sympathovagal balance in cardiac control of NREM sleep (38). These studies suggest that the Per3 VNTR is also related to sleep per se, and raise the additional possibility that it may also be associated with abnormal autonomic physiology during sleep.
In contrast to the Viola at al study, Goel et al compared Per3 genotypes and a variable denoted “equivalent inter-individual vulnerability to sleep restriction” (39). They observed that Per3 variation does not contribute to the neurobehavioral effects of chronic sleep loss. The differences between these studies could be due to smaller sample size recruited by Viola et al. Further analysis of this question is warranted.
Significant associations between the Per3 VNTR and DSPS have been noted by two other groups, with the 5-repeat allele being associated with increased risk in a University of Surrey study and the 4-repeat conferring risk in a Brazilian sample (33), (35). Earlier, a Japanese group reported only on haplotype based associations, i.e., groups of polymorphism tagging a chromosomal segment (40). In this study, the haplotype associated with DSPS carried a 4-repeat VNTR allele, consistent with the University of Surrey study. The differing associations between the United Kingdom and the Brazilian samples raise a number of possible interpretations. First, it is possible that there is no true genetic association between DSPS and the VNTR. Such a scenario does not, however, negate the associations with the other sleep related traits outlined above. Second, it is possible that an association with the VNTR does exist, but is impacted by additional variation at flanking polymorphisms. This possibility highlights the need to evaluate other Per3 polymorphisms in addition to the VNTR to enable haplotype-based associations.
Thus, the Per3 VNTR is associated with diurnal preference, but the associations have not been detected consistently. Smaller, unreplicated studies suggest that the Per35/5 homozygous state is also associated with several markers of sleep homeostasis, and may impact individual differences in the sleep-loss-induced decrement in performance. The latter associations may reflect an effect on sleep homeostasis. The molecular mechanisms for the associations are unknown, but may be related to alterations in phosphorylation of the Per3 protein product. These studies also illustrate the utility of a two-stage approach in which self-report and behavioral measures are used initially, followed by in-depth laboratory evaluations.
Associations with other circadian gene polymorphisms have also been reported (Table 1). Another interesting candidate gene of Period family is Per2. Toh et al(2001) have shown that DNA variation causing non-synonymous change in Ser 662 Glycine in human Per2 is associated with FASPS (22). A mis-sense mutation at the Casein Kinase 1 Epsilon (CK1ξ) gene may be protective against DSPS (41). Others have reported an association of DSPS with an exonic polymorphism of the arylalkylamine N-acetyltransferase (AANAT) gene, which is a rate-limiting enzyme in melatonin synthesis. However, some exploratory studies have not detected significant associations with sleep disorders at the melatonin 1a or 1b receptor gene (42). Another prominent example of circadian gene association with sleep is DEC2 gene. Recently He at al., identified a point mutation in the DEC2 gene segregating in a family with two affected members exhibiting short sleep times (14). They also demonstrated that rare the DNA variation in the gene DEC2 alters sleep duration in a transgenic mouse model.
Non-circadian genes implicated in sleep regulation
Very few studies have tested associations with genes that do not directly participate in the feedback loops regulating the molecular circadian clock, though some genes have been implicated from studies of model organisms. This is because not all the genetic components/players involved in regulating sleep are known. Most of the studies involving non-circadian genes were originally designed in Drosophila models; these studies merit investigations in humans. For example, the dopamine transporter (DAT, SLC6A3) may have a crucial role in sleep regulation, based on studies of knockout mice and Drosophila (43). Kume et al reported that DAT mutant drosophila have abnormally high levels of activity, reduced rest (sleep) and decreased arousal threshold (44). Using drosophila strains with mutant Serotonin receptor subtype- d5-HT1A receptor, Yuan et al reported reduced sleep amount and poor consolidation (45). These studies indicate a role for serotonin in promoting sleep through distinct subtypes of serotonin receptors. Immune related proteins may also promote sleep (46). In animal models, interleukin-2 and interlukin-15 alter the amplitude of EEG waves in NREM sleep (47). In Drosophila, Relish, a homologue of an immune response gene also alters sleep. Drosophila with reduced expression, but not lacking, Relish expression exhibited decreased levels of night time sleep (48). Indeed, a significant replicable association has been shown between narcolepsy and an immune response gene at the TCR locus in humans (21). Other studies using Drosophila mutants have implicated potassium channel homologues (Shaker) and its associated subunits (Hyperkinetic) in sleep regulation (49),(50). These investigators observed that mutants of Shaker and Hyperkinetic gene have reduced sleep amounts. Interestingly, Hyperkinetic mutants also showed impaired memory, indicating association between poor sleep and poor memory. Recently, Seugnet et al reported that insomnia-like (ins-l) gene is one of the regulators of sleep in Drosophila (51). They observed that ins-l flies have reduced sleep amount (60 minutes per day), show evidence of difficulty in initiating and maintaining sleep, and also show signs of daytime cognitive impairment. Other associations with sleep modulation have also been reported, e.g, SLEEPLESS (52), CREB (53), pKA (54) and EGF (55). Variations in human homologues of such genes may be worth analysis in genetic association studies.
Genome wide association studies (GWAS)
The key advantage of GWAS over candidate gene association studies is the simultaneous analysis of a set of representative polymorphisms across the entire genome. Thus, the relative impact of different genomic regions can be assessed simultaneously in the same sample. On the other hand, the likelihood of false positive results is high, if appropriate corrections for multiple corrections are not applied (56). False negative results are also possible in underpowered samples and loci with relatively small impact may thus be discarded incorrectly. Thus, DNA markers with ‘true’ associations may not be identified.
Currently available polymorphism arrays for GWAS do not include a representative set of rare variants. This has fueled on ongoing debate concerning the likely impact of rare versus common genetic variants on genetically complex traits (1), (57). The contribution of common or rare variants manifestly depends on the trait under consideration, but is impossible to predict their relative contributions when gene mapping studies are launched. Many gene mappers have opted for GWAS using common SNPs (i.e., those with minor allele frequencies ~ 1–5% or greater), because the sample sizes required to detect associations with such polymorphisms are smaller than the samples required to find relatively rare causative mutations. However, GWAS completed for many common diseases using common SNPs explain only a relatively small proportion of the genetic variance (58). Therefore, others have argued that GWAS using relatively rare mutations should now be conducted. Indeed, recent reports suggesting that relatively large and rare sub-microscopic chromosomal aberrations such as copy number variations (CNVs) may confer risk for many common diseases (59). Comprehensive evaluation of variants in gene exons using a genome wide ‘exome sequencing’ strategy is coming to the fore for the rare mutation strategy as it offers the opportunity of analyzing variants that are very likely to be functional (60). One drawback of the exome sequencing approach is that it does not include non-coding regions of the genomes that may also be functional, e.g., promoter sequences, other regulatory domains and variants impacting alternative splicing. The issue of common versus rare causative variants is likely to be resolved once comprehensive genome sequencing is attainable at reasonable cost.
Cost is a major concern regarding GWAS, as thousands of individuals may need to be analyzed eventually. Hence one could analyze sleep related variables collected opportunistically during other ongoing studies. This was the approach taken by a recent study by Gottleib and colleagues (61). Questionnaire data on sleep habits and sleepiness collected from participants in the Frammingham Heart Study offspring cohort were analyzed in conjunction with a GWAS conducted among 700 individuals. The design of the study also enabled simultaneous linkage analyses. Consistent with other studies, significant heritability was noted for sleep related traits. Suggestive association was reported between bedtime and a non-synonymous exonic single nucleotide polymorphism (SNP) at NPSR1. This non-synonymous SNP encodes a gain of function mutation of the Neuropeptide S receptor, whose endogenous ligand Neuropeptide S (NPS) is a potent promoter of wakefulness. The less frequent allele (variant) of this SNP was associated with delayed mean bedtime. The investigators also proposed that intronic variation in the Phosphodiesterase 4 (PDE4D) gene, a cAMP-specific phosphodiesterase might be associated with sleepiness. There is no direct evidence to prove a functional role of PDE4D, but its effect on intracellular levels of cAMP in brain might affect sleepiness. While the approach outlined by Gottlieb et al provides significant cost savings in terms of genotype assays, it is very important to replicate such results independently. Replication is a sine qua non for any genetic association study, but it is particularly important for retrospective analyses such as those used in the Frammingham Heart Study.
The cost-effective approach used by Gottlieb and colleagues could also be used for disorders that manifest with sleep abnormalities (61). GWAS have already been reported for several such disorders, including Restless leg syndrome (RLS) (20), Attention deficit disorder, Autism and Bipolar disorder (1). Associations between Attention-deficit/hyperactivity disorder (ADHD) and polymorphisms of two circadian genes, namely Per3 and Brain-derived neurotrophic factor (BDNF) have already been reported (62), (63).
The associations with RLS are noteworthy because this periodic limb movement disorder is also associated with sleep disruption. Importantly, the RLS studies also indicate that it is possible to find plausible genetic associations with self reported measures. While linkage studies of RLS did not provide insights about causative variants, the recent GWAS have highlighted SNPs that may have functional effects (20), (64). Using postmortem brain samples and lymphoblastoid cell lines from subjects, Xiong et al observed that haplotypes of associated intronic markers of the gene MEIS1 contributed to reduced expression of this gene. MEIS1 is known to be involved in a transcriptional regulatory pathway that helps to establish motor neuron pool, connectivity and limb development in model animals (64).
Another disorder of interest is Angelman syndrome (AS). Approximately 20–80% of patients with this Mendelian neurodevelopmental disorder have a wide array of sleep problems, including decreased sleep time and abnormal sleep-wake phases (65). As the causative mutations for AS have been localized to chromosome 15q11–q13, genes localized to this region may well modulate sleep.
Conclusions
Sleep and circadian rhythms are complex and inter-connected physiological processes. Relative to the remarkable progress made in identifying the genetic basis of circadian rhythms and some specific sleep disorders, efforts to identify genetic variants associated with normal variation in sleep have progressed more slowly. Gene mapping studies of normal sleep variation, as well as sleep related disorders may provide novel insights into the mechanisms regulating sleep. The design for gene mapping studies depends critically on the traits of interest. Genetic association studies have come to the fore to study genetically complex traits. They may be suitable for sleep related studies. Candidate gene association studies have been popular for other disorders with well-established physiological bases. Such studies have been conducted in relation to sleep, but have tended to focus on circadian genes. Association of other plausible genes that are not obviously related to circadian function may be worthwhile. As GWAS enable analysis of representative variants across the genome, they have obvious appeal for variables for which the biological basis is not understood well. GWAS may therefore provide useful information for sleep related traits, provided attention is paid to quality control and analytic issues. It may not be possible to conduct GWAS in relation to certain sleep related traits that require time consuming evaluations in the sleep laboratory. Such traits may be reserved for in depth analysis once appropriate genes have been identified using proxy variables, such as self-report behavioral measures. In view of the cost of GWAS, it may be economical to analyze sleep related traits already collected from participants in completed or ongoing GWAS not only for sleep related abnormalities but also among other studies that have overlapping sleep related traits in them.
Practice points
Gene mapping studies could be used to:
- identify genetic causes of heritable sleep disorders.
- identify persons at increased risk for such disorders.
- help us to understand the biological basis of normal sleep variation.
Research agenda
- Gene mapping studies should focus on normal sleep variation, in addition to sleep disorders.
- Whole genome association studies (GWAS) may be pertinent for understanding mechanisms governing sleep.
- In view of their cost, it may be worthwhile to analyze sleep related variables available as part of GWAS that have been completed for disorders with concurrent sleep abnormalities.
Glossary of Terms
- PSG
Polysomnography
- EEG
Electroencephalography
- NREM
Non-rapid eye movement
- REM
Rapid eye movement
- BRCA1
Breast Cancer 1, gene
- Per2
Period 2, gene
- CK1δ
Casein Kinase Delta, gene
- DSPS
Delayed sleep phase syndrome
- FASPS
Familial advanced sleep phase syndrome
- LD
Linkage Disequilibrium
- APOE
Apolipoprotein E, gene
- Per3
Period 3, gene
- VNTR
Variable number of tandem repeat
- M/E
morningness/eveningness
- OR
Odds ratio
- CI
Confidence Interval
- SWS
Slow wave sleep
- HRV
Heart rate variability
- CK1ξ
Casein Kinase 1 Epsilon, gene
- AANAT
Arylalkylamine N-acetyltransferase, gene
- DEC2
Differentially Expressed in Chondrocytes 2, gene
- HLA DR1
Human leukocyte antigen DR1
- DAT
Dopamine transporter, gene
- SLC6A3
Solute carrier family 6 member 3, gene
- TCR
T-cell Receptor
- ins-l
insomnia-like, gene
- cAMP
cyclic adenosine monophosphate
- CREB
cAMP Response ElemenT-Binding Protein, gene
- pKA
Protein Kinase A-interacting protein, gene
- EGF
Epidermal Growth Factor, gene
- GWAS
Genome wide association studies
- CNV
Copy number variation
- NPSR1
Neuropeptide S receptor 1, gene
- NPS
Neuropeptide S
- SNP
Single nucleotide polymorphism
- PDE4D
Phosphodiesterase 4, gene
- BDNF
Brain-derived neurotrophic factor, gene
- RLS
Restless leg syndrome
- ADHD
Attention-deficit/hyperactivity disorder
- AS
Angelman syndrome
- MEIS1
Myeloid ecotropic viral integration site 1 homolog
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mikhil N. Bamne, Email: bamnemn@upmc.edu, Department of Psychiatry, 441 Western Psychiatric Institute and Clinic, 3811 O’Hara St. University of Pittsburgh, School of Medicine, Pittsburgh PA-15213.USA. Phone: 412-246-6354. fax:412-246-650
Hader Mansour, Email: manseurha@upmc.edu, Department of Psychiatry, 441 Western Psychiatric Institute and Clinic, 3811 O’Hara St. University of Pittsburgh, School of Medicine, Pittsburgh PA-15213. USA. Phone: 412-246-6348. fax:412-246-650
Timothy H. Monk, Email: monkth@upmc.edu, Sleep and Chronobiology Center, Department of Psychiatry, Western Psychiatric Institute and Clinic, 3811 O’Hara St. University of Pittsburgh, School of Medicine, Pittsburgh PA-15213. Phone: 412-2 46-6401. fax: 412) 246-5300
Daniel J. Buysse, Email: buyssedj@upmc.edu, Sleep and Chronobiology Center, Department of Psychiatry, Western Psychiatric Institute and Clinic, 3811 O’Hara St. University of Pittsburgh, School of Medicine, Pittsburgh PA-15213. Phone: 412-246-6413 fax: 412-246-5300.
Vishwajit L. Nimgaonkar, Email: nimga@upmc.edu, Department of Psychiatry & Human Genetics, 441 Western Psychiatric Institute and Clinic, 3811 O’Hara St. University of Pittsburgh, School of Medicine, Pittsburgh PA-15213. Phone: 412-246-6353 fax: 412-246-6350
References
- 1.Altshuler D, Daly MJ, Lander ES. Genetic mapping in human disease. Science. 2008;322(5903):881–8. doi: 10.1126/science.1156409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw PJ, Franken P. Perchance to dream: solving the mystery of sleep through genetic analysis. J Neurobiol. 2003;54(1):179–202. doi: 10.1002/neu.10167. [DOI] [PubMed] [Google Scholar]
- 3.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 4.Nofzinger EA. Functional neuroimaging of sleep. Semin Neurol. 2005;25(1):9–18. doi: 10.1055/s-2005-867070. [DOI] [PubMed] [Google Scholar]
- 5.Ohayon MM. From wakefulness to excessive sleepiness: what we know and still need to know. Sleep Med Rev. 2008;12(2):129–41. doi: 10.1016/j.smrv.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90(8):4510–5. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- 7.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–80. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andretic R, Franken P, Tafti M. Genetics of sleep. Annu Rev Genet. 2008;42:361–88. doi: 10.1146/annurev.genet.42.110807.091541. [DOI] [PubMed] [Google Scholar]
- 9.Luciano JV, Algarabel S. Individual differences in self-reported thought control: the role of the repressive coping sytle. Psicothema. 2006;18(2):228–31. [PubMed] [Google Scholar]
- 10.de Castro JM. The influence of heredity on self-reported sleep patterns in free-living humans. Physiol Behav. 2002;76(4–5):479–86. doi: 10.1016/s0031-9384(02)00699-6. [DOI] [PubMed] [Google Scholar]
- 11.Gregory AM, Rijsdijk FV, Eley TC. A twin-study of sleep difficulties in school-aged children. Child Dev. 2006;77(6):1668–79. doi: 10.1111/j.1467-8624.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- 12.Klei L, Reitz P, Miller M, Wood J, Maendel S, Gross D, et al. Heritability of morningness-eveningness and self-report sleep measures in a family based sample of 521 Hutterites. Chronobiology International. 2005;22(6):1041–1054. doi: 10.1080/07420520500397959. [DOI] [PubMed] [Google Scholar]
- 13.Dauvilliers Y, Morin C, Cervena K, Carlander B, Touchon J, Besset A, et al. Family studies in insomnia. J Psychosom Res. 2005;58(3):271–8. doi: 10.1016/j.jpsychores.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Ying He CRJ, Fujiki Nobuhiro, Xu Ying, Guo Bin, Holder Jimmy L, Jr, Rossner Moritz J, Nishino Seiji, Fu Ying-Hui. The Transcriptional Repressor DEC2 Regulates Sleep Length in Mammals. Science. 2009;325(5942):866–870. doi: 10.1126/science.1174443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visscher PM. Sizing up human height variation. Nat Genet. 2008;40(5):489–90. doi: 10.1038/ng0508-489. [DOI] [PubMed] [Google Scholar]
- 16.Buysse D. Insomnia state of the science: an evolutionary, evidence-based assessment. Sleep. 2005;28(9):1045–6. [PubMed] [Google Scholar]
- 17.Collins A, Morton NE. Mapping a disease locus by allelic association. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(4):1741–5. doi: 10.1073/pnas.95.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall JM, Lee MK, Newman B, Morrow JE, Anderson LA, Huey B, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250(4988):1684–9. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- 19.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 20.Winkelmann J, Schormair B, Lichtner P, Ripke S, Xiong L, Jalilzadeh S, et al. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39(8):1000–6. doi: 10.1038/ng2099. [DOI] [PubMed] [Google Scholar]
- 21.Hallmayer J, Faraco J, Lin L, Hesselson S, Winkelmann J, Kawashima M, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41(6):708–11. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291(5506):1040–3. doi: 10.1126/science.1057499. Order. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Padiath QS, Shapiro RE, Jones CR, Wu SC, Saigoh N, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434(7033):640–4. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 24.Franken P, Thomason R, Heller HC, O’Hara BF. A non-circadian role for clock-genes in sleep homeostasis: a strain comparison. BMC Neurosci. 2007;8:87. doi: 10.1186/1471-2202-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merikangas KR, Spence MA, Kupfer DJ. Linkage studies of bipolar disorder: methodologic and analytic issues. Report of MacArthur Foundation Workshop on Linkage and Clinical Features in Affective Disorders. Archives of General Psychiatry. 1989;46(12):1137–41. doi: 10.1001/archpsyc.1989.01810120079012. [DOI] [PubMed] [Google Scholar]
- 26.Chiang DY, Getz G, Jaffe DB, O’Kelly MJ, Zhao X, Carter SL, et al. High-resolution mapping of copy-number alterations with massively parallel sequencing. Nat Methods. 2009;6(1):99–103. doi: 10.1038/nmeth.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavalli-Sforza LL, Menozzi P, Piazza A. The history and geography of human genes. Princeton: Princeton University Press; 1994. [Google Scholar]
- 28.Spielman RS, Ewens WJ. The TDT and other family-based tests for linkage disequilibrium and association [editorial] American Journal of Human Genetics. 1996;59(5):983–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Devlin B, Bacanu SA, Roeder K. Genomic Control to the extreme. Nat Genet. 2004;36(11):1129–30. doi: 10.1038/ng1104-1129. [DOI] [PubMed] [Google Scholar]
- 30.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 31.Weisgraber KH, Mahley RW. Human apolipoprotein E: the Alzheimer’s disease connection. Faseb J. 1996;10(13):1485–94. doi: 10.1096/fasebj.10.13.8940294. [DOI] [PubMed] [Google Scholar]
- 32.Curtis AM, Fitzgerald GA. Central and peripheral clocks in cardiovascular and metabolic function. Ann Med. 2006;38(8):552–9. doi: 10.1080/07853890600995010. [DOI] [PubMed] [Google Scholar]
- 33.Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26(4):413–5. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 34.Jones KH, Ellis J, von Schantz M, Skene DJ, Dijk DJ, Archer SN. Age-related change in the association between a polymorphism in the PER3 gene and preferred timing of sleep and waking activities. J Sleep Res. 2007;16(1):12–6. doi: 10.1111/j.1365-2869.2007.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereira DS, Tufik S, Louzada FM, Benedito-Silva AA, Lopez AR, Lemos NA, et al. Association of the length polymorphism in the human Per3 gene with the delayed sleep-phase syndrome: does latitude have an influence upon it? Sleep. 2005;28(1):29–32. [PubMed] [Google Scholar]
- 36.Viola AU, Archer SN, James LM, Groeger JA, Lo JC, Skene DJ, et al. PER3 Polymorphism Predicts Sleep Structure and Waking Performance. Curr Biol. 2007;17(7):613–8. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 37.Groeger JA, Viola AU, Lo JC, von Schantz M, Archer SN, Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. 2008;31(8):1159–67. [PMC free article] [PubMed] [Google Scholar]
- 38.Viola AU, James LM, Archer SN, Dijk DJ. PER3 polymorphism and cardiac autonomic control: effects of sleep debt and circadian phase. Am J Physiol Heart Circ Physiol. 2008;295(5):H2156–63. doi: 10.1152/ajpheart.00662.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goel N, Banks S, Mignot E, Dinges DF. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS One. 2009;4(6):e5874. doi: 10.1371/journal.pone.0005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebisawa T, Uchiyama M, Kajimura N, Mishima K, Kamei Y, Katoh M, et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2001;2(4):342–6. doi: 10.1093/embo-reports/kve070. Order. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takano A, Uchiyama M, Kajimura N, Mishima K, Inoue Y, Kamei Y, et al. A missense variation in human casein kinase I epsilon gene that induces functional alteration and shows an inverse association with circadian rhythm sleep disorders. Neuropsychopharmacology. 2004;29(10):1901–9. doi: 10.1038/sj.npp.1300503. [DOI] [PubMed] [Google Scholar]
- 42.Ebisawa T, Uchiyama M, Kajimura N, Kamei Y, Shibui K, Kim K, et al. Genetic polymorphisms of human melatonin 1b receptor gene in circadian rhythm sleep disorders and controls. Neurosci Lett. 2000;280(1):29–32. doi: 10.1016/s0304-3940(99)00981-7. [DOI] [PubMed] [Google Scholar]
- 43.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21(5):1787–94. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25(32):7377–84. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16(11):1051–62. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 46.Krueger JM, Majde JA. Humoral links between sleep and the immune system: research issues. Ann N Y Acad Sci. 2003;992:9–20. doi: 10.1111/j.1749-6632.2003.tb03133.x. [DOI] [PubMed] [Google Scholar]
- 47.Kubota T, Brown RA, Fang J, Krueger JM. Interleukin-15 and interleukin-2 enhance non-REM sleep in rabbits. Am J Physiol Regul Integr Comp Physiol. 2001;281(3):R1004–12. doi: 10.1152/ajpregu.2001.281.3.R1004. [DOI] [PubMed] [Google Scholar]
- 48.Williams JA, Sathyanarayanan S, Hendricks JC, Sehgal A. Interaction between sleep and the immune response in Drosophila: a role for the NFkappaB relish. Sleep. 2007;30(4):389–400. doi: 10.1093/sleep/30.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, et al. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434(7037):1087–92. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- 50.Bushey D, Huber R, Tononi G, Cirelli C. Drosophila Hyperkinetic mutants have reduced sleep and impaired memory. J Neurosci. 2007;27(20):5384–93. doi: 10.1523/JNEUROSCI.0108-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seugnet L, Suzuki Y, Thimgan M, Donlea J, Gimbel SI, Gottschalk L, et al. Identifying sleep regulatory genes using a Drosophila model of insomnia. J Neurosci. 2009;29(22):7148–57. doi: 10.1523/JNEUROSCI.5629-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koh K, Joiner WJ, Wu MN, Yue Z, Smith CJ, Sehgal A. Identification of SLEEPLESS, a sleep-promoting factor. Science. 2008;321(5887):372–6. doi: 10.1126/science.1155942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20(24):9187–94. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu MN, Ho K, Crocker A, Yue Z, Koh K, Sehgal A. The effects of caffeine on sleep in Drosophila require PKA activity, but not the adenosine receptor. J Neurosci. 2009;29(35):11029–37. doi: 10.1523/JNEUROSCI.1653-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC, et al. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294(5551):2511–5. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- 56.Pearson TA, Manolio TA. How to interpret a genome-wide association study. Jama. 2008;299(11):1335–44. doi: 10.1001/jama.299.11.1335. [DOI] [PubMed] [Google Scholar]
- 57.Dermitzakis ET, Clark AG. Genetics. Life after GWA studies. Science. 2009;326(5950):239–40. doi: 10.1126/science.1182009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cook EH, Jr, Scherer SW. Copy-number variations associated with neuropsychiatric conditions. Nature. 2008;455(7215):919–23. doi: 10.1038/nature07458. [DOI] [PubMed] [Google Scholar]
- 60.Ng SB, Buckingham KJ, Lee C, Bigham AW, Tabor HK, Dent KM, et al. Exome sequencing identifies the cause of a mendelian disorder. Nat Genet. 2009 doi: 10.1038/ng.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gottlieb DJ, O’Connor GT, Wilk JB. Genome-wide association of sleep and circadian phenotypes. BMC Med Genet. 2007;8 (Suppl 1):S9. doi: 10.1186/1471-2350-8-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lasky-Su J, Neale BM, Franke B, Anney RJ, Zhou K, Maller JB, et al. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1345–54. doi: 10.1002/ajmg.b.30867. [DOI] [PubMed] [Google Scholar]
- 63.Neale BM, Lasky-Su J, Anney R, Franke B, Zhou K, Maller JB, et al. Genome-wide association scan of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1337–44. doi: 10.1002/ajmg.b.30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiong L, Catoire H, Dion P, Gaspar C, Lafreniere RG, Girard SL, et al. MEIS1 intronic risk haplotype associated with restless legs syndrome affects its mRNA and protein expression levels. Hum Mol Genet. 2009;18(6):1065–74. doi: 10.1093/hmg/ddn443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Didden R, Korzilius H, Smits MG, Curfs LM. Sleep problems in individuals with Angelman syndrome. Am J Ment Retard. 2004;109(4):275–84. doi: 10.1352/0895-8017(2004)109<275:SPIIWS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 66.Katzenberg D, Young T, Finn L, Lin L, King DP, Takahashi JS, et al. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998;21(6):569–76. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- 67.Serretti A, Benedetti F, Mandelli L, Lorenzi C, Pirovano A, Colombo C, et al. Genetic dissection of psychopathological symptoms: insomnia in mood disorders and CLOCK gene polymorphism. Am J Med Genet. 2003;121B(1):35–8. doi: 10.1002/ajmg.b.20053. [DOI] [PubMed] [Google Scholar]
- 68.Mishima K, Tozawa T, Satoh K, Saitoh H, Mishima Y. The 3111T/C polymorphism of hClock is associated with evening preference and delayed sleep timing in a Japanese population sample. Am J Med Genet B Neuropsychiatr Genet. 2005;133B(1):101–4. doi: 10.1002/ajmg.b.30110. [DOI] [PubMed] [Google Scholar]
- 69.Robilliard DL, Archer SN, Arendt J, Lockley SW, Hack LM, English J, et al. The 3111 Clock gene polymorphism is not associated with sleep and circadian rhythmicity in phenotypically characterized human subjects. J Sleep Res. 2002;11(4):305–12. doi: 10.1046/j.1365-2869.2002.00320.x. [DOI] [PubMed] [Google Scholar]
- 70.Iwase T, Kajimura N, Uchiyama M, Ebisawa T, Yoshimura K, Kamei Y, et al. Mutation screening of the human Clock gene in circadian rhythm sleep disorders. Psychiatry Res. 2002;109(2):121–8. doi: 10.1016/s0165-1781(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 71.Pedrazzoli M, Ling L, Finn L, Kubin L, Young T, Katzenberg D, et al. A polymorphism in the human timeless gene is not associated with diurnal preferences in normal adults. Sleep Res Online. 2000;3(2):73–6. [PubMed] [Google Scholar]
- 72.Carpen JD, Archer SN, Skene DJ, Smits M, von Schantz M. A single-nucleotide polymorphism in the 5′-untranslated region of the hPER2 gene is associated with diurnal preference. J Sleep Res. 2005;14(3):293–7. doi: 10.1111/j.1365-2869.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- 73.Carpen JD, von Schantz M, Smits M, Skene DJ, Archer SN. A silent polymorphism in the PER1 gene associates with extreme diurnal preference in humans. J Hum Genet. 2006;51(12):1122–5. doi: 10.1007/s10038-006-0060-y. [DOI] [PubMed] [Google Scholar]
- 74.Katzenberg D, Young T, Lin L, Finn L, Mignot E. A human period gene (HPER1) polymorphism is not associated with diurnal preference in normal adults. Psychiatr Genet. 1999;9(2):107–9. doi: 10.1097/00041444-199906000-00011. [DOI] [PubMed] [Google Scholar]
- 75.Jones KH, Ellis J, von Schantz M, Skene DJ, Dijk DJ, Archer SN. Age-related change in the association between a polymorphism in the PER3 gene and preferred timing of sleep and waking activities. J Sleep Res. 2007;16(1):12–16. doi: 10.1111/j.1365-2869.2007.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johansson C, Willeit M, Smedh C, Ekholm J, Paunio T, Kieseppa T, et al. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology. 2003;28(4):734–9. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]