Abstract
The polyamines spermidine and spermine, and their precursor putrescine, are required for cell growth and cellular functions. The high levels of tissue polyamines are implicated in carcinogenesis. The major sources of exogenous polyamines are diet and intestinal luminal bacteria in gastrointestinal (GI) tissues. Both endocytic and solute carrier-dependent mechanisms have been described for polyamine uptake. Knocking down of caveolin-1 protein increased polyamine uptake in colon cancer-derived HCT116 cells. Dietary supplied putrescine was accumulated in GI tissues and liver in caveolin-1 knockout mice more than wild-type mice. Knocking out of nitric oxide synthase (NOS2), which has been implicated in the release of exogenous polyamines from internalized vesicles, abolished the accumulation of dietary putrescine in GI tissues. Under conditions of reduced endogenous tissue putrescine contents, caused by treatment with the polyamine synthesis inhibitor difluoromethylornithine (DFMO), small intestinal and colonic mucosal polyamine contents increased with dietary putrescine levels, even in mice lacking NOS2. Knocking down the solute carrier transporter SLC3A2 in HCT116-derived Hkh2 cells reduced the accumulation of exogenous putrescine and total polyamine contents in DFMO treated cells, relative to non-DFMO-treated cells. These data demonstrate that exogenous putrescine is transported into GI tissues by caveolin-1- and NOS2-dependent mechanisms, but that the solute carrier transporter SLC3A2 can function bidirectionally to import putrescine under conditions of low tissue polyamines.
Keywords: caveolin-1, NOS2, SLC3A2, gastrointestinal tissue
polyamines are small organic compounds that have two or more amino groups and are found in almost all organisms (6, 13). The major polyamines found in cells are spermidine and spermine, and their precursor putrescine. Among these, putrescine and spermidine are essential factors for cell growth (4). Polyamines can bind to anions such as DNA, RNA, and ATP and can thereby regulate their functions (9). The major sources of exogenous polyamines come from diet and luminal bacteria (11). An antibiotic treatment to remove microbial flora activity (7) or polyamine-free diet (15) increased the polyamine-depleting effect of the polyamine biosynthetic enzyme inhibitor difluoromethylornithine (DFMO). These results indicate that transport makes a significant contribution to cellular polyamine levels.
We have identified the amino acid transporter SLC3A2 as a polyamine export protein in colon cancer-derived cells (24). SLC3A2 associated with the polyamine catabolic enzyme spermidine/spermine N1-acetyltransferase, SAT1, and catalyzed the export of acetylated polyamines by the polyamine/arginine exchange reaction. As for polyamine uptake, we have reported that polyamine uptake was mediated by caveolae-dependent endocytosis in colon cancer-derived cells (16).
Caveolae are flask-shaped invaginations of plasma membrane with a diameter of 50–100 nm. They have been implicated in endocytosis and signal transductions (10, 12). Caveolin-1 is a major structural protein of caveolae in nonmuscle cells and negatively regulates the caveolae-dependent endocytosis by stabilizing caveolae structure (19). In a previous study, we showed that knocking down caveolin-1, using an anti-sense RNA method, increased polyamine uptake activity in colon cancer-derived HCT116 cells (16).
Polyamine transport is well characterized in Escherichia coli and Saccharomyces cerevisiae (8, 20–23). There are several polyamine transport proteins classified as ATP binding cassette transporters and proton potential-dependent solute carriers. In mammalian cells, no transporters like these are identified. The importance of caveolar endocytosis-dependent polyamine transport in animal is not clear.
In this report, we examined the significance of caveolae-dependent endocytosis in polyamine uptake in vivo using genetically engineered mice. We also investigated the role of SLC3A2 in polyamine transport in colon-derived cells. We found that putrescine uptake in gastrointestinal tissues was mediated by caveolar endocytosis- and nitric oxide synthase (NOS2)-dependent mechanisms and, under specific conditions, SLC3A2 catalyzed putrescine uptake.
MATERIALS AND METHODS
Cell culture.
The human colorectal carcinoma cell lines HCT116, transfected with mock vector (HCT116/Mock) or caveolin-1 antisense (HCT116/Cav-1 A. S.), were a kind gift from Dr. B. Sloane and Dr. D. Cadavello-Medved (3). The Hkh2 cell line transfected with short hairpin RNA (shRNA) for SLC3A2 was isolated previously (24). These cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. Puromycin was supplied at 0.5 μg/ml in the HCT116/Mock and HCT116/Cav-1 A. S. culture media. Cells were maintained in a humidified incubator at 37°C with 5% CO2.
Animals.
The wild-type B6129SF2/J, caveolin-1 knockout STOCK Cav1tm1Mls/J, and NOS2 knockout C57BL/6-NOS2tmlLau/J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All the animals were raised in cages under nonsterile microisolator conditions and fed with the AIN-93G diet (Harlan Teklad, Indianapolis, IN). Putrescine and/or d,l-α-difluoromethylornithine (DFMO) was supplemented in drinking water at 1%. After 2 wk, mice were euthanized by CO2 inhalation and the small intestine and colon were collected.
Measurement of polyamine content in mice tissues.
Mice tissues (20 mg) were homogenated in 0.2 N HClO4 and acid soluble fractions were separated by reverse-phase ion pair HPLC. Polyamines were detected as described by Seiler and Knodgem (17). Total protein contents were determined by the bicinchoninic acid (BCA) protein assay reagent (Pierce, Rockford, IL).
Polyamine transport assay in cells.
For kinetic analysis, polyamine uptake activity was measured as described previously (24) with use of [3H]putrescine (37 MBq/mmol, GE Healthcare), [3H]spermidine (37 MBq/mmol, GE Healthcare), or [14C]spermine (37 MBq/mmol, GE Healthcare) as substrates. For putrescine accumulation assay, 106 cells were cultured in the presence of 5 mM [3H]putrescine (37 MBq/mmol) with or without 5 mM DFMO and 50 μM 1400W (N-([3-(aminomethyl)phenyl]methyl)ethanimidamide dihydrochloride; Sigma) for 2 days. Cells were washed twice with PBS containing 10 mM putrescine and lysed in 0.5 N NaOH. Radioactivity was counted by use of a Beckman LS 5000TD scintillation counter. Total cellular protein content was determined by the BCA protein assay reagent.
Western blot analysis.
Cells and mouse tissues were washed with buffered saline and homogenized in lysis buffer (10 mM Tris·HCl, pH 8.0 containing 10 μg/ml aprotinin, 500 μM sodium orthovanadate, 10 μg/ml phenylmethylsulfonyl fluoride). After brief centrifugation, supernatant was used for Western blot analysis. Protein (40 μg) was separated on a 10% polyacrylamide gel and transferred electrophoretically to a Hybound-C nitrocellulose membrane (Amersham, Arlington Heights, IL). Caveolin-1, NOS2, NOS3, SLC3A2, flotilin 1, and β-tubulin were detected by ECL Western blotting detection system (GE Healthcare) using anti-caveolin-1 (1:2,000 dilution, Santa Cruz Biotechnology), anti-NOS2 (1:2,500 dilution, BD Transduction Laboratories), anti-NOS3 (1:2,500 dilution, BD Transduction Laboratories), anti-SLC3A2 (1:1,000 dilution, Santa Cruz Biotechnology), anti-flotilin 1 (1:1,000 dilution, BD Transduction Laboratories), and anti-β-tubulin (1:10,000 dilution, Santa Cruz Biotechnology) as primary antibodies.
RESULTS
Caveolin-1 negatively regulates polyamine uptake activity.
We have reported that caveolae-dependent endocytosis played an important role in polyamine uptake (16). To obtain the detailed information, we determined kinetic characteristics of polyamine uptake. Figure 1 shows polyamine uptake activities of HCT116 cells transfected with mock vector (HCT116/Mock) and caveolin-1 antisense (HCT116/Cav-1 A. S.). Caveolin-1-dependent transport of polyamines was observed in HCT116 cells (Fig. 1), which putrescine transported more than spermidine, which was transported more than spermine in a molar basis. The effect of caveolin-1 on spermine transport was less than that for either putrescine or spermidine, but still statistically significant (P < 0.05, Fig. 1C). Km and Vmax values for polyamine uptake are summarized in Table 1. Km values for putrescine, spermidine, and spermine uptake were at the same levels in two cell lines (P > 0.1). Vmax values for putrescine and spermidine uptake were significantly higher in HCT116/Cav-1 A. S. cells, compared with HCT116/Mock cells. An increase in Vmax value for spermine uptake in HCT116/Cav-1 A. S. cells was small but statistically significant (P < 0.01). These results indicated that caveolin-1 negatively regulated polyamine uptake by decreasing uptake rate. Caveolin-1 did not change the affinity for polyamines. The knockdown of caveolin-1 in HCT116/Cav-1 A. S. cells was confirmed by Western blot analysis (Fig. 1D).
Fig. 1.
Effect of caveolin-1 on polyamine transport. Putrescine (A), spermidine (B), and spermine (C) uptake activities in HCT116/Mock (○) and HCT116/Cav-1 A. S. cells (●) were measured as described in materials and methods. Values are means ± SE of triplicate determinations. Curve fitting was calculated by using the Microsoft Excel program. D: caveolin-1 protein levels in HCT116/Mock and HCT116/Cav-1 A. S. cells. Flotilin 1 levels are shown as a loading control. *P < 0.05 against HCT116/Mock.
Table 1.
Km and Vmax values for polyamine uptake
| Cells | Substrate | Km, μM | Vmax, nmol•min−1•mg protein−1 |
|---|---|---|---|
| HCT116/Mock | Putrescine | 542.15 ± 21.12 | 1.11 ± 0.21 |
| Spermidine | 6.44 ± 0.60 | 0.12 ± 0.01 | |
| Spermine | 2.93 ± 0.07 | 0.09 ± 0.01 | |
| HCT116/Cav-1 A. S. | Putrescine | 606.96 ± 24.17 | 3.04 ± 0.39* |
| Spermidine | 6.84 ± 1.12 | 0.30 ± 0.02* | |
| Spermine | 2.78 ± 0.02 | 0.11 ± 0.01* |
Values are means ± SE of triplicate determinations.
P < 0.01 against HCT116/Mock.
Caveolae-dependent endocytosis and NOS2 play significant roles in polyamine transport in vivo.
We examined the significance of caveolae-dependent endocytosis in polyamine uptake in vivo using caveolin-1 knockout mice. Dietary polyamine uptake was analyzed by measuring polyamine contents in the small intestine, colon, and liver after supplementation of 1% putrescine in drinking water for 2 wk. DFMO was used to inhibit synthesis of endogenous polyamines.
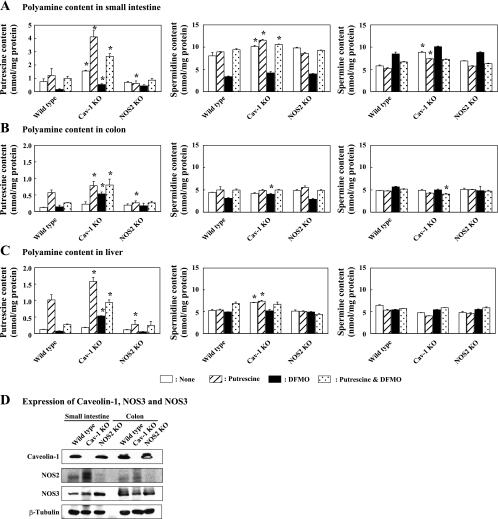
We examined the role of NOS2 in polyamine transport, as others have suggested that nitric oxide (NO)-dependent cleavage of heparan sulfate was required for release of polyamines from endocytic vesicles (1). As shown in Fig. 2, putrescine, spermidine, and spermine contents were high in the small intestine of caveolin-1 knockout mice compared with wild-type and NOS2 knockout mice without treatment. The supplementation of 1% putrescine in drinking water increased putrescine contents in the small intestine, colon, and liver of wild-type mice. In caveolin-1 knockout mice, an accumulation of putrescine was significantly greater in all tissues compared with wild-type mice (P < 0.05). The accumulation of putrescine was significantly low in NOS2 knockout mice (P < 0.05 against wild type). These results indicated that the uptake of putrescine in the small intestine and colon were mediated mainly by caveolar endocytosis and a NOS2-dependent mechanism and affected liver polyamine levels. DFMO reduced putrescine content in the small intestine of wild-type and caveolin-1 knockout mice. On the other hand, DFMO significantly increased putrescine content in the colon and liver of caveolin-1 knockout mice (P < 0.05). The spermidine contents in the small intestine of wild-type, caveolin-1 knockout, and NOS2 knockout mice were reduced by DFMO treatment. DFMO treatment increased spermine content in the small intestine. The combination of putrescine and DFMO restored putrescine and spermidine contents in the small intestine, colon, and liver of all kinds of mice. The knockout of caveolin-1 and NOS2 was confirmed by Western blot analysis. As shown in Fig. 2D, the expression of caveolin-1 and NOS2 was not detected in caveolin-1 or NOS2 knockout mice, respectively. It was also found that NOS2 expression in the small intestine and colon was upregulated in the caveolin-1 knockout mouse. The expression level of NOS3 in the small intestine was increased in the NOS2 knockout mouse.
Fig. 2.
Effect of caveolin-1 and NOS2 knockout on putrescine transport in vivo. Wild-type, caveolin-1 knockout, and NOS2 knockout mice were treated with 1% putrescine (hatched bar), 1% difluoromethylornithine (DFMO; solid bar), and the combination of 1% putrescine and 1% DFMO (dotted bar) for 2 wk and polyamine contents in the small intestine (A), colon (B), and liver (C) were determined. Open bar, no treatment. Values are means ± SE of determinations in 3 mice. *Statistically significant (P < 0.05) against wild type. D: protein levels of caveolin-1, NOS2, and NOS3 in the small intestine and colon. β-Tubulin levels are shown as a loading control.
SLC3A2 mediates polyamine uptake in DFMO-treated cells.
As shown in Fig. 2, the combination of putrescine and DFMO restored putrescine and spermidine contents not only in wild-type and caveolin-1 knockout mice but also NOS2 knockout mice. Putrescine alone did not increase tissue polyamine levels in NOS2 knockout mice. These results suggested that, in DFMO-treated tissues, putrescine uptake was mediated by a NOS2-independent mechanism. We have reported that the cationic amino acid transporter SLC3A2 mediated putrescine export by putrescine/arginine antiport activity (24). We tested whether SLC3A2 can mediate uptake of putrescine in the reverse direction in DFMO-treated cells. As shown in Fig. 3A, 5 mM DFMO increased the accumulation of exogenous putrescine in mock-transfected cells. This increase of putrescine accumulation was significantly lower in SLC3A2 shRNA transfected cells compared with mock-transfected cells. Inhibition of NOS2 activity by using NOS2-specific inhibitor 1400W (5) decreased the accumulation of exogenous putrescine in both cells. DFMO increased the accumulation of putrescine in mock-transfected cells even in the presence of 1400W. This effect was significantly decreased in SLC3A2 shRNA transfected cells. These results indicated that SLC3A2 mediated the uptake of putrescine in DFMO-treated cells. The expression level of SLC3A2 was significantly decreased by shRNA transfection (Fig. 3B). The levels of caveolin-1 and NOS2 were not affected by SLC3A2 shRNA (Fig. 3B).
Fig. 3.
Effect of SLC3A2 on putrescine transport. Hkh2 cells transfected short hairpin RNA (shRNA) for SLC3A2 (solid bar) and mock vector (open bar) were cultured with 5 mM [3H]putrescine in the presence and absence of 5 mM DFMO and 50 μM 1400W for 2 days. [3H]putrescine accumulation was measured as described in materials and methods. Values are shown as means ± SE of triplicate determinations. *P < 0.01. B: SLC3A2, caveolin-1, and NOS2 protein levels in mock and SLC3A2 shRNA transfected cells. Flotilin 1 was shown as a loading control.
DISCUSSION
In this study, we found that polyamine transport in gastrointestinal tissues was mediated by both endocytic and solute carrier transport mechanisms. Knocking down of caveolin-1 increased Vmax for polyamine uptake but did not affect Km values (Table 1). These values indicated that induction of caveolar endocytosis by caveolin-1 knockdown increased the frequency and/or the number of polyamine internalization but did not change the affinity of polyamine binding to the cell surface. An increase in putrescine accumulation in caveolin-1 knockout mice indicated that the uptake of putrescine occurred via caveolar endocytosis. Putrescine uptake was diminished in NOS2 knockout mice. It has been suggested by others that NO was required for the release of polyamines from intracellular vesicles (1). Our results indicate that caveolar endocytosis and NO production, which is catalyzed by NOS2, played a major role in putrescine uptake in gastrointestinal tissues.
The decrease in tissue putrescine and spermidine contents caused by DFMO was restored by putrescine in wild-type mice as expected. Surprisingly, dietary putrescine also restored intestinal and colonic spermidine pools in NOS2 knockout mice (Fig. 2). This result implied that dietary putrescine was being taken up by an endocytosis-independent mechanism and subsequently metabolized into spermidine. The results in Fig. 3 indicate that SLC3A2 can function as a putrescine importer under conditions of high extracellular and low intracellular putrescine. As shown in Fig. 2, DFMO decreased tissue polyamine levels. Supplementation of putrescine to DFMO-treated tissues and cells forms a concentration gradient across the plasma membrane. It is possible that this concentration gradient drives SLC3A2 to function as a putrescine importer, rather than an exporter. Recently it was reported that bidirectional transport of amino acids by the solute carrier transporter containing SLC3A2 played a regulatory role in autophagy (14).
Inhibition of polyamine synthesis with DFMO caused putrescine contents in the colon and liver to increase, compared with the nontreated group in caveolin-1 knockout mice (Figs. 2, B and C). The same treatment caused putrescine contents to decrease in the small intestine (Fig. 2A). These results suggested that DFMO treatment increased uptake (2) of colonic luminal polyamines, possibly produced by luminal bacteria. This result suggests that colonic luminal bacterial polyamines can contribute significantly to tissue polyamine levels.
The expression of NOS2 in caveolin-1 knockout mice was increased compared with wild-type mice (Fig. 2C). It was reported that the expression of NOS2 was inhibited by caveolin-1 in neuroblastoma cells (18). The mechanism and the role of polyamines in the regulation of NOS2 expression are unknown and should be addressed in further studies. A compensatory increase in intestinal but not colonic NOS3 expression in the absence of NOS2 was observed as reported previously by us (25). NOS3 was mainly localized to the nucleus and cytoplasmic expression was low in NOS2 knockout mice (25). Because of this cellular distribution, polyamine uptake was likely not influenced by NOS3.
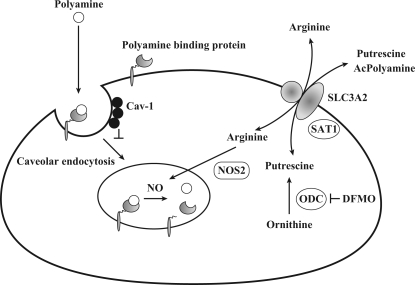
Polyamine transport mechanisms are summarized in Fig. 4. Polyamine uptake in gastrointestinal tissues was mainly mediated by caveolar endocytosis and a NOS2-dependent mechanism. Putrescine and acetylated polyamines are exported by SLC3A2 via a diamine/arginine exchange activity (24). In certain putrescine concentration gradients, SLC3A2 can catalyze the uptake of dietary putrescine by a reverse reaction. The transport of dietary and luminal bacterial polyamines significantly contributes to the tissue polyamine levels.
Fig. 4.
Model of polyamine transport in animal cells. The regulation of cellular polyamine levels by transport is depicted. The polyamine binds to polyamine binding protein(s) and is internalized by caveolar endocytosis, which is negatively regulated by caveolin-1 (Cav-1). The nitric oxide (NO) produced by NOS2 releases polyamine from polyamine binding protein(s). Putrescine and acetylated polyamines (AcPolyamine), which produced by spermidine/spermine N-acetyltransferase (SAT1), are exported by SLC3A2. When cells are treated with the polyamine biosynthesis inhibitor DFMO, SLC3A2 can catalyze the uptake of putrescine by a reverse reaction. ODC, ornithine decarboxylase.
GRANTS
This work was supported, in whole or in part, by National Institutes of Health Grants CA123065 and CA095060.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. B. Sloane and Dr. D. Cadavello-Medved, Department of Pharmacology, Barbara Karmanos Cancer Institute, Wayne State University, School of Medicine, Detroit, MI for providing HCT116/Mock and HCT116/Cav-1 A. S. cells. We also thank Dr. Shirasawa, Research Institute, International Medical Center of Japan, for kindly supplying Hkh2 cells.
REFERENCES
- 1.Belting M, Mani K, Jonsson M, Cheng F, Sandgren S, Jonsson S, Ding K, Delcros JG, Fransson LA. Glypican-1 is a vehicle for polyamine uptake in mammalian cells: a pivotal role for nitrosothiol-derived nitric oxide. J Biol Chem 278: 47181–47189, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Casero RAJ, Frydman B, Stewart TM, Woster PM. Significance of targeting polyamine metabolism as an antineoplastic strategy: unique targets for polyamine analogues. Proc West Pharmacol Soc 48: 24–30, 2005 [PubMed] [Google Scholar]
- 3.Cavallo-Medved D, Mai J, Dosescu J, Sameni M, Sloane BF. Caveolin-1 mediates the expression and localization of cathepsin B, pro-urokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cells. J Cell Sci 118: 1493–1503, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SS. A Guide to the Polyamines. Oxford, UK: Oxford University Press, 1998 [Google Scholar]
- 5.Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJ, Knowles RG. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem 272: 4959–4963, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Hamana K, Matsuzaki S. Polyamines as a chemotaxonomic marker in bacterial systematics. Crit Rev Microbiol 18: 261–283, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Hessels J, Kingma AW, Ferwerda H, Keij J, van den Berg GA, Muskiet FA. Microbial flora in the gastrointestinal tract abolishes cytostatic effects of alpha-difluoromethylornithine in vivo. Int J Cancer 43: 1155–1164, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Igarashi K, Ito K, Kashiwagi K. Polyamine uptake systems in Escherichia coli. Res Microbiol 152: 271–278, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Igarashi K, Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochem Biophys Res Commun 271: 559–564, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Kurzchalia TV, Parton RG. Membrane microdomains and caveolae. Curr Opin Cell Biol 11: 424–431, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Larqué E, Sabater-Molina M, Zamora S. Biological significance of dietary polyamines. Nutrition 23: 87–95, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Lisanti MP, Tang Z, Scherer PE, Kubler E, Koleske AJ, Sargiacomo M. Caveolae, transmembrane signalling and cellular transformation. Mol Membr Biol 12: 121–124, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Morgan DM, Wallace HM. Polyamines in clinical and basic science: introductory remarks. Biochem Soc Trans 22: 845–846, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136: 521–534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quemener V, Moulinoux JP, Havouis R, Seiler N. Polyamine deprivation enhances antitumoral efficacy of chemotherapy. Anticancer Res 12: 1447–1453, 1992 [PubMed] [Google Scholar]
- 16.Roy UK, Rial NS, Kachel KL, Gerner EW. Activated K-RAS increases polyamine uptake in human colon cancer cells through modulation of caveolar endocytosis. Mol Carcinog 47: 538–553, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiler N, Knodgen B. High-performance liquid chromatographic procedure for the simultaneous determination of the natural polyamines and their monoacetyl derivatives. J Chromatogr 221: 227–235, 1980 [DOI] [PubMed] [Google Scholar]
- 18.Shen J, Lee W, Li Y, Lau CF, Ng KM, Fung ML, Liu KJ. Interaction of caveolin-1, nitric oxide, and nitric oxide synthases in hypoxic human SK-N-MC neuroblastoma cells. J Neurochem 107: 478–487, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Stan RV. Structure of caveolae. Biochim Biophys Acta 1746: 334–348, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Uemura T, Kashiwagi K, Igarashi K. Uptake of putrescine and spermidine by Gap1p on the plasma membrane in Saccharomyces cerevisiae. Biochem Biophys Res Commun 328: 1028–1033, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Uemura T, Kashiwagi K, Igarashi K. Polyamine uptake by DUR3 and SAM3 in Saccharomyces cerevisiae. J Biol Chem 282: 7733–7741, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Uemura T, Tachihara K, Tomitori H, Kashiwagi K, Igarashi K. Characteristics of the polyamine transporter TPO1 and regulation of its activity and cellular localization by phosphorylation. J Biol Chem 280: 9646–9652, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Uemura T, Tomonari Y, Kashiwagi K, Igarashi K. Uptake of GABA and putrescine by UGA4 on the vacuolar membrane in Saccharomyces cerevisiae. Biochem Biophys Res Commun 315: 1082–1087, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Uemura T, Yerushalmi HF, Tsaprailis G, Stringer DE, Pastorian KE, Hawel L, Byus CV, Gerner EW. Identification and characterization of a diamine exporter in colon epithelial cells. J Biol Chem 283: 26428–26435, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yerushalmi HF, Besselsen DG, Ignatenko NA, Blohm-Mangone KA, Padilla-Torres JL, Stringer DE, Cui H, Holubec H, Payne CM, Gerner EW. The role of NO synthases in arginine-dependent small intestinal and colonic carcinogenesis. Mol Carcinog 45: 93–105, 2006. [DOI] [PubMed] [Google Scholar]