Abstract
Background
Patients with schizophrenia may be impaired at remembering inter-item and item-context relationships (relational memory), even when memory for items is intact. Here, we applied the novel approach of using eye movements to assess integrity of item and relational memory in schizophrenia. This method does not rely on introspection and may be more readily translated to animal models than traditional behavioral methods.
Methods
Sixteen healthy controls and sixteen patients were administered a scene memory task while eye movements were monitored. During testing, participants indicated whether the scenes were unchanged, contained a new item (item manipulation), had a change in item location (relational manipulation), or were new. It was predicted that memory would be disproportionately impaired when relational changes were made.
Results
Results confirmed that tasks were equally difficult, and showed that patients were impaired identifying all scene types. These behavioral impairments were associated with more severe disorganization and negative symptoms. Eye movement results were more specific. Both groups looked disproportionately at critical regions of repeated versus novel scenes – an effect of scene repetition. However, in contrast to predictions, patients showed equivalent eye-movement-based memory impairment whether changes were relational or item-based.
Conclusions
This is the first experiment to demonstrate that eye movements can be used to investigate item and relational memory in schizophrenia. The eye movement procedure was well tolerated and was more specific than behavioral measures with respect to memory impairment. Results suggest that eye movements may be of use in clinical trials and translational studies employing animal models.
Keywords: Schizophrenia, Episodic Memory, Eye Tracking, Associative Memory, Relational Memory, Item Memory
Introduction
Episodic memory refers to formation and retrieval of lasting memories for events (1). Although multiple cognitive deficits are observed in schizophrenia, a meta-analysis found the largest effects for episodic memory (2,3), suggesting a disproportionately severe deficit in this domain (4,5). Recent studies demonstrated that episodic memory is strongly related to patients’ function in everyday life (6-8) and that existing medications do little to ameliorate memory deficits (9-11). Accordingly, it is essential to develop procedures to characterize memory in individuals with schizophrenia that can be used in development of new cognitive enhancing agents (12).
Relational memory may be an important target for treatment development, as there is some evidence that patients with schizophrenia have relatively spared memory for items and disproportionately impaired memory for relationships between items and the context in which they were encountered (13,14). This relational processing deficit was first noticed on verbal list learning tasks such as the CVLT (15) in which patients’ failure to use semantic relationships to cluster items during encoding contributed to overall retrieval deficits (16). Problems with relational memory can also explain the tendency of patients to retrieve items on the basis of familiarity, often failing to recollect related contextual information (17-19; see 20 for exception). For instance, patients are less impaired on item recognition, and demonstrate significant impairments on associative recognition tasks (21-25).
These findings suggest that relational encoding and retrieval deficits are candidate mechanisms for episodic memory dysfunction in schizophrenia, and appropriate targets for pharmaceutical intervention. However, relational memory tasks in humans are not easily translated to animals, posing a significant barrier to development of new pharmaceutical agents. For instance, tests involving subjective reports of recollection cannot be used in studies with rodents or nonhuman primates (although see 26), and even objective tests of relational memory (e.g., associative or source recognition) place heavy demands on meta-cognitive and decision making processes, and may not be easily translated to animals. Furthermore, behavioral measures can be confounded by levels of motivation, task comprehension, and response-mapping difficulties (27), and experimenters often fail to equate difficulty levels across experimental conditions. These confounding factors raise the possibility that differential deficits are secondary to the greater difficulty and discriminating power of the relational tasks (28). Accordingly, it is important to develop methods to equate task difficulty and assess item and relational memory in a manner suitable for patients and nonhuman animals.
In the current study, eye movements were used as an indirect measure of memory for scenes. Effects of memory on eye movement behavior have been documented in several investigations (29-33), and these methods have been used successfully to study memory in nonhuman primates (34) and infants as young as nine months who cannot yet verbalize remembered content (35). Eye movements have also been used to identify spatial working memory (WM) deficits in schizophrenia patients with tasks originally developed for nonhuman primates (36,37). Accordingly, eye movements may provide a particularly sensitive measure of episodic memory, even in cognitively impaired patients and nonhuman animals.
Using a novel paradigm based on work by Ryan and colleagues (30), we investigated memory for scenes and memory for constituent scene elements (individual items or item-location relationships). At test, eye movements were monitored while participants determined whether scenes were new, old and unchanged, old but contained a novel item (‘item manipulation’), or old but contained an item that had changed spatial locations (‘relational manipulation’). As in previous work with healthy participants, we predicted that controls would spend more time viewing regions of scenes that had been manipulated, relative to corresponding regions of repeated/unchanged scenes. We hypothesized that patients, like controls, would spend more time viewing regions of scenes in which an item change occurred, but would fail to look disproportionately at regions where a relational change had occurred.
Methods
Participants
Thirty-seven participants were studied, and the final sample included 16 medicated patients with schizophrenia and 16 healthy controls. Four patients and one healthy participant were excluded because peripheral factors (e.g., eye glasses) prevented calibration of eye position. Participants were matched at the group level for age, gender, handedness and parental education (Table 1). The study was approved by the IRB at the University of California Davis. Details on medication, exclusion criteria, and diagnostic and clinical assessments are in the Supplement.
Table 1.
Participant Demographics
| Healthy Control Group (n=16) |
Patients with Schizophrenia (n=16) |
p-value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 26.4 | 6.1 | 28.4 | 8.2 | ns |
| Gender (% male) | 62.5 | 68.8 | ns | ||
| Handedness (% right) | 87.5 | 93.8 | ns | ||
| WRAT | 113.5 | 6.2 | 106.6 | 8.8 | ns |
| Education (years) | 15.6 | 1.5 | 14.2 | 2.4 | ns |
| Parental Education (years) | 15.8 | 2.2 | 14.4 | 3.1 | ns |
| SANS | 39.1 | 16.8 | |||
| SAPS | 14.9 | 17.1 | |||
| BPRS | 40.6 | 8.7 | |||
Note: ns = no significant group difference at p<.05, two-tailed
Stimuli & Design
Sixty-four rendered scenes, sized to 800×600 pixels, were developed using Punch! Home Design Software. Three versions of each scene were created – the original, a version containing an item manipulation, and a version containing a relational manipulation – producing a total of 192 stimuli. One item in each original scene was designated a “critical item” and in manipulated scenes it was either: (1) replaced with a different exemplar (i.e., item manipulation), or (2) moved to a different, albeit equally plausible, spatial location (i.e., relational manipulation). Each critical item was presented in the context of just one scene, and critical items moved equally often from left (in the original scene) to right (in the manipulated scene) as right to left when the change was relational.
Two “orienting questions” were created, one for the item condition and another for the relational condition. Questions were crafted to encourage processing of either the critical item embedded in the scene (i.e. item condition), or the spatial relationship between that item and some other scene element (i.e. relational condition). These questions ensured that attention was directed to critical aspects of the scene that might be manipulated during the test phase (see Figure 1).
Figure 1.
Experimental Methods. (A-B) Illustration of first (A) and second (B) study blocks. During the second block, each picture was preceded by an orienting question crafted to encourage processing of item-specific or relational information. (C) Illustration of test block. After each scene, participants were required to identify the picture type and rate their confidence. Eye movements were monitored throughout.
Procedure
After informed consent, instructions were provided, and each participant successfully completed a practice session. Eye position was calibrated at the beginning of each experimental block using a 3×3 spatial array, and the onset of each trial was experimenter-initiated contingent upon fixation of a centrally presented crosshair. Study and test procedures are illustrated in Figure 1.
Study Block 1
Participants were shown 48 scenes, each for eight seconds. They were instructed to study each scene carefully, paying close attention to items embedded in the scene and their spatial locations.
Study Block 2
The same 48 scenes were presented in a different random order. Each scene was preceded by a question orienting viewer’s attention to the item that might subsequently be replaced with a different item or moved to a different location during test block. The orienting question was presented in the center of the screen for four seconds, and the scene was presented immediately thereafter for five seconds. Participants were instructed to read the question and respond “yes”, “no”, or “don’t know”, via button press, as quickly as possible.
Test Block
Sixty-four scenes (16 novel, 16 repeated, 16 with an item manipulation, and 16 with a relational manipulation) were presented in random order and remained on screen for six seconds. After each scene disappeared, participants pressed a button indicating whether the scene was 1) old and unchanged, 2) old, with a new item, 3) old, but one of the items had changed position, or 4) new. Participants were then prompted to rate confidence on a scale from one (“just guessing”) to three (“absolutely certain”). The confidence scale remained in view until the response, and all responses were self-paced.
Eye Tracking Acquisition and Analysis
Eye position was monitored at a rate of 120 Hz using an Applied Science Laboratories model 504 remote eye tracker. For scenes assigned to the item condition, the proportion of total viewing time allocated to a single region of interest (ROI) surrounding the critical item provided a dependent measure of memory. For scenes assigned to the relational condition there were two ROIs (an empty region and a filled region), and viewing time directed to these regions was examined two ways. First, the proportion of total combined viewing time directed to the empty and filled regions was calculated. This combined viewing time was used in direct comparisons of the item condition and the relational condition. Second, the filled and empty regions were examined separately. Additional detail about data collection and quantification are provided in the Supplement.
Results
Behavioral Performance
Orienting Question Accuracy
Controls made correct responses to orienting questions more often than patients, though both groups responded correctly on the majority of trials [93.23% correct, SD=4.72 and 84.51% correct, SD=6.27, respectively; F(1,30)=19.76, p<.001]. Critically, there were no effects of scene type [F(2,60)=1.35, p>.05] or any group by scene type interaction [F(2,60)=.66, p>.05)]. Thus, although patients were less accurate in responding to orienting questions, lack of scene type effects indicates that any subsequent differences in patients’ performance or eye movements across conditions cannot be attributed to orienting question accuracy.
Scene Identification Accuracy
Performance was evaluated by subtracting false alarm rates from hit rates. A repeated measures ANOVA indicated that patients performed worse than controls [F(1,30)=16.41, p<.001], but that performance differences between conditions were similar across groups (main effect of condition: [F(3,90)=25.03, p<.001]; non-significant group x condition interaction: [F(3,90)=.184, p>.05]. Post-hoc comparisons showed that all participants were better at identifying novel scenes than repeated scenes [controls: t(15)=6.50, Bonferroni corrected p<.001; patients: t(15)=3.09, Bonferroni corrected p<.05], novel scenes than scenes containing an item change [controls: t(15)=6.36, Bonferroni corrected p<.001; patients: t(15)=5.70, p<.001], and novel scenes than scenes containing a relational change [controls: t(15)= 4.08, Bonferroni corrected p<.01; patients: t(15)=3.42, p<.05]. There were no differences in performance across the remaining three scene types for either group [controls: t’s(15)≤1.73, p>.05; patients: t’s(15) ≤2.31, p>.05]. Statistics were also performed using a signal detection index (d’), and results were replicated [main effect of group: F(1,30)=18.34, p<.001; main effect of condition: F(3,90)=45.79, p<.001; non-significant group x condition interaction: F(3,90)=1.86, p>.05]. Hit rates, false alarm rates, and d’ scores are provided in Table 2.
Table 2.
Mean hit rates, false alarm rates, and d’ scores on the 4-alternative forced-choice test for participants in the control group and for schizophrenia patients. Standard deviations are presented in the parentheses
| Repeated Scenes |
Item Change | Relational Change |
Novel Scenes | |
|---|---|---|---|---|
| Control Group: | ||||
| Hit Rate | .816 (.157) | .727 (.184) | .746 (.217) | .941 (.098) |
| FA Rate | .103 (.067) | .083 (.073) | .052 (.050) | .018 (.025) |
| d’ | 2.39 | 2.24 | 2.53 | 3.67 |
|
Schizophrenia
Patients: |
||||
| Hit Rate | .621 (.209) | .488 (.254) | .582 (.231) | .758 (.180) |
| FA Rate | .150 (.064) | .132 (.094) | .132 (.089) | .103 (.092) |
| d’ | 1.48 | 1.19 | 1.49 | 2.24 |
Confidence Ratings
Confidence ratings were calculated separately for correct and incorrect responses. Both groups were more confident when scenes were correctly identified [controls: t(15)=8.06, p<.001; schizophrenia Patients: t(15)=8.06, p<.001]. Confidence ratings for correct responses were also examined for effects of scene type, group, or group by scene type interaction. This revealed a main effect of scene type [F(3,90)=11.90, p<.001], with no group [F(3,90)=.91, p>.05] or group by scene type interaction [F(3,90)=.40, p>.05]. Participants were less confident when making correct responses for matching scenes than novel scenes [t(31)=3.68, p<.005], scenes containing an item change [t(31)=6.21, p<.001], or scenes containing a relational change [t(31)=4.35, p<.001; all Bonferroni corrected]. Because there were so few confident incorrect trials, this analysis could not be performed for incorrect responses.
Eye-Movement-Based Memory Assessment
Inclusion of repeated and novel scenes allowed us to calculate two eye-movement-based measures of memory: 1) Memory for Repetition was operationalized as differences in viewing time directed to critical regions of repeated versus novel scenes. Because attention had been drawn to critical regions of repeated scenes by orienting questions, these regions were expected to elicit more viewing than the same regions of novel scenes. This index provided a general measure of memory to confirm that participants were attending to critical scene regions and did not have any fundamental eye movement deficits. 2) Memory for Detail was operationalized as differences in viewing time directed to critical regions of manipulated versus repeated/unchanged scenes. Because repeated and manipulated scenes were subject to the same encoding conditions (i.e. both associated with an orienting question), and differed only in previous viewing history (i.e. whether or not the scene was manipulated at test), any significant differences could be attributed to memory for the originally studied item or item-location relationship. This index provided a specific measure of memory for scene detail (i.e. either memory for the previously studied item or item-location relationship). These measures are illustrated in Figure 2 for the relational memory condition.
Figure 2.
Eye movement data from three different control participants superimposed on scenes presented during test blocks assigned to the relational memory condition. The top row illustrates the study scenes with two different locations of the critical item. The bottom row illustrates eye movements during the test phase when participants either viewed the scene for the first time (Novel), viewed the exact same scene as during the study phase (Repeated), or viewed a new version of the scene in which the critical item was in a different location (Manipulated). Circles represent fixations; with the size of each circle proportional to the amount of viewing time directed to that part of the scene. Red lines represent transitions from one fixation to the next. Yellow boxes illustrate the locations of empty and filled regions of interest for illustrative purposes, and were not part of the test stimuli. Test phase fixations illustrate the increase in viewing time directed to the filled region of repeated versus novel scenes – an effect of memory for scene repetition, and the increase in viewing directed to filled and empty regions of manipulated versus repeated scenes – an effect of memory for scene detail.
Results of a repeated measures ANOVA investigating effects of group (patient, control), condition (item, relational) and memory index (repetition, detail) showed main effects of group [F(1,30)=13.86, p<.001)] and condition [F(1,30)=16.58, p<.001], as well as a group by memory index interaction [F(1,30)=10.37, p<.003]. No other main effects or interactions were significant [F’s≤3.72, all p’s>.05]. Therefore, between-groups repeated measures ANOVAs were performed separately for the repetition and detail indices described above (see Figure 3).
Figure 3.
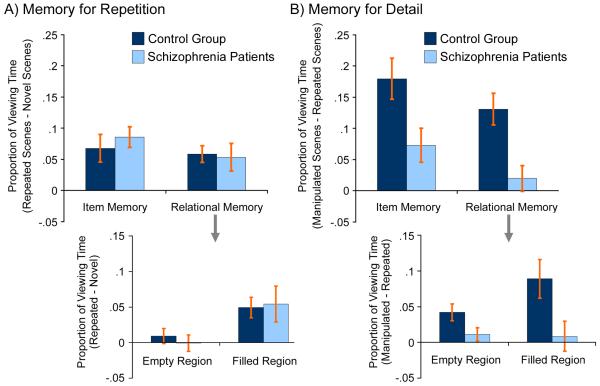
Eye-movement-based memory effects in patients and control participants. (a) Memory for Repetition is intact in schizophrenia. Bar graphs illustrate differences in the proportion of total viewing time directed to critical regions of repeated minus novel scenes for both experimental conditions. Both groups of participants looked disproportionately at critical regions of repeated (vs. novel) scenes, with no significant between-group difference in patterns of viewing. This effect of memory for scene repetition was likely due to the fact that attention had been drawn to critical regions of repeated scenes by orienting questions used during the study phase. For the relational condition, results were calculated collapsed across filled and empty regions (top figure), and were calculated separately for these ROIs (bottom figure). The empty region of repeated scenes did not attract disproportionate viewing by either group, a result that is attributable to the fact that for repeated scenes this region had always been empty. (b) Memory for Detail is impaired in schizophrenia. Bar graphs illustrate differences in the proportion of total viewing time directed to critical regions of manipulated minus repeated scenes for both experimental conditions. Schizophrenia patients were impaired on eye-movement-based memory measures for both experimental conditions. Impairments were evident for the relational memory condition whether viewing of the critical regions was collapsed (top figure), or was evaluated separately for the empty and filled regions (bottom figure). Although patients were impaired in both memory conditions, these graphs also illustrate that patients successfully increased viewing of the critical region of manipulated (vs. repeated) scenes assigned to the item memory condition. This did not occur for the relational memory condition, indicating some preservation of eye-movement-memory effects in schizophrenia patients for item but not for relational memory representations.
Memory for Repetition
ANOVA revealed equivalent increases in the proportion of viewing time directed to critical regions of repeated (vs. novel) scenes in patients and controls [non-significant group and group x condition effects: F(1,30)≤.30, p>.05], with no differences across conditions [F(1,30)=.97, p>.05]. Separate evaluation of the critical regions of scenes assigned to the relational memory condition confirmed that there were no group differences for either empty or filled regions [t(30)=.66, p>.05 and t(30)=.17, p>.05, respectively].
To confirm that there were significant repetition effects on eye movement behavior in both groups, we evaluated whether difference scores (proportion of viewing time directed to ROIs of repeated minus novel scenes) were greater than zero. As predicted, healthy controls looked disproportionately at the critical regions of repeated (vs. novel) scenes in both item and relational conditions [t(15)=3.14, p<.005 and t(15)=4.46, p<.001, respectively]. For the relational memory condition this disproportionate viewing effect was selectively evident for the filled [t(15)=3.52, p<.005], but not the empty region [t(15)=.91, p>.05]. This result makes intuitive scene because the item in the filled region of repeated scenes had been the target of the orienting question, whereas the empty region had always been empty, providing no reason to direct viewing to that part of the scene.
This same pattern was seen in patients. Disproportionate viewing of the critical regions of repeated (vs. novel) scenes was evident for both the item and relational conditions [t(15)=5.39, p<.001 and t(15)=2.48, p<.01, respectively], and separate evaluation of the critical regions of relational scenes showed disproportionate viewing of the filled [t(15)=2.22, p<.05], but not the empty region [t(15)=.07, p>.05]. Combined ANOVA and t-test results suggest that, like controls, patients had retained information about previous exposure to repeated scenes, and that this information was sufficient to guide eye movements to potentially informative scene regions. Results are illustrated in Figure 3a.
Memory for Detail
ANOVA revealed that controls spent more time viewing the critical regions of manipulated (vs. repeated) scenes than patients [main effect of group: F(1,30)=15.05, p<.001]. Contrary to predictions, this difference was of similar magnitude for the item and the relational memory manipulations [non-significant group x condition interaction: F(1,30)=.01, p>.05]. Separate evaluation of the empty and filled regions of relational scenes confirmed the main effect of group [t(30)=2.10, p<.05 and t(30)=2.42, p<.05, respectively]. The ANOVA also revealed that differences in viewing time directed to critical regions of manipulated scenes were larger for scenes containing an item change than for scene containing a relational change [main effect of condition: F(1,30)=4.52, p<.05].
Difference scores (proportion of viewing time directed to ROIs of manipulated minus repeated scenes) were examined to confirm effects of memory on eye movement behavior for both groups and both conditions. These analyses are important because they indicate whether or not there was any evidence for memory in patients’ eye movement behavior, despite their overall deficit. Planned comparisons confirmed eye-movement-based memory effects in healthy participants, with disproportionate viewing directed to the critical regions of scenes that had been manipulated [item change: t(15)=5.65, p<.001; relational change: t(15)=5.29, p<.001]. There was no difference in the magnitude of these viewing time effects across conditions [t(15)=1.26, p>.05]. Separate evaluation of the empty and filled regions provided converging evidence for relational memory effects on eye movement behavior [t(15)=3.64, p<.001 and t(15)=3.40, p<.005, respectively]. This result is notable because increased viewing of the (now) empty region could only be attributed to participants’ memory for the scene when that region was previously filled.
Schizophrenia patients also looked disproportionately at the manipulated regions of scenes that contained an item change [t(15)=2.77, p<.01] showing some residual retention of specific item information based on previous exposure to those scenes. They did not, however, look disproportionately at manipulated regions of scenes that contained relational changes [t(15)=.99, p>.05]. Separate evaluation of the empty and filled regions confirmed the absence of relational memory effects in patients’ eye movements [t(15)=1.22, p>.05 and t(15)=.42, p>.05, respectively]. These combined results suggest that although patients were impaired in both memory conditions, there is evidence that they retained some memory for item information. The same was not true for the relational memory condition, which showed no evidence of eye-movement-based memory effects. Results are illustrated in Figure 3b.
Correlational Analysis
Pearson correlations were calculated investigating relationships between behavioral and eye movement measures in both groups, and with positive, negative and disorganization symptoms (38) in patients. In controls, higher d’ performance in the item condition was associated with increased viewing of item changes in the scenes (r=.54, p<.05). This relationship was not significant in patients (r=.26, p>.05), but there were no differences between groups (Fisher’s Z=.86, p>.05). Correlations between performance and clinical symptoms revealed no relationships with positive symptoms. However, less severe negative symptoms were associated with better d’ performance on repeated scenes (r=−.59, p<.05), scenes containing an item change (r=−.54, p<.05), and scenes containing a relational change (r=−.53, p<.05). Less severe disorganization was also related to better d’ performance on repeated (r=−.59, p<.05) and relational change scenes (r=−.70, p<.005).
Discussion
The current investigation examined memory for scenes and memory for constituent scene elements in schizophrenia using a novel eye movement paradigm. Results for healthy volunteers were consistent with previous findings (30), in showing increased viewing of critical regions of scenes where there was either a new item or a change in item location. Furthermore, eye movement and performance effects were of equivalent magnitude for the item and relational memory conditions in healthy participants, supporting the utility of this paradigm to investigate hypothesized differential deficits in relational versus item memory in schizophrenia. Contrary to predictions, patients showed reduced eye-movement-based memory effects for both item and relational memory manipulations. However, reduced memory for scene detail did not appear secondary to a generalized deficit in eye movement behavior, as patients successfully increased viewing of critical regions of repeated versus novel scenes. This specificity in the eye movement data contrasts with direct behavioral measures, which showed a generalized deficit in patient performance across all of the scene types. These results suggest that eye movement paradigms provide a sensitive measure of memory in healthy controls and schizophrenia patients, and therefore, have the potential to reveal patterns of spared and impaired performance that may not be evident in behavioral responses.
We predicted that eye-movement-based memory effects would be selectively disrupted by schizophrenia when spatial relationships among items were changed. Instead, results showed that these effects were disrupted to a similar extent whether changes were relational or item-based. One potential explanation for this discrepancy is that previously reported differential deficits in relational memory (14,21) were secondary to greater difficulty and discriminating power (28) of relational memory tasks, and that by matching difficulty across memory conditions the current paradigm eliminated these previously artifactual results. For example, in a meta-analysis examining these two memory processes (21) all the associative memory studies utilized tasks requiring recollection of either source, temporal order or original item pairings, whereas item memory studies employed simple old/new recognition tests that could be performed based on a sense of familiarity. Another potential explanation for the lack of a group by condition interaction is that the current item task was not process pure. Because of the complex nature of the visual scenes it is possible that individuals employed relational strategies in the item memory condition, and that patients were less likely to use these strategies given their previously noted difficulties with strategy generation (16,17). For example, controls may have remembered that the vacuum cleaner seen during the study phase in Figure 1 was the same color as the sky in the painting on the wall – and used this relational information to improve memory for the previously studied item. We believe that this second possibility is the more likely explanation as results suggest some preservation of item memory in the patient sample. As noted in our planned comparisons, and as illustrated in Figure 3b, patients spent more time viewing the critical regions of manipulated versus repeated scenes in the item memory condition, but failed to do so for relational memory. Furthermore, the lack of a group by condition interaction does not appear to be a consequence of small sample size as power for the interaction term was estimated at 21%. This means that approximately 64 participants would have been required per group to reach significance. Work is underway to revise the task with the aim of reducing the likelihood that relational encoding strategies will be invoked during the item condition while maintaining equivalent task difficulty to further investigate this important differential deficit question.
It is worth considering potential limitations of the present study. The sample consisted of clinically stable patients with relatively mild symptoms (see Table 1). It is unclear whether results would generalize to a more acutely ill sample. Additionally, patients were medicated (primarily second-generation anti-psychotics), although it is unlikely that the expression of eye-movement-based memory effects(or lack thereof) was related to medication effects because reflexive saccades are intact in both medicated and unmedicated patients (39). Several reports have shown that patients with schizophrenia have impaired smooth pursuit eye movements (40), and show increased error rates on anti-saccade tasks (41-43) raising potential concerns that results might have reflected a more fundamental eye movement deficit. However, there is consistent evidence that medicated and un-medicated patients show normal saccade latency, gain, and final eye position in reflexive saccade tasks (39), and smooth pursuit deficits were not associated with eye movement effects in earlier working memory studies (37). This suggests that aforementioned smooth pursuit and anti-saccade deficits are related to higher-level cognitive demands and do not reflect a fundamental deficit in oculomotor control. Moreover, if schizophrenia patients suffer from a more fundamental eye movement deficit, one would expect to see a complete absence of memory effects in eye movements. This was not the case, as intact effects of scene repetition were evident in patients’ eye movement behavior.
In summary, present results show that eye movement monitoring is a sensitive measure of memory in patients with schizophrenia. Worse behavioral performance on this task was related to increased negative symptoms and disorganization, suggesting that treatments to improve episodic memory may have a positive clinical impact and that changes in eye movement behavior may be used to index treatment effects. Future studies could build on present results by obtaining measures of functional outcome, by combining eye tracking and functional imaging in humans, and by adapting indirect eye-movement-based memory paradigms for use with animal models to evaluate the integrity of memory processes. This translational approach holds great promise for identifying neural mechanisms of spared and impaired memory in schizophrenia.
Supplementary Material
Acknowledgments
Portions of this work were presented at the 2008 Annual Meeting of the Society for Neuroscience, Washington, D.C. DEH was supported by an NIMH fellowship F32MH075513, and JDR by an NIMH R01MH084895. Thanks goes to Dr. Laurel Beckett for statistical consultation, and to our participants for their time and effort.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Tulving E. Elements of episodic memory. Oxford University Press, USA; New York: 1985. [Google Scholar]
- 2.Aleman A, Hijman R, de Haan EHF, Kahn RS. Memory impairment in schizophrenia: A meta-analysis. American Journal of Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 3.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 4.McKenna PJ, et al. Amnesic syndrome in schizophrenia. Psychological Medicine. 1990;20:967–972. doi: 10.1017/s0033291700036667. [DOI] [PubMed] [Google Scholar]
- 5.Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, et al. Neuropsychological function in schizophrenia: Selective impairment in memory and learning. Archives of General Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- 6.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophrenia Bulletin. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 8.Milev P, Tamlyn D, Lund CE, Mortimer AM, Hammond S, Baddeley AD. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: A longitudinal first-episode study with 7-year follow-up. American Journal of Psychiatry. 2005;162:495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- 9.Harvey PD, Keefe RS. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry. 2001;158:176–184. doi: 10.1176/appi.ajp.158.2.176. [DOI] [PubMed] [Google Scholar]
- 10.Keefe RSE, Sweeney JA, Hongbin G, Hamer RM, Perkins DO, McEvoy JP, Lieberman JA. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164:1061–1071. doi: 10.1176/ajp.2007.164.7.1061. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- 12.Carter CS, Barch DM. Cognitive Neuroscience-Based Approaches to Measuring and Improving Treatment Effects on Cognition in Schizophrenia: The CNTRICS Initiative. Schizophrenia Bulletin. 2007;33:1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ragland JD, Cools R, Frank M, Pizzagalli DA, Preston A, Ranganath C, Wagner AD. CNTRICS final task selection: long-term memory. Schizophr Bull. 2009;35:197–212. doi: 10.1093/schbul/sbn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranganath C, Minzenburg MJ, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol Psychiatry. 2008;64:18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test: Research Edition, Adult Version. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- 16.Iddon JL, McKenna PJ, Sahakian BJ, Robbins TW. Impaired generation and use of strategy in schizophrenia: evidence from visuospatial and verbal tasks. Psychological Medicine. 1998;28:1049–1062. doi: 10.1017/s0033291798006758. [DOI] [PubMed] [Google Scholar]
- 17.Bonner-Jackson A, Yodkovik N, Csernansky JG, Barch DM. Episodic memory in schizophrenia: The influence of strategy use on behavior and brain activation. Psychiatry Research: Neuroimaging. 2008;164:1–15. doi: 10.1016/j.pscychresns.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thoma P, Zopplet D, Wiebel B, Daum I. Recollection and familiarity in negative schizophrenia. Neuropsychologia. 2005;44:430–435. doi: 10.1016/j.neuropsychologia.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 19.vanErp TGM, Lesh TA, Knowlton BJ, Bearden CE, Hardt M, Karlsgodt KH, et al. Remember and know judgments during recognition in chronic schizophrenia. Schizophrenia Research. 2008;100:181–190. doi: 10.1016/j.schres.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss AP, Goff DC, Duff M, Roffman JL, Schacter DL. Distinguishing familiarity-based from source-based memory performance in patients with schizophrenia. Schizophrenia Research. 2008;99:208–217. doi: 10.1016/j.schres.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achim AM, Lepage M. Is associative recognition more impaired than item recognition in Schizophrenia? A meta-analysis. Brain Cogn. 2003;53:121–124. doi: 10.1016/s0278-2626(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 22.Danion JM, Rizzo L, Bruant A. Functional mechanisms underlying impaired recognition memory and conscious awareness in patients with schizophrenia. Arch Gen Psychiatry. 1999;56:639–644. doi: 10.1001/archpsyc.56.7.639. [DOI] [PubMed] [Google Scholar]
- 23.Lepage M, Menear M, Montoya A, Achim AM. Associative interference does not affect recognition memory in schizophrenia. Schizophrenia Research. 2005;80:185–196. doi: 10.1016/j.schres.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Lepage M, Montoya A, Pelletier M, Achim AM, Menear M, Lal S. Associative memory encoding and recognition in schizophrenia: An event-related fMRI study. Biol Psychiatry. 2006;60:1215–1223. doi: 10.1016/j.biopsych.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 25.Luck D, Montoya A, Menear M, Achim AM, Lal S, Lepage M. Selective pair recognition memory impairment with no response bias in schizophrenia. Psychiatry Research. 2009;169:39–42. doi: 10.1016/j.psychres.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 26.Sauvage MM, Fortin NJ, Owens CB, Yonelinas AP, Eichenbaum H. Recognition memory: opposite effects of hippocampal damage on recollection and familiarity. Nat Neurosci. 2008;11:16–18. doi: 10.1038/nn2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luck SJ, Gold JM. The translation of cognitive paradigms for patient research. Schizophrenia Bulletin. 2008;34:629–644. doi: 10.1093/schbul/sbn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapman LJ, Chapman JP. The Measurement of Differential Deficit. Journal of Psychiatric Research. 1978;14:303–311. doi: 10.1016/0022-3956(78)90034-1. [DOI] [PubMed] [Google Scholar]
- 29.Althoff RR, Cohen NJ. Eye-movement-based memory effect: A re-processing effect in face perception. JEP:LMC. 1999;25:1–14. doi: 10.1037//0278-7393.25.4.997. [DOI] [PubMed] [Google Scholar]
- 30.Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychological Science. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- 31.Hannula DE, Ryan JD, Tranel D, Cohen NJ. Rapid onset relational memory effects are evident in eye movement behavior, but not in hippocampal amnesia. Journal of Cognitive Neuroscience. 2007;19:1690–1705. doi: 10.1162/jocn.2007.19.10.1690. [DOI] [PubMed] [Google Scholar]
- 32.Hannula DE, Ranganath C. The eyes have it: Hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan JD, Hannula DE, Cohen NJ. The obligatory effects of memory on eye movements. Memory. 2007;15:508–525. doi: 10.1080/09658210701391022. [DOI] [PubMed] [Google Scholar]
- 34.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey‟s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 35.Richmond J, Nelson CA. Relational memory during infancy: evidence from eye tracking. Dev Sci. 2009;12:549–556. doi: 10.1111/j.1467-7687.2009.00795.x. [DOI] [PubMed] [Google Scholar]
- 36.Reilly JL, Harris MSH, Keshavan MS, Sweeny JA. Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Archives of General Psychiatry. 2006;63:1189–1197. doi: 10.1001/archpsyc.63.11.1189. [DOI] [PubMed] [Google Scholar]
- 37.Park S, Holzman PS. Association of working memory deficit and eye tracking dysfunction in schizophrenia. Schizophrenia Research. 1993;11:55–61. doi: 10.1016/0920-9964(93)90038-k. [DOI] [PubMed] [Google Scholar]
- 38.Barch DM, Carter CS, MacDonald AW, Braver TS, Cohen JD. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. Journal of Abnormal Psychology. 2003;112:132–143. [PubMed] [Google Scholar]
- 39.Hutton SB, Crawford TJ, Puri BK, Duncan LJ, Chapman M, Kennard C, et al. Smooth pursuit and saccadic abnormalities in first-episode schizophrenia. Psychological Medicine. 1998;28:685–692. doi: 10.1017/s0033291798006722. [DOI] [PubMed] [Google Scholar]
- 40.Levy DL, Dorus E, Shaughnessy R, Yasillo NJ. Pharmacologic evidence for specificity of pursuit dysfunction to schizophrenia: Lithium carbonate associated with abnormal pursuit. Archives of General Psychiatry. 1985;42:335–341. doi: 10.1001/archpsyc.1985.01790270021002. [DOI] [PubMed] [Google Scholar]
- 41.Everling S, Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- 42.Fukushima J, Fukushima K, Chiba S, Tanaka S, Yamashita I, Kato M. Disturbances of voluntary control of saccadic eye movements in schizophrenic patients. Biological Psychiatry. 1988;23:670–677. doi: 10.1016/0006-3223(88)90050-9. [DOI] [PubMed] [Google Scholar]
- 43.Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology. 2006;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.