Abstract
Heart disease is the leading cause of morbidity and mortality. Cardiac gene transfer may serve as a novel therapeutic approach. This investigation was undertaken to compare cardiac tropisms of adeno-associated virus (AAV) serotypes 1, 6, 7, 8, and 9. Neonatal mice were injected with 2.5 × 1011 genome copies (GC) of AAV serotype 1, 6, 7, 8, or 9 expressing LacZ under the control of the constitutive chicken β-actin promoter with cytomegalovirus enhancer promoter via intrapericardial injection and monitored for up to 1 year. Adult rats were injected with 5 × 1011 GC of the AAV vectors via direct cardiac injection and monitored for 1 month. Cardiac distribution of LacZ expression was assessed by X-Gal histochemistry, and β-galactosidase activity was quantified in a chemiluminescence assay. Cardiac functional data and biodistribution data were also collected in the rat. AAV9 provided global cardiac gene transfer stable for up to 1 year that was superior to other serotypes. LacZ expression was relatively cardiac specific, and cardiac function was unaffected by gene transfer. AAV9 provides high-level, stable expression in the mouse and rat heart and may provide a simple alternative to the creation of cardiac-specific transgenic mice. AAV9 should be used in rodent cardiac studies and may be the vector of choice for clinical trials of cardiac gene transfer.
Introduction
Adeno-associated virus (AAV) is an ideal gene therapy vector because its low immunogenicity favors persistent transgene expression. The immune response evoked by cardiac AAV injection is negligible and not significantly elevated over the baseline response that occurs after treatment with saline or naked plasmid (Wright et al., 2001). This is in contrast to the profound immune response elicited by other viral vectors, such as adenovirus, herpesvirus, and to some extent, lentivirus (Wright et al., 2001; Vandendriessche et al., 2007). As a result, AAV vectors are capable of providing safe, long-term gene transfer in animal models to several organs, including liver, skeletal muscle, and heart (Gao et al., 2002; Arruda et al., 2005; Woo et al., 2005).
AAV is especially suited to serve as a gene therapy vector for cardiac diseases, which generally follow a chronic course and would therefore require safe, persistent transgene expression. Indeed, a phase 1/2 clinical trial using AAV1 to deliver the SERCA2a gene to patients with congestive heart failure (CHF) has already been proposed (Hajjar et al., 2008), and others are sure to follow. However, there are many other genes that may demonstrate clinical benefit in CHF and several other novel AAV serotypes that may transduce the heart more efficiently than AAV1 (Gao et al., 2002, 2004).
Rodent models offer a relatively quick and inexpensive system in which to screen and evaluate the therapeutic potential of such genes and serotypes before advancing to large animal and clinical trials. With respect to serotype, initial studies in the literature were conducted with AAV2 simply because this was the first serotype to be engineered into a vector (Carter, 2004). However, once additional serotypes were isolated (Gao et al., 2002, 2004), pseudotyped vectors soon went into production (Hildinger et al., 2001) and were evaluated for differential tissue tropism. In the mouse, an initial screen of AAV1–AAV5 identified AAV1 as the most cardiotropic serotype (Du et al., 2004), but later, more comprehensive studies that included AAV6–AAV9 all concur that AAV9 is the most cardiotropic serotype for the murine heart (Inagaki et al., 2006; Pacak et al., 2006; Vandendriessche et al., 2007; Zincarelli et al., 2008). However, although these studies were able to identify the most potent AAV serotype for cardiac gene transfer, none focused on combining highly efficient gene transfer with a delivery method that would limit systemic exposure. In the rat, AAV8 was identified as the serotype most efficient for cardiac gene transfer (Palomeque et al., 2007), but this study only evaluated AAV1–AAV8. In fact, a direct comparison of AAV9 with other serotypes has not been performed in the rat heart.
Our goal in this study was to compare the cardiac tropism of AAV1, which may soon be used in a clinical trial for heart failure (Hajjar et al., 2008), with those of the novel AAV serotypes 6, 7, 8, and 9 in the mouse and rat. To expand on the existing literature in the mouse, we delivered the virus by a subxiphoid injection technique to target the pericardial space in an effort to limit systemic exposure (Zhang et al., 1999). In the rat, this is the first direct comparison of AAV9 with other AAV serotypes in the heart. In both species, we sought to maximize transgene expression and performed a dose–response study to identify the minimal dose required for global delivery. Mice were monitored for up to 1 year to evaluate stability of expression, and rats underwent both hemodynamic and biodistribution analysis to determine the safety profile of AAV-mediated cardiac gene transfer.
Materials and Methods
Vector design and production
Each vector was designed to express the nuclear-localized LacZ reporter gene under the control of the constitutive chicken β-actin promoter with cytomegalovirus (CMV) enhancer (CB promoter). Vectors were produced according to the previously described pseudotyping protocol by the Vector Core of the University of Pennsylvania (Philadelphia, PA) (Gao et al., 2002). Briefly, recombinant AAV genomes containing AAV2 inverted terminal repeats (ITRs) were packaged by triple transfection of 293 cells with a cis-plasmid containing the LacZ transgene, an adenovirus helper plasmid, and a chimeric trans-plasmid containing the AAV2 rep gene fused to the capsid gene of the AAV serotype of interest.
Animal use and vector delivery protocol
All animals were handled in compliance with National Institutes of Health (Bethesda, MD) and institutional guidelines that were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Neonatal mice were injected with vector as previously described (Zhang et al., 1999). Briefly, 4- to 5-day-old mice (n = 4 per group) underwent cryoanesthesia, and a puncture was made at the left costoxiphoid angle of the anterior chest with a 33-gauge Hamilton needle. To avoid direct injection into the myocardium, microbore tubing (Tygon, I.D. 0.02 in.; Saint-Gobain Performance Plastics, Bridgewater, NJ) was threaded over the needle to leave 3 mm exposed at the end. This subxiphoid approach positions the needle beneath the sternum and anterior to the heart. Fifty microliters containing the AAV vector in normal saline was then injected into the pericardial space. Pups were subsequently rewarmed under a heat lamp and returned to their mothers for further care.
Adult rats (300 g, n = 4 per group) underwent left thoracotomy after intubation and mechanical ventilation, and 250 μl containing the AAV vector was injected directly into the myocardium of the left ventricular free wall in five equal aliquots from the base to apex. Animals were allowed to recover until euthanasia at 4 weeks. A subset of rats (n = 3 per group from AAV8 and AAV9) underwent functional analysis at 4 weeks, before euthanasia. These rats were subjected to two-dimensional (2-D) echocardiography followed by acquisition of pressure–volume loops using a 2F conductance catheter (Millar Instruments, Houston, TX). For placement of the conductance catheter, rats underwent sternotomy after intubation and mechanical ventilation, and the catheter was inserted into the left ventricular cavity via a stab incision through the apex of the heart.
Analysis of LacZ expression and vector biodistribution
Distribution of transgene expression in mouse and rat tissues was determined by staining with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) as previously described (Zhang et al., 1999). β-Galactosidase activity was quantified by Tropix Galacto-Light Plus assay (Applied Biosystems, Foster City, CA). For biodistribution analysis, samples were snap frozen in liquid nitrogen. After DNA extraction, genome copy titers were quantified by TaqMan polymerase chain reaction (PCR) (Applied Biosystems) using primers and probes designed against the LacZ transgene. An uninjected control was analyzed to confirm specificity of the assay.
Statistical analysis
Mean values from each experimental group were compared by one-way analysis of variance (ANOVA) with Student–Newman–Keuls post-hoc analysis.
Results
Evaluation of cardiac gene transfer by AAV serotypes in the mouse
AAV serotypes 1, 6, 7, 8, and 9 were evaluated for their ability to provide gene transfer to the mouse heart. Neonatal mice (day 4–5) were injected via the pericardial cavity with 2.5 × 1011 genome copies (GC) of AAV-CB-LacZ and killed at 6 weeks to analyze transgene expression by X-Gal staining. All serotypes were capable of providing highly efficient global cardiac gene transfer at this dose of vector (Fig. 1). AAV8 and AAV9 were also efficient at transducing the diaphragm (Fig. 1). Expression was low in the liver for all serotypes examined (Fig. 1).
FIG. 1.

Representative photomicrographs of sections from mouse heart, diaphragm, and liver 6 weeks after intrapericardial injection of 50 μl containing 2.5 × 1011 GC of AAV-CB-LacZ of the indicated serotype. Sections have been stained with X-Gal and counterstained with eosin. Scale bars: 1 mm for heart, 200 μm for liver and diaphragm.
Dose response of AAV serotypes in the mouse heart
Because all serotypes examined appeared to have similar cardiac tropism at the initial vector dose, a dose–response study was performed next. Two additional groups of mice were injected with AAV-CB-LacZ as described previously and monitored for 6 weeks: one at a dose of 2.5 × 1010 GC and another at a dose of 2.5 × 109 GC. AAV9 continued to provide high-level, global cardiac gene transfer at the intermediate dose, whereas expression mediated by the other serotypes declined sharply (Fig. 2a). At the low dose, AAV9 was still able to provide moderate cardiac transgene expression, whereas expression mediated by the other serotypes was negligible (Fig. 2a). In addition, although cardiac transgene expression continued to be high at the intermediate dose, expression in the liver and diaphragm was barely detectable (data not shown). A quantitative β-galactosidase assay was performed on cardiac tissue extracts from the intermediate group, and the results of this assay confirmed the X-Gal staining: activity in the AAV9-treated hearts was approximately 1 log higher than in the other serotypes (Fig. 2b).
FIG. 2.
Dose response of LacZ expression in the mouse heart 6 weeks after intrapericardial injection of 50 μl containing the indicated dose of AAV-CB-LacZ. (a) Representative photomicrographs of mouse heart stained with X-Gal and counterstained with eosin. Scale bars: 200 μm. (b) Graph displaying β-galactosidase activity as determined by quantitative chemiluminescence assay of samples from mice treated with the intermediate dose. Columns and error bars represent means and SD. Note that AAV9 is superior to the other serotypes evaluated at the intermediate dose (*p < 0.05 vs. other serotypes).
Time course of AAV9 expression in the mouse heart
Because AAV9 appears to be the serotype most tropic for the mouse heart, we next evaluated the stability of transgene expression in this group over time. Mice were treated with high-dose AAV9-CB-LacZ as described previously, and monitored for up to 1 year with euthanasia occurring at 1 week, 2 weeks, 3 weeks, 6 weeks, 7 months, and 1 year. Transgene expression was global and highly efficient at all time points examined (Fig. 3). Expression was detectable by 1 week and reached a peak by 2 weeks that was stable through 6 weeks. By 7 months and 1 year, numerous LacZ-positive cells were still present throughout the heart although their frequency was somewhat reduced (Fig. 3). Results of a quantitative β-galactosidase assay showed that enzyme activity had decreased by approximately 5-fold from 6 weeks to 1 year (data not shown).
FIG. 3.
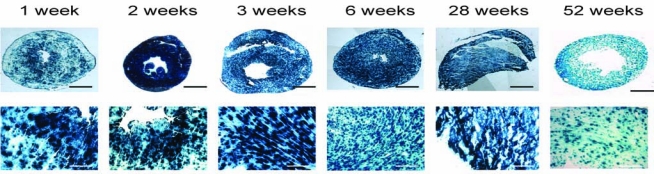
Time course of LacZ expression after intrapericardial injection of 50 μl containing 2.5 × 1011 GC of AAV9-CB-LacZ. Shown are representative photomicrographs of sections stained with X-Gal and counterstained with eosin. Scale bars: 1 mm for low magnification (top row), 200 μm for high magnification (bottom row).
Cardiac tropism of AAV serotypes in the rat
AAV serotypes 1, 7, 8, and 9 were next evaluated for their ability to provide gene transfer to the rat heart. AAV6 was not evaluated in the rat because it performed similarly to AAV1 in the mouse heart in this study and has been reported previously to perform similarly to AAV1 in the rat heart (Palomeque et al., 2007). Adult rats (8 weeks old) underwent left thoracotomy with direct injection of 5 × 1011 GC of AAV-CB-LacZ into the myocardium and were killed at 4 weeks for analysis of LacZ expression by X-Gal staining. AAV9 provided highly efficient, global transgene expression to the targeted region of the heart (left ventricular free wall), whereas expression was minimal after injection with other serotypes (Fig. 4).
FIG. 4.

Representative photomicrographs of sections from rat heart 4 weeks after direct myocardial injection into the left ventricular free wall of 250 μl containing 5 × 1011 GC of AAV-CB-LacZ of the indicated serotype in five equal aliquots. Sections have been stained with X-Gal and counterstained with eosin. Scale bars: 2.4 mm.
Dose response of AAV serotypes in the rat
To determine whether AAV9 would continue to provide high-level gene transfer at a lower vector dose, rats were injected with 5 × 1010 GC of AAV-CB-LacZ as described previously and killed at 4 weeks. AAV9 was able to provide moderate gene transfer at this lower dose, but LacZ expression was barely detectable in the hearts of rats treated with the other serotypes (Fig. 5a). A quantitative β-galactosidase assay was performed on cardiac tissue extracts from the high-dose group, and the results of this assay confirmed the X-Gal staining: activity in the AAV9 hearts was 5- to 10-fold higher than in the other serotypes (Fig. 5b).
FIG. 5.
Dose response of LacZ expression in the rat heart 4 weeks after direct myocardial injection of 250 μl of the indicated dose of AAV-CB-LacZ in five equal aliquots. (a) Representative photomicrographs of rat heart stained with X-Gal and counterstained with eosin. Scale bars: 200 μm. (b) Graph displaying β-galactosidase activity as determined by quantitative chemiluminescence assay of samples from rats treated with the high dose. Columns and error bars represent means and SD. Note that AAV9 is superior to the other serotypes evaluated (*p < 0.05 vs. other serotypes).
Cardiac function after cardiac gene transfer in the rat
To determine whether cardiac gene transfer would have deleterious effects on cardiac function, rats treated with the high dose of the two most highly efficient serotypes, AAV8 and AAV9, underwent echocardiography (echo) and hemodynamic assessment with a Millar pressure–volume conductance catheter before euthanasia at 4 weeks and were compared with uninjected controls. The echo data displayed in Table 1 show that there is no significant difference in cardiac function among the groups in terms of fractional shortening (FS) or ejection fraction (EF). There was also no significant difference in cardiac geometry among the three groups, although an unusually large animal in the AAV8 group did cause a trend toward increased cardiac mass and chamber dimensions in this group (Table 1). The hemodynamic data displayed in Fig. 6 show that there is no significant difference in pressure–volume relationships among the three groups. Therefore, cardiac gene transfer did not adversely affect the cardiac cycle either in terms of diastolic filling or systolic pressure generation.
Table 1.
Cardiac Function as Assessed by Two-Dimensional Echocardiography at 4 Weeksa
| HR (bpm) | IVSd (cm) | LVIDd (cm) | LVFW (cm) | LVIDs (cm) | FS (%) | EDV (ml) | ESV (ml) | EF (%) | LV mass (g) | CO (liters/min) | SV (ml) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Naïve | 267 ± 15b | 0.17 ± 0.02 | 0.72 ± 0.03 | 0.18 ± 0.07 | 0.42 ± 0.02 | 41 ± 4.2 | 0.83 ± 0.11 | 0.19 ± 0.03 | 77 ± 4.6 | 1.3 ± 0.10 | 0.17 ± 0.04 | 0.64 ± 0.12 |
| AAV9 | 274 ± 51 | 0.18 ± 0.02 | 0.73 ± 0.03 | 0.16 ± 0.02 | 0.44 ± 0.08 | 39 ± 10 | 0.86 ± 0.09 | 0.22 ± 0.12 | 74 ± 13 | 1.3 ± 0.06 | 0.17 ± 0.04 | 0.63 ± 0.12 |
| AAV8 | 259 ± 3.5 | 0.16 ± 0.06 | 0.80 ± 0.06 | 0.29 ± 0.13 | 0.52 ± 0.07 | 35 ± 6.1 | 1.13 ± 0.25 | 0.34 ± 0.12 | 69 ± 9.0 | 1.9 ± 0.96 | 0.20 ± 0.05 | 0.78 ± 0.19 |
Abbreviations: CO, cardiac output; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; FS, fractional shortening; HR, heart rate; IVSd, interventricular septum diastolic diameter; LV, left ventricle; LVFW, left ventricular free wall; LVIDd, left ventricular inner dimension at diastole; LVIDs, left ventricular inner dimersion at systole; SV, stroke volume.
P = NS for all comparisons.
Values are reported as means ± SD.
FIG. 6.
Cardiac hemodynamic measurements in rats 4 weeks after direct myocardial injection of 5 × 1011 GC of AAV8-and AAV9-CB-LacZ compared with uninjected control. Representative pressure–volume loops were recorded via a Millar conductance catheter. No significant differences in pressure or volume were observed. RVU, relative volume units.
Biodistribution of gene expression and vector genomes in the rat
Biodistribution studies were next performed to determine the extent of LacZ expression and vector genome presence in noncardiac tissues at 4 weeks in the high-dose groups treated with AAV8 and AAV9. LacZ expression was largely restricted to the heart after direct myocardial injection of both serotypes (Fig. 7a). A minimal number of positive cells was detected in the liver, and expression was virtually absent in other tissues examined (Fig. 7a). Vector genomes were detected in all tissues examined, with the highest number being found in the heart and liver (Fig. 7b). The number of genomes detected in the heart and liver were similar and were 2 to 3 logs more abundant than those found in other tissues (Fig. 7b).
FIG. 7.
Biodistribution of LacZ expression and vector genomes 4 weeks after direct injection of 5 × 1011 GC of AAV8- and AAV9-CB-LacZ into the rat heart. (a) Representative photomicrographs of LacZ expression in several organs examined. Sections were stained with X-Gal and counterstained with eosin. Scale bars: 200 μm. (b) Graph displaying vector genome distribution in several organs examined via TaqMan PCR. He, heart; Li, liver; Lu, lung; Br, brain; Te, testis; Ki, kidney; Sp, spleen; St, stomach; Ga, gastrocnemius. Columns and error bars represent means and SD.
Discussion
The goal of this investigation was to determine the relative cardiac tropisms of AAV serotypes 1, 6, 7, 8, and 9 in the mouse and rat. We also sought to use a delivery technique that would maximize cardiac gene transfer while minimizing systemic exposure to vector. In the mouse arm of the study, we found that injection of AAV into the pericardial space of neonates via a subxiphoid approach is an effective method for achieving highly efficient, global cardiac gene transfer. At a high vector dose (2.5 × 1011), all serotypes examined were capable of providing high-level, global cardiac gene transfer of the LacZ reporter gene with low hepatic expression. AAV8 and AAV9 also effectively transduced the diaphragm at this dose. However, at an intermediate dose (2.5 × 1010), AAV9 was the only serotype that continued to provide high-level gene transfer to the heart. In addition, at the intermediate dose, expression was limited almost entirely to the heart, with only a minimal number of positive cells detectable in the diaphragm and liver.
Although several other groups have demonstrated that AAV9 is the most cardiotropic serotype in the mouse (Inagaki et al., 2006; Pacak et al., 2006; Bostick et al., 2007; Vandendriessche et al., 2007; Zincarelli et al., 2008), none has focused on combining high-level gene transfer with a delivery method that would limit potentially dangerous systemic exposure. We were able to achieve global, cardiac-specific gene transfer at a dose that was approximately 5-fold lower than was possible after tail vein injection (Inagaki et al., 2006). This allowed us not only to minimize extracardiac vector exposure and gene transfer but also to reduce the animal's total viral load. In addition, our time course study demonstrated that AAV9-mediated gene transfer after intrapericardial injection has a quick onset and is relatively stable for at least 1 year.
This intrapericardial injection approach has been used previously to deliver adenovirus to the mouse heart, and although LacZ transgene expression was efficient at 3 days, it was virtually nonexistent in the myocardium by 2 months (Zhang et al., 1999). This approach has also been used to deliver single-stranded AAV1 (ssAAV1) and self-complementary AAV1 (scAAV1) to the mouse heart; however, GFP expression mediated by ssAAV1 was barely detectable at 11 days and minimal at 21 days (Andino et al., 2007). Although the use of scAAV led to faster onset of expression and higher expression, scAAV limits therapeutic applications because packaging capacity is reduced by half (McCarty et al., 2001, 2003; Choi et al., 2005). For example, the SERCA gene, which has been proposed for use in a clinical trial (Hajjar et al., 2008), is too large to package into scAAV. As a result, we believe that our strategy using ssAAV9 offers significant advantages over these previous approaches.
The potential applications of our technique are numerous. The highly efficient and stable gene transfer mediated by AAV9 makes it ideal for use as a cardiac gene transfer vector because most cardiac diseases follow a chronic course. In addition, because the mice are injected as neonates and because vector dose can be adjusted to limit expression to the heart, this intrapericardial injection technique can be used as a simple alternative to the creation of cardiac-specific transgenic or knockout (using short hairpin RNA [shRNA]; Andino et al., 2008) lines. This would be especially useful in the case of a gene whose manipulation during embryonic development produces a lethal phenotype. Alternatively, this gene transfer technique could be used to screen potentially therapeutic transgenes in many of the widely available mouse models of cardiac disease to identify candidates for large animal trials. Finally, by using the high vector dose, one could simultaneously treat both the heart and diaphragm, a technique that may prove useful in the mdx mouse model of Duchenne muscular dystrophy (Yue et al., 2003).
In the second arm of our study we evaluated in adult rats the AAV serotypes that we had screened in the mouse arm, to determine whether AAV9 would continue to be superior as a cardiac gene transfer vector in a larger animal. To the best of our knowledge we are the first group to perform a direct comparison of AAV9 with other serotypes in the adult rat heart. The most comprehensive study found that AAV8 was superior to AAV serotypes 1–7 (Palomeque et al., 2007), whereas others showed the superiority of AAV6 over AAV2 (Kawamoto et al., 2005) and of AAV1 over AAV2 and AAV5 (Schirmer et al., 2007). We did not test AAV6 in the rat because it performed similarly to AAV1 in mice in this study and because it has been previously reported to perform similarly to AAV1 in the rat (Palomeque et al., 2007). This is not surprising because AAV1 and AAV6 are part of the same clade and therefore share >95% sequence homology in their capsids (Gao et al., 2002, 2004).
We report here that AAV9 provides highly efficient, global gene transfer to the left ventricular free wall of the adult rat after direct injection into the myocardium in five equally spaced aliquots. This level of gene transfer exceeds that provided by the other serotypes evaluated by approximately 1 log and is superior to the gene transfer achieved by another investigator using a vascular delivery method (Miyagi et al., 2008). AAV9-mediated gene expression was also specific to the heart after direct injection. Although vector genomes were detected in all tissues examined, only a minimal number of LacZ-positive cells was detected in the liver, and positive cells were absent from the multiple other tissues examined. AAV9 may have an advantage in mediating cardiac gene expression because of differential viral internalization and/or nuclear uncoating, as was determined previously for other AAV serotypes (Sipo et al., 2007), but further investigation is necessary to confirm this hypothesis. Finally, AAV9-mediated cardiac gene transfer via direct myocardial injection in the rat appears to be safe, as no differences in cardiac function were noted between AAV9-injected rats and uninjected rats by either echocardiography or Millar conductance catheter.
Our results indicate that AAV9 should be the vector of choice for studies involving cardiac gene transfer to the rat heart. This is important because as larger animals, rats offer the opportunity to evaluate potentially therapeutic genes in a more clinically relevant model. For example, it is technically more feasible to create models of ischemic cardiomyopathy via coronary artery ligation or models of pressure overload cardiomyopathy via aortic banding in the rat rather than in the mouse, and these rat models are well established in the literature (Pleger et al., 2007; Sakata et al., 2007). However, investigators are not using AAV9 in these models, and as a result, are achieving suboptimal gene transfer efficiency, which may be causing them to underestimate or miss the beneficial effects of potentially therapeutic genes.
AAV9 is the most cardiotropic serotype in the mouse and rat and should be used in investigations involving cardiac gene transfer in these animals. We have described techniques that allow global, cardiac-specific gene transfer in these species. If desired, cardiac specificity could be further enhanced by transcriptional and/or transductional targeting of vectors (Godecke, 2006; Muller et al., 2006, 2007). AAV9 may be the vector of choice for clinical trials in the heart, but large animal and nonhuman primate studies should first be initiated to evaluate the cardiac performance of AAV9 in these higher species.
Acknowledgments
This work was supported by a grant from the NHLBI (P01-HL059407 to J.M.W. and H.L.S.) and by TG-HL-007748 to L.T.B.
Author Disclosure Statement
J.M.W. and G.P.G. are inventors on patents that have been licensed to various biopharmaceutical companies. No competing financial interests exist for L.T.B., K.M., M.M., J.S., D.W., and H.L.S.
References
- Andino L.M. Conlon T.J. Porvasnik S.L. Boye S.L. Hauswirth W.W. Lewin A.S. Rapid, widespread transduction of the murine myocardium using self-complementary adeno-associated virus. Genet. Vaccines Ther. 2007;5:13. doi: 10.1186/1479-0556-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andino L.M. Takeda M. Kasahara H. Jakymiw A. Byrne B.J. Lewin A.S. AAV-mediated knockdown of phospholamban leads to improved contractility and calcium handling in cardiomyocytes. J. Gene Med. 2008;10:132–142. doi: 10.1002/jgm.1131. [DOI] [PubMed] [Google Scholar]
- Arruda V.R. Stedman H.H. Nichols T.C. Haskins M.E. Nicholson M. Herzog R.W. Couto L.B. High K.A. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. 2005;105:3458–3464. doi: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostick B. Ghosh A. Yue Y. Long C. Duan D. Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther. 2007;14:1605–1609. doi: 10.1038/sj.gt.3303029. [DOI] [PubMed] [Google Scholar]
- Carter B.J. Adeno-associated virus and the development of adeno-associated virus vectors: A historical perspective. Mol. Ther. 2004;10:981–989. doi: 10.1016/j.ymthe.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Choi V.W. Samulski R.J. McCarty D.M. Effects of adeno-associated virus DNA hairpin structure on recombination. J. Virol. 2005;79:6801–6807. doi: 10.1128/JVI.79.11.6801-6807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L. Kido M. Lee D.V. Rabinowitz J.E. Samulski R.J. Jamieson S.W. Weitzman M.D. Thistlethwaite P.A. Differential myocardial gene delivery by recombinant serotype-specific adeno-associated viral vectors. Mol. Ther. 2004;10:604–608. doi: 10.1016/j.ymthe.2004.06.110. [DOI] [PubMed] [Google Scholar]
- Gao G. Vandenberghe L.H. Alvira M.R. Lu Y. Calcedo R. Zhou X. Wilson J.M. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G.P. Alvira M.R. Wang L. Calcedo R. Johnston J. Wilson J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godecke A. AAV vector re-targeting: A small step on the way to cardiac-specific gene transfer. Cardiovasc. Res. 2006;70:6–8. doi: 10.1016/j.cardiores.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Hajjar R.J. Zsebo K. Deckelbaum L. Thompson C. Rudy J. Yaroshinsky A. Ly H. Kawase Y. Wagner K. Borow K. Jaski B. London B. Greenberg B. Pauly D.F. Patten R. Starling R. Mancini D. Jessup M. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J. Card. Fail. 2008;14:355–367. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Hildinger M. Auricchio A. Gao G. Wang L. Chirmule N. Wilson J.M. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J. Virol. 2001;75:6199–6203. doi: 10.1128/JVI.75.13.6199-6203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K. Fuess S. Storm T.A. Gibson G.A. McTiernan C.F. Kay M.A. Nakai H. Robust systemic transduction with AAV9 vectors in mice: Efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S. Shi Q. Nitta Y. Miyazaki J. Allen M.D. Widespread and early myocardial gene expression by adeno-associated virus vector type 6 with a β-actin hybrid promoter. Mol. Ther. 2005;11:980–985. doi: 10.1016/j.ymthe.2005.02.009. [DOI] [PubMed] [Google Scholar]
- McCarty D.M. Monahan P.E. Samulski R.J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- McCarty D.M. Fu H. Monahan P.E. Toulson C.E. Naik P. Samulski R.J. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- Miyagi N. Rao V.P. Ricci D. Du Z. Byrne G.W. Bailey K.R. Nakai H. Russell S.J. McGregor C.G. Efficient and durable gene transfer to transplanted heart using adeno-associated virus 9 vector. J. Heart Lung Transplant. 2008;27:554–560. doi: 10.1016/j.healun.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller O.J. Leuchs B. Pleger S.T. Grimm D. Franz W.M. Katus H.A. Kleinschmidt J.A. Improved cardiac gene transfer by transcriptional and transductional targeting of adeno-associated viral vectors. Cardiovasc. Res. 2006;70:70–78. doi: 10.1016/j.cardiores.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Muller O.J. Katus H.A. Bekeredjian R. Targeting the heart with gene therapy-optimized gene delivery methods. Cardiovasc. Res. 2007;73:453–462. doi: 10.1016/j.cardiores.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Pacak C.A. Mah C.S. Thattaliyath B.D. Conlon T.J. Lewis M.A. Cloutier D.E. Zolotukhin I. Tarantal A.F. Byrne B.J. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ. Res. 2006;99:e3–e9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- Palomeque J. Chemaly E.R. Colosi P. Wellman J.A. Zhou S. Del Monte F. Hajjar R.J. Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo. Gene Ther. 2007;14:989–997. doi: 10.1038/sj.gt.3302895. [DOI] [PubMed] [Google Scholar]
- Pleger S.T. Most P. Boucher M. Soltys S. Chuprun J.K. Pleger W. Gao E. Dasgupta A. Rengo G. Remppis A. Katus H.A. Eckhart A.D. Rabinowitz J.E. Koch W.J. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007;115:2506–2515. doi: 10.1161/CIRCULATIONAHA.106.671701. [DOI] [PubMed] [Google Scholar]
- Sakata S. Lebeche D. Sakata N. Sakata Y. Chemaly E.R. Liang L.F. Tsuji T. Takewa Y. Del Monte F. Peluso R. Zsebo K. Jeong D. Park W.J. Kawase Y. Hajjar R.J. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J. Mol. Cell Cardiol. 2007;42:852–861. doi: 10.1016/j.yjmcc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer J.M. Miyagi N. Rao V.P. Ricci D. Federspiel M.J. Kotin R.M. Russell S.J. McGregor C.G. Recombinant adeno-associated virus vector for gene transfer to the transplanted rat heart. Transpl. Int. 2007;20:550–557. doi: 10.1111/j.1432-2277.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- Sipo I. Fechner H. Pinkert S. Suckau L. Wang X. Weger S. Poller W. Differential internalization and nuclear uncoating of self-complementary adeno-associated virus pseudotype vectors as determinants of cardiac cell transduction. Gene Ther. 2007;14:1319–1329. doi: 10.1038/sj.gt.3302987. [DOI] [PubMed] [Google Scholar]
- Vandendriessche T. Thorrez L. Acosta-Sanchez A. Petrus I. Wang L. Ma L., L. De Waele L. Iwasaki Y. Gillijns V. Wilson J.M. Collen D. Chuah M.K. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J. Thromb. Haemost. 2007;5:16–24. doi: 10.1111/j.1538-7836.2006.02220.x. [DOI] [PubMed] [Google Scholar]
- Woo Y.J. Zhang J.C. Taylor M.D. Cohen J.E. Hsu V.M. Sweeney H.L. One year transgene expression with adeno-associated virus cardiac gene transfer. Int. J. Cardiol. 2005;100:421–426. doi: 10.1016/j.ijcard.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Wright M.J. Wightman L.M. Lilley C. De Alwis M. Hart S.L. Miller A. Coffin R.S. Thrasher A. Latchman D.S. Marber M.S. In vivo myocardial gene transfer: Optimization, evaluation and direct comparison of gene transfer vectors. Basic Res. Cardiol. 2001;96:227–236. doi: 10.1007/s003950170053. [DOI] [PubMed] [Google Scholar]
- Yue Y. Li Z. Harper S.Q. Davisson R.L. Chamberlain J.S. Duan D. Microdystrophin gene therapy of cardiomyopathy restores dystrophin–glycoprotein complex and improves sarcolemma integrity in the mdx mouse heart. Circulation. 2003;108:1626–1632. doi: 10.1161/01.CIR.0000089371.11664.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.C. Woo Y.J. Chen J.A. Swain J.L. Sweeney H.L. Efficient transmural cardiac gene transfer by intrapericardial injection in neonatal mice. J. Mol. Cell. Cardiol. 1999;31:721–732. doi: 10.1006/jmcc.1998.0905. [DOI] [PubMed] [Google Scholar]
- Zincarelli C. Soltys S. Rengo G. Rabinowitz J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]






