Summary
Background
Lactosylceramide (LacCer) is a member of the glycosphingolipid family, which has been implicated in the atherogenic process. The goal of this study was to determine the effects and molecular mechanisms of LacCer on endothelial cells in porcine coronary arteries and human coronary endothelial cells (HCAECs).
Materials/Methods
The vessel rings and HCAECs were treated with different concentrations of LacCer for 24 hours. Vasomotor function was studied using a myograph tension system in response to thromboxane A2 analog U46619, to bradykinin and sodium nitroprusside (SNP). Superoxide anion production was determined using lucigenin enhanced chemiluminescence. The expression of endothelial nitric oxide synthase (eNOS), NADPH oxidase subunit NOX4 and catalase was determined by real-time PCR.
Results
LacCer (0.1, 1 and 10 μM) significantly decreased endothelium-dependent vasorelaxation (bradykinin) in porcine coronary artery rings in a concentration-dependent manner compared with untreated controls (P<0.05). High concentration of LacCer (10 μM) also reduced endothelium-independent vasorelaxation (SNP). However, LacCer did not affect vessel contraction (U46619). Antioxidant selenomethionine (SeMet) effectively reversed LacCer-induced endothelial dysfunction in the vessel rings. Furthermore, LacCer significantly increased superoxide anion production in the vessel rings in a concentration-dependent manner compared with untreated controls (P<0.05). In response to LacCer treatment, NOX4 mRNA levels were significantly increased, while the expression of catalase and eNOS was significantly decreased in HCAECs compared with controls (P<0.05).
Conclusions
LacCer causes endothelial dysfunction with potential mechanisms of the down-regulation of eNOS and increase of oxidative stress due to the activation of NADPH oxidase and inhibition of internal antioxidant catalase. This study suggests that LacCer may represent a risk factor to the vascular system and antioxidant SeMet may have clinical applications for prevention of vascular disease.
Keywords: Lactosylceramide, endothelial dysfunction, eNOS, Coronary artery, Oxidative stress, NOX4, calatase
BACKGROUND
Lactosylceramide (LacCer), a bioactive sphingolipid, is an important second-messenger in multiple atherosclerotic processes [1]. Schissel et al., who found a 10- to 50-fold higher content of LacCer in low density lipoproteins (LDL) of atherosclerotic lesions when compared with plasma LDL [2]. The accumulation of LacCer and other sphingolipids noted in atherosclerosis appears to influence the atherogenic process by affecting lipoprotein metabolism, and lipid efflux. However, the molecular mechanisms of the action of LacCer in the vascular system remain largely unknown although many investigations of the potential role of LacCer on the vascular disease formation have been carried out [1,2].
Bioavailability of nitric oxide (NO) is a critical factor to maintain normal vascular functions including vasomotor reactivity, anti-thrombosis state, barrier function and non-adhesive state to inflammation cells [3]. In the endothelium, endothelial nitric synthase (eNOS) converts L-arginine to L-citrulline and NO. Active NO levels are largely regulated by eNOS gene expression or its activity [4]. Many cardiovascular risk factors could negatively affect NO levels by different mechanisms. Reactive oxygen species including superoxide anion could react with NO and reduce NO bioavailability [5]. A decrease in the relative bioavailability of NO not only impairs endothelium-dependent vasorelaxation, but also activates other mechanisms that play an important role in atherogenesis [6]. However, it is not clear whether LacCer could affect vasomotor reaction and eNOS expression and ROS production in the vascular system.
The objective of this study was to determine the effects and molecular mechanisms of LacCer on endothelial cells in porcine coronary arteries and human coronary endothelial cells (HCAECs). This study may advance our understanding of new risk factors for the vascular disease formation and suggest new strategies for treatment or prevention of vascular disease.
MATERIALS AND METHODS
Chemicals and Reagents
Dimethyl sulfoxide (DMSO), thromboxane A2 analog U46619, bradykinin, sodium nitroprusside (SNP), seleno-L-methionine (SeMet), and Tri-reagent kit were obtained from Sigma Chemical (St. Louis, MO, USA). Dulbecco’s Modified Eagle Medium (DMEM) was obtained from Life Technologies (Grand Island, NY, USA). Bovine LacCer was purchased from EMD Chemicals Inc. (San Diego, CA, USA). Lucigenin was obtained from Molecular Probes (Eugene, OR, USA).
Tissue harvest and cell culture
Fresh porcine hearts were harvested from farm pigs (6- to 8-mo-old males) at a local slaughterhouse as previously described from our studies [7–10]. Briefly, porcine right coronary arteries were carefully dissected and cut into 5-mm rings. Several rings from each heart were allocated into groups including controls (DMEM), those treated with LacCer (0.01, 0.1, 1 and 10 μM) and those treated with LacCer plus antioxidant SeMet (20 μM). HCAECs, purchased from Cambrex (San Diego, CA, USA), were used. Once cells grew to 80–90% confluence in 6-well culture plates, they were treated with DMEM as control or with LacCer (0.01, 0.1, 1 and 10 μM) for 24 hours at 37°C. Cells were then harvested, and total mRNA was extracted for real-time PCR study.
Myograph analysis
The myograph tension system used in our laboratory has been previously described [7–10]. Briefly, the rings were cultured in the medium for 24 hours and then were suspended between the wires of the organ bath chamber (myograph system 700MO; Myo Technology, Aarhus N, Denmark) in 6 mL of Krebs solution. After equilibration, each ring was pre-contracted with 20 μL U46619 (10−7 M). After 60–90 minutes of contraction, the relaxation concentration-response curve was generated by adding 60 μL of five cumulative additions of the endothelium-dependent vasodilator bradykinin (10−9, 10−8, 10−7, 10−6, and 10−5 M) every 3 minutes. In addition, 60 μL of SNP (10−6 M) was added to the organ bath, and the endothelium-independent vasorelaxation was recorded.
Detection of superoxide anion
Levels of superoxide anion produced by endothelial cells were detected using the lucigenin-enhanced chemiluminescence method as previously described in our studies [7–10]. Briefly, the rings were cut open longitudinally and trimmed into 5 × 5-mm pieces. An assay tube (12 × 75 mm) was filled with 500 μL of buffer and 25 μL of lucigenin (final concentration 5 μM). The vessel segments were placed endothelium side down in the tubes so signals from the endothelial layer could be recorded. Time-based reading of the luminometer was recorded. The data, in relative light units ((RLU) per second for each sample, were averaged between 5 and 10 minutes. Values of blank tubes containing the same reagents as the vessel ring samples were subtracted from their corresponding vessel samples. The area of each vessel segment was measured with a caliper and was used to normalize the data for each sample. Final data were presented as RLU per second per square millimeter.
Real-time PCR
Endothelial cells of porcine coronary arteries were collected by scraping the luminal surface with surgical blades. HCAECs were collected from the cultures by trypsin digestion. Total RNA was isolated by use of Tri-Reagent following the manufacturer’s instructions. The mRNA levels of human eNOS, NADPH oxidase subunit NOX4 and catalase as well as glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were determined by real-time PCR analysis as previous described in our studies [7–10]. Sample cycle threshold (Ct) values were determined from plots of relative fluorescence units versus PCR cycle number during exponential amplification so that sample measurement comparisons were possible. The eNOS mRNA levels in each sample were calculated as 2^(40-Ct) and further normalized to GAPDH expression as 2^(Ct[GAPDH] − Ct[gene of interest])
Statistical analysis
Statistical analysis was performed on the Data Analysis tool of the Microsoft Excel program (Microsoft Office 2007, Microsoft, Seattle, WA, USA). Data were expressed as means ± SE. Significant difference of data between the control and treated groups was determined by the paired Student’s t-test (two-tail). The final data points of all contractions and relaxations among different groups were also analyzed by ANOVA test. P<0.05 was considered statistically significant.
RESULTS
LacCer decreases endothelium-dependent vasorelaxation in porcine coronary arteries
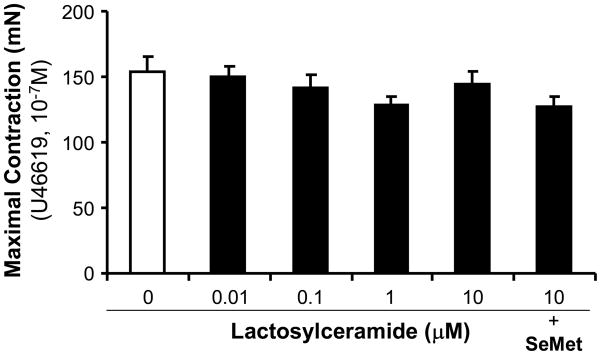
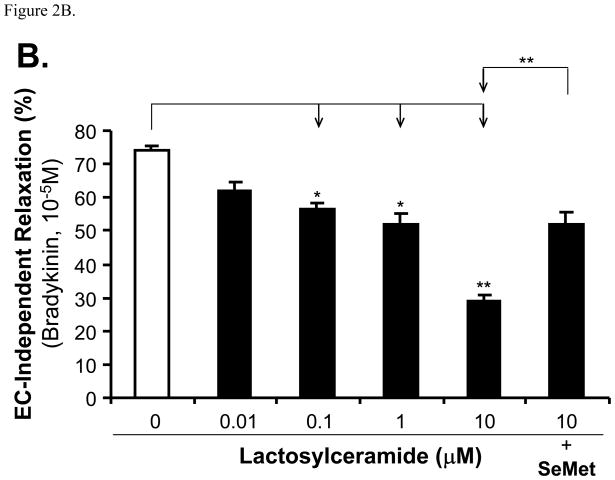
Porcine coronary artery rings were cultured overnight with DMEM as control or treated with different LacCer (0.01, 0.1, 1 and 10 μM). Contraction was achieved by using the thromboxane analog U46619 (10−7 M). The maximum contraction in all groups did not show statistical differences compared with controls (Figure 1). The endothelium-dependent vasorelaxation was induced by cumulative concentrations of bradykinin (Figure 2A). For example, LacCer treatment (0.1, 1 and 10 μM) significantly reduced the vasorelaxation in response to bradykinin (10−5 M) by 24, 30 and 61%, respectively, compared with untreated vessel rings (Figure 2B). Finally, the endothelium-independent vasorelaxation was induced with SNP (10−5 M). With this treatment, only highest concentration of LacCer (10 μM)-treated vessel rings showed 35% reduction compared with control vessels. Other concentrations of LacCer treatments did not show any difference of endothelium-independent vasorelaxation (Figure 3). Antioxidant SeMet was used in combination with LacCer (10 μM) and effectively blocked LacCer-induced decrease in endothelium-dependent and endothelium-independent vasorelaxation (Figure 2 and Figure 3).
Figure 1.
Effects of LacCer and SeMet on contractility in porcine coronary artery rings. The vessel rings were treated with different concentrations of LacCer (0.01 to 10 μM) with or without SeMet (20 μM) for 24 hours. The vasomotor reactivity was analyzed with a myograph system. The maximal contraction was recorded in response to thromboxane A2 analog U46619 (10−7 M). n = 8.
Figure 2.
Effects of LacCer and SeMet on endothelium-dependent vasorelaxation in porcine coronary artery rings. The vessel rings were pre-contracted with U46619 (10−7 M), and then subjected for endothelium-dependent vaorelaxation in response to accumulated concentrations of bradykinin (10−9 to 10−5 M). The percentage of tension change was recorded. A. Vessel responses to serial concentrations of bradykinin (10−9 to 10−5 M). B. Vessel responses to serial concentrations of bradykinin (10−5 M). n=8. *P<0.05.
Figure 3.
Effects of LacCer and SeMet on endothelium-independent vasorelaxation in porcine coronary artery rings. The vessel rings were pre-contracted with U46619 (10−7 M), and then subjected for endothelium-independent vaorelaxation in response to sodium nitroprusside (SNP, 10−5 M). The percentage of tension change was recorded. n=8. *P<0.05.
LacCer increases superoxide anion production in porcine coronary arteries
To determine whether oxidative stress could be involved in LacCer-induced endothelial dysfunction, superoxide anion levels were detected by using the lucigenin-enhanced chemiluminescence assay. Porcine coronary artery rings were incubated overnight with DMEM (control) and different LacCer concentrations (0.1, 1 and 10 μM). LacCer at concentrations of 0.1 and 10 μM significantly increased superoxide anion production by 118 and 159%, respectively, compared with controls (P<0.05, Figure 4).
Figure 4.
Effect of LacCer on superoxide anion production in porcine coronary artery rings. The vessel rings were treated with different concentrations of LacCer (0.01, 0.1 and 10 μM) for 24 hours. Superoxide anion levels at the endothelial layer of the rings were determined by the lucigenin-enhanced chemiluminescence method. n=4. *P<0.01 and **P<0.01
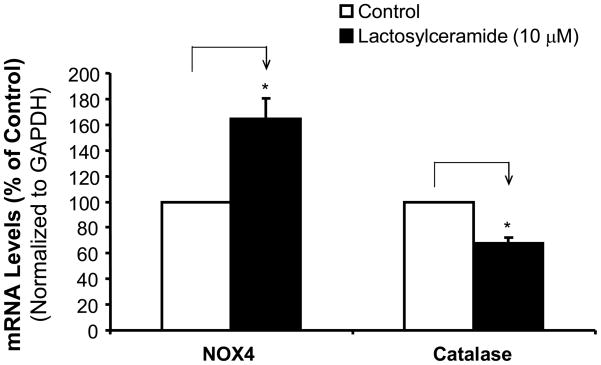
LacCer increases NOX4 mRNA levels, while decreasing catalase mRNA levels in HCAECs
To determine potential sources of ROS production, we determine the expression of ROS generating enzyme NADPH oxidase subunit NOX4 and intracellular antioxidant enzyme catalase in HCAECs. The cells were treated with LacCer (10 μM) for 24 hours, the mRNA levels of NOX4 and catalase were determined by real time PCR. Interestingly, LacCer significantly increased NOX4 mRNA levels by 61% compared with untreated control cells (P<0.05), while LacCer decreased catalase mRNA levels by 32% compared with controls in HCAECs (P<0.05, Figure 5).
Figure 5.
Effects of LacCer on mRNA levels of NOX4 and catalase in HCAECs. The cells were treated with LacCer (10 μM) for 24 hours, and the mRNA levels of NOX4 and catalase were determined by real time PCR and normalized to GAPDH mRNA levels. n=4. *P<0.05.
LacCer decreases eNOS expression in HCAECs
HCAECs were treated with LacCer with increasing concentrations (0.01, 0.1, 1 and 10 μM) for 24 hours. The mRNA levels of eNOS were determined by real time PCR. LacCer treatment significantly reduced eNOS mRNA levels in HCAECs in a concentration-dependent manner (Figure 6). For example, LacCer at concentrations of 0.1, 1 and 10 μM significantly reduced eNOS mRNA levels by 42, 61 and 67%, respectively, compared with untreated control cells (P<0.05, Figure 6).
Figure 6.
Effects of LacCer on eNOS mRNA levels in HCAECs. The cells were treated with different concentrations of LacCer for 24 hours and the eNOS mRNA levels were determined by real time PCR and normalized to GAPDH mRNA levels. n=4. *P<0.05 and **P<0.01.
DISCUSSION
In the present study, we demonstrate that LacCer significantly decreases endothelium-dependent vasorelaxation and oxidative stress in porcine coronary arteries in a concentration-dependent manner, and that antioxidant SeMet effectively blocks LacCer-induced endothelial dysfunction. In addition, we also demonstrate that LacCer is able to increase the expression of ROS generating enzyme NADPH oxidase subunit NOX4, while it decreases the expression of intracellular antioxidant enzyme catalase and eNOS in HCAECs. We have discovered new functions of LacCer in the vascular system, which may contribute to the vascular lesion formation.
NO, produced by eNOS, is one of the most important regulatory mechanisms in vascular homeostasis such as endothelium-dependent vasorelaxation. Loss of NO bioavailability due to reduced synthesis and increased scavenging by ROS is a major cause of endothelial dysfunction in vascular disease states. Our data clearly demonstrate that LacCer causes endothelial dysfunction and downregulates eNOS expression in the vascular system. Several lines of investigations have shown that different types of ceramides play an important role in NO related endothelial functions in the vascular system although controversies exist. Treatment with C2-ceramide significantly reduced endothelium-dependent vasorelaxation in small bovine coronary arteries in response to bradykinin. This effect was NO specific because C-ceramide had no further inhibitory effect on the vasorelaxation in response to bradykinin in the presence of eNOS inhibitor (L-NAME) [11]. However, Mogami et al. found that exogenous sphingomyelinase-induced endothelium-dependent relaxation via eNOS in bovine coronary arteries and that C8 ceramide also had vasodilatory effects, albeit less profound [12]. Cell culture studies have shown that ceramide reduces the release of bioactive NO in human umbilical vein endothelial cells [13, 14].
Overwhelming evidence demonstrates that ceramide-induced oxidative stress may be the major reason for its effects on the reduction of NO bioavailability and endothelial dysfunction. We had also noted a significant increase in superoxide anion production in LacCer-treated porcine coronary arteries in a concentration-dependent manner, and antioxidant SeMet effectively blocked LacCer-induced endothelial dysfunction in porcine coronary arteries. Thus, dietary SeMet may be of therapeutic application in treatment or prevention of LacCer-associated vascular disease. Other reports also showed that ceramide and/or other sphingolipids stimulate the production of superoxide anion in vascular cells [15–17]. Accordingly, pretreatment of the arteries with tiron, a chemical mimetic of SOD that is capable of removing superoxide anion could prevent ceramide-induced decreases in NO levels and endothelial dysfunction in small coronary arteries [18]. Superoxide production was noted to be stimulated 4-fold when aortic smooth muscle cells were incubated with LacCer [19]. Similarly, Yeh et al. noted that LacCer was able to induce superoxide generation via a plasma membrane associated NADPH oxidase [20]. Zhang and colleagues found that ceramide caused a time-dependent rise in superoxide anion relative to controls and that this was attenuated by treatment with NADPH oxidase inhibitor. The activation of NADPH oxidase and the ensuing increase in superoxide anion are likely important clinical events as they are implicated in the pathophysiology of cardiovascular diseases such as atherosclerosis [21]. We essentially noted that the treatment with LacCer not only significantly increased the expression of NADPH oxidase subunit NOX4, while decreased intracellular antioxidant enzyme catalase mRNA levels, which may be the mechanisms of LacCer-induced oxidative stress in the vascular system.
In conclusion, the current study demonstrates that LacCer has the ability to decrease endothelium-dependent vasorelaxation in porcine coronary arteries. Not surprisingly, LacCer also increased superoxide anion production. This is likely a consequence of the increase in NOX4 and decrease in catalase. Overall, all these effects initiated by LacCer culminate in the decreased eNOS expression and NO bioavailability, which may greatly contribute to the vascular disease formation.
Acknowledgments
Source of support: This work was partially supported by research grants from the National Institutes of Health (Lin: HL076345 and Chen: HL65916 and HL72716) and by the Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA.
References
- 1.Bismuth J, Lin P, Yao Q, Chen C. Ceramide: a common pathway for atherosclerosis? Atherosclerosis. 2008;196:497–504. doi: 10.1016/j.atherosclerosis.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schissel SL, Tweedie-Hardman J, Rapp JH, Graham G, Williams KJ, Tabas I. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J Clin Invest. 1996;98:1455–64. doi: 10.1172/JCI118934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Z, Ming XF. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clin Med Res. 2006;4:53–65. doi: 10.3121/cmr.4.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bredt DS. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res. 1999;31:577–96. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- 5.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 6.Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105:32S–9S. doi: 10.1016/s0002-9343(98)00209-5. [DOI] [PubMed] [Google Scholar]
- 7.Chai H, Zhou W, Lin P, Lumsden A, Yao Q, Chen C. Ginsenosides block HIV protease inhibitor ritonavir-induced vascular dysfunction of porcine coronary arteries. Am J Physiol Heart Circ Physiol. 2005;288:H2965–71. doi: 10.1152/ajpheart.01271.2004. [DOI] [PubMed] [Google Scholar]
- 8.Dhadwal AK, Wang X, Annambhotla S, Lin PH, Yao Q, Chen C. Capsaicin blocks HIV protease inhibitor ritonivir-induced vascular dysfunction in porcine pulmonary arteries. Med Sci Monit. 2009;15:BR1–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Chai H, Lin P, Yao Q, Chen C. Roles and mechanisms of HIV protease inhibitor ritonavir and other anti-HIV drugs in endothelial dysfunction of porcine pulmonary arteries and human pulmonary artery endothelial cells. Am J Path. 2009;174:771–81. doi: 10.2353/ajpath.2009.080157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Chai H, Wang X, Jiang J, Jamaluddin MS, Liao D, Zhang Y, Wang H, Bharadwaj U, Zhang S, Li M, Lin P, Yao Q. Soluble CD40 ligand induces endothelial dysfunction in human and porcine coronary artery endothelial cells. Blood. 2008;112:3205–16. doi: 10.1182/blood-2008-03-143479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang DX, Zou AP, Li PL. Ceramide reduces endothelium-dependent vasodilation by increasing superoxide production in small bovine coronary arteries. Circ Res. 2001;88:824–31. doi: 10.1161/hh0801.089604. [DOI] [PubMed] [Google Scholar]
- 12.Mogami K, Kishi H, Kobayashi S. Sphingomyelinase causes endothelium-dependent vasorelaxation through endothelial nitric oxide production without cytosolic Ca(2+) elevation. FEBS Letters. 2005;579:393–7. doi: 10.1016/j.febslet.2004.11.100. [DOI] [PubMed] [Google Scholar]
- 13.Igarashi J, Thatte HS, Prabhakar P, Golan DE, Michel T. Calcium-independent activation of endothelial nitric oxide synthase by ceramide. Proc Natl Acad Sci U S A. 1999;96:12583–8. doi: 10.1073/pnas.96.22.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulotta S, Barsacchi R, Rotiroti D, Borgese N, Clementi E. Activation of the endothelial nitric-oxide synthase by tumor necrosis factor-alpha. A novel feedback mechanism regulating cell death. J Biol Chem. 2001;276:6529–36. doi: 10.1074/jbc.M006535200. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Junk P, Huwiler A, Burkhardt C, Wallerath T, Pfeilschifter J, Forstermann U. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation. 2002;106:2250–6. doi: 10.1161/01.cir.0000035650.05921.50. [DOI] [PubMed] [Google Scholar]
- 16.Harada-Shiba M, Kinoshita M, Kamido H, Shimokado K. Oxidized low density lipoprotein induces apoptosis in cultured human umbilical vein endothelial cells by common and unique mechanisms. J Biol Chem. 1998;273:9681–7. doi: 10.1074/jbc.273.16.9681. [DOI] [PubMed] [Google Scholar]
- 17.Bhunia AK, Han H, Snowden A, Chatterjee S. Redox-regulated signaling by lactosylceramide in the proliferation of human aortic smooth muscle cells. J Biol Chem. 1997;272:15642–9. doi: 10.1074/jbc.272.25.15642. [DOI] [PubMed] [Google Scholar]
- 18.Hein TW, Kuo L. LDLs impair vasomotor function of the coronary microcirculation: role of superoxide anions. Circ Res. 1998;83:404–14. doi: 10.1161/01.res.83.4.404. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee S. Sphingolipids in atherosclerosis and vascular biology. 1. Arterioscler Thromb Vasc Biol. 1998;18:1523–33. doi: 10.1161/01.atv.18.10.1523. [DOI] [PubMed] [Google Scholar]
- 20.Yeh LH, Kinsey AM, Chatterjee S, Alevriadou BR. Lactosylceramide mediates shear-induced endothelial superoxide production and intercellular adhesion molecule-1 expression. J Vas Res. 2001;38:551–9. doi: 10.1159/000051091. [DOI] [PubMed] [Google Scholar]
- 21.Williams RD, Wang E, Merrill AH., Jr Enzymology of long-chain base synthesis by liver: characterization of serine palmitoyltransferase in rat liver microsomes. Arch Biochem Biophys. 1984;228:282–91. doi: 10.1016/0003-9861(84)90069-9. [DOI] [PubMed] [Google Scholar]