Abstract
By altering chromatin structure, histone acetyltransferases (HATs) act as transcriptional regulators. We observed in a model of primary neurons that histone acetylation levels decreased at the onset of apoptosis. The CREB-binding protein (CBP) is a HAT of particular interest because it also acts as a co-activator controlling, among others, CREB-dependent transcriptional activity. It has been demonstrated that CREB exerts neuroprotective functions, but the fate of CBP during neuronal apoptosis remained unclear till now. This work provided evidence that CBP is specifically targeted by caspases and calpains at the onset of neuronal apoptosis, and CBP was futher identified as a new caspase-6 substrate. This ultimately impinged on the CBP/p300 HAT activity that decreased with time during apoptosis entry, whereas total cellular HAT activity remained unchanged. Interestingly, CBP loss and histone deacetylation were observed in two different pathological contexts: amyloid precursor protein-dependent signaling and amyotrophic lateral sclerosis model mice, indicating that these modifications are likely to contribute to neurodegenerative diseases. In terms of function, we demonstrated that fine-tuning of CBP HAT activity is necessary to ensure neuroprotection.
Keywords: apoptosis/caspase-6/CBP/HAT/neurodegeneration
Introduction
Apoptosis is a form of programmed cell death that primarily occurs physiologically during development and in the adult, guiding the fate of individual cells or organs (Brill et al., 1999). Nevertheless, it is thought that apoptosis contributes to neuronal loss during neurodegenerative diseases, and several studies have shown cellular hallmarks of apoptosis in diseases such as Alzheimer’s disease (AD) or amyotrophic lateral sclerosis (ALS) (Honig and Rosenberg, 2000). Apoptosis is an active cell death characterized by classical morphological and biochemical changes (McConkey, 1998) operated by the caspase family of enzymes. They are responsible for irreversible protein degradations implicated in neuroprotection (Honig and Rosenberg, 2000).
By definition, programmed cell death occurs with transcriptional modifications leading to activation of pro-apoptotic genes, and repression of genes involved in neuroprotection. Transcription/translation inhibitors prevent or delay apoptosis in response to a wide range of insults (Schulz et al., 1996; D’Mello, 1998). However, the fine mechanisms of transcriptional regulation occurring during apoptosis are still obscure, particularly because the global nuclear condensation observed during apoptosis should lead to a broad transcriptional repression. Indeed, the nucleosomal structure limits transcriptional activation (Cheung et al., 2000), and mechanisms of post-translational chromatin modifications such as histone acetylation exist to counteract the repressive nucleosomal environment (Th’ng, 2001). The idea that histone acetylations were directly associated with transcriptional regulation has emerged in recent years (Gregory et al., 2001). These particular post-translational modifications occur on conserved lysine residues of the N-terminal tails of core histones, and induce chromatin opening by perturbing higher order chromatin folding. Histone acetylations are performed by histone acetyltransferases (HATs) (Gregory et al., 2001) and the reversed deacetylation process by histone deacetylases (HDACs) (De Ruijter et al., 2003). Therefore, by affecting chromatin folding, HATs and HDACs control both DNA accessibility and transcriptional activation (Eberharter and Becker, 2002). Moreover, these enzymes can also acetylate/deacetylate non-histone proteins such as transcription factors, thus adding another connection to transcriptional regulation (Gregory et al., 2001; De Ruijter et al., 2003).
So far, different HAT families have been characterized, among which notably the global co-activators CBP and p300, the GNAT superfamily, which includes the PCAF protein (CBP/p300-associated factor), the MYST family, nuclear receptor co-activators, TAF II p250 [TATA box-binding protein (TBP)-associated factor II p250] and the TFIIIC family, more related to transcriptional initiation (for a review see Sterner and Berger, 2000). When studying neuronal apoptosis, the CREB-binding protein (CBP) (Chrivia et al., 1993; Kwok et al., 1994; Bannister and Kouzarides, 1996) is of particular interest. Not only does CBP display HAT functions, making it a potential key player in chromatin availability for transcription, but it also acts as a transcriptional co-activator (Chan and La Thangue, 2001; Vo and Goodman, 2001) by binding phospho-CREB, a transcription factor largely implicated in neuroprotection (Lonze and Ginty, 2002; Lonze et al., 2002). Several observations suggest a role for CBP in central nervous system (CNS) development: cbp homozygous mutant mice show complete embryonic lethality and display open neural tube defects (Yao et al., 1998) and mutations within the cbp gene have been detected in Rubinstein–Taybi patients displaying CNS abnormalities (Petrij et al., 1995; Kalkhoven et al., 2003).
In this report, we investigated the interesting possibility that CBP participates in neuroprotection. We therefore examined the fate of CBP in different models of neuronal cell death: an in vitro model of K+-deprived cerebellar granule neuron (CGN) apoptosis (D’Mello et al., 1993; Boutillier et al., 1999), an in vitro model of cortical neurons in which cell death is induced by activation of the amyloid precursor protein (APP) signaling pathway (Mbebi et al., 2002), and an in vivo model of ALS: the G86R mutant SOD-1 mice displaying motoneuron degeneration (Ripps et al., 1995). We show here that apoptosis is specifically accompanied by a decrease in CBP/p300 proteins, followed by a global histone deacetylation. We demonstrated that CBP can be targeted by caspases and calpains during the onset of neuronal apoptosis, and futher identified it as a new caspase-6 substrate. Interestingly, CBP/p300 overexpression studies showed that they display neuroprotective functions, in part through their HAT domain. More particularly, loss of CBP and a subsequent decrease in histone acetylation were found in the three models under investigation, underlying common traits in neurodegenerative processes.
Results
Histones are deacetylated during the onset of neuronal apoptosis
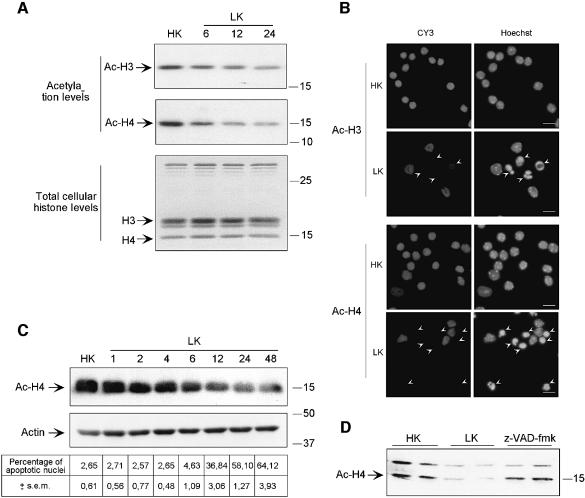
Acetylated histone levels were followed by western blot analysis during neuronal apoptosis. Acetylated H3 (Ac-H3) and H4 (Ac-H4) histone levels progressively decreased during K+ deprivation of CGNs (LK) (Figure 1A, upper panels), whereas total amounts of cellular histones were not modified (Figure 1A, lower panel). This acetylation decrease was confirmed further by immunostaining and Hoechst labeling (Figure 1B). In survival conditions (HK), nuclei that appeared large and round shaped displayed a bright Ac-H3 and Ac-H4 immunoreactivity. In apoptotic conditions (LK), condensed or fragmented nuclei displayed little or no Ac-H3 and Ac-H4 immunostaining. To investigate whether histone deacetylation was correlated with apoptosis entry, we performed a more precise time course of K+ deprivation on H4 histone acetylation status coupled to an evaluation of apoptotic nuclei. Figure 1C shows that histone deacetylation occurred prior to nuclear condensation, since Ac-H4 levels clearly decreased at 6 h of LK treatment, a time at which no significant changes in the percentage of apoptotic nuclei were detectable. It is noteworthy that acetylated H4 levels are even slightly lower than control at 2 and 4 h of LK treatment. Interestingly, Ac-H4 deacetylation was partly reversed by the general caspase inhibitor z-VAD-fmk (Figure 1D). Altogether, these results show that histone deacetylation is an early event within the apoptotic pathway, that occurs through caspase-dependent mechanisms.
Fig. 1. Acetylation levels decrease at the onset of apoptosis, by a caspase-dependent mechanism. (A) Cerebellar granule neurons (CGNs) were K+ deprived (LK) during 6–24 h, or maintained in survival conditions (HK). Histone acetylation levels were monitored by western blot analysis using specific antibodies directed against the acetylated forms of H3 histone (Ac-H3) and H4 histone (Ac-H4) (upper panel). The total amount of histones was controlled after revelation of acid-extracted histone proteins on a Coomassie blue-stained gel (lower panel). The results shown are representative of five independent experiments. (B) Representative examples of Ac-H3 and Ac-H4 immunostainings and Hoechst staining. Arrows indicate condensed or fragmented nuclei; scale bar = 10 µm. (C) Correlation between histone acetylation decrease and percentage of apoptotic nuclei. Ac-H4 forms were analyzed by western blot after K+ deprivation (LK) during short (1, 2, 4 and 6 h) and long (12, 24 and 48 h) periods of time or maintained in survival conditions (HK). The percentage of apoptotic nuclei was evaluated at each time point by scoring nuclei presenting chromatin condensation or nuclear fragmentation after Hoechst labeling (lower panel). Data shown are representative of three independent experiments. (D) Ac-H4 immunoblot was performed on protein extracts from K+-deprived CGNs (LK) during 15 h, treated or not with the general caspase inhibitor z-VAD-fmk (50 µM) or maintained in survival conditions (HK). Results shown in duplicate are representative of three independent experiments.
CBP/p300 HAT activity specifically decreases during apoptosis
To investigate the possible cause of histone deacetylation during neuronal apoptosis, we investigated the fate of different HAT families by western blot analyses. Four HATs were tested: CBP, p300, TAF II p250 and PCAF. We found a strong decrease of CBP levels as soon as 6 h of LK treatment (Figure 2A). p300 levels also dropped between 6 and 12 h of K+ deprivation (Figure 2A). In contrast, neither TAF II p250 nor PCAF levels were changed upon K+ deprivation (Figure 2A), suggesting a selective decrease of the CBP/p300 HAT family of proteins during apoptosis. We then evaluated the consequences of CBP/p300 loss on either CBP/p300 HAT activity or global HAT activity (Figure 2B). The assay was tuned using a recombinant HAT protein, CBP (a generous gift from Dr J.R.Lundblad, Portland, OR), as positive control. CBP/p300 HAT activity decreased by 73% after 12 h of K+ deprivation and by 88% after 24 h, a decrease partly reversible by z-VAD-fmk (Figure 2B). Interestingly, global HAT activity was not altered by LK treatment (Figure 2B). This could probably be reflected by the fact that some HATs such as TAF II p250 can acetylate histones in an in vitro assay (Kuo and Allis, 1998), whereas they would have less affinity in living cells, when nucleosomal histones are in the chromatin context. Altogether, these results show a specific loss of CBP/p300 during neuronal apoptosis by a caspase-dependent mechanism, resulting in a drop in their HAT activity which could be responsible for histone deacetylation.
Fig. 2. CBP/p300 HAT activity specifically decreases during apoptosis. (A) CBP, p300, TAF II p250 and PCAF levels were monitored on protein extracts prepared from K+-deprived CGNs (LK) for 6–48 h or not (HK). Actin was used as internal control for loading. Results shown are representative of four independent experiments. (B) HAT activity was determined on extracts from CGNs treated or not with LK (12 and 24 h), or LK/z-VAD-fmk (12 h, 50 µM) after HAT immunoprecipitation with a mixture of antibodies recognizing CBP and p300 proteins (black histograms) or not (gray histograms). A control for HAT activity was performed with 100 ng of recombinant CBP protein (open histogram). Data from three independent experiments performed in duplicate represent means ± SEM. ***P < 0.001 (versus HK) and ###P < 0.001 (versus LK).
CBP is degraded during apoptosis
Further investigations were performed on CBP, that is potentially involved in neuroprotection. Semi-quantitative RT–PCR analysis revealed that cbp expression was not modified during K+ deprivation relative to the housekeeping gene g3ph (Figure 3A), suggesting that the decrease of CBP levels was likely to be due to a proteolytic processing. We therefore tested different protease inhibitors on CGN for their ability to reverse CBP degradation (Figure 3B). The proteasome inhibitor PSI had no effect on CBP levels, whereas z-VAD-fmk partly reversed the CBP decrease. The calpain inhibitor I, ALLN, did not reverse CBP degradation but allowed us to show a cleavage fragment migrating at an apparent mol. wt of ∼175 kDa (CBP-I, Figure 3B). The use of a CBP N-terminus-directed antibody in these conditions allowed the detection of another cleavage fragment migrating at ∼130 kDa (CBP-II, data not shown). These results suggest that CBP might be cleaved by caspases during apoptosis, giving two cleavage fragments further processed by calpains.

Fig. 3. The CBP protein is cleaved by caspases and further processed by calpains. (A) cbp and g3pdh semi-quantitative RT–PCR analysis was performed on total RNA extracts isolated from CGNs after a K+ deprivation time course. (B) CBP western blot performed on K+-deprived (LK, 15 h) CGNs in the presence or absence PSI (1 μM), z-VAD-fmk (50 μM) or ALLN (5 μM), or CGNs maintained in neuroprotective conditions (HK). ALLN allows the detection of a cleavage fragment, CBP-I, migrating at an apparent mol. wt of 175 kDa. Results are representative of at least four independent experiments.
CBP is a caspase-6 substrate
To identify further the caspase(s) involved in CBP proteolysis during apoptosis, we investigated caspase activities using fluorogenic substrates. Caspase-3 is the main executioner caspase of the CNS and more particularly of CGN apoptosis (D’Mello, 1998; Boutillier et al., 2000). Caspase-6 was described as an initiator caspase, responsible for caspase-3 activation in K+/serum-deprived CGNs (Allsopp et al., 2000). We found that caspase-6 was moderately but rapidly activated (1 h) after K+ deprivation (Figure 4A). As expected, caspase-3 was strongly activated at later time points. Specific inhibitors for these caspases were then tested on CGN for their ability to reverse CBP degradation (Figure 4B). We found that z-DEVD-fmk, a caspase-3 inhibitor, partly reversed the decrease in CBP levels, whereas the caspase-6 inhibitor z-VEID-fmk was much more efficient (Figure 4B), suggesting that caspase-6 could be responsible for CBP degradation. To investigate this hypothesis further, we tested the effects of recombinant caspase-3 and -6 on CBP cleavage in an in vitro assay (Figure 4C). We used human double minute 1 (HDM1), the human MDM2 homolog, as a control for caspase-3 cleavage (Chen et al., 1997). Recombinant [35S]methionine-labeled CBP and HDM1 were synthesized in a rabbit reticulocyte lysate and incubated with increasing amounts of caspase-3. Caspase-3 was not able to cleave CBP (Figure 4C, left panel), even when 10 U of recombinant protein were used (data not shown). In the same assay, caspase-3 cleaved HDM1 in a dose-dependent manner, giving an expected fragment migrating at 68 kDa (Figure 4C, right panel, HDM1-I) (Chen et al., 1997) and being reversed by both z-DEVD-fmk and Ac-VAD-CHO. Recombinant caspase-6 cleaved [35S]methionine-labeled CBP in a dose-dependent manner, producing two cleavage fragments migrating at apparent mol. wts of 175 and 130 kDa, respectively (Figure 4C, left panel). This cleavage could be reversed by both z-VEID-fmk and Ac-VAD-CHO. It is noteworthy that cleavage fragments observed by western blot in CGNs undergoing apoptosis (CBP-I, Figure 3B, and CBP-II, data not shown) and those obtained with the caspase cleavage assay (Figure 4C, left panel) seem to migrate at identical molecular weights, suggesting that CBP could indeed be targeted by endogenous caspase-6 in CGNs. Collectively, these data suggest that CBP is preferentially cleaved by caspase-6 in the early events of CGN apoptosis.
Fig. 4. CBP is a caspase-6 substrate. (A) Caspase-6 and -3 activity measurements were performed on extracts from K+-deprived CGNs during 1–6 h, using the fluorogenic substrates Ac-VEID-AMC and Ac-DEVD-AMC, respectively. (B) CGNs were K+ deprived (LK, 15 h) in the presence or absence of z-DEVD-fmk (50 μM) or z-VEID-fmk (50 or 100 μM), or maintained in neuroprotective conditions (HK), and CBP was analyzed by western blot. Results shown are representative of three independent experiments. (C) [35S]methionine-labeled CBP and HDM1 recombinant proteins were produced in vitro in a rabbit reticulocyte lysate and tested in an in vitro capsase cleavage assay with recombinant capsase-3 and -6. CBP is not processed by caspase-3 but is cleaved in a dose-dependent manner (1, 2.5 and 5 U, as noted) by recombinant capsase-6. Two cleavage fragments (CBP-I and CBP-II) can be detected, with apparent mol. wts of ∼175 and 130 kDa, respectively; when performed with 5 U of caspase-6, this cleavage can be reversed by z-VEID-fmk (50 μM) and Ac-VAD-CHO (50 μM). HDM1 is used as positive control for caspase-3 cleavage, giving an expected cleavage fragment of 68 kDa (HDM1-I). HDM1 is not cleaved by the recombinant caspase-6 in our experimental conditions.
Acetylated histone and CBP levels decrease in two other models of neuronal cell death
To determine whether histone deacetylation and CBP degradation were common traits of neurodegeneration, we investigated their levels in an in vitro model of embryonic cortical neuron cell death (Mbebi et al., 2002) and in an in vivo model of ALS (Ripps et al., 1995; Gonzalez de Aguilar et al., 2000).
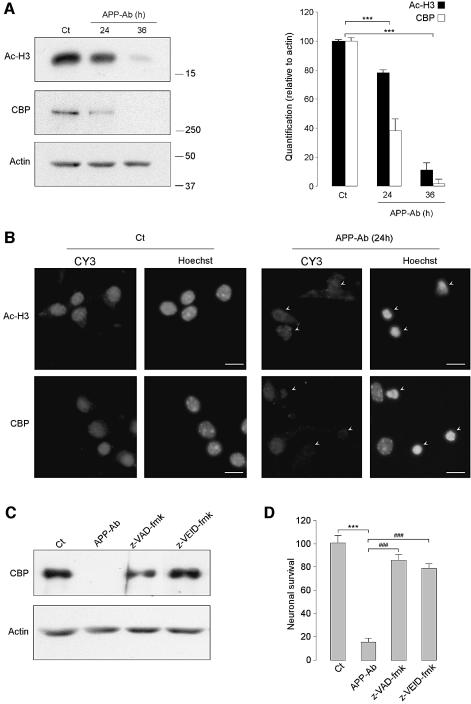
Embryonic cortical neuron death was induced by activation of the APP signaling pathway with an antibody directed against the extracellular domain of APP (APP-Ab) (Mbebi et al., 2002). Western blot analyses revealed that Ac-H3 and CBP levels decreased during cortical neuron death (Figure 5A). CBP and Ac-H3 levels were quantified relative to actin levels and are represented as histograms (right panel). These results were confirmed by immunohistochemistry (Figure 5B): Ac-H3 and CBP immunoreactivities were easily detected in healthy neurons displaying large nuclei (Ct) and strongly decreased in dying neurons treated with APP-Ab. The use of z-VEID-fmk further suggested the implication of caspase-6 in this model as it was able to reverse CBP degradation, as did z-VAD-fmk (Figure 5C). Moreover, both z-VEID-fmk and z-VAD-fmk were also able to prevent cortical neuronal death induced by APP-Ab (Figure 5D). Altogether, these results show that histone acetylation and CBP levels also decreased in this cell death model, in a caspase-6-dependent manner.
Fig. 5. Acetylated H3 histone and CBP levels decrease during cell death induced by activation of the APP signaling pathway in primary cortical neurons. (A) Protein extracts were prepared from embryonic cortical neurons treated during 24 or 36 h with an anti-APP antibody (APP-Ab), or maintained in survival conditions (Ct) and were analyzed further by western blot with anti-Ac-H3, anti-CBP and anti-actin antibodies (left panel). Bands were quantified and the levels of CBP and Ac-H3 are represented as histograms, relative to the actin amounts (right panel). Controls are arbitrary set to 100. Data represent means ± SEM. ***P < 0.001 (versus Ct). (B) Cortical neurons were treated for 24 h with APP-Ab or not (Ct), and Ac-H3 and CBP immunostainings were revealed with a Cy3-coupled secondary antibody (CY3). Neurons were also stained with the Hoechst dye 33342 to visualize nuclear morphology (Hoechst). Scale bar = 10 μm; arrows indicate the condensed or fragmented nuclei. (C and D) Cortical neurons were treated for 36 h with anti-APP antibody (APP-Ab), with or without z-VAD-fmk (50 μM) or z-VEID-fmk (100 μM), or maintained in survival conditions (Ct). Results shown are representative of three independent experiments. (C) Western blot analyses of CBP. (D) Neuronal survival was measured by MTT assay. Data represent means ± SEM. ***P < 0.001 (versus Ct) and ###P < 0.001 (versus APP-Ab).
The in vivo mice model of ALS, G86R mutant SOD-1 mice (Ripps et al., 1995; Gonzalez de Aguilar et al., 2000), represents a progressive neurodegenerative disorder inducing motoneuron death (Hand and Rouleau, 2002). CBP and H3 histone acetylation levels were monitored by western blots in the lumbar spinal cord of 3.5-month-old G86R mice, a time point at which motoneurons die (Figure 6A, left panel). Wild-type (WT) littermates were used as control. Quantification is shown on the right. We found that the CBP protein level decreased >70% in G86R compared with WT mice, but failed to detect any changes in total Ac-H3 amounts. Nevertheless, immunohistochemistry performed on lumbar spinal cord slides revealed some differences (Figure 6B). Nuclei of motoneurons were easily detected, as they appeared larger than those of other cells such as glial cells and interneurons (Casanovas et al., 2001). We found that Ac-H3 immunoreactivity was present in all cell types, but strongly decreased in motoneuron nuclei of G86R mice compared with WT (Figure 6B). This could explain why the decrease between G86R and WT mice could not be detected by western blot since the global amount of Ac-H3 was not that affected relative to the whole tissue. In contrast, CBP immunoreactivity was detected mainly in motoneurons and strongly decreased in G86R mice (Figure 6B), confirming results readily obtained by western blot analyses.
Fig. 6. Decrease of acetylated H3 histone and CBP protein levels in the lumbar spinal cord of G86R mutant SOD-1 ALS mice. (A) Ac-H3 and CBP immunoblot analysis of protein extracts prepared from lumbar spinal cord of wild-type (WT) and transgenic G86R mice (left). Total Ac-H3 protein amounts did not vary between the WT and G86R mice (quantification on the right). The results shown were obtained in two independent experiments from four WT and four G86R mice. Data represent means ± SEM. ***P < 0.001 (versus WT). (B) Ac-H3 and CBP immunostaining of 1.5 µm sections from lumbar spinal cord of WT and G86R mice. Immunoreactivities were revealed by Cy3-coupled secondary antibody. Ac-H3 and CBP immunoreactivities specifically decreased in motoneuron nuclei (shown by arrow), that apper larger than nuclei of other cell types, of G86R mice compared with WT. The same results were obtained in two independent experiments from three different zones of lumbar spinal cord of each animal (three WT and three G86R mice). Scale bar = 25 µm.
Altogether, the results obtained in these different models suggest that the decrease in histone acetylation and CBP levels may be a common trait of neurodegeneration.
Fine-tuning of HAT activity is necessary for neuronal survival
Proteins involved in neuroprotection are often degraded at the onset of apoptosis. To understand whether CBP loss of function has an important role during neuronal apoptosis, we tested the ability of CBP overexpression to counteract LK-induced CGN apoptosis (Figure 7B). Typical photographs of the results obtained are shown in Figure 7A–C, and quantification is represented at the bottom of each panel (Figure 7B and 7C). In control conditions (p2N3T empty vector, Figure 7A), neuronal survival in the transfected neuronal population is evaluated at 74% in survival conditions (HK, left panel) and only 30% in apoptotic conditions (LK, right panel). We found that CBP overexpression significantly protected neurons from apoptosis: survival was increased (65%) compared with the control (30%), as depicted in Figure 7B (upper panel). Interestingly, overexpression of p300 produced effects similar to CBP (Figure 7B), suggesting that protection occurs through similar functions. We repeated this experiment with a mutant of CBP, in which the HAT domain was deleted (CBPΔHAT). The percentage of healthy nuclei obtained with CBPΔHAT was significantly reduced when compared with CBP (Figure 7B), suggesting that the neuroprotective effects induced by CBP overexpression were indeed mediated in part through its HAT activity.
Fig. 7. CBP/p300-driven HAT overexpression protects from cell death in apoptotic conditions and induces cell death in survival conditions. CGNs were transiently co-transfected with pEGFP vector, together with the empty p2N3T vector (A) or vectors containing genes encoding CBP, p300 or CBP mutated within the HAT domain sequence (CBPΔHAT) (B and C). After 10 h of transfection, neurons were K+ deprived (LK) or not (HK) for 12 h, fixed and stained with Hoechst dye 33342. Results shown are representative of an independent experiment performed in duplicate and repeated 10 times. Hoechst-labeled nuclei are visualized in blue; fluorescent transfected cells are visualized in green. Arrows indicate transfected cells, scale bars = 10 mm. (A) In control conditions, neurons transfected with the empty vector remained healthy in survival conditions (HK, left), but commonly degenerated in apoptotic conditions (LK, right). (B) In apoptotic conditions (LK), neurons transfected with CBP or p300 displayed healthy nuclei compared with the control (HK, A). Healthy and apoptotic nuclei were scored in every transfection conditions (∼2500 neurons scored/condition) and the percentage of healthy nuclei was represented as histograms. The data represent means ± SEM (n = 10). ***P < 0.001 (versus empty vector) and ###P < 0.001 (versus vector containing cbp). (C) In survival conditions, overexpression of CBP and p300 induced nuclear fragmentation (upper panel) and cell death (lower panel), compared with the empty vector, whereas CBPΔHAT had no significant effect.
However, in survival conditions (HK), neurons transfected with CBP presented apoptotic nuclei, sometimes condensed but mainly fragmented (Figure 7C, upper panel). Quantitated results showed that CBP overexpression allowed only 33% survival in HK, which was significantly reduced compared with that obtained with the empty vector (74%) (Figure 7C, lower panel). This suggests that CBP overexpression is lethal for neurons maintained in survival conditions. p300 overexpression also induced cell death and chromatin fragmentation. We found that CBP overexpression-induced cell death was likely to be driven by its HAT function, as CBPΔHAT was without effect on neuronal survival (72% compared with 74% obtained with the empty vector).
Taken together, these results argue for a major role for CBP in neuroprotection by finely tuning specific acetylations through its HAT function.
Discussion
The findings presented in this report show that early CBP/p300 degradation and subsequent decrease in HAT activity represent critical steps in neuronal apoptosis. We identified caspase-6 as the effector caspase responsible for CBP degradation. Because we showed that the decrease of CBP and acetylated histone levels occurred in different models of neuronal cell death, this could be an important hallmark of neuronal apoptosis, common to several neuropathologies.
CBP degradation normally occurs through the proteasome pathway, but our data support the existence of alternative pathways in which CBP is targeted to specific apoptotic enzymes during neurodegeneration. Among the specific proteases involved in neuronal apoptosis, both calpains and caspases seemed to be implicated, but only caspase inhibitors could prevent CBP loss. However, calpain blockade allowed us to show a cleavage of the CBP protein, suggesting that calpain degradation indeed occurred in our apoptotic conditions, but chronologically after caspase degradation. Caspase-3 is one of the major executioner caspases involved in the CNS in general (Troy and Salvesen, 2002) and in K+ deprivation-induced apoptosis of CGNs in particular (Boutillier et al., 2000). Surprisingly, caspase-3 was not able to degrade CBP in an in vitro caspase cleavage assay, whereas it could cleave the HDM1 protein as reported (Chen et al., 1997). Interest ingly, Allsopp and colleagues described, in the same model of CGN primary cultures, that following both K+ and serum starvation, caspase-6 was activated and necessary for caspase-3 activation. In our experimental model, we found that caspase-6 was activated as soon as 1–2 h after K+ deprivation and it is likely that it could also be implicated in caspase-3 activation such as that described by Allsopp et al. (2000). Caspase-6 was identified further as the effector able to cleave CBP in vitro, and most probably also in vivo, as the caspase-6-like inhibitor z-VEID-fmk strongly reversed LK- and APP-Ab-mediated CBP degradation. The mechanisms by which caspase-6 would itself be activated are still unclear. It is noteworthy that not many specific substrates of caspase-6 have been reported, these being usually structural proteins, among which only a few are caspase-6-only substrates: lamin A, which participates in nuclear structure and must be degraded to produce chromatin condensation (Ruchaud et al., 2002); and SATB1, an AT-rich DNA-binding protein which is cleaved early during T-cell apoptosis (Galande et al., 2001). Our results add CBP to this short list. We concluded that there is a single cleavage site operated by caspase-6, occurring between the bromo- and the HAT- domains of the CBP protein. Whether CBP is indeed cleaved at this site in vivo is currently under investigation.
Interestingly, these findings were observed in two other models. One deals with APP signaling, activation of which caused cortical neuronal death, either by revealing an APP-dependent death signaling pathway or, alternatively, by blocking the proposed neuroprotective functions of APP (Xu et al., 1999; Burton et al., 2002; Mbebi et al., 2002). The other is an in vivo mouse model of ALS disease causing motoneuronal death (Ripps et al., 1995). The implication of caspase-6 in these models has not been demonstrated yet. Interestingly, as we show that z-VEID-fmk could reverse both CBP degradation and APP-Ab-mediated cortical neuron death, caspase-6 could indeed be an effector caspase in this model. Moreover, caspase-6 proenzyme as well as the p10 active caspase-6 fragment have been evidenced in pathological adult human AD brain tissue, making it likely that caspase-6 was implicated in AD (LeBlanc et al., 1999). However, despite the fact that we also detected the p10 active caspase-6 fragment in our model, we failed to detect any regulation upon APP-Ab treatment (data not shown). Whether loss of CBP by caspase-6 is implicated in AD is an interesting possibility that remains to be established. The implication of caspase-6 in ALS is less clear. So far, using models of different lineages of SOD mutant mice, caspase-1 and -3 have been reported to be the main caspases activated during motoneuron degeneration (Pasinelli et al., 1998, 2000). In our experimental conditions, neither caspase-1, even when used at up to 10 U (our unpublished results), nor caspase-3 (Figure 4) were able to degrade CBP. However, the studies performed by Pasinelli et al. (1998, 2000) did not exclude the participation of other caspase(s) and it is thus possible that caspase-1 and -3 are not the only caspases involved in motoneuron degeneration. Nevertheless, we have demonstrated a specific loss of CBP in motoneurons of an ALS mice model, but whether this is operated by caspase-6, an as yet unidentified caspase, calpains or other degradation pathways, such as the proteasome pathway, is still unknown.
Our results raise the possibility that CBP/p300 loss of function could be a hallmark of neurodegeneration. This is in line with the recent results obtained by Steffan et al. (2000) in a Drosophila model of polyglutamine disease. This group recently reported that a CBP dysfunction occurred through the binding of the polyglutamine-containing domain of the Htt protein directly to its acetyltransferase domain, thus blocking CBP-mediated transcription. This mutated protein could also bind PCAF. More recently, another group reported that translocation of the polyglutamine-containing Htt protein to the nucleus selectively enhanced CBP ubiquitylation and degradation by the ubiquitin–proteasome pathway (Jiang et al., 2003). This strongly supports our findings that CBP/p300 degradation and subsequent loss of HAT function are essential steps towards neurodegeneration, regardless of what the destruction mechanisms are (degradation by caspases or calpains or by the proteasome pathway).
However, our findings that PCAF and TAF II p250 were not altered by K+ deprivation demonstrate a higher degree of specificity in the apoptotic signaling pathway, meaning that it is probably not the acetylations in general that are impaired following apoptosis entry, but specific CBP/p300-dependent acetylations. We thus expect that not only the acetylation status of histones H3 and H4 will decrease as shown here, but probably other acetylations of as yet unidentified non-histone proteins. CBP and p300 share a high degree of homology (Chan and La Thangue, 2001; Vo and Goodman, 2001). For instance, they both bind the transcription factor CREB. It is now largely acknowledged that CREB-dependent transcription plays a key role in neuroprotection (reviewed in Lonze and Ginty, 2002), making it a good candidate implicated in these mechanisms. CBP/p300 degradation would lead to an irreversible decrease in CREB-dependent transcription in apoptotic conditions, thus compromising neuroprotection (Figure 8). Still, fundamental differences between CBP and p300 have also been described. For example, full activation of CREB-dependent transcription by CBP also depends on a secondary regulatory event, CBP phosphorylation at Ser301, an event that does not occur for p300 (Impey et al., 2002). Therefore, this suggests that survival could also be attributable to the acetylase function of these proteins.
Fig. 8. Schematic representation of the fate of CBP and CREB under survival or cell death conditions. Neuronal electrical activity present in HK induces Ca2+ entry, which allows calcium/calmodulin protein kinase IV (CamKIV) activation and subsequent CREB and CBP proteins phosphorylation (Chawla et al., 1998; Hu et al., 1999; Impey et al., 2002). Phospho-CREB binds CBP allowing CREB-dependent gene transcription and basal H3 and H4 histone acetylation levels via the CBP HAT domain. These conditions are favorable to neuronal survival. K+ deprivation (LK) blocks Ca2+ entry and CamKIV activation. CREB and CBP are dephosphorylated and degraded. Therefore, CREB-mediated gene transcription is inhibited, CBP HAT activity decreases, inducing a global histone deacetylation, and neurons irreversibly go towards death. CBP overexpression in survival conditions would result in histone hyperacetylation, through an increase in HAT activity. If CREB acts as a limiting factor (?), CREB-dependent gene transcription should not be modified under CBP overexpression conditions. Histone acetylation levels can also be increased artificially with HDAC inhibitors. Both situations are correlated with cell death, probably because of high histone acetylation levels and aberrant chromatin-remodeling events. Taken together, these findings point to the importance of the HAT/HDAC balance in neuronal survival.
On the other hand, experimentally increasing acetylations with HDAC inhibitors such as trichostatin A or sodium butyrate does not rescue cells from apoptosis. In fact, not only is this treatment not sufficient per se to protect against neuronal cell death induced in CGNs (data not shown), but it is also pro-apoptotic when given in neuroprotective conditions (Boutillier et al., 2003). How ever, this is not the case in a Drosophila model of polyglutamine disease, in which administration of these HDAC inhibitors reduced animal lethality (Steffan et al., 2001). This points to the need to identify the molecular events leading to CBP loss of function (degradation versus competition in the HAT domain). It is noteworthy that overexpression of CBP/p300 resulted in increased cell death, attributable to the HAT function. Interestingly, we have published findings recently that HDAC inhibitor-evoked hyperacetylations induced a caspase-dependent programmed cell death in primary CGNs (Boutillier et al., 2003). It is thus likely that high levels of CBP/p300 also resulted in aberrant chromatin remodeling. Clearly, these results demonstrate that a fine-tuning of HAT activity is required to ensure neuroprotection (Figure 8).
Altogether, these data strongly support the loss of function of CBP/p300 HAT activity during apoptosis and neurodegenerative diseases, by a novel mechanism dependent on caspase-6 activation. Animals bearing caspase-6 gene disruption show no obvious morphological differences (Ruchaud et al., 2002; T.S.Zheng, personal communication). Thus, the role of caspase-6 during apoptosis may have been underestimated so far. Programmed cell death through caspase activation may not necessarily commit the neuron to die immediately, but could cause neuronal dysfunction through altered proteolytic processing of key neuronal proteins during neuropathologies. We hypothesize that caspase-6 could have discrete but selective substrates such as CBP or p300, the chronic degradation of which during a neurodegenerative disease would not only irreversibly slow down CREB-dependent transcription, but also alter cellular acetylation levels of histone and non-histone proteins. On a long-term basis, this would result in increased neuronal sensitivity to other signals, thus seriously compromising neuronal survival.
Materials and methods
Peptides, recombinant proteins and antibodies
Protease inhibitors z-DEVD-fmk, z-VEID-fmk, z-VAD-FMK, Ac-VAD-CHO, ALLN, PSI and recombinant caspase-6 were purchased from Calbiochem (La Jolla, CA), and recombinant caspase-1 from Alexis Biochemical (San Diego, CA). Anti-CBP Ct, anti-Ac-H3, anti-Ac-H4 antibodies, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and recombinant caspase-3 were obtained from Upstate Biotechnology (Lake Placid, NY). Anti-p300, anti-TAF II p250 and anti-PCAF antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin was a kind gift from Dr D.Aunis (Strasbourg, France). HRP-conjugated secondary goat anti-rabbit IgG was purchased from Pierce (Rockford, IL).
Neuronal primary cultures
CGNs from 7-day-old mice (fvb strain) were cultured as previously described (Boutillier et al., 1999). Experiments were performed after 6 days in vitro (DIV), except transfection experiments (3 DIV). HK is defined medium with final [K+] = 30 mM and LK, [K+] = 5 mM.
Cortical neurons from fvb mice embryos (E18) were cultured as described previously (Mbebi et al., 2002). Experiments were performed after 4 DIV. To induce cell death, the APP-Ab antibody was used at a final concentration of 8 µg/ml.
SOD G86R mice
Transgenic mice with the missense mutation G86R (human G85R equivalent) in SOD-1 (Ripps et al., 1995), showing a very early stage of hind leg paralysis (between 100 and 105 days old), were used as an in vivo model of ALS. WT littermates were used as control. All experiments were carried out according to current EC regulations. For immunocytochemistry, animals were anesthetized intraperitoneally with urethane (2 mg/g of body weight) and perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS). Lumbar spinal cords were dissected and fixed for 24 h at 4°C. Tissues were then dehydrated in graded ethanol and embedded in araldite. Semi-thin sections were cut and collected on gelatin-coated slides. After araldite removal, sections were stained by immunofluorescence methods. For western blot analysis, animals were killed by decapitation and lumbar spinal cords were rapidly dissected and stored in liquid nitrogen. After tissue homogenization, lysates were centrifuged, protein determination was performed on supernatants and 70 µg of total proteins were analyzed.
Cellular histone extraction
Total cellular histones were acid extracted according to Nakajima et al. (1998) and 10 µg were separated on 13% polyacrylamide gels. Gels were either stained with Coomassie brilliant blue R-250 to visualize total amounts of cellular histones or processed further by western blot analyses.
Western blot analysis
Western blots were performed as previously described (Boutillier et al., 1999) with typically 50–70 µg of total cell extracts run on 7, 10 or 13% SDS–acrylamide gels for CBP, actin or histone detection, respectively. Specific bands were detected by enhanced chemiluminescence (ECL; Amersham).
HAT assay
HAT assay was performed using the kit purchased from Upstate Biotechnology either on CBP/p300-immunoprecipitated extracts or not, according to the manufacturer’s procedures. HAT activity was read in an LS6500 Multi-Purpose Scintillation Counter (Beckman Coulter, Fullerton, CA).
Morphological evaluation of apoptosis
Cells were fixed with 4% paraformaldehyde in PBS for 15 min at room temperature and incubated for 30 min with the Hoechst dye 33342 (1 µg/ml). Stained nuclei were scored by blind analysis, those presenting condensed or fragmented chromatin being counted as apoptotic.
Immunocytochemistry
Immunocytochemistry was performed as previously described. Cy3 and Hoechst staining were visualized with a Leitz microscope. Photographs were taken with Nikon Digital Camera Dxm1200.
RT–PCR analysis
Total RNA was extracted from neurons by the guanidinium thiocyanate–phenol–chloroform method. A 2 µg aliquot was reverse-transcribed using 200 U of MMLV reverse transcriptase, and PCR analysis was performed (Promega, Madison, WI) as previously described (Boutillier et al., 1999). Cycle parameters were 1 min at 94°C, 1 min at 60°C and 1 min at 72°C for 22 cycles. Ethidium bromide-stained PCR products were separated on 2% agarose gels, visualized and quantified using a Gel Doc 2000 (Bio-Rad, Hercules, CA).
Mouse CBP primers: reverse: 5′-TCAAGTTTGGTGGCTGTTGA-3′, forward: 5′-TTGCCTCCCTTTGAAAAATG-3.
Caspase activity measurements
Caspase activities were determined by measuring the release of fluorescent cleavage products from the caspase tetrapeptide substrates as described previously (Boutillier et al., 2000). The caspase-specific substrates Ac-DEVD-AMC and Ac-VEID-AMC were purchased from Sigma (St Louis, MO). Fluorescence was monitored at excitation 360 nm, emission 465 nm, using a Perkin-Elmer HTS 7000 Bioassay reader (Foster City, CA). A standard curve of fluorescence versus free AMC was used to calculate the amount of substrate cleaved from fluorescent units.
In vitro synthesis of recombinant proteins and cleavage assay
In vitro synthesis of recombinant [35S]methionine-labeled CBP and HDM1 was performed using the TNT® Coupled Reticulocyte Lysate Systems kit (L4611) purchased from Promega, according to the manufacturer’s procedures. [35S]Methionine was purchased from Perkin Elmer (Boston, MA). The in vitro cleavage assay was performed on 2 µl of the recombinant protein mixture. Neo-synthesized proteins were incubated for 20 min at 37°C with recombinant caspase-1, -3 or -6 (1, 2.5, 5 or 10 U) with or without caspase inhibitor (z-DEVD-FMK, z-VEID-FMK, Ac-VAD-CHO, 50 µM). Reactions were stopped with 5× Laemmli buffer, boiled, and products were analyzed by 7% SDS–PAGE.
Colorimetric MTT assay
After 4 DIV, cortical neurons cultured in 48-well plates were treated with an anti-APP antibody as noted, in the absence or presence of z-VAD-fmk (50 µM) or z-VEID-fmk (100 µM). After 36 h, MTT assays were performed as described in Boutillier et al. (1999).
Co-transfections
Neuronal gene transfections were performed as reported previously (Boutillier et al., 2000). Neurons were grown on glass coverslips and co-transfected at 3 DIV for 30 min with 1 µg/ml of expression vector: p2N3T, p2N3T-CBP, p2N3T-CBPΔHAT (generous gifts from Drs A.Harel-Bellan and S.Ait-Si-Ali, Villejuif, France) or pcDNA3-p300 (generous gift from Dr J.R.Lundblad, Portland, OR) and 0.2 µg of pEGFP vector. Plates were spun for 5 min at 1500 r.p.m. Apoptotic nuclei were revealed by Hoechst staining and transfected cells were visualized with a Leitz microscope using a fluorescein isothiocyanate (FITC) filter set.
Statistics
Data were expressed as the mean ± SEM. Statistical significance was determined by one-way ANOVA followed by Newman–Keuls’ multiple comparison test. Differences were considered as significant at P < 0.05.
Acknowledgments
Acknowledgements
The authors wish to thank Drs J.R.Lundblad (Portland, OR), A.Harel-Bellan and S.Ait-Si-Ali (Villejuif, France) for the kind gift of CBP vectors, Dr J.Chen (New Orleans, LO) for HDM1, Drs J.R.Lundblad (Portland, OR) and K.Fisher (San Fransisco, CA) for critical reading of the manuscript, M.J.Ruivo for her technical assistance and Dr F.René for the care of the G86R SOD mice. The laboratory is supported by grants from the French Cancer Research Association (ARC no. 4306 and 7653), AREMANE and the Amyotrophic Lateral Sclerosis Association.
References
- Allsopp T.E., McLuckie,J., Kerr,L.E., Macleod,M., Sharkey,J. and Kelly,J.S. (2000) Caspase 6 activity initiates caspase 3 activation in cerebellar granule cell apoptosis. Cell Death Differ., 7, 984–993. [DOI] [PubMed] [Google Scholar]
- Bannister A.J. and Kouzarides,T. (1996) The CBP co-activator is a histone acetyltransferase. Nature, 384, 641–643. [DOI] [PubMed] [Google Scholar]
- Boutillier A.L., Kienlen-Campard,P. and Loeffler,J.P. (1999) Depolarization regulates cyclin D1 degradation and neuronal apoptosis: a hypothesis about the role of the ubiquitin/proteasome signalling pathway. Eur. J. Neurosci., 11, 441–448. [DOI] [PubMed] [Google Scholar]
- Boutillier A.L., Trinh,E. and Loeffler,J.P. (2000) Caspase-dependent cleavage of the retinoblastoma protein is an early step in neuronal apoptosis. Oncogene, 19, 2171–2178. [DOI] [PubMed] [Google Scholar]
- Boutillier A.L., Trinh,E. and Loeffler,J.P. (2003) Selective E2F-dependent gene transcription is controlled by histone deacetylase activity during neuronal apoptosis. J. Neurochem., 84, 814–828. [DOI] [PubMed] [Google Scholar]
- Brill A., Torchinsky,A., Carp,H. and Toder,V. (1999) The role of apoptosis in normal and abnormal embryonic development. J. Assist. Reprod. Genet., 16, 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton T.R., Dibrov,A., Kashour,T. and Amara,F.M. (2002) Anti-apoptotic wild-type Alzheimer amyloid precursor protein signaling involves the p38 mitogen-activated protein kinase/MEF2 pathway. Brain Res. Mol. Brain Res., 108, 102–120. [DOI] [PubMed] [Google Scholar]
- Casanovas A., Ribera,J., Hager,G., Kreutzberg,G.W. and Esquerda,J.E. (2001) c-jun regulation in rat neonatal motoneurons postaxotomy. J. Neurosci. Res., 63, 469–479. [DOI] [PubMed] [Google Scholar]
- Chan H.M. and La Thangue,N.B. (2001) p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci., 114, 2363–2373. [DOI] [PubMed] [Google Scholar]
- Chawla S., Hardingham,G.E., Quinn,D.R. and Bading,H. (1998) CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science, 281, 1505–1509. [DOI] [PubMed] [Google Scholar]
- Chen L., Marechal,V., Moreau,J., Levine,A.J. and Chen,J. (1997) Proteolytic cleavage of the mdm2 oncoprotein during apoptosis. J. Biol. Chem., 272, 22966–22973. [DOI] [PubMed] [Google Scholar]
- Cheung P., Allis,C.D. and Sassone-Corsi,P. (2000) Signaling to chromatin through histone modifications. Cell, 103, 263–271. [DOI] [PubMed] [Google Scholar]
- Chrivia J.C., Kwok,R.P., Lamb,N., Hagiwara,M., Montminy,M.R. and Goodman,R.H. (1993) Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature, 365, 855–859. [DOI] [PubMed] [Google Scholar]
- D’Mello S.R. (1998) Molecular regulation of neuronal apoptosis. Curr. Top. Dev. Biol., 39, 187–213. [DOI] [PubMed] [Google Scholar]
- D’Mello S.R., Galli,C., Ciotti,T. and Calissano,P. (1993) Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc. Natl Acad. Sci. USA, 90, 10989–10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ruijter A.J., Van Gennip,A.H., Caron,H.N., Kemp,S. and Van Kuilenburg,A.B. (2003) Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem. J., 370, 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberharter A. and Becker,P.B. (2002) Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep., 3, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galande S., Dickinson,L.A., Mian,I.S., Sikorska,M. and Kohwi-Shigematsu,T. (2001) SATB1 cleavage by caspase 6 disrupts PDZ domain-mediated dimerization, causing detachment from chromatin early in T-cell apoptosis. Mol. Cell. Biol., 21, 5591–5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez de Aguilar J.L., Gordon,J.W., Rene,F., de Tapia,M., Lutz-Bucher,B., Gaiddon,C. and Loeffler,J.P. (2000) Alteration of the Bcl-x/Bax ratio in a transgenic mouse model of amyotrophic lateral sclerosis: evidence for the implication of the p53 signaling pathway. Neurobiol. Dis., 7, 406–415. [DOI] [PubMed] [Google Scholar]
- Gregory P.D., Wagner,K. and Horz,W. (2001) Histone acetylation and chromatin remodeling. Exp. Cell Res., 265, 195–202. [DOI] [PubMed] [Google Scholar]
- Hand C.K. and Rouleau,G.A. (2002) Familial amyotrophic lateral sclerosis. Muscle Nerve, 25, 135–159. [DOI] [PubMed] [Google Scholar]
- Honig L.S. and Rosenberg,R.N. (2000) Apoptosis and neurologic disease. Am. J. Med., 108, 317–330. [DOI] [PubMed] [Google Scholar]
- Hu S.C., Chrivia,J. and Ghosh,A. (1999) Regulation of CBP-mediated transcription by neuronal calcium signaling. Neuron, 22, 799–808. [DOI] [PubMed] [Google Scholar]
- Impey S. et al. (2002) Phosphorylation of CBP mediates transcriptional activation by neural activity and CaM kinase IV. Neuron, 34, 235–244. [DOI] [PubMed] [Google Scholar]
- Jiang H., Nucifora,F.C.,Jr, Ross,C.A. and DeFranco,D.B. (2003) Cell death triggered by polyglutamine-expanded huntingtin in a neuronal cell line is associated with degradation of CREB-binding protein. Hum. Mol. Genet., 12, 1–12. [DOI] [PubMed] [Google Scholar]
- Kalkhoven E., Roelfsema,J.H., Teunissen,H., Den Boer,A., Ariyurek,Y., Zantema,A., Breuning,M.H., Hennekam,R.C. and Peters,D.J. (2003) Loss of CBP acetyltransferase activity by PHD finger mutations in Rubinstein–Taybi syndrome. Hum. Mol. Genet., 12, 441–450. [DOI] [PubMed] [Google Scholar]
- Kuo M.H. and Allis,C.D. (1998) Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays, 20, 615–626. [DOI] [PubMed] [Google Scholar]
- Kwok R.P., Lundblad,J.R., Chrivia,J.C., Richards,J.P., Bachinger,H.P., Brennan,R.G., Roberts,S.G., Green,M.R. and Goodman,R.H. (1994) Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature, 370, 223–226. [DOI] [PubMed] [Google Scholar]
- LeBlanc A., Liu,H., Goodyer,C., Bergeron,C. and Hammond,J. (1999) Caspase-6 role in apoptosis of human neurons, amyloidogenesis and Alzheimer’s disease. J. Biol. Chem., 274, 23426–23436. [DOI] [PubMed] [Google Scholar]
- Lonze B.E. and Ginty,D.D. (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron, 35, 605–623. [DOI] [PubMed] [Google Scholar]
- Lonze B.E., Riccio,A., Cohen,S. and Ginty,D.D. (2002) Apoptosis, axonal growth defects and degeneration of peripheral neurons in mice lacking CREB. Neuron, 34, 371–385. [DOI] [PubMed] [Google Scholar]
- Mbebi C., See,V., Mercken,L., Pradier,L., Muller,U. and Loeffler,J.P. (2002) Amyloid precursor protein family-induced neuronal death is mediated by impairment of the neuroprotective calcium/calmodulin protein kinase IV-dependent signaling pathway. J. Biol. Chem., 277, 20979–20990. [DOI] [PubMed] [Google Scholar]
- McConkey D.J. (1998) Biochemical determinants of apoptosis and necrosis. Toxicol. Lett., 99, 157–168. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Kim,Y.B., Terano,H., Yoshida,M. and Horinouchi,S. (1998) FR901228, a potent antitumor antibiotic, is a novel histone deacetylase inhibitor. Exp. Cell Res., 241, 126–133. [DOI] [PubMed] [Google Scholar]
- Pasinelli P., Borchelt,D.R., Houseweart,M.K., Cleveland,D.W. and Brown,R.H.,Jr (1998) Caspase-1 is activated in neural cells and tissue with amyotrophic lateral sclerosis-associated mutations in copper–zinc superoxide dismutase. Proc. Natl Acad. Sci. USA, 95, 15763–15768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinelli P., Houseweart,M.K., Brown,R.H.,Jr and Cleveland,D.W. (2000) Caspase-1 and -3 are sequentially activated in motor neuron death in Cu,Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA, 97, 13901–13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrij F. et al. (1995) Rubinstein–Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature, 376, 348–351. [DOI] [PubMed] [Google Scholar]
- Ripps M.E., Huntley,G.W., Hof,P.R., Morrison,J.H. and Gordon,J.W. (1995) Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA, 92, 689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchaud S., Korfali,N., Villa,P., Kottke,T.J., Dingwall,C., Kaufmann,S.H. and Earnshaw,W.C. (2002) Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation. EMBO J., 21, 1967–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J.B., Weller,M. and Klockgether,T. (1996) Potassium deprivation-induced apoptosis of cerebellar granule neurons: a sequential requirement for new mRNA and protein synthesis, ICE-like protease activity and reactive oxygen species. J. Neurosci., 16, 4696–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan J.S. et al. (2000) The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc. Natl Acad. Sci. USA, 97, 6763–6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan J.S. et al. (2001) Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature, 413, 739–743. [DOI] [PubMed] [Google Scholar]
- Sterner D.E. and Berger,S.L. (2000) Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev., 64, 435–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Th’ng J.P. (2001) Histone modifications and apoptosis: cause or consequence? Biochem. Cell Biol., 79, 305–311. [PubMed] [Google Scholar]
- Troy C.M. and Salvesen,G.S. (2002) Caspases on the brain. J. Neurosci. Res., 69, 145–150. [DOI] [PubMed] [Google Scholar]
- Vo N. and Goodman,R.H. (2001) CREB-binding protein and p300 in transcriptional regulation. J. Biol. Chem., 276, 13505–13508. [DOI] [PubMed] [Google Scholar]
- Yao T.P. et al. (1998) Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell, 93, 361–372. [DOI] [PubMed] [Google Scholar]
- Xu X., Yang,D., Wyss-Coray,T., Yan,J., Gan,L., Sun,Y. and Mucke,L. (1999) Wild-type but not Alzheimer-mutant amyloid precursor protein confers resistance against p53-mediated apoptosis. Proc. Natl Acad. Sci. USA, 96, 7547–7552. [DOI] [PMC free article] [PubMed] [Google Scholar]