Abstract
Endotoxin (LPS) and sepsis decrease mammalian target of rapamycin (mTOR) activity in skeletal muscle, thereby reducing protein synthesis. Our study tests the hypothesis that inhibition of branched-chain amino acid (BCAA) catabolism, which elevates circulating BCAA and stimulates mTOR, will blunt the LPS-induced decrease in muscle protein synthesis. Wild-type (WT) and mitochondrial branched-chain aminotransferase (BCATm) knockout mice were studied 4 h after Escherichia coli LPS or saline. Basal skeletal muscle protein synthesis was increased in knockout mice compared with WT, and this change was associated with increased eukaryotic initiation factor (eIF)-4E binding protein-1 (4E-BP1) phosphorylation, eIF4E·eIF4G binding, 4E-BP1·raptor binding, and eIF3·raptor binding without a change in the mTOR·raptor complex in muscle. LPS decreased muscle protein synthesis in WT mice, a change associated with decreased 4E-BP1 phosphorylation as well as decreased formation of eIF4E·eIF4G, 4E-BP1·raptor, and eIF3·raptor complexes. In BCATm knockout mice given LPS, muscle protein synthesis only decreased to values found in vehicle-treated WT control mice, and this ameliorated LPS effect was associated with a coordinate increase in 4E-BP1·raptor, eIF3·raptor, and 4E-BP1 phosphorylation. Additionally, the LPS-induced increase in muscle cytokines was blunted in BCATm knockout mice, compared with WT animals. In a separate study, 7-day survival and muscle mass were increased in BCATm knockout vs. WT mice after polymicrobial peritonitis. These data suggest that elevating blood BCAA is sufficient to ameliorate the catabolic effect of LPS on skeletal muscle protein synthesis via alterations in protein-protein interactions within mTOR complex-1, and this may provide a survival advantage in response to bacterial infection.
Keywords: branched-chain amino acids, raptor, mTOR, skeletal muscle, eIF3
gram-negative bacterial infection, when sustained, leads to muscle atrophy, which is due, in part, to coordinated decreases in both myofibrillar and sacroplasmic protein synthesis (35, 53). However, live bacteria are not essential for the development of this catabolic condition as endotoxin (e.g., LPS) decreases skeletal muscle protein synthesis in a time- and dose-dependent manner (30, 33). Both sepsis and LPS impair muscle protein synthesis by decreasing translational efficiency, in general, and peptide-chain initiation, in particular, as opposed to reducing the number of ribosomes (30, 43, 55). Central to the sepsis- and LPS-induced decrease in translation initiation in muscle is the inhibition of the serine (Ser)/threonine (Thr) kinase mammalian target of rapamycin (mTOR), the activity of which is typically characterized by the reduced phosphorylation of two downstream substrates, eukaryotic initiation factor (eIF)-4E binding protein-1 (4E-BP1) and ribosomal protein S6 kinase-1 (30, 33, 43). A comparable decrease in mTOR activity is also observed in myotubes cultured with LPS and interferon-γ (11). The activity of mTOR regulates protein synthesis by integrating and transducing diverse extracellular cues from growth factors [e.g., IGF-I] and nutrients (e.g., amino acids), as well as the cellular energy status and environmental stresses (7). Of the two distinct intracellular mTOR complexes (mTORC), the mTORC-1, consisting of mTOR, raptor (regulatory-associated protein of mTOR), G protein β-subunit-like protein (GβL), proline-rich Akt substrate 40 kDa (PRAS40), and DEP domain containing 6 (DEPDC), is most important in regulating protein synthesis (21). Moreover, alterations in various protein-protein interactions within mTORC1 markedly affect mTOR activity (14, 18, 24).
Muscle protein synthesis is accelerated by amino acid availability, in general, and the indispensable branched-chain amino acids (BCAA) valine, leucine, and isoleucine, in particular (4, 37, 38). Of these, leucine stimulates muscle protein synthesis with the greatest efficiency by increasing substrate delivery and stimulating signal transduction pathways controlling translation initiation (1, 37). Recently, She et al. (47) generated a mouse model in which the gene encoding the mitochondrial branched-chain aminotransferase isozyme (BCATm) was disrupted. BCATm catalyzes the first step in BCAA metabolism, which transfers the α-amino group of the BCAA to α-ketoglutarate, thereby forming the three respective branched-chain α-keto acids. Because of its wide tissue distribution, this enzyme represents an important regulator of leucine signaling and interorgan exchange of BCAA metabolites (20). As a result of the knockout (KO) of BCATm, mice demonstrate an impaired peripheral BCAA catabolism accompanied by elevated plasma BCAA concentrations. Importantly, the plasma branched-chain α-keto acids, which produce toxic neurological manifestations (51), are not increased in BCATm KO mice (47). Therefore, these null mice were used in the present study to address the hypothesis that a sustained elevation in the plasma BCAA concentration produced by inhibition of BCAA catabolism and the subsequent stimulation of mTOR will ameliorate or prevent the reduced muscle protein synthesis observed in response to LPS. As LPS represents an acute septic-like insult, an additional study was also performed to determine whether elevating BCAA would convey a long-term survival advantage to mice in response to polymicrobial bacterial infection, which was more chronic in duration.
MATERIALS AND METHODS
Animals and experimental protocol.
The animal protocols were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine and adhere to the National Institutes of Health guidelines for the use of experimental animals. Studies were performed on male KO and wild-type (WT) C57BL6 littermates between 12 and 14 wk of age. BCATm KO mice have elevated circulating BCAA concentrations, but they exhibit no increase in α-keto acids in either plasma or muscle (47). These KO mice, however, do possess the cytosolic isoform of BCAT present in neurons and brain (12), which can potentially lead to the excessive local production of BCAA metabolites and neurological derangements (48). To prevent the toxic accumulation of keto acids within neural tissues, both BCATm KO and WT mice had free access to both normal chow diet (model 2018,; Global 18% protein rodent diet; Harlan Teklad, Indianapolis, IN) and an isonitrogenous and isocaloric purified BCAA-free diet (model 510081; Dyets, Bethlehem, PA) (36, 47). Male WT mice consume ∼55% and 45% of the normal chow and BCAA-free diet, respectively, whereas KO mice consume 24% and 76% of the two diets, respectively. Importantly, total food consumption and energy intake between the WT and BCATm KO mice does not differ (36, 47). Food was removed from all mice at 0700 h, and experiments were performed between 0900 and 1200 h. BCATm and WT mice administered either saline or LPS were alternated to compensate for differences in the period of time mice were without food.
The in vivo rate of protein synthesis in the gastrocnemius-plantaris complex (hereafter referred to as skeletal muscle) and heart (ventricle only) was determined in WT and BCATm KO mice 4 h after either intraperitoneal injection of 0.9% saline (e.g., basal time-matched controls) or a nonlethal dose of LPS (Escherichia coli 026:B6, 25 μg/mouse; Sigma, St. Louis, MO). The LPS dose and timing were based on preliminary studies in mice demonstrating a reproducible reduction in muscle protein synthesis (data not shown). Protein synthesis was determined using the flooding dose technique, exactly as described (54). Mice were injected intraperitoneally with [3H]-l-phenylalanine (150 mM, 30 μCi/ml; 1 ml/100 g body wt), and blood was collected 15 min later for determining the plasma phenylalanine concentration and radioactivity. Thereafter, skeletal and cardiac muscle were rapidly excised, freeze-clamped, and then stored at −70°C. Tissues were processed exactly as described (33, 53). The rate of protein synthesis was calculated by dividing the amount of radioactivity incorporated into protein by the plasma phenylalanine-specific radioactivity, and the advantages and disadvantages of this method have been reviewed (13). The specific radioactivity of the plasma phenylalanine was measured by HPLC analysis of supernatant from TCA extracts of plasma. In addition, samples of fresh muscle were homogenized for Western blot analysis and immunoprecipitation of selected proteins as described below.
Western blot analysis.
Fresh tissue was homogenized in ice-cold homogenization buffer consisting of (in mmol/l): 20 HEPES (pH 7.4), 2 EGTA, 50 sodium fluoride, 100 potassium chloride, 0.2 EDTA, 50 β-glycerophosphate, 1 DTT, 0.1 phenylmethane-sulphonylfluoride, 1 benzamidine, and 0.5 sodium vanadate (30, 33, 36). Protein was determined after centrifugation and equal amounts of protein per sample were subjected to standard SDS-PAGE, using antibodies obtained from Cell Signaling (Beverly, MA), unless otherwise specified. Specifically, Western blot analysis was performed for total and phosphorylated (Thr246) PRAS40 (Biosource, Camarillo, CA), total and phosphorylated (Ser792) raptor, and total and phosphorylated 4E-BP1 (Thr 37/46; Bethyl Laboratories, Montgomery, TX). In addition, total GβL (also called mLST8), DEPTOR (Millipore, Billerica, MA), and eIF3f or eIF3b (Abcam, Cambridge, MA) were determined by Western blot analysis. Blots were developed with enhanced chemiluminescence Western blotting reagents (Supersignal Pico; Pierce, Rockford, IL). Dried blots were exposed to X-ray film to achieve a signal within the linear range, and film was then scanned (Microtek ScanMaker IV) and quantified using Scion Image 3b software (Scion, Frederick, MD).
The 4E-BP1·eIF4E and eIF4G·eIF4E complexes were quantified as described previously (30, 36). Briefly, eIF4E was immunoprecipitated from aliquots of supernatants by using an anti-eIF4E monoclonal antibody (Drs. Jefferson and Kimball; Hershey, PA). Antibody-antigen complexes were collected by using magnetic beads subjected to SDS-PAGE and quantified as above.
To maintain the protein-protein interactions within mTORC1, a portion of muscle was homogenized in 3[(3-cholamidopropyl)dimethylammonio]-propanesulfonic acid (CHAPS) buffer consisting of (in mmol/l): 40 HEPES (pH 7.5), 120 NaCl, 1 EDTA, 10 pyrophosphate, 10 β-glycerophosphate, 50 sodium fluoride, 1.5 sodium vanadate, 0.3% CHAPS, and 1 protease inhibitor cocktail tablet (14). The homogenate was mixed on a platform rocker and clarified by centrifugation. An aliquot of the resulting supernatant was combined with anti-raptor, anti-mTOR or anti-eIF3b antibody, and immune complexes were isolated with goat anti-rabbit BioMag IgG beads (PerSeptive Diagnostics, Cambridge, MA). Beads were collected, washed with CHAPS buffer, precipitated by centrifugation, and subjected to SDS-PAGE and analyzed as above.
Ribonuclease protection assays.
Total RNA was extracted from tissues in a mixture of phenol and guanidine thiocyanate (TRI Reagent; Molecular Research Center, Cincinnati, OH) by using the manufacturer's protocol. Riboprobes were synthesized from a multiprobe mouse cytokine template set (rCK-1; Pharmingen, San Diego, CA) by using an in vitro transcription kit (Pharmingen). Primer sequences for TNF-α, IL-6, nitric oxide synthase (NOS)-2, and inhibitor of NF-κB-δ (IκBζ) have been reported (41). The labeled riboprobe was hybridized with RNA overnight by using a ribonuclease protection assay. Protected RNAs were separated by using a 5% acrylamide gel, and dried gels were exposed to a phosphorimager screen (Molecular Dynamics, Sunnyvale, CA). The resulting data were quantified by using ImageQuant and normalized to L32.
Determination of plasma metabolites and hormone concentrations.
The plasma concentrations of insulin and IGF-I were determined by enzyme-linked immunosorbent assays (Linco Research, St. Charles, MO and Immunodiagnostic Systems, Fountain Hills, AZ, respectively). BCAAs were determined using reverse-phase HPLC after precolumn derivatization of amino acids with phenylisothiocyanate.
Sepsis survival study.
In a second study, the effect of BCATm deletion and elevated BCAA concentrations on survival was determined in septic mice. For this study, polymicrobial peritonitis was produced by cecal ligation and puncture (CLP), as described previously (41). Briefly, mice were anesthetized using isoflorane (Abbott Laboratories, North Chicago, IL). The abdomen was shaved, the skin was prepared with povidone iodine, and a midline incision (1.5 cm) was made below the diaphragm. The cecum was isolated, ligated, punctured twice with a 22-gauge needle, and a small amount of cecal material extruded to ensure patency. The cecum was returned to the abdomen, the muscle incision closed with 6-0 surgical suture (Ethicon, Somerville, NJ), and metal wound clips were used to close the skin incision. Before suturing the skin, two to three drops of lidocaine (Abbott Laboratories) were administered to the wound for analgesia. All mice were housed individually after surgery, and each received 1 ml of warmed (37°C) 0.9% sterile saline containing 0.05 mg/kg of buprenorphine (Reckitt Benckiser Pharmaceuticals, Richmond, VA) administered subcutaneously every 12 h for the remainder of the experimental protocol. Sham controls were subjected to the same surgical laparotomy and cecal isolation, but the cecum was neither ligated nor punctured. The survival study was performed in a manner whereby the investigators were blinded to the animal genotype and/or sepsis treatment until completion of the study. Survival was assessed every 12 h for 7 days. Although the primary endpoint of this second experiment was survival, any mouse determined to be moribund was anesthetized and killed instead of waiting for spontaneous death (6, 41). Before and after surgery, animals had unrestricted access to food and water, and food consumption was measure daily. At the end of the observation period, whole body lean mass was measured using an 1H-NMR system (model LF90; Bruker Minispec, Woodlands, TX) as previously reported (47). Mice were then anesthetized, the gastrocnemius and heart were removed, and their wet weight was determined.
Statistical analysis.
Data for each condition are summarized as means ± SE where the number of mice per treatment group is indicated in the legend of the figure or table. Statistical evaluation of the data was performed using two-way ANOVA with post hoc Student-Neuman-Keuls test when the interaction was significant. Differences in survival were analyzed by a Kaplan-Meier survival plot and the log-rank statistic. Differences were considered significant when P < 0.05.
RESULTS
Plasma BCCA and hormone concentrations.
Under basal postabsorptive conditions, BCATm KO mice had elevated plasma concentrations for each BCAA [leucine (∼10-fold), isoleucine (∼6-fold), and valine (∼5-fold)] compared with time-matched pair-fed WT mice (Table 1). LPS did not significantly alter the plasma concentration of leucine, isoleucine, or valine in either WT or BCATm KO mice. There was no statistically significant interaction between gene deletion and LPS, so the BCAA concentrations did not differ between BCAT KO and WT mice injected with LPS.
Table 1.
Effect of LPS on plasma concentrations of branched-chain amino acids, insulin and IGF-I in wild-type (WT) and mitochondrial branched-chain amino acid knockout (BCATm KO) mice
| Saline Controls |
LPS-Treated |
|||
|---|---|---|---|---|
| WT | BCATm KO | WT | BCATm KO | |
| No./group | 12 | 15 | 15 | 13 |
| Leucine, μmol/l | 63 ± 9a | 662 ± 174b | 55 ± 8a | 687 ± 202b |
| Isoleucine, μmol/l | 84 ± 8a | 561 ± 119b | 71 ± 11a | 504 ± 145b |
| Valine, μmol/l | 123 ± 15a | 722 ± 103b | 118 ± 19a | 744 ± 113b |
| Insulin, ng/ml | 0.61 ± 0.08a | 0.56 ± 0.06a | 1.37 ± 0.23b | 1.49 ± 0.26b |
| IGF-I, ng/ml | 755 ± 44 | 739 ± 38 | 667 ± 46 | 688 ± 51 |
Values are means ± SE; Means in a row with superscripts without a common letter are statistically different, P < 0.05.
The plasma insulin concentration did not differ between WT and BCATm KO mice under basal conditions (Table 1). In contrast, hyperinsulinemia was detected in both WT and BCATm KO mice after LPS, and the increment in plasma insulin was comparable in both groups. Neither BCATm deletion nor LPS administration significantly altered the plasma IGF-I concentration.
Muscle protein synthesis.
In vivo-determined protein synthesis in skeletal muscle from BCATm KO mice was increased 25%, compared with WT control values under basal (e.g., no LPS) conditions (Fig. 1, top). LPS decreased muscle protein synthesis 43% in WT mice and 33% in BCATm KO mice, compared with their respective control values. As a result, the protein synthetic rate was not different in muscle from WT mice under basal conditions and BCATm KO mice after LPS. In contrast to skeletal muscle, protein synthesis in heart was not altered by either the deletion of the BCATm gene and/or LPS administration (Fig. 1, bottom).
Fig. 1.
Effect of LPS on in vivo protein synthesis in skeletal muscle (top) and heart (bottom) from wild-type (WT) and mitochondrial branched-chain aminotransferase (BCATm) knockout (KO) mice. Phe, [3H]-L-phenylalanine. Values in bar graph are means ± SE; n = 10 mice per group. Values with different letters are significantly different from each other (P < 0.05).
4E-BP1 phosphorylation and eIF4F complex formation.
We quantified the phosphorylation of 4E-BP1 as a marker of mTOR kinase activity, because this protein is a known mTOR substrate (7). The extent of 4E-BP1 phosphorylation was increased 60% in skeletal muscle of BCATm KO mice, compared with time-matched control values (Fig. 2A). Conversely, LPS decreased 4E-BP1 phosphorylation in both groups, but the extent of 4E-BP1 dephosphorylation was greater in WT than BCATm KO mice. These changes were independent of a change in total 4E-BP1 content, as assessed by quantitation of the α-, β-, and γ-isoforms of the protein in muscle homogenates among the four groups [WT-control = 5,460 ± 445 arbitrary units (AU), BCATm KO-control = 5,520 ± 566 AU, WT-LPS = 5,402 ± 298 AU, and BCATm KO-LPS = 5,606 ± 522 AU; P = not significant (NS)]. The hyperphosphorylation of 4E-BP1 leads to the redistribution of eIF4E from the inactive eIF4E·4E-BP1 to the active eIF4E·eIF4G complex (15). However, under control conditions, the amount of eIF4E·4E-BP1 complex in skeletal muscle did not differ between WT and BCATm KO mice (Fig. 2B). LPS appeared to selectively increase eIF4E·4E-BP1 content in WT, but not in KO mice. As anticipated, the binding of eIF4E to eIF4G was increased in BCATm KO mice under control conditions compared with WT control values (Fig. 2C). Conversely, the amount of eIF4E·eIF4G was reduced in both groups after LPS, but the absolute amount of the complex was lower in WT than KO mice. The above-mentioned changes in eIF4E distribution were independent of a change in total eIF4E in skeletal muscle among the four experimental groups (data not shown).
Fig. 2.
Effect of LPS on 4E binding protein-1 (4E-BP1) phosphorylation and the distribution of eukaryotic initiation factor (eIF)-4E (eIF4E) in skeletal muscle of WT and BCATm KO mice. A: bar graph quantitating the densitometric analysis of Western blot analysis for phosphorylated 4E-BP1 in total muscle homogenate. In the total homogenate, there was no effect of gene deletion (e.g., KO) or LPS on the total amount eIF4E, eIF4G, or 4E-BP1 (data not shown). Additionally, eIF4E was immunoprecipitated from the homogenate and then immunoblotted for 4E-BP1 (B), eIF4G (C), and eIF4E [data not shown; P = not significant (NS)]. For all bar graphs, the value from the control WT group was set at 1.0 arbitrary unit (AU). Values in bar graph are means ± SE; n = 9–10 mice per group. Values with different letters are significantly different from each other (P < 0.05).
To confirm the above-mentioned data for myocardial protein synthesis, we also determined 4E-BP1 phosphorylation. Our data indicate that Thr37/46 phosphorylation of 4E-BP1 was not statistically altered by either BCATm gene deletion and/or LPS administration in heart (data not shown), and additional analysis of mTORC1 in heart was not pursued because of this lack of change.
mTORC-1.
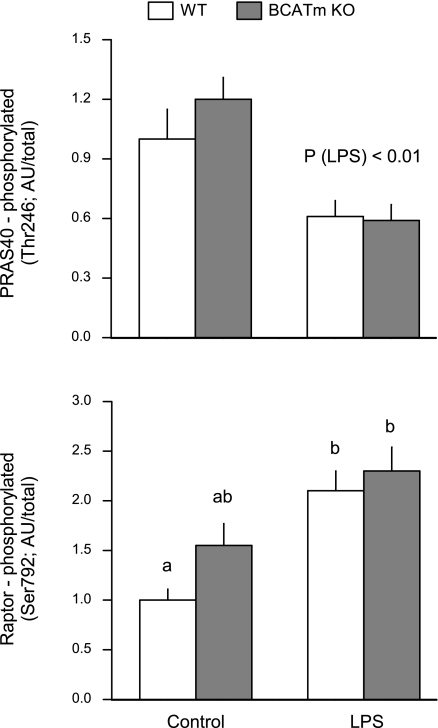
BCATm deletion and/or LPS were without effect on the total amount of raptor, PRAS40, GβL, and DEPTOR (data not shown), four proteins that together with mTOR comprise mTORC1 (14, 24). Although PRAS40 phosphorylation did not differ between WT and BCATm KO mice under control conditions, LPS decreased PRAS40 phosphorylation by 40% in both groups (Fig. 3, top). Furthermore, raptor phosphorylation increased twofold in both WT and KO mice in response to LPS (Fig. 3, bottom).
Fig. 3.
Effect of LPS on proline-rich Akt substrate 40 kDa (PRAS40) and raptor phosphorylation in skeletal muscle of WT and BCATm KO mice. Bar graphs represent, quantitation of the densitometric analysis of all immunoblots for either phosphorylated PRAS40 (top) or raptor (bottom) in which the value from the control WT group was set at 1.0 AU. No difference in total PRAS40 or raptor among groups (data not shown). Values in bar graph are means ± SE; n = 8–10 mice per group. Values with different letters are significantly different from each other (P < 0.05).
Neither BCATm gene deletion nor LPS altered the amount of mTOR or PRAS40 bound to raptor (Fig. 4, A and B, respectively). In contrast, the ability of 4E-BP1 to bind raptor tended to be increased (P < 0.08) under control conditions in KO mice, compared with WT controls (Fig. 4C). In response to LPS, the amount of the 4E-BP1·raptor complex was reduced in muscle from both WT and KO mice, but the decrease was greater in LPS-treated WT mice. This change in 4E-BP1·raptor binding was independent of the amount of raptor in the immunoprecipitate (data not shown). Finally, BCATm KO mice demonstrated an increased binding of raptor with eIF3 under control conditions (Fig. 4D). The amount of the raptor·eIF3 complex in muscle was decreased by LPS in WT mice, but not in BCATm KO mice.
Fig. 4.
Effect LPS on mamalian target of rapamycin (mTOR), 4E-BP1, and PRAS40 binding with raptor in skeletal muscle of WT and BCATm KO mice. Raptor was immunoprecipitated, and the amount of mTOR (A), 4E-BP1 (B) and PRAS40 (C) bound to the raptor immunoprecipitate was determined. The amount of raptor in the immunoprecipitate did not differ among the 4 groups (data not shown). D: eIF3f was immunoprecipitated and the amount of raptor bound was determined by immunoblotting. The amount of eIF3 in the immunoprecipitate did not differ between groups (data not shown). Bar graphs, represent quantitation of the densitometric analysis of all immunoblots where the value from the control WT group was set at 1.0 AU per either total raptor or eIF3 in immunoprecipitate. Values in bar graph are means ± SE; n = 8–10 mice per group. Values with different letters are significantly different from each other (P < 0.05).
Muscle cytokines.
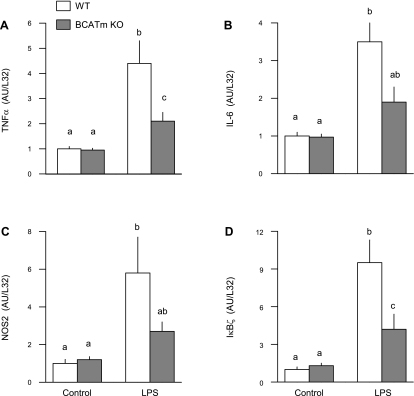
As part of the innate immune system, whole muscle and cultured myocytes secrete a number of inflammatory cytokines that can impair protein synthesis (8). Therefore, TNF-α IL-6, NOS2, and IkBζ mRNA expression were determined. The muscle mRNA content for these immunomodulators did not differ between WT and BCATm KO mice under control conditions (Fig. 5). As anticipated, LPS increased muscle mRNA expression of each modulator in WT mice. However, this LPS-induced increase was smaller in magnitude for each of the assessed inflammatory mediators.
Fig. 5.
Effect of LPS on muscle cytokine mRNA content in WT and BCATm KO mice. The mRNA content for TNF-α (A), IL-6 (B), nitric oxide synthase-2 (NOS2; C), and IκBζ (D) was determined by ribonuclease protection assay. Bar graphs, values are means ± SE; n = 8–10 mice per group. Values with different letters are significantly different from each other (P < 0.05).
Sepsis survival study.
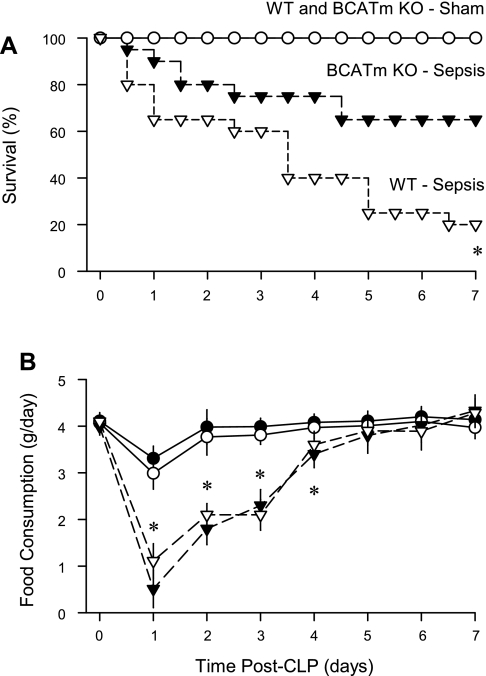
To determine whether the elevation in leucine and other BCAA, as well as the reduced inflammatory response, would translate to a survival advantage in BCATm KO mice, 7-day survival was determined by using a standard CLP model of peritonitis. This model is generally recognized to mimic the clinical scenario with greater fidelity than the injection of LPS, which is more appropriate in studying the acute phase of bacterial infection (3). The survival of both WT and BCATm KO mice under control conditions (e.g., following sham surgery) was 100% (Fig. 6A). WT mice exhibited only 20% survival at 7 days after CLP, and this was significantly (P < 0.05) lower than the 65% survival rate observed in BCATm KO mice after CLP.
Fig. 6.
Survival and food consumption of WT and BCATm KO mice to peritonitis produced by cecal ligation and puncture (CLP). A: survival was determined in all 4 experimental groups over a 7-day period. *P < 0.05, compared with BCATm KO + sepsis group. B: food consumption was determined daily for all 4 groups. *P < 0.05, septic groups, compared with nonseptic control values. There was no significant difference in food consumption between WT-septic and BCATm KO-septic mice at any time point.
Both groups of septic mice showed a 60–80% decrease in food consumption the first day after CLP, compared with time-matched control values (Fig. 6B). Food consumption gradually increased and was not different from time-matched control values on days 5–7. Hence, a difference in food consumption cannot explain the survival advantage seen in the BCATm KO mice, compared with WT mice, in response to CLP.
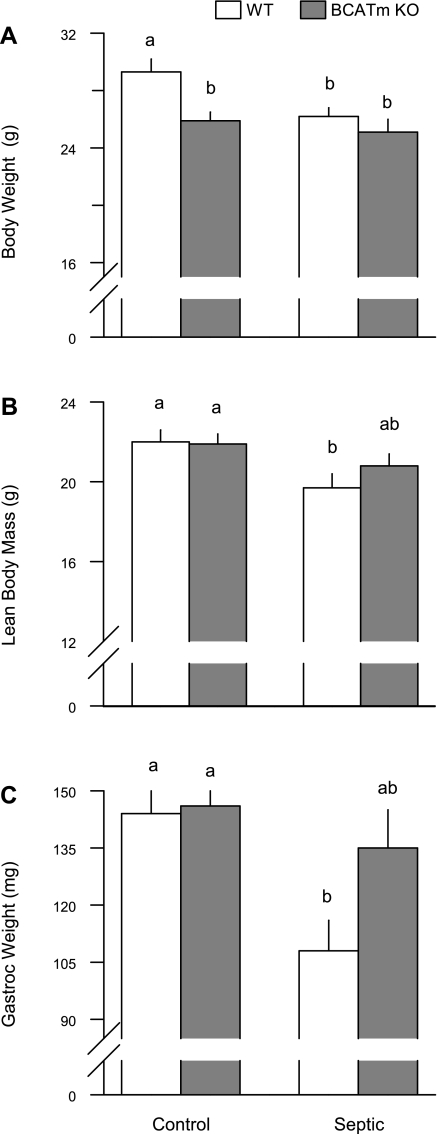
The body weight of the BCATm KO mice immediately prior to the induction of sepsis was 9% lower than WT control values (28.4 ± 0.8 vs. 25.5 ± 0.4 g; P < 0.05), consistent with previous reports (36, 47). In the sham control mice, a comparable difference in body weight was detected at the end of the 7-day observation period (Fig. 7A). Sepsis induced by CLP decreased body weight 10% on day 7 in WT mice, but no decrement in body weight was detected in BCATm KO mice. Whole body lean body mass determined by 1H-NMR did not differ between WT and BCATm KO mice prior to CLP (22.1 ± 0.1 and 21.9 ± 0.1 g, respectively; P = NS). However, similar to the change in body weight, the sepsis-induced decrease in lean body mass of 11% in WT mice was not seen in BCATm KO mice (Fig. 7B). Likewise, the directly determined wet weight of the gastrocnemius did not differ between WT and BCATm KO mice under nonseptic control conditions, but muscle weight was selectively decreased in WT and intermediate in the BCATm KO mice in response to CLP (Fig. 7C). In contrast, the wet weight of the heart did not differ among any of the four experimental groups (data not shown).
Fig. 7.
Body weight, lean body mass, and gastrocnemius weight in WT and BCATm KO mice 7 days after sepsis produced by CLP. Bar graphs, values are means ± SE; n = 7–8 mice per group. Values with different letters are significantly different from each other (P < 0.05).
DISCUSSION
Deletion of BCATm increased the plasma concentration of each BCAA severalfold in the basal (e.g., no LPS) postabsorptive condition. This increase was associated with an elevated rate of protein synthesis and increased phosphorylation of 4E-BP1 in skeletal, but not cardiac, muscle. The phosphorylation of 4E-BP1 on Thr37/46 by mTORC1 primes 4E-BP1 for additional phosphorylation events that lead to the redistribution of eIF4E from the inactive eIF4E·4E-BP1 complex to the active eIF4E·eIF4G complex produced by leucine or growth factors (29). In the present study, while we failed to observe the anticipated reciprocal reduction in the formation of the inactive 4E-BP1·eIF4E complex, we did detect increased binding of eIF4G with eIF4E (e.g., active eIF4F complex) in the basal state in BCATm KO mice. A similar anabolic effect is seen in perfused hindlimb, incubated muscle, and cultured myocytes, and it is predominantly mediated by the elevation in leucine, as opposed to either valine or isoleucine (1, 4, 23, 25). These data also support the concept that leucine per se is a direct-acting nutrient signal for stimulating protein synthesis, because leucine is transported into but not metabolized by muscle of BCATm KO mice (38, 47). Furthermore, whereas the acute administration of leucine produces a robust increase in plasma insulin (1, 29), the insulin concentration of WT and BCATm KO mice did not differ. Collectively, these data suggest the anabolic effect of BCATm deletion in the postabsorptive state is largely leucine-dependent and insulin-independent.
Our subsequent analyses provided a more detailed characterization of mTORC1 and related signaling elements in BCATm KO mice. In this regard, raptor is the unique adaptor protein in mTORC1 that binds and presents substrates to mTOR (14, 18, 24). In the present study, basal raptor phosphorylation tended to be increased in BCATm KO mice, although this change did not achieve statistical significance. As catabolic, not anabolic, stimuli have been reported to increase raptor phosphorylation (11, 44), this response was unexpected. Likewise, the similar extent of basal PRAS40 phosphorylation in WT and BCATm KO mice was not anticipated because the acute elevation of plasma leucine increases PRAS40 phosphorylation in striated muscle (45). Because PRAS40 is a downstream substrate of Akt, the unaltered PRAS40 phosphorylation in the present study may be the result of the comparable basal insulin levels between the two groups. Collectively, these data suggest that a change in either raptor or PRAS40 phosphorylation (Ser792 and Thr246, respectively) is unlikely to mediate the increased muscle protein synthesis detected in BCATm KO mice under basal conditions.
Alterations in protein-protein interactions within mTORC1 markedly influence mTOR kinase activity (7, 14, 24). In contrast to other catabolic conditions, no difference in the amount of mTOR·raptor binding was detected between BCATm and WT mice in the control condition. Furthermore, there was no change in the binding of PRAS40 to raptor in BCATm KO mice. Again, this finding was unexpected as binding of PRAS40 with mTORC1 increases under in vivo conditions of nutrient and growth factor withdrawal (e.g., mTOR inhibition) (52). In contrast, there was a marked elevation in the amount of 4E-BP1 binding to raptor. Raptor binds to the TOR signaling motif found in all known substrates of mTORC1, including 4E-BP1 and PRAS40 (40). This response is consistent with the ability of raptor to function as a scaffold protein and the necessity of 4E-BP1 recruitment to the mTOR·raptor complex for optimal stimulation of protein synthesis (18). Moreover, compared with WT mice, the amount of the raptor·eIF3 complex was increased in BCATm KO mice under basal conditions. In this regard, eIF3 is a multisubunit protein complex whose interaction with the eIF4F·mRNA complex facilitates translation initiation (17). Collectively, these data imply that raptor may be central to integrating various signals for mTOR regulation.
Administration of LPS decreases skeletal muscle protein synthesis in rats and humans (33, 55). This defect is mediated by decreased mTOR kinase activity as evidenced by 4E-BP1 hypophosphorylation and the subsequent decrease in eIF4E·eIF4G, the latter of which is necessary for active eIF4F complex formation as described above. Direct evidence has been reported regarding the importance of a functional eIF4F complex in regulating basal protein synthesis (19). Comparable changes in muscle protein synthesis and eIF4E distribution have been reported in skeletal muscle in response to peritonitis and overproduction of TNF-α (29, 32). In contradistinction to the response in rats (33), in mice LPS selectively decreased protein synthesis in skeletal muscle, but not heart, and the reason for this species-specific response is not apparent. Our work also provides novel data related to the effect of LPS on mTORC1 complex formation in skeletal muscle. For example, LPS increased raptor phosphorylation, a response similar to that seen in other catabolic conditions in vivo (44) and in myocytes cultured with LPS (11). The increased raptor phosphorylation is consistent with stimulation by AMP-activated protein kinase, which increases in muscle in response to sepsis and LPS and inhibits mTOR kinase activity (10, 42). However, because enhanced raptor phosphorylation was detected in BCATm mice under both basal conditions (where muscle protein synthesis was increased) and in LPS-treated WT mice (where muscle protein synthesis was decreased), we interpret these data to suggest this particular phosphorylation event is not central to regulating muscle mTOR kinase activity. PRAS40 also binds raptor and is generally believed to be a translational repressor, so that its overexpression decreases mTOR activity (26). LPS did not alter total PRAS40 but did decrease its phosphorylation, which is consistent with the response observed in myocytes cultured with LPS (10). Although a change in PRAS40 phosphorylation can influence its binding to raptor, the extent of PRAS40-raptor complex formation did not differ in WT mice after LPS administration.
We also report that LPS acutely decreases the amount of both 4E-BP1 and eIF3 bound to raptor, without altering the amount of the mTOR-raptor complex. These data are consistent with the scaffold function of raptor and the necessary recruitment of 4E-BP1 prior to its phosphorylation and release (18). Hence, the decreased amount of 4E-BP1·raptor corroborates the observed LPS-induced decrease in 4E-BP1 phosphorylation seen in the total muscle homogenate. Furthermore, these changes are consistent with reports in cell systems exposed to catabolic agents and conditions (56). LPS also decreased the binding of raptor with eIF3 in skeletal muscle, which has not been previously reported. Finally, the above-mentioned LPS-induced changes in muscle protein synthesis and mTORC1 appear independent of altered BCAA, insulin, and IGF-I concentrations in the systemic circulation.
We hypothesized that despite the presence of muscle leucine resistance after LPS and during sepsis (16, 29), increasing the circulating leucine concentration would antagonize the LPS-induced decrease in skeletal muscle protein synthesis. Indeed, the inhibitory effects of LPS on protein synthesis per se and several of the protein-protein interactions within mTORC1, specifically the binding of both 4E-BP1 and eIF3 with raptor, were partially ameliorated in BCATm KO mice, compared with WT animals. Muscle is now recognized to be an important component of the innate immune system (9), and increased concentrations of various inflammatory mediators in the blood and muscle decrease muscle protein synthesis and mTOR activity (22, 34), which we speculate might serve to conserve or shift energy substrates for enhanced immune function. Therefore, it is noteworthy that the LPS-induced increase in muscle inflammation, as evidenced by the elevated mRNA content for TNF-α, IL-6, NOS2, and IκKζ was blunted in BCATm KO mice, compared with WT animals. The mechanism mediating this suppressive effect is not apparent, but a similar anti-inflammatory effect of essential amino acids (including leucine) has been reported (46). These results suggest the inhibitory effect of LPS can be offset by increasing the availability of leucine and/or BCAA and infer that the LPS-induced defect in muscle protein synthesis is, in part, dependent upon leucine availability and intracellular metabolism of BCAA.
The present study focused on the synthetic side of the protein balance equation. However, it is well recognized that muscle wasting accompanying sepsis is, in part, due to an elevated rate of protein degradation (49, 50, 58, 59). Data surrounding alterations in muscle protein breakdown in BCATm KO mice are limited and difficult to interpret. For example, She et al. (47) reported an increased urinary 3-methylhistidine-to-creatinine ratio in BCATm KO mice, compared with WT control values. While such data are suggestive of an increased myofibrillar degradation, other tissues in addition to muscle can potentially contribute to the enhanced excretion of 3-methylhistidine (39). Additionally, the muscle mRNA content of both atrogin-1 and MuRF1 determined by ribonuclease protection assay did not differ between BCATm KO and WT control mice (C. H. Lang, unpublished observations). While these two proteins have been implicated in muscle wasting in other catabolic states (2), our observations are limited because the protein content for these two atrogenes was not determined. Finally, indices of muscle proteolysis have not been assessed in BCATm KO mice after LPS or sepsis. Hence, a systematic analysis is required to determine whether changes in muscle protein breakdown may also contribute to the preservation of muscle mass in BCATm KO mice in response to sepsis.
We acknowledge that the administration of LPS produces a severe and acute inflammatory insult that mimics the early phase of bacterial infection (3). While this inflammatory model has limitations, the protein metabolic changes produced by LPS (at 4 h) and CLP-induced sepsis (at 24 h) are remarkably consistent (29, 30, 32, 33, 35). Hence, to broaden our data interpretation, we also examined changes in survival as well as body and muscle weight in WT and BCATm KO mice after 7 days of peritonitis. Our data clearly show that the sepsis-induced reductions in body weight, lean body mass, and muscle weight observed in WT mice are largely absent in BCATm KO mice, and this phenotype was associated with improved survival in response to CLP. At this time, it is not possible to ascribe these beneficial responses directly to the inhibition of BCAA metabolism, the increase in circulating BCAA concentrations and/or some other unique feature of the BCATm KO mouse.
Perspectives and Significance
The systemic inflammatory response accompanying sepsis leads to the erosion of lean body mass by stimulating protein degradation and impairing protein synthesis in skeletal muscle under basal conditions (35, 50). Moreover, sepsis blunts the normal anabolic effect of leucine on muscle protein accretion (29). This sepsis-induced muscle atrophy is generally acknowledged to increase morbidity and mortality, as well as slow rehabilitation of this patient population. To date, some therapeutic modalities directed at inhibiting excessive cytokine or glucocorticoid action, or enhancing anabolic hormone availability, have been successfully employed to prevent or minimize muscle wasting in animal models (28, 31, 41, 49, 58, 59). However, equivocal data have been reported in studies providing metabolic support to critically ill patients using BCAA in general or leucine in particular (5). These discordant findings may be due to differences in the relative amount of BCAA or leucine vs. other nutrients, route of nutritional support (enteral vs. parenteneral), underlying nutritional status of the patients (well nourished vs. malnourished), and/or the severity and type of critical illness (27). The present investigation used BCATm KO mice to probe the potential beneficial effect of a sustained elevation in the circulating BCAA concentrations in septic rats under well-controlled conditions difficult to achieve in the clinical arena. Our data indicate enhanced survival and amelioration of muscle wasting in BCATm KO mice in response to CLP, and extrapolation of our data from LPS-treated mice suggests the muscle effect was due, in part, to an improved rate of protein synthesis mediated via enhanced mTOR signaling. However, because the circulating BCAA concentrations in BCATm KO mice are increased as a result of decreased BCAA catabolism in nonneuronal tissues (20), we cannot exclude this as a possible mechanism for the improvement in survival and muscle protein balance after sepsis. Regardless of the exact mechanism, it is tempting to speculate that future development of small molecule inhibitors of BCATm based on the known crystal structure of this enzyme (57) might represent a novel therapeutic strategy.
GRANTS
This work was supported, in part, by National Institutes of Health Grants GM-38032, GM-39277, and DK-062880.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the expert technical assistance of Anne Pruznak, Danuta Huber, Jay Nystrom, Rachel Fogle, and Gina Deiter.
REFERENCES
- 1.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130: 2413–2419, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov 4: 854–865, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Buse MG, Reid SS. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest 56: 1250–1261, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bandt JP, Cynober L. Therapeutic use of branched-chain amino acids in burn, trauma, and sepsis. J Nutr 136: 308S–313S, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Demers G, Griffin G, De Vroey G, Haywood JR, Zurlo J, Bedard M. Animal research. Harmonization of animal care and use guidance. Science 312: 700–701, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Dunlop EA, Tee AR. Mammalian target of rapamycin complex 1: signalling inputs, substrates and feedback mechanisms. Cell Signal 21: 827–835, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Frost RA, Lang CH. Regulation of muscle growth by pathogen-associated molecules. J Anim Sci 86: E84–E93, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am J Physiol Regul Integr Comp Physiol 283: R698–R709, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Frost RA, Nystrom GJ, Lang CH. Endotoxin and interferon-γ inhibit translation in skeletal muscle cells by stimulating nitric oxide synthase activity. Shock 32: 416–426, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frost RA, Nystrom GJ, Lang CH. Endotoxin and interferon-γ inhibit translation in skeletal muscle cells by stimulating nitric oxide synthase activity. Shock 32: 416–426, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Espinosa MA, Wallin R, Hutson SM, Sweatt AJ. Widespread neuronal expression of branched-chain aminotransferase in the CNS: implications for leucine/glutamate metabolism and for signaling by amino acids. J Neurochem 100: 1458–1468, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Garlick PJ, McNurlan MA, Essen P, Wernerman J. Measurement of tissue protein synthesis rates in vivo: a critical analysis of contrasting methods. Am J Physiol Endocrinol Metab 266: E287–E297, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177–189, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Hara K, Yonezawa K, Kozlowski MT, Sugimoto T, Andrabi K, Weng QP, Kasuga M, Nishimoto I, Avruch J. Regulation of eIF-4E BP1 phosphorylation by mTOR. J Biol Chem 272: 26457–26463, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Hasselgren PO, James JH, Warner BW, Hummel RP, III, Fischer JE. Protein synthesis and degradation in skeletal muscle from septic rats. Response to leucine and α-ketoisocaproic acid. Arch Surg 123: 640–644, 1988 [DOI] [PubMed] [Google Scholar]
- 17.Hinnebusch AG. eIF3: a versatile scaffold for translation initiation complexes. Trends Biochem Sci 31: 553–562, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123: 569–580, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Huang BP, Wang Y, Wang X, Wang Z, Proud CG. Blocking eukaryotic initiation factor 4F complex formation does not inhibit the mTORC1-dependent activation of protein synthesis in cardiomyocytes. Am J Physiol Heart Circ Physiol 296: H505–H514, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Hutson SM, Wallin R, Hall TR. Identification of mitochondrial branched chain aminotransferase and its isoforms in rat tissues. J Biol Chem 267: 15681–15686, 1992 [PubMed] [Google Scholar]
- 21.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6: 1122–1128, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Janssen SP, Gayan-Ramirez G, Van den BA, Herijgers P, Maes K, Verbeken E, Decramer M. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation 111: 996–1005, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Jurasinski C, Gray K, Vary TC. Modulation of skeletal muscle protein synthesis by amino acids and insulin during sepsis. Metabolism 44: 1130–1138, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163–175, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Kimball SR, Shantz LM, Horetsky RL, Jefferson LS. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem 274: 11647–11652, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, Roth RA. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem 278: 10189–10194, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Kudsk KA. Immunonutrition in surgery and critical care. Annu Rev Nutr 26: 463–479, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Lang CH, Fan J, Cooney R, Vary TC. IL-1 receptor antagonist attenuates sepsis-induced alterations in the IGF system and protein synthesis. Am J Physiol Endocrinol Metab 270: E430–E437, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Lang CH, Frost RA. Differential effect of sepsis on ability of leucine and IGF-I to stimulate muscle translation initiation. Am J Physiol Endocrinol Metab 287: E721–E730, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Lang CH, Frost RA. Endotoxin disrupts the leucine-signaling pathway involving phosphorylation of mTOR, 4E-BP1, and S6K1 in skeletal muscle. J Cell Physiol 203: 144–155, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Lang CH, Frost RA. Glucocorticoids and TNFα interact cooperatively to mediate sepsis-induced leucine resistance in skeletal muscle. Mol Med 12: 291–299, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang CH, Frost RA. Sepsis-induced suppression of skeletal muscle translation initiation mediated by tumor necrosis factor-α. Metabolism 56: 49–57, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Lang CH, Frost RA, Jefferson LS, Kimball SR, Vary TC. Endotoxin-induced decrease in muscle protein synthesis is associated with changes in eIF2B, eIF4E, and IGF-I. Am J Physiol Endocrinol Metab 278: E1133–E1143, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Lang CH, Frost RA, Nairn AC, MacLean DA, Vary TC. TNF-α impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab 282: E336–E347, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab 293: E453–E459, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Lang CH, Lynch CJ, Vary TC. Alcohol-induced IGF-I resistance is ameliorated in mice deficient for mitochondrial branched-chain aminotransferase. J Nutr 140: 932–938, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li JB, Jefferson LS. Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochim Biophys Acta 544: 351–359, 1978 [DOI] [PubMed] [Google Scholar]
- 38.Lynch CJ, Halle B, Fujii H, Vary TC, Wallin R, Damuni Z, Hutson SM. Potential role of leucine metabolism in the leucine-signaling pathway involving mTOR. Am J Physiol Endocrinol Metab 285: E854–E863, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Nishizawa N, Noguchi T, Hareyama S, Funabiki R. Fractional flux rates of Nt-methylhistidine in skin and gastrointestine: the contribution of these tissues to urinary excretion of Nt-methylhistidine in the rat. Br J Nutr 38: 149–151, 1977 [DOI] [PubMed] [Google Scholar]
- 40.Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, Hara K, Tanaka N, Avruch J, Yonezawa K. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem 278: 15461–15464, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Nystrom G, Pruznak A, Huber D, Frost RA, Lang CH. Local insulin-like growth factor I prevents sepsis-induced muscle atrophy. Metabolism 58: 787–797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nystrom GJ, Lang CH. Sepsis and AMPK activation by AICAR differentially regulate FoxO-1, -3 and -4 mRNA in striated muscle. Int J Clin Exp Med 1: 50–63, 2008 [PMC free article] [PubMed] [Google Scholar]
- 43.Orellana RA, Kimball SR, Nguyen HV, Bush JA, Suryawan A, Thivierge MC, Jefferson LS, Davis TA. Regulation of muscle protein synthesis in neonatal pigs during prolonged endotoxemia. Pediatr Res 55: 442–449, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Pruznak AM, Kazi AA, Frost RA, Vary TC, Lang CH. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-d-ribonucleoside prevents leucine-stimulated protein synthesis in rat skeletal muscle. J Nutr 138: 1887–1894, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez CC, Demeulder B, Ginion A, Bayascas JR, Balligand JL, Alessi DR, Vanoverschelde JL, Beauloye C, Hue L, Bertrand L. Activation of the cardiac mTOR/p70(S6K) pathway by leucine requires PDK1 and correlates with PRAS40 phosphorylation. Am J Physiol Endocrinol Metab 298: E761–E769, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Schuster H, Blanc MC, Neveux N, Bonnefont-Rousselot D, Le TA, De Bandt JP, Cynober L. Protective effects of regulatory amino acids on ischemia-reperfusion injury in the isolated perfused rat liver. Scand J Gastroenterol 41: 1342–1349, 2006 [DOI] [PubMed] [Google Scholar]
- 47.She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab 6: 181–194, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skvorak KJ. Animal models of maple syrup urine disease. J Inherit Metab Dis 32: 229–246, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Smith IJ, Alamdari N, O'Neal P, Gonnella P, Aversa Z, Hasselgren PO. Sepsis increases the expression and activity of the transcription factor Forkhead Box O 1 (FOXO1) in skeletal muscle by a glucocorticoid-dependent mechanism. Int J Biochem Cell Biol 42: 701–711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith IJ, Lecker SH, Hasselgren PO. Calpain activity and muscle wasting in sepsis. Am J Physiol Endocrinol Metab 295: E762–E771, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr 68: 72–81, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Vander HE, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Vary TC, Kimball SR. Sepsis-induced changes in protein synthesis: differential effects on fast- and slow-twitch muscles. Am J Physiol Cell Physiol 262: C1513–C1519, 1992 [DOI] [PubMed] [Google Scholar]
- 54.Vary TC, Lang CH. Assessing effects of alcohol consumption on protein synthesis in striated muscles. Methods Mol Biol 447: 343–355, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Vesali RF, Cibicek N, Jakobsson T, Klaude M, Wernerman J, Rooyackers O. Protein metabolism in leg muscle following an endotoxin injection in healthy volunteers. Clin Sci (Lond) 118: 421–427, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Harris TE, Roth RA, Lawrence JC., Jr PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem 282: 20036–20044, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Yennawar NH, Conway ME, Yennawar HP, Farber GK, Hutson SM. Crystal structures of human mitochondrial branched chain aminotransferase reaction intermediates: ketimine and pyridoxamine phosphate forms. Biochemistry 41: 11592–11601, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Zamir O, Hasselgren PO, Kunkel SL, Frederick J, Higashiguchi T, Fischer JE. Evidence that tumor necrosis factor participates in the regulation of muscle proteolysis during sepsis. Arch Surg 127: 170–174, 1992 [DOI] [PubMed] [Google Scholar]
- 59.Zamir O, O'Brien W, Thompson R, Bloedow DC, Fischer JE, Hasselgren PO. Reduced muscle protein breakdown in septic rats following treatment with interleukin-1 receptor antagonist. Int J Biochem 26: 943–950, 1994 [DOI] [PubMed] [Google Scholar]