Abstract
Fatty acid β-oxidation (FAO) and oxidative phosphorylation (OXPHOS) are key pathways involved in cellular energetics. Reducing equivalents from FAO enter OXPHOS at the level of complexes I and III. Genetic disorders of FAO and OXPHOS are among the most frequent inborn errors of metabolism. Patients with deficiencies of either FAO or OXPHOS often show clinical and/or biochemical findings indicative of a disorder of the other pathway. In this study, the physical and functional interactions between these pathways were examined. Extracts of isolated rat liver mitochondria were subjected to blue native polyacrylamide gel electrophoresis (BNGE) to separate OXPHOS complexes and supercomplexes followed by Western blotting using antisera to various FAO enzymes. Extracts were also subjected to sucrose density centrifugation and fractions analyzed by BNGE or enzymatic assays. Several FAO enzymes co-migrated with OXPHOS supercomplexes in different patterns in the gels. When palmitoyl-CoA was added to the sucrose gradient fractions containing OXPHOS supercomplexes in the presence of potassium cyanide, cytochrome c was reduced. Cytochrome c reduction was completely blocked by myxothiazol (a complex III inhibitor) and 3-mercaptopropionate (an inhibitor of the first step of FAO), but was only partially inhibited by rotenone (a complex I inhibitor). Although palmitoyl-CoA and octanoyl-CoA provided reducing equivalents to OXPHOS-containing supercomplex fractions, no accumulation of their intermediates was detected. In contrast, short branched acyl-CoA substrates were not metabolized by OXPHOS-containing supercomplex fractions. These data provide evidence of a multifunctional FAO complex within mitochondria that is physically associated with OXPHOS supercomplexes and promotes metabolic channeling.
Keywords: Energy Metabolism, Enzyme Mechanisms, Fatty Acid Metabolism, Fatty Acid Oxidation, Mitochondrial Metabolism, Respiratory Chain, Supercomplexes, Acyl-CoA Dehydrogenases, Metabolic Channeling
Introduction
Mitochondria are the site of three of the most important energy generating pathways in humans: oxidative phosphorylation (OXPHOS),2 fatty acid β-oxidation (FAO), and the tricarboxylic acid cycle. OXPHOS is carried out by >150 structural and enzymatic proteins embedded within the inner mitochondrial membrane organized into five functional electron transport chain (ETC) complexes I–V. Blue native gel electrophoresis (BNGE) shows that the ETC complexes associate into higher order structures consisting of complexes I, III, and IV in varying stoichiometries termed supercomplexes. Two supercomplexes species have been identified in Saccharomyces cerevisiae consisting of dimeric complex III with one or two copies of monomeric complex IV (III2+IV1–2) (1, 2). In mammalian and plant studies, a supercomplex containing a dimeric complex III has been shown to associate through the inner mitochondrial membrane to an arm of complex I (I1+III2) (3, 4). Additionally, a supercomplex consisting of one complex I, dimeric complex III, and one to four copies of complex IV (I1+III2+IV1–4) was identified in rat muscle (5) and bovine heart mitochondria (3, 6). Dimeric ATP synthase has been reported to be critical in formation of the mitochondrial inner membrane cristae (7, 8). Three-dimensional models based on electron micrographs of mammalian I1III2,I1III2IV1 and yeast III2IV4 supercomplexes were recently published (6, 9). Association into higher order supercomplexes likely mediates substrate channeling leading to enhanced stability, higher electron transfer rates, greater catalytic efficiency, and sequestration of reactive intermediates (6).
At least 25 enzymes and transport proteins are involved in mitochondrial FAO, which is predominantly responsible for the oxidation of fatty acids of carbon chain length 20 or fewer (10). Fatty acids are transferred across the mitochondrial membrane as carnitine esters by carnitine palmitoyl transferases 1 and 2 in conjunction with the carnitine-acylcarnitine translocase. The four reactions of the β-oxidation cycle, catalyzed by acyl-CoA dehydrogenases (ACADs), enoyl-CoA hydratases, l-3-hydroxyacyl-CoA dehydrogenase, and 3-ketoacyl-CoA thiolase, sequentially remove two carbons until the acyl-CoA is converted to acetyl-CoA molecules. The FAO proteins have been postulated to carry out their function in the context of a multienzyme complex, but this has not been well characterized. The observation that intact mitochondria oxidize fatty acids at a faster rate than disrupted mitochondria is suggestive of functional organization of the enzymes of the FAO pathway (15). Enoyl-CoA hydratase, l-3-hydroxyacyl-CoA dehydrogenase, and 3-ketoacyl-CoA thiolase have been shown to bind to the inner mitochondrial membrane. Additionally, l-3-hydroxyacyl-CoA dehydrogenase interacts with ETC complex I, and 3-ketoacyl-CoA thiolase interacts with citrate synthase (11, 12).
FAO provides reducing equivalents directly to the ETC through two biochemical reactions. In the first reaction, reducing equivalents from ACADs are transferred via electron transfer flavoprotein (ETF) to ETF:ubiquinone oxidoreductase (also known as ETF dehydrogenase), resulting in reduction of coenzyme Q (CoQ). In the second reaction, NAD is reduced by 3-hydroxyacyl-CoA dehydrogenase to form NADH, the substrate for complex I. Despite this functional relationship, evidence for a physical interaction between the two pathways is scant. Submitochondrial particles have been shown to catalyze the transfer of electrons from ETF the complex III through ETF-ETF dehydrogenase and the CoQ pool (13), and a protein complex containing the ACADs, ETF, ETF dehydrogenase, and complex III has been identified but poorly characterized (12). ETF and ETF dehydrogenase have been shown to form a stable complex, and long chain 3-hydroxyacyl-CoA dehydrogenase has been shown to interact directly with ETC complex I at the inner mitochondria membrane (14–16). Finally, we have recently shown that very long chain acyl-CoA dehydrogenase (a member of the ACAD gene family) interacts directly with the mitochondrial membrane in a way that provides a possible direct physical connection between FAO and OXPHOS (17, 18).
Given the localization of components of the ETC and FAO to the inner mitochondrial membrane, the existence of ETC supercomplexes, and the close functional interaction of ETC and FAO, we hypothesized that the proteins of FAO physically associate with each other as well as with the ETC supercomplexes to optimize the efficiency of energy metabolism. In this study, we have used sucrose gradient centrifugation and BNGE to isolate ETC supercomplexes from rat liver mitochondria and demonstrated their association with several enzymes of FAO using highly specific assays. Our results provide direct evidence for the existence of a multifunctional FAO complex that is physically and functionally associated with ETC supercomplexes.
EXPERIMENTAL PROCEDURES
Preparation of Mitochondria from Rat Liver
Freshly isolated rat liver was immediately homogenized in a tissue blender at high speed for 20 s at 4 °C in a buffer containing 25 mm Tris-HCl, pH 7.5, 100 mm KCl, 0.4 m sucrose, and protease inhibitor mixture (Sigma). The homogenate was centrifuged at 900 × g for 10 min at 4 °C, and the pellet was discarded. The supernatant containing mitochondria was subjected to centrifugation at 14,000 × g for 15 min, the pellet was washed once with the homogenization buffer, and the mitochondria were collected by centrifugation under the same conditions and resuspended in the same buffer.
BNGE
Isolated mitochondria (100 μl, 1 mg of protein) were lysed by the addition of 200 μl of high purity digitonin solution (1:4 g of protein/g of digitonin) prepared as follows. 5 mg of digitonin (MP Biomedicals, Solon, OH) was dissolved in 200 μl of 30 mm HEPES buffer, pH 7.4, containing 150 mm potassium acetate and 10% glycerol, heated at 95 °C, then cooled on ice. The mitochondria/digitonin solution (final protein concentration 3 mg/ml) was incubated for 20 min on ice, a Coomassie Blue solution (5% Coomassie Blue G250 in 750 mm 6-aminocaproic acid) was added (1/20 v/v), and the reaction mixture was centrifuged at 14,000 × g for 30 min at 4 °C. The supernatant was directly loaded onto a 4–15% Tris-HCl Ready Gel (Bio-Rad), pH 8.8, and subjected to electrophoresis in 25 mm Tris-glycine buffer (without SDS) at 80 v constant voltage for 4 h, at 4 °C. Following electrophoresis, gels were stained with Bio-Safe Coomassie Blue G250 (Bio-Rad) for 30 min and destained with water. For second dimension separation, a strip of the gel corresponding to a single sample well was rotated 90 degrees and placed on a 12% SDS-polyacrylamide gel and subjected to electrophoresis as described (19). Following electrophoresis, the gel was visualized either with silver staining or Western blotting as below.
Western Blotting
Following BNGE or SDS-PAGE, proteins in the gel were electrophoretically transferred to PVDF membrane for 2 h as described (20). The membrane was blocked for 1 h at room temperature in 20 ml phosphate buffered saline containing 0.1% Tween 20 and 7% powdered milk. The primary antiserum was diluted 1:500–1000 in the same buffer, whereas the secondary antisera were diluted 1:2000–5000 (goat anti-rabbit IgG conjugated to alkaline phosphatase) and 1:5000 (goat anti-rabbit IgG conjugated to horseradish peroxidase).
Sucrose Gradient Centrifugation
Approximately 1.2 ml of digitonin-lysed mitochondria were loaded onto a 10–50% sucrose gradient in 50 mm phosphate buffer, pH 7.2, containing 0.2% digitonin. Samples were then subjected to centrifugation at 40,000 rpm (∼120,000 × g) using a Beckman Ti70 rotor for 19 h. The gradient was collected starting from the bottom in 12 2-ml fractions using a peristaltic pump.
In Situ Gel Staining for ETC Enzyme Activity
Strips corresponding to the sample wells were excised from the blue native gels and incubated with reaction buffer systems specific for ETC complex I, II, IV, or V. The activity staining procedures were essentially as described (21).
Enzyme Activity Assays
ACAD activity was measured with the anaerobic ETF fluorescence reduction assay using an LS50B fluorescence spectrophotometer from PerkinElmer Life Sciences with a heated cuvette block set to 32 °C as previously described (21–23). For standard reactions, the final substrate concentration was 50 μm. One unit of activity is defined as the amount of enzyme necessary to completely reduce 1 μmol of ETF in 1 min.
ETC complex I activity was measured in 50 mm phosphate buffer, pH 7.4, 50 μm NADH, 1 mm KCN, and 50 μm oxidized CoQ as described (24, 25). The reaction was detected by monitoring the decrease in absorbance at 340 nm and quantified using a molar extinction coefficient of 6.2 mm−1·cm−1. Complex III activity (defined as cytochrome c reduction) was measured in 50 mm phosphate buffer, pH 7.4, 1 mm KCN, 30 μm cytochrome c, 20 μm DBH2, monitoring the increase in absorbance at 550 nm (26, 27). Complex IV activity was measured in 50 mm phosphate buffer, pH 7.4, 6 μm ferrocytochrome c, 100 mm KCl. The reaction was monitored at 550 nm (28).
FAO-ETC Bridging Assay
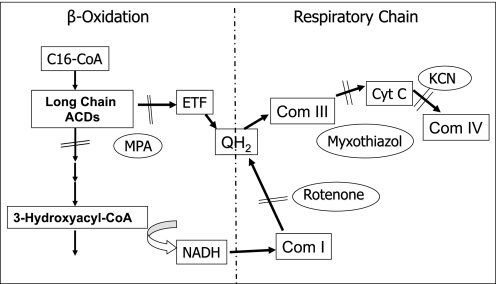
This reaction reflects the interaction of FAO and ETC as measured by reduction of cytochrome c in response to the addition of an acyl-CoA substrate to the reaction mixture. The basic reaction scheme was as follows: an aliquot of sample (usually the sucrose gradient supercomplex fraction) was added to reaction buffer (50 mm phosphate, pH 8.2, containing 50 μm acyl-CoA substrate, 3 μm ETF, 20 μm oxidized CoQ, 30 μm cytochrome c, 1 mm KCN) to give a final volume of 0.7 ml. The reaction was started by the addition of ETF and monitored for the reduction of cytochrome c as indicated by an increase in absorbance at 550 nm using a Beckman DU7500 spectrophotometer (29). Stearyl-CoA, palmitoyl-CoA, octanoyl-CoA, or butyryl-CoA was utilized as substrate to test the long, medium, and short chain specificity of the assay, respectively. A variety of enzymatic inhibitors were added to the base reaction to characterize the contribution of reducing equivalents from reduced ETF and NADH to cytochrome c reduction. Myxothiazol and antimycin A inhibit complex III. Rotenone is a specific inhibitor of complex I. 3-Mercaptopropionic acid (MPA) inhibits long chain ACADs (30). Kinetic parameters for cytochrome c reduction were calculated using a nonlinear regression algorithm as previously described (29, 31).
High Pressure Liquid Chromatography (HPLC) FAO Flux Assay
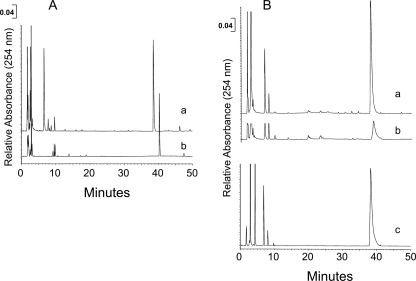
The ability of a sample to perform all of the functions of the entire FAO pathway was tested by following depletion of an acyl-CoA substrate and accumulation of various cycle intermediates via HPLC. Sucrose gradient fractions (2 μg of protein) or purified recombinant ACAD (2–5 ng of protein) was incubated with 50–300 μm butyryl-, octanoyl-, or palmitoyl-CoA at room temperature for 0–50 min. The reaction contained 1 mm pig ETF, 1.5 mm NAD, 0.3 mm CoASH, and 0.2 mm ATP. The reaction was stopped at 5 min intervals by the addition of 40 ml of 2 n HCl, and then immediately neutralized with 40 ml of 2 n NaOH in 200 mm MES. Following clearing of the sample in a bench top microcentrifuge for 10 min, 150 μl of sample was injected into a Luna (Phenomenex, Torrance, CA) 3 μm C18(2), 100 Å, 4.6 × 150-mm, 3-μm column (for palmitoyl-CoA reactions) or a SunFire (Phenomenex) C18, 3.5-μm column (for octanoyl- and butyryl-CoA reactions) equilibrated with 50 mm NH4PO4, pH 5.5, with 9% acetonitrile. The CoA esters that bound to the columns were eluted with a 9–45% linear gradient of acetonitrile in 50 mm NH4PO4, pH 5.5, at 0.9 ml/min over 50 min (Luna column) and 25 min (SunFire column). The presence of CoA ester intermediates in the eluent was monitored by absorbance at 254 nm and compared with the retention times of acyl-CoA ester standards eluted under identical conditions.
RESULTS
Characterization of the Association of ETC and FAO Pathway Components Using BNGE
To examine the association of ETC supercomplexes with FAO components, we separated extracts from digitonin-permeabilized rat liver mitochondria by BNGE and tested for the presence of FAO enzymes by Western blotting. Permeabilization conditions were selected to emphasize preservation of the ETC supercomplexes (1, 6). Following BNGE, a Coomassie Blue banding pattern characteristic of the ETC complexes and supercomplexes was observed (Fig. 1A). A complex I stain identified the top three Coomassie-stained bands as containing complex I activity (Fig. 2A). The two highest relative molecular mass bands (∼1200–2000 kDa) correspond to the previously described mobility of ETC supercomplexes, whereas the third band (∼1000 kDa) is the appropriate size for individual complex I (6). The remaining bands were readily identified by migration and specific activity stains as the other individual ETC complexes V (∼750 kDa), III (∼500 kDa), IV (∼230 kDa), and II (∼120 kDa) in order of migration (1, 6).
FIGURE 1.
Identification of ETC supercomplexes by BNGE. A, rat liver mitochondria were permeabilized with digitonin and resolved by BNGE followed by Coomassie staining to visualize ETC bands. Molecular mass and ETC activity stains identify the top two bands as ETC supercomplexes (SC). The migration of the individual ETC complexes is also indicated. B, permeabilized rat liver mitochondria were subjected to sucrose density gradient centrifugation, and the resulting 10 fractions were separated by BNGE and visualized with Coomassie Blue. The supercomplexes and complex I appeared in the first three fractions. These fractions were pooled and designated as supercomplex (SC) containing fraction in subsequent experiments.
FIGURE 2.
Supercomplexes react with antibodies against FAO proteins. A, relative migration of SC bands and complex I on a blue native gel. Complex I activity stain reacts with the individual complex I band as well as the SC bands. B, the complexes were transferred from the blue native gel to a nylon membrane and Western blotted with antibodies against FAO enzymes including the acyl-CoA dehydrogenases (very long chain acyl-CoA dehydrogenase (VLCAD), long chain acyl-CoA dehydrogenase (LCAD), medium chain acyl-CoA dehydrogenase (MCAD)), their redox partner ETF, and the mitochondrial trifunctional protein (TFP). The supercomplex (SC) and complex I bands reacted variably with these antibodies. In contrast, an antibody against isovaleryl-CoA dehydrogenase, an acyl-CoA dehydrogenase active in amino acid degradation rather than FAO, did not react with either the SC or complex I bands. C and D, the gel strip from A was treated with Laemmli sample buffer for 30 min, placed at the top of a 12.5% SDS-PAGE gel, subjected to electrophoresis, and either treated with silver staining (C) or Western blotted with purified VLCAD antibody (D). The scanned images were superimposed to identify the location of VLCAD among ETC complexes and supercomplexes (arrows). I, V, III, and IV: complexes I, V, III, and IV, respectively.
The ETC complexes and supercomplex bands separated by BNGE were transferred by electrophoresis to a nylon membrane and immunoblotted individually with antisera to the FAO enzymes. Variable patterns of staining of the supercomplex and complex I bands were observed (Fig. 2B). In contrast, antiserum to isovaleryl-CoA dehydrogenase, a member of the structurally homologous ACAD family that also uses ETF as its electron acceptor but is active in leucine metabolism, did not react with the three high molecular mass ETC bands. Thus, the FAO enzymes, but not closely related metabolic enzymes from other pathways, co-migrate with complex I and ETC supercomplexes on BNGE. Two-dimension gel electrophoresis with separation by SDS-PAGE following BNGE gave a pattern consistent with those previously published (Fig. 2C). Western blotting of this gel confirmed the association of very long ACAD (Fig. 2D) and long ACAD (data not shown) with supercomplexes and complex I. Because IVD antiserum was not found to react with the supercomplex fractions following single dimension BNGE, two-dimensional gel experiments were not pursued.
Characterization of the Association of ETC and FAO Pathway Components Using Sucrose Gradient Centrifugation Fractionation
Digitonin-permeabilized rat liver mitochondria were subjected to sucrose gradient centrifugation, and the gradient fractions were analyzed by BNGE (Fig. 1B). The three highest density fractions containing ETC supercomplexes and complex I were combined for further experiments. The proteins present in the pooled supercomplex fraction were separated by SDS-PAGE and subjected to Western blotting with antisera to the various FAO proteins. Very long chain ACAD, long chain ACAD, medium chain ACAD, and ETF along with short chain ACAD were identified in the pooled supercomplex fraction, and all migrated parallel to their corresponding purified recombinant proteins (Fig. 3). The membrane was also tested with antiserum to isovaleryl-CoA dehydrogenase, which detected no antigen in the high Mr fractions, confirming that the other highly homologous ACADs in the supercomplex fractions were not simply contamination with mitochondrial matrix proteins.
FIGURE 3.
Verification of the presence of FAO enzymes in ETC supercomplexes by SDS-PAGE and Western blotting. Permeabilized rat liver mitochondria were fractionated by sucrose density centrifugation. The supercomplex (SC)-containing fractions were pooled and resolved by SDS-PAGE followed by Western blotting for very long chain acyl-CoA dehydrogenase (VLCAD), long chain acyl-CoA dehydrogenase (LCAD), medium chain acyl-CoA dehydrogenase (MCAD), short chain acyl-CoA dehydrogenase (SCAD), ETF, and isovaleryl-CoA dehydrogenase (IVD). Purified recombinant proteins (P) were used as positive control for each blot. All of the FAO proteins were present in the supercomplex fractions whereas the closely related enzyme isovaleryl-CoA dehydrogenase was not. (ETF is a heterodimer, thus there are two bands representing the α and β subunits.)
High levels of ACAD activity were detected in the supercomplex containing fractions with straight chain acyl-CoA substrates ranging between 4 and 20 carbons in length (Fig. 4). In contrast, activity toward the short branched chain substrates isovaleryl-CoA, isobutyryl-CoA, 2-methylbutyryl-CoA, and glutaryl-CoA was very low. Again, the absence of all of the FAO ACADs but not those associated with branched chain amino acid metabolism confirms the specificity of the association of the former with supercomplexes.
FIGURE 4.
ETC supercomplexes demonstrate enzymatic activity for ACADs involved in FAO but not for ACADs involved in amino acid degradation. Permeabilized rat liver mitochondria were fractionated by sucrose density centrifugation. The supercomplex-containing fractions were pooled and used for ACAD activity assays with straight chain acyl-CoA substrates for very long chain ACAD and long chain ACAD (C20–C14), medium chain acyl-CoA dehydrogenase (C12–C8), and short chain ACAD (C6–C4). Considerable activity was detected with these substrates. In contrast, only trace activity was detected with the branched chain acyl-CoA substrates for isovaleryl-CoA dehydrogenase (iC5), isobutyryl-CoA dehydrogenase (iC4), short branched chain ACAD (2-methylbutyryl-CoA (2MeC4)), or glutaryl-CoA dehydrogenase (Glutaryl). Columns represent means ± S.D (error bars) of triplicate assays with the corresponding acyl-CoA substrate.
Evidence for Metabolic Channeling of FAO in the Mr Gradient Fractions
Because a variety of FAO enzymes were identified in the ETC supercomplex gradient fractions, we investigated the ability of these fractions to support coordinated flux through all four reactions necessary for complete β-oxidation. A HPLC-based assay was used to monitor the utilization of palmitoyl-CoA and the appearance of FAO reaction intermediates. In this assay, purified recombinant very long chain ACAD converted palmitoyl-CoA (Fig. 5A, curve a) to its enoyl-CoA product (Fig. 5A, curve b). When the supercomplex containing fractions were substituted for purified enzyme in the reaction, the palmitoyl-CoA substrate peak still decreased with time, but minimal accumulation of β-oxidation intermediates occurred (Fig. 5B, curves a and b). When MPA, a specific inhibitor of the long chain ACADs, was added to the reaction mixture, the palmitoyl-CoA concentration was stable throughout the reaction time (Fig. 5B, curve c). The supercomplex-containing fractions likewise oxidized octanoyl-CoA and butyryl-CoA. Thus, not only are the enzymes of FAO all present in association with the ETC complexes, they appear to be functionally organized to allow substrate channeling from one enzyme to the next in analogy to that seen with OXPHOS.
FIGURE 5.
Supercomplex-containing fractions show FAO substrate channeling. The pooled supercomplex fraction was incubated with palmitoyl-CoA, and the reaction products were analyzed by HPLC. A, curve a, palmitoyl-CoA at 50 μm is shown. Retention time is 38.5 min. Curve b, palmitoyl-CoA was incubated with 2 of μg purified very long chain ACAD in the presence of ETF, NAD+, CoASH, and ATP. The retention time for the generated C16-enoyl-CoA is 40.5 min. These were used as standards for subsequent reactions with the supercomplex fractions. B, supercomplex fraction (2.0 μg of protein) was incubated with 0.5 nm palmitoyl-CoA in the presence of ETF, NAD+, CoASH, and ATP. Curve a, zero time shows the initial amount of palmitoyl-CoA. Curve b, after 20 min the amount of palmitoyl-CoA was dramatically reduced, but there was no accumulation of enoyl-CoA or other intermediate products, indicating efficient channeling of the FAO substrates by the supercomplex fraction. Curve c, supercomplex fraction was preincubated for 10 min with the long chain ACAD/very long chain ACAD inhibitor MPA prior to reaction with palmitoyl-CoA in the presence of ETF, NAD+, CoASH, and ATP. MPA effectively inhibited the metabolism of palmitoyl-CoA by the supercomplex-containing fraction.
Evidence of Electron Transfer from FAO to ETC in the High Molecular Mass Sucrose Fractions
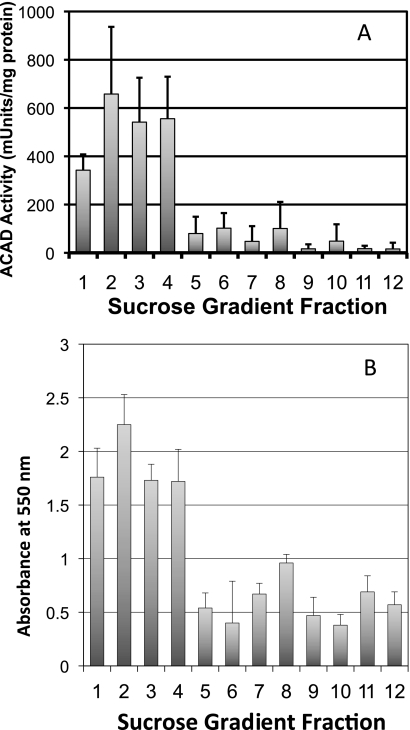
We postulated that the physical association of the multifunctional FAO complex and the ETC supercomplexes in the sucrose gradients might represent a functional unit that promotes metabolic channeling of electrons directly from FAO to the ETC. To test this, we utilized a bridging assay that starts with an FAO substrate and measures reduction of cytochrome c in ETC (Scheme 1 and Fig. 6). When palmitoyl-CoA was used as substrate in the reaction mixture in the presence of excess cytochrome c and CoQ (along with KCN to inhibit electron transfer from cytochrome c to complex IV), the sucrose gradient fractions containing ETC supercomplexes efficiently promoted reduction of cytochrome c (Fig. 6). Addition of exogenous NAD greatly enhanced the reaction. Cytochrome c reduction was dependent on the addition of ETF and was inhibited completely by myxothiazol, an inhibitor of ETC complex III (Fig. 6A). Adding rotenone (a complex I inhibitor) to the reaction mixture reduced the rate of cytochrome c reduction by about half, indicating that only part of the electron flow to cytochrome c was through complex I when acyl-CoA substrate was in excess (Fig. 6B). Thus, the remaining reducing equivalents flowing to cytochrome c must be a result of the ACAD reaction with entry of electrons to the ETC through ETF and ETF-CoQ-oxidoreductase. In keeping with this finding, bridging activity was completely inhibited by the addition of the long chain ACAD inhibitor MPA (Fig. 6C). As a control, the activity of complex I in the presence of MPA was shown to be intact by the ability of NADH to reduce cytochrome c (Fig. 6C). The bridging activity was of similar magnitude when octanoyl-CoA was used as substrate. ACAD activity toward palmitoyl-CoA was most prominent in the high density fractions that contain the ETC supercomplexes (first four fractions; Fig. 7A). The lower density fractions corresponding to the expected molecular mass of the individual ACADs (∼140–170 kDa) exhibited approximately one-tenth of the ACAD activity of the ETC supercomplex fractions. The high density fractions also exhibited the highest bridging activity (Fig. 7B). These results, together with the data presented in Figs. 2 and 3, indicate that fatty acid oxidation and the ETC are both physically and functionally linked.
SCHEME 1.
Relationship between FAO and the ETC. FAO provides reducing equivalents directly to the ETC through two mechanisms. In the first mechanism, reducing equivalents from ACADs are transferred via ETF to ETF:ubiquinone oxidoreductase (also known as ETF dehydrogenase), resulting in reduction of CoQ, the substrate for complex III. In the second mechanism, NAD is reduced by 3-hydroxyacyl-CoA dehydrogenase to form NADH, the substrate for complex I. 3-Mercaptopropionic acid (MPA) inhibits the long chain ACADs. Myxothiazol and rotenone are inhibitors of complex III and complex I, respectively. KCN inhibits the electron transfer from cytochrome c to complex IV.
FIGURE 6.
ETC supercomplexes bridge the activities of FAO and the ETC. A, supercomplexes isolated by sucrose density gradient centrifugation were incubated with palmitoyl-CoA and cytochrome c. The addition of exogenous ETF was required to drive the flow of electrons from palmitoyl-CoA through the ETC to cytochrome c, as measured by an increase in absorbance at 550 nm. Myxothiazol, a complex III inhibitor, blocks the reaction. B, complex I inhibitor rotenone reduces bridging activity by about half by blocking the contribution of electrons from the 3-hydroxyacyl-CoA dehydrogenase step of fatty acid oxidation (see Scheme 1). C, MPA, an inhibitor of long chain ACAD and very long chain ACAD, completely inhibited the bridging activity. The ETC was still functional and responsive to stimulation by exogenous NADH. Arrowheads indicate the time at which each reagent was added to the reaction.
FIGURE 7.
ACAD enzymatic activity and FAO-ETC bridging co-separate in sucrose gradient fractions. A, ACAD activity was measured in sucrose gradient fractions using palmitoyl-CoA as substrate. The highest activity was in fractions 1–4, which contain ETC supercomplexes. B, bridging activity was also the highest in the supercomplex-containing fractions. Bridging was measured as palmitoyl-CoA reduction of cytochrome c reduction (absorbance at 550 nm). Columns represent means ± S.D. (error bars) of triplicate assays.
To examine the kinetic of the bridging reaction, assays were performed with variable palmitoyl-CoA concentrations. The apparent Km of the bridging reaction was calculated to be 14.3 μm, only ∼5 times higher than the in vitro Km of purified recombinant human very long chain ACAD and thus is in a range that is physiologically relevant.
DISCUSSION
FAO is a major energy-producing pathway that provides electrons directly to the ETC for ATP production and is especially critical for cardiac tissue, which uses fatty acids for about 80% of its energy needs. Biochemical abnormalities seen in patients with clinical deficiencies of the ETC support a close relationship between FAO and ETC. Many of these patients accumulate metabolites in blood and urine suggestive of dysfunction of FAO (32–35). Moreover, ∼25% of fibroblast cultures from patients with complex I deficiency show a concomitant decrease in the ability to oxidize palmitate (36). Our results indicate that disruption of larger metabolic supercomplexes containing both the ETC and FAO machinery is a viable mechanism for this phenomenon. Mitochondrial FAO has been previously postulated to occur in the context of a multiprotein complex that promotes metabolic channeling, but this has been poorly characterized, and little evidence exists to physically link FAO to the respiratory chain (37–40).
To examine this possibility of a physical interaction between the two pathways further, we isolated high molecular mass ETC supercomplexes from digitonin-permeabilized mitochondria and then examined the supercomplexes for the presence of FAO enzymes. The results confirm that multiple ACADs, their electron acceptor ETF, ETF dehydrogenase, and trifunctional protein all associate with ETC supercomplexes. This association is not likely to be an artifact of matrix protein contamination for several reasons. All of the activities were present in sufficient quantity such that ACAD enzymatic activity could be robustly measured in the ETC supercomplexes and less so in the fractions corresponding to their isolated molecular masses. This pattern was reversed for the highly homologous ACADs involved in branched-chain amino acid metabolism (that also use ETF as their electron acceptor). Moreover, in vitro assays indicate that all of the necessary FAO components were present to mediate oxidation of acyl-CoAs ranging in length from 4–20 carbons with no accumulation of pathway intermediates. The exception was ETF, which we detected in supercomplex fractions by Western blotting, but was not present in sufficient quantity to drive the bridging assay. With the addition of exogenous ETF, electron flow from palmitoyl-CoA through FAO and the ETC to cytochrome c was very efficient, displaying a Km in the low μm range. Finally, the ACADs involved in branched chain amino acid metabolism did not associate with the complex, even though they are highly homologous to the FAO ACADs. In total, these results provide compelling evidence for the existence of a FAO complex that is physically associated with ETC supercomplexes within mitochondria.
Many functional macromolecular complexes such as the ribosome and splicosome are composed of a relatively consistent constellation of proteins. Although ETC complexes and supercomplexes fall into this category, the FAO multienzyme complex may be more dynamic. CPT-2, very long chain ACAD, ACAD9, and trifunctional protein, are membrane-bound proteins (39). In contrast, medium chain ACAD, long chain ACAD, and short chain ACAD are all considered as “soluble” matrix proteins. In our studies, very long chain ACAD and ACAD9 were found primarily in the supercomplex-containing gradient fractions. However, the soluble matrix ACADs appeared in both the supercomplex-containing fractions as well as in lower density fractions consistent with the know size of the individual tetramers. Thus, it seems likely that the “soluble” ACADs are distributed between membrane-bound and unbound forms depending upon the conditions used for fractionation. We postulate that the membrane-associated FAO enzymes form the core of the MFAO complex whereas the other enzymes interact more loosely with this core. It is possible that other closely related pathways such as the tricarboxylic acid cycle might also be associated with this complex, but this requires further study.
Interestingly, it has recently been shown that very long chain ACAD, long chain ACAD, and short chain ACAD are all subject to acetylation on surface lysine residues (41). Furthermore, long chain ACAD interacts with and is deacetylated by sirtuin-3, a mitochondrial lysine deacetylase. Sirtuin-3 knock-out mice have a metabolic phenotype similar to FAO disorders (41, 42). We speculate that acetylation, which alters protein surface charge by modifying lysines, may modulate the formation and interaction of FAO complex components. Alterations in such a metabolic microenvironment could explain the subtle dysfunction in energy metabolism that we, and others, have demonstrated in complex disease states such as obesity and type 2 diabetes (43, 44).
In summary, we have used sucrose gradient centrifugation and BNGE to provide evidence for the existence of a multifunctional FAO complex within mitochondria that is physically associated with OXPHOS supercomplexes and promotes metabolic channeling. Characterization of the interactions within and between these complexes provides the opportunity to explore new mechanisms for cellular regulation of energy metabolism.
Footnotes
- OXPHOS
- oxidative phosphorylation
- ACAD
- acyl-CoA dehydrogenase
- BNGE
- blue native gel electrophoresis
- CoQ
- coenzyme Q
- ETC
- electron transport chain
- ETF
- electron transfer flavoprotein
- FAO
- fatty acid oxidation
- MPA
- mercaptopropionic acid.
REFERENCES
- 1.Schägger H., Pfeiffer K. (2000) EMBO J. 19, 1777–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruciat C. M., Brunner S., Baumann F., Neupert W., Stuart R. A. (2000) J. Biol. Chem. 275, 18093–18098 [DOI] [PubMed] [Google Scholar]
- 3.Schägger H. (2001) IUBMB Life 52, 119–128 [DOI] [PubMed] [Google Scholar]
- 4.Eubel H., Heinemeyer J., Sunderhaus S., Braun H. P. (2004) Plant Physiol. Biochem. 42, 937–942 [DOI] [PubMed] [Google Scholar]
- 5.Krause F., Reifschneider N. H., Goto S., Dencher N. A. (2005) Biochem. Biophys. Res. Commun. 329, 583–590 [DOI] [PubMed] [Google Scholar]
- 6.Schäfer E., Seelert H., Reifschneider N. H., Krause F., Dencher N. A., Vonck J. (2006) J. Biol. Chem. 281, 15370–15375 [DOI] [PubMed] [Google Scholar]
- 7.Arnold I., Pfeiffer K., Neupert W., Stuart R. A., Schägger H. (1998) EMBO J. 17, 7170–7178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paumard P., Vaillier J., Coulary B., Schaeffer J., Soubannier V., Mueller D. M., Brèthes D., di Rago J. P., Velours J. (2002) EMBO J. 21, 221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinemeyer J., Braun H. P., Boekema E. J., Kouril R. (2007) J. Biol. Chem. 282, 12240–12248 [DOI] [PubMed] [Google Scholar]
- 10.Wanders R. J. A. (2001) in The Metabolic and Molecular Basis of Inherited Disease (Scriver C., Beaudet A. L., Sly W., Valle D. eds) 8th Ed., pp. 3219–3256, McGraw-Hill, New York [Google Scholar]
- 11.Sumegi B., Gilbert H. F., Srere P. A. (1985) J. Biol. Chem. 260, 188–190 [PubMed] [Google Scholar]
- 12.Sumegi B., Porpaczy Z., Alkonyi I. (1991) Biochim. Biophys. Acta 1081, 121–128 [DOI] [PubMed] [Google Scholar]
- 13.Frerman F. E. (1987) Biochim. Biophys. Acta 893, 161–169 [DOI] [PubMed] [Google Scholar]
- 14.Parker A., Engel P. C. (2000) Biochem. J. 345, 429–435 [PMC free article] [PubMed] [Google Scholar]
- 15.Kispal G., Sumegi B., Alkonyi I. (1986) J. Biol. Chem. 261, 14209–14213 [PubMed] [Google Scholar]
- 16.Sumegi B., Srere P. A. (1984) J. Biol. Chem. 259, 15040–15045 [PubMed] [Google Scholar]
- 17.Goetzman E. S., Wang Y., He M., Mohsen A. W., Ninness B. K., Vockley J. (2007) Mol. Genet. Metab. 91, 138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAndrew R. P., Wang Y., Mohsen A. W., He M., Vockley J., Kim J. J. (2008) J. Biol. Chem. 283, 9435–9443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reifschneider N. H., Goto S., Nakamoto H., Takahashi R., Sugawa M., Dencher N. A., Krause F. (2006) J. Proteome Res. 5, 1117–1132 [DOI] [PubMed] [Google Scholar]
- 20.Schägger H., Cramer W. A., von Jagow G. (1994) Anal. Biochem. 217, 220–230 [DOI] [PubMed] [Google Scholar]
- 21.Van Coster R., Smet J., George E., De Meirleir L., Seneca S., Van Hove J., Sebire G., Verhelst H., De Bleecker J., Van Vlem B., Verloo P., Leroy J. (2001) Pediatr. Res. 50, 658–665 [DOI] [PubMed] [Google Scholar]
- 22.Vockley J., Mohsen A. W., Binzak B., Willard J., Fauq A. (2000) Methods Enzymol. 324, 241–258 [DOI] [PubMed] [Google Scholar]
- 23.Mohsen A. W., Anderson B. D., Volchenboum S. L., Battaile K. P., Tiffany K., Roberts D., Kim J. J., Vockley J. (1998) Biochemistry 37, 10325–10335 [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Howton M. M., Beattie D. S. (1995) Biochemistry 34, 7476–7482 [DOI] [PubMed] [Google Scholar]
- 25.Fang J., Wang Y., Beattie D. S. (2001) Eur J. Biochem. 268, 3075–3082 [DOI] [PubMed] [Google Scholar]
- 26.Kniazeva M., Crawford Q. T., Seiber M., Wang C. Y., Han M. (2004) PLoS Biol. 2, E257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraser J. L., Antonioli D. A., Chopra S., Wang H. H. (1995) Mod. Pathol. 8, 65–70 [PubMed] [Google Scholar]
- 28.Prochaska L. J., Bisson R., Capaldi R. A., Steffens G. C., Buse G. (1981) Biochim. Biophys. Acta 637, 360–373 [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Obungu V., Beattie D. S. (1998) Arch. Biochem. Biophys. 352, 193–198 [DOI] [PubMed] [Google Scholar]
- 30.Uchiyama A., Aoyama T., Kamijo K., Uchida Y., Kondo N., Orii T., Hashimoto T. (1996) J. Biol. Chem. 271, 30360–30365 [DOI] [PubMed] [Google Scholar]
- 31.Trumpower B. L. (1990) J. Biol. Chem. 265, 11409–11412 [PubMed] [Google Scholar]
- 32.Jethva R., Bennett M. J., Vockley J. (2008) Mol. Genet. Metab. 95, 195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enns G. M., Bennett M. J., Hoppel C. L., Goodman S. I., Weisiger K., Ohnstad C., Golabi M., Packman S. (2000) J. Pediatr. 136, 251–254 [DOI] [PubMed] [Google Scholar]
- 34.Vockley J., Rinaldo P., Bennett M. J., Matern D., Vladutiu G. D. (2000) Mol. Genet. Metab. 71, 10–18 [DOI] [PubMed] [Google Scholar]
- 35.Bennett M. J., Weinberger M. J., Sherwood W. G., Burlina A. B. (1994) J. Inher. Metab. Dis. 17, 283–286 [DOI] [PubMed] [Google Scholar]
- 36.Venizelos N., von Döbeln U., Hagenfeldt L. (1998) J. Inher. Metab. Dis. 21, 409–415 [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa M., Mikami Y., Usukura J., Iwasaki H., Shinagawa H., Morikawa K. (1997) Biochem. J. 328, 815–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishikawa M., Tsuchiya D., Oyama T., Tsunaka Y., Morikawa K. (2004) EMBO J. 23, 2745–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nada M. A., Rhead W. J., Sprecher H., Schulz H., Roe C. R. (1995) J. Biol. Chem. 270, 530–535 [DOI] [PubMed] [Google Scholar]
- 40.Yu W., Liang X., Ensenauer R. E., Vockley J., Sweetman L., Schulz H. (2004) J. Biol. Chem. 279, 52160–52167 [DOI] [PubMed] [Google Scholar]
- 41.Ahn B. H., Kim H. S., Song S., Lee I. H., Liu J., Vassilopoulos A., Deng C. X., Finkel T. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 14447–14452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., Mann M. (2009) Science 325, 834–840 [DOI] [PubMed] [Google Scholar]
- 43.Kelley D. E., Goodpaster B., Wing R. R., Simoneau J. A. (1999) Am. J. Physiol. Endocrinol. Metab. 277, E1130–E1141 [DOI] [PubMed] [Google Scholar]
- 44.Goodpaster B. H., Wolfe R. R., Kelley D. E. (2002) Obes. Res. 10, 575–584 [DOI] [PubMed] [Google Scholar]