Summary
Endothelial cells form cell-cell adhesive structures, called adherens and tight junctions, which maintain tissue integrity, but must be dynamic for leukocyte transmigration during the inflammatory response and cellular remodeling during angiogenesis. This review will focus on Vascular Endothelial (VE)-cadherin, an endothelial-specific cell-cell adhesion protein of the adherens junction complex. VE-cadherin plays a key role in endothelial barrier function and angiogenesis, and consequently VE-cadherin availability and function are tightly regulated. VE-cadherin also participates directly and indirectly in intracellular signaling pathways that control cell dynamics and cell cycle progression. Here we highlight recent work that has advanced our understanding of multiple regulatory and signaling mechanisms that converge on VE-cadherin and have consequences for endothelial barrier function and angiogenic remodeling.
Introduction
Endothelial cells form the vasculature and are the major barrier between the blood and the rest of the body. These specialized cells regulate the exchange of solutes and fluids between the blood and tissue and control entry of leukocytes into the surrounding tissue. The endothelium is also the site for angiogenesis, which involves extension and remodeling of blood vessels. Essential for all these functions is the ability of endothelial cells to properly regulate cell-cell adhesions between themselves and neighboring cells. Endothelial dysfunction is often a result of altered permeability of the endothelial cell monolayer and is a hallmark of many pathological and disease states, including atherosclerosis, diabetes, hypertension, inflammation, and tumor metastasis [1,2].
Endothelial cells present a special challenge for the regulation of cell-cell adhesion. On the one hand, the integrity of cell-cell adhesion within the monolayer must be maintained for proper barrier function. On the other hand, cell-cell adhesion must be sufficiently plastic to allow passage of leukocytes as well as growth and development of blood vessels. This review will focus on VE-cadherin, the endothelial-specific transmembrane component of the adherens junction complex [3], which has emerged as a master regulator of endothelial cell-cell adhesive properties.
Endothelial cell junctions
Endothelial cells utilize two types of adhesion complexes to mediate cell-cell interactions, adherens and tight junctions (Figure 1) [4]. Adherens junctions participate in multiple functions, including establishment and maintenance of cell-cell adhesion, actin cytoskeleton remodeling, intracellular signaling, and transcriptional regulation. Tight junctions regulate monolayer permeability, and play a greater role in endothelial cells that maintain stringent barriers, such as those that constitute the blood-brain barrier. Adherens junction assembly and organization precedes formation of tight junctions. In endothelial cells, adherens and tight junctions are intermingled, whereas in epithelial cells the tight junction is localized apical to the adherens junction
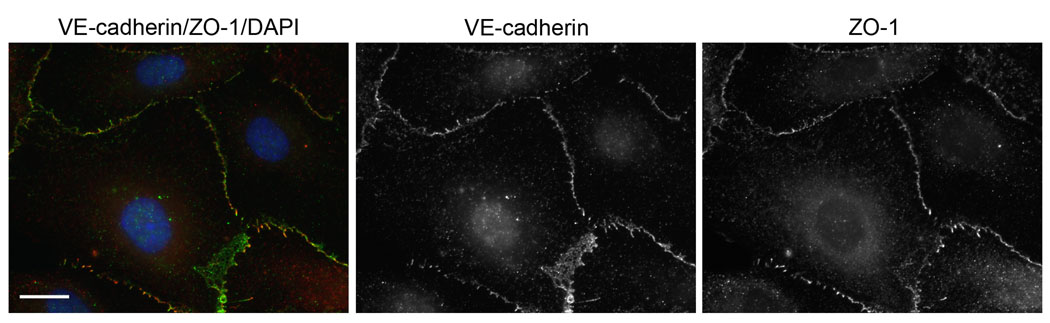
Figure 1.
Human umbilical vein endothelial cells stained for VE-cadherin (adherens junctions, green), ZO-1 (tight junctions, red), and DAPI (nucleus, blue). Scale bar, 20 microns.
The protein compositions of adherens and tight junctions in endothelial cells are similar to those in epithelial cells [5], with a few exceptions. The transmembrane component of endothelial adherens junction is VE-cadherin (Cadherin-5, CD144), a type-II endothelial-restricted classical cadherin [3], which binds through its cytoplasmic tail to members of the armadillo repeat family of proteins, including p120-catenin, β-catenin, and plakoglobin. p120-Catenin and β-catenin can also shuttle to the nucleus to regulate gene expression. α-Catenin associates indirectly with VE-cadherin by binding to β-catenin and plakoglobin. In addition to these core components of the adherens junction, several other proteins bind VE-cadherin and regulate its activity (discussed below). Endothelial cells also express high levels of Neuronal (N)-cadherin, but it is not clustered at cell-cell contacts between endothelial cells [6,7].
Mouse models in which the VE-cadherin gene was inactivated demonstrate the importance of VE-cadherin for endothelial cell biology. VE-cadherin-deficient mice die in mid-gestation as a result of major defects in vascular remodeling. The primitive vascular plexus initially forms, but beyond E9.25 these vessels regress and disintegrate [8,9]. Similarly, in zebrafish embryos, initial vascular network formation occurs following morpholino-mediated depletion of VE-cadherin, but vessels do not form connections necessary for lumen formation and collapse [10]. VE-cadherin function blocking antibodies disrupt cell-cell adhesion, increase permeability, and enhance transmigration of leukocytes [11,12]. Together, these studies show that VE-cadherin participates in multiple aspects of endothelial cell biology, and is indispensable for maturation, extension, and remodeling of vessels that are characteristic of angiogenesis. Further evidence that the overall integrity of the adherens junction is important for endothelial cell function is supported by conditional inactivation of β-catenin [13] and p120-catenin [14] in mouse endothelial cells. In both cases, vascular defects lead to embryonic death at E11.5–13.5. Other in vivo models of cell-cell adhesion components have been made and are discussed in a recent review [15].
Tight junctions are composed of transmembrane proteins that include claudins, occludins, and junctional adhesion molecules (JAMs). These membrane proteins associate with cytoplasmic proteins including, zonula occludens (ZO), AF-6/afadin, and PAR-3. Of the transmembrane components, claudin-5 is specifically expressed in endothelial cells [16]. Claudin-5 knockout mice die within 10 hours of birth due to a size-selective disruption in blood-brain barrier permeability, but surprisingly overall morphology of the vasculature and ultrastructure of tight junctions is normal [17]. Disruption of claudin-5 also causes increased monolayer permeability in tissue culture cells, but does not alter VE-cadherin localization. On the other hand, VE-cadherin regulates expression of claudin-5 [18••]. VE-cadherin clustering at cell-cell contacts attenuates activity of the forkhead transcriptional repressor FoxO1, resulting in upregulation of claudin-5 mRNA. FoxO1 regulates expression of genes involved in vascular development and remodeling, and FoxO1 null embryos die due to defects in these processes [19,20]. Downregulation of FoxO1 in response to VE-cadherin clustering occurs through two mechanisms: 1) activation of phosphatidylinositol-3 kinase (PI3K)-Akt signaling cascade, which induces phosphorylation and inactivation FoxO1, and 2) reduction in levels of nuclear β-catenin, a binding partner and enhancer of FoxO1 activity [18••]. Thus, in addition to adhesive properties, the role of VE-cadherin as a transducer of intracellular signals is critical to maintain proper endothelial cell function.
VE-cadherin regulation
VE-cadherin is essential for controlling endothelial monolayer permeability and angiogenesis. There are many mechanisms that regulate VE-cadherin including, modulating VE-cadherin activity through phosphorylation and controlling the amount of VE-cadherin available for engagement at adherens junctions at both the protein and mRNA level (Figure 2A).
Figure 2.
Schematic of VE-cadherin regulation and signaling. Numbers correspond to labeling on figure. For simplicity, only p120-catenin and β-catenin are shown bound to VE-cadherin; plakoglobin and α-catenin have been omitted. (A) Mechanisms that modulate VE-cadherin activity include: (1) changes in VE-cadherin gene expression through activity of Erg/Ets-1, TAL-1/SCL, and Twist/Slug/Snail transcription factors; (2) trafficking via Golgi-associated protein cPLA2α; (3) transport along actin filaments via myosin-X; (4) stabilization at the plasma membrane by p120-catenin, which is enhanced by FGF; (5) phosphorylation induced by permeability factors (e.g. VEGF, TNF-α, PAF, thrombin, histamine, IL-8) which signal through various kinases to promote disassembly of the adherens junction complex and/or VE-cadherin internalization; (6) phosphorylation induced by leukocytes which promotes disassembly of the adherens junction complex; and (7) inhibition of phosphorylation by PTPs or Ang1. (B) Mechanisms by which VE-cadherin can transduce intracellular signals include: (8) indirect binding to VEGF-R2, which prevents its phosphorylation, internalization, and signaling to MAPK; (9) binding to and assembly of TGF-β receptor complex, which enhances Smad-dependent transcription; signaling through small GTPases including (10) Rho/ROCK to promote actomyosin contraction, (11) Rac1/Tiam1, and (12) Rap1/Epac; and indirect regulation of gene transcription by (13) limiting the amount of p120-catenin and β-catenin that can translocate to the nucleus and (14) inhibiting FoxO1, which leads to an increase in claudin-5 mRNA.
Phosphorylation of VE-cadherin
It is generally accepted that phosphorylation of VE-cadherin leads to destabilization of the adherens junction complex and increased monolayer permeability, although which residues are phosphorylated and important for modifying VE-cadherin activity is less clear.
Several soluble factors such as vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), platelet-activating factor (PAF), thrombin, and histamine induce tyrosine phosphorylation of VE-cadherin, which correlates with an increase in vascular permeability [21]. These factors also lead to phosphorylation of β-catenin, p120-catenin, and plakoglobin, suggesting that phosphorylation of other components of the adherens junction complex promotes disruption of cell-cell contacts. The molecular mechanism(s) down-stream of these soluble factors that result in VE-cadherin phosphorylation, and how this leads to increased permeability is currently an area of intense investigation.
VEGF was originally identified as a potent factor for inducing endothelial permeability [22], and was shown subsequently to induced tyrosine phosphorylation of the adherens junction complex [23]. VEGF stimulation results in Src tyrosine kinase-mediated phosphorylation of VE-cadherin on Y685 [24]. Mice lacking Src or treated with Src inhibitors do not show an increase in vascular permeability in response to VEGF [25,26], suggesting that Src-mediated phosphorylation of VE-cadherin induced by VEGF is a major regulatory pathway that modifies the structural integrity of cell-cell contacts.
VEGF also induces tyrosine phosphorylation of additional residues on VE-cadherin. VEGF stimulation promoted Rac1-dependent production of reactive oxygen species, resulting in phosphorylation of VE-cadherin on Y658 and Y731. This results in the disorganization of cell-cell contacts and increased monolayer permeability [27]. TNF-α stimulation also leads to tyrosine phosphorylation of Y658 and Y731 mediated by a signaling cascade initiated by PI3K p100α, including activation of proline-rich tyrosine kinase 2 (Pyk2) and Rac1/Tiam1 [28]. Y658 or Y731 phosphorylation disrupts VE-cadherin association with p120-catenin or β-catenin, respectively [29].
Leukocyte docking to endothelial monolayers also triggers tyrosine phosphorylation of VE-cadherin, an effect mediated by signals induced upon engagement of inter-cellular adhesion molecule (ICAM)-1 [30•,31•]. Activation of Src and Pyk2 in response to ICAM-1 engagement results in phosphorylation of Y658 and Y731 on VE-cadherin. Inhibition of these kinases, or mutation of these residues to non-phosphorylatable amino acids, impairs transmigration of leukocytes [31•]. However, another study showed ICAM-1 engagement promotes phosphorylation of Y645, Y731 and Y733, but not Y658 [30•]. Despite these discrepancies, it seems clear that leukocytes alter the phosphorylation state of VE-cadherin as a general mechanism to weaken cell-cell contacts and promote their efficient transmigration.
Several protein tyrosine phosphatases (PTPs) associate with and dephosphorylate VE-cadherin. These PTPs include vascular endothelial receptor-type PTP (VE-PTP), whose expression is restricted to endothelial cells [32,33]. VE-PTP activity enhances VE-cadherin-mediated cell-cell adhesion, which results in a decrease in endothelial barrier permeability [32]. VE-PTP mutant [34] or knockout mice [35] die in mid-gestation with defects in angiogenic remodeling, similar to the VE-cadherin-deficient mice. Thus, the balance between PTP and tyrosine kinase activities is important to regulate the level of VE-cadherin phosphorylation and thereby the degree of endothelial permeability. Interestingly, leukocytes trigger the dissociation of VE-PTP from VE-cadherin [36], further supporting the idea that leukocytes induce changes in the phosphorylation state of VE-cadherin to enhance their transmigration.
While much of the focus has been on understanding the effects of tyrosine phosphorylation of VE-cadherin, serine phosphorylation also plays an important role. VEGF and interleukin-8 (IL-8) both promote phosphorylation of VE-cadherin on S665, which results in increased barrier permeability due to internalization of VE-cadherin [37••,38]. Mutation of this serine residue to a non-phosphorylatable amino acid prevents internalization of VE-cadherin in response to either VEGF or IL-8. Interestingly, these two soluble factors both signal through Rac1, which leads to p21-activated kinase (PAK)-mediated phosphorylation of S665. While VEGF stimulation activates Rac1 through Src-dependent phosphorylation of Vav2 [37••], IL-8 activates Rac1 through CXC chemokine receptor 2 (CXCR2) and activation of PI3Kγ [38]. In summary, tyrosine and serine phosphorylation of VE-cadherin directly affects VE-cadherin stability and protein interactions that, in turn, affect endothelial permeability.
VE-cadherin availability at the cell surface
Cells modulate their adhesive state by controlling the amount of cadherin localized at the cell surface. The direct association of p120-catenin with VE-cadherin prevents clathrin-dependent endocytosis of VE-cadherin [39,40]. The interaction of p120-catenin with VE-cadherin is also necessary for maintaining endothelial barrier function [41] and for proper angiogenic remodeling [14]. Fibroblast growth factor (FGF) signaling, which promotes stabilization of the vasculature and decreases monolayer permeability, increases p120-catenin association with VE-cadherin [42], supporting the idea that the interaction of p120-catenin with VE-cadherin enhances monolayer integrity. Furthermore, overexpression of p120-catenin reduces transmigration of leukocytes mainly due to decreased tyrosine phosphorylation of VE-cadherin on Y658 [43•]. Y658 is located in the binding site for p120-catenin and its phosphorylation prevents p120-catenin association with VE-cadherin [27,29]. Phosphorylation of Y658 by Src and Pyk2 promotes efficient transmigration of leukocytes [31•]. By competing with kinases that phosphorylate VE-cadherin, p120-catenin may prevent VE-cadherin internalization from the plasma membrane and enhance monolayer barrier function.
Interaction of VE-cadherin with β-arrestin also regulates the amount of VE-cadherin at the plasma membrane. In contrast to p120-catenin binding, β-arrestin promotes internalization of VE-cadherin into clathrin-coated vesicles in response to VEGF-induced, PAK-mediated phosphorylation of S665 on VE-cadherin [37••]. IL-8 stimulation also leads to PAK-mediated phosphorylation of S665 and internalization of VE-cadherin, but it is not known whether this is mediated by β-arrestin binding [38]. As VEGF (and possibly IL-8) signaling promotes β-arrestin-mediated internalization of VE-cadherin [37••] and FGF signaling promotes p120-catenin-mediated stabilization of VE-cadherin [42], changes in the balance between these pathways may determine permeability properties of the endothelial monolayer. Angiopoietin-1 (Ang1), a proangiogenic factor known to promote stabilization of the vasculature [44], also prevents VEGF-induced phosphorylation of S665 and internalization of VE-cadherin through the inhibition of Src activity [45•]. Thus, Ang1 represents another signaling mechanism to counteract the increase in endothelial permeability by VEGF.
Although the amount of VE-cadherin at the cell surface is regulated at the level of endocytosis, little is know about exocytosis of newly synthesized VE-cadherin. Recently, Golgi-associated phospholipase A2α (cPLA2α) has been implicated in trafficking VE-cadherin to the cell surface [46]. Disruption of cPLA2α results in disorganization of VE-cadherin at the plasma membrane and accumulation of VE-cadherin in intracellular vesicles and the Golgi. Interestingly, the localization of cPLA2α to the Golgi is enhanced as cell monolayers mature, while disruption of VE-cadherin results in dissociation of cPLA2α from the Golgi [46]. This suggests a positive feedback loop mediated by VE-cadherin clustering and/or signaling promotes continued transport of newly synthesized VE-cadherin to the cell surface, thereby maintaining structural integrity of the adherens junction.
The association of VE-cadherin with the motor protein myosin-X also promotes VE-cadherin trafficking to the plasma membrane [47•]. In subconfluent endothelial cells, myosin-X binds and transports VE-cadherin along filopodial actin filaments, leading to accumulation of VE-cadherin at filopodia tips. When these filopodia contact a neighboring cell, VE-cadherin engages in homophilic interactions with the opposing cell forming nascent cell-cell contacts. Blocking the motor activity of myosin-X prevents VE-cadherin transport and formation of early cell-cell contacts [47•]. Thus, endothelium wound repair, which requires establishment of nascent cell-cell contacts between actively migrating cells, may require myosin-X mediated transport of VE-cadherin.
VE-cadherin expression
Several transcription factors regulate VE-cadherin gene expression. ETS transcription factors are expressed early in development and are important for vasculogenesis and angiogenesis [19]. Members of this family, including Erg and Ets-1, bind and enhance VE-cadherin promoter activity [48,49]. Depletion of Erg disrupts VE-cadherin-mediated cell-cell adhesion, increases apoptosis, and decreases vessel formation [50]. The TAL-1/SCL transcription factor, which was originally found to be a key regulator of hematopoiesis but is now known to be equally important for vascular development [51], also binds and activates the VE-cadherin promoter and is required for formation of capillary-like structures in vitro [52].
Exposure of human endothelial cell monolayers to breast cancer cells or conditioned media leads to decreased expression and mis-localization of VE-cadherin [53]. Members of the Twist/Slug/Snail family are responsible for this transcriptional repression due to direct binding to the VE-cadherin promoter [54]. Interestingly, in epithelial cells transcription of Slug is induced by β-catenin signaling, the main effector of the Wnt pathway [55]. Wnt signaling is emerging as an important factor for endothelial cell biology [56], but it has not been reported whether VE-cadherin is a direct transcriptional target of Wnt signaling. However, Wnt-induced upregulation of Slug, which leads to repression of VE-cadherin, may prolong and/or enhance Wnt signaling-mediated affects on endothelial cells by increasing the amount of free β-catenin available for transcription.
VE-cadherin signaling
In addition to adhesive properties, VE-cadherin is an important signaling molecule. Signaling via VE-cadherin influences endothelial cell behavior by modulating activity of growth factor receptors, intracellular messengers, and proteins that regulate gene transcription (Figure 2B).
VE-cadherin engagement with growth factor receptors and intracellular messengers
VE-cadherin associates with two growth factor receptors - VEGF receptor 2 (VEGF-R2) and transforming growth factor-β (TGF-β) receptor. In confluent cells, VE-cadherin binds VEGF-R2 indirectly through β-catenin. This prevents VEGF-induced VEGF-R2 tyrosine phosphorylation and internalization into clathrin-coated vesicles, and reduces mitogen-activated protein kinase (MAPK) activation and proliferative signals [57,58]. In contrast, TGF-β anti-proliferative and anti-migratory signals are enhanced upon VE-cadherin binding to the TGF-β receptor complex. VE-cadherin promotes assembly of the TGF-β receptor complex into an active receptor complex capable of phosphorylating and activating Smad-dependent transcription [59••]. Thus, the effect of VE-cadherin on these two receptors is opposing (inhibition of VEGF-R2, activation of TGF-β receptor complex), but both result in stabilization of the vasculature. It is unknown whether the phosphorylation state of VE-cadherin affects its association with either VEGF-R2 or TGF-β receptor complex, but this could be one potential mechanism to tip the balance between these growth factors, allowing the transition between quiescent and activated cell states.
There is a growing list of intracellular messengers regulated by VE-cadherin, many of which are involved in GTPase signaling. VE-cadherin signals via RhoC to activate Rho kinase (ROCK) and myosin light-chain 2 (MLC2) phosphorylation. This promotes actomyosin contractility, but suppresses VEGF/VEGF-R2-mediated Rac1-dependent angiogenic sprouting. Inhibition of ROCK results in loss of VE-cadherin from the plasma membrane, suggesting a positive feedback loop between VE-cadherin clustering and ROCK activation may maintain integrity of cell-cell contacts [60]. Previous studies also demonstrated VE-cadherin signaling through RhoA-ROCK enhances actomyosin-mediated contractions [61,62]. However, VE-cadherin clustering may also inhibit Rho, while activating Rac1/Tiam1 [63]. Whether VE-cadherin activates Rho or Rac may depend on which upstream signaling messages are received.
VE-cadherin has a reciprocal relationship with the small GTPase Rap1. Signaling through Rap1/Epac decreases monolayer permeability by enhancing VE-cadherin-mediated cell-cell adhesion. VE-cadherin clustering in turn activates Rap1 by creating a scaffold for recruitment of the Rap1 activator PDZ-GEF1 [64]. Another effector of Rap1, cerebral cavernous malformation-1 (CCM-1), also stabilizes VE-cadherin at cell-cell contacts, thereby increasing monolayer integrity and promoting proper formation and maintenance of the vascular lumen [65,66]. Mutations in CCM-1 result in a human disease associated with defective endothelial cell-cell junctions and abnormal vasculature [2]. Thus, the Rap1-VE-cadherin signaling axis appears critical for proper endothelial cell function.
VE-cadherin regulation of transcription
As noted above, VE-cadherin-mediated cell-cell adhesion can indirectly regulate gene transcription by suppressing the transcriptional repressor FoxO1, leading to upregulation of claudin-5 mRNA [18••]. In the adherens junction complex, VE-cadherin associates with p120-catenin and β-catenin, both of which are well known mediators of gene transcription. Endothelial cells treated with the permeability-inducing factor thrombin show translocation of p120-catenin and β-catenin into the nucleus and induction of β-catenin target genes [67], suggesting the assembly of p120-catenin and β-catenin into the adherens junction complex may reduce availability and signaling capabilities of these proteins. However, disrupting VE-cadherin-mediated endothelial cell-cell adhesion by Ca2+ chelation does not induce β-catenin translocation or transcription [68], indicating that simply disrupting cell-cell adhesion is not sufficient to induce gene transcription. Therefore, thrombin (and other factors) may trigger additional signaling events (e.g. phosphorylation of adherens junction proteins) that promote the nuclear signaling functions of these proteins.
In endothelial cells, p120-catenin associates with the transcription factor Kaiso and suppresses its activity [69], similar to its role in non-endothelial cells [70]. The consequences of this interaction on vascular development have not been examined.
Several studies indicate a role for β-catenin-mediated Wnt transcription in endothelial cell biology and vascular development. Using a transgenic reporter mouse, Wnt/β-catenin signaling was shown to be active in endothelial cells [71], and Wnt/β-catenin signaling is critical for blood-brain barrier function [72•,73•]. In addition, several human diseases associated with vascular defects are linked to Wnt/β-catenin pathway, further implicating the importance of this signaling axis for proper endothelial cell function [56]. Interestingly, there is evidence for cross-talk between VEGF and Wnt/β-catenin signaling pathways. β-Catenin signaling enhances expression of VEGF/VEGF-R2 and promotes endothelial cell proliferation and formation of capillary-like structures [74]. Whether VE-cadherin plays a role in regulating transcriptional activity of p120-catenin and β-catenin remains to be determined.
Concluding Remarks
VE-cadherin plays a key role in all branches of endothelial cell biology. Through both its adhesive and signaling properties, VE-cadherin maintains a careful balance between intercellular junction plasticity and integrity, a requirement for endothelial cells to maintain proper barrier function of blood vessels while still being capable of dynamically responding to inflammatory and growth factor signals. Deciphering the molecular mechanism(s) of different regulatory and signaling pathways that act on, or are initiated by VE-cadherin, and understanding how they contribute to endothelial cell biology, is an important area for future research. These studies may lead to the discovery of new therapeutic targets for the many inherited and pathological diseases associated with endothelial dysfunction.
Acknowledgements
The authors apologize for inability to cite original sources in some instances due to restrictions in the number of references allowed. This work was supported by NIH grant GM035527 to WJN, and a post-doctoral fellowship from Jane Coffin Childs Fund for Medical Research to ESH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of the review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 2.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Lampugnani MG, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco LP, Dejana E. A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J Cell Biol. 1992;118:1511–1522. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salomon D, Ayalon O, Patel-King R, Hynes RO, Geiger B. Extrajunctional distribution of N-cadherin in cultured human endothelial cells. J Cell Sci. 1992;102(Pt 1):7–17. doi: 10.1242/jcs.102.1.7. [DOI] [PubMed] [Google Scholar]
- 7.Gerhardt H, Wolburg H, Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev Dyn. 2000;218:472–479. doi: 10.1002/1097-0177(200007)218:3<472::AID-DVDY1008>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 9.Gory-Faure S, Prandini MH, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, Huber P. Role of vascular endothelial-cadherin in vascular morphogenesis. Development. 1999;126:2093–2102. doi: 10.1242/dev.126.10.2093. [DOI] [PubMed] [Google Scholar]
- 10.Montero-Balaguer M, Swirsding K, Orsenigo F, Cotelli F, Mione M, Dejana E. Stable vascular connections and remodeling require full expression of VE-cadherin in zebrafish embryos. PLoS One. 2009;4:e5772. doi: 10.1371/journal.pone.0005772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotsch U, Borges E, Bosse R, Boggemeyer E, Simon M, Mossmann H, Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J Cell Sci. 1997;110(Pt 5):583–588. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- 13.Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, et al. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oas RG, Xiao K, Summers S, Wittich KB, Chiasson CM, Martin WD, Grossniklaus HE, Vincent PA, Reynolds AB, Kowalczyk AP. p120-Catenin is required for mouse vascular development. Circ Res. 2010;106:941–951. doi: 10.1161/CIRCRESAHA.109.207753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyqvist D, Giampietro C, Dejana E. Deciphering the functional role of endothelial junctions by using in vivo models. EMBO Rep. 2008;9:742–747. doi: 10.1038/embor.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. •• These authors show VE-cadherin clustering enhances expression of claudin-5 by inhibiting activity of the transcriptional repressor FoxO1, suggesting VE-cadherin can modulate endothelial cell adhesive properties by indirectly regulating gene transcription.
- 19.Dejana E, Taddei A, Randi AM. Foxs and Ets in the transcriptional regulation of endothelial cell differentiation and angiogenesis. Biochim Biophys Acta. 2007;1775:298–312. doi: 10.1016/j.bbcan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Ikeda K, et al. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 21.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 22.Senger DR, Galli SJ, DvorakDvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 23.Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998;111(Pt 13):1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- 24.Wallez Y, Cand F, Cruzalegui F, Wernstedt C, Souchelnytskyi S, Vilgrain I, Huber P. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene. 2007;26:1067–1077. doi: 10.1038/sj.onc.1209855. [DOI] [PubMed] [Google Scholar]
- 25.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 26.Weis S, Shintani S, Weber A, Kirchmair R, Wood M, Cravens A, McSharry H, Iwakura A, Yoon YS, Himes N, et al. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J Clin Invest. 2004;113:885–894. doi: 10.1172/JCI20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem. 2009;284:25602–25611. doi: 10.1074/jbc.M109.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cain RJ, Vanhaesebroeck B, Ridley AJ. The PI3K p110alpha isoform regulates endothelial adherens junctions via Pyk2 and Rac1. J Cell Biol. 2010;188:863–876. doi: 10.1083/jcb.200907135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem. 2005;280:31906–31912. doi: 10.1074/jbc.M505568200. [DOI] [PubMed] [Google Scholar]
- 30. Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, et al. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci. 2008;121:29–37. doi: 10.1242/jcs.022681. • see annotation below
- 31. Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. • These two papers demonstrate that leukocyte adhesion induces phosphorylation of VE-cadherin, permitting efficient transmigration. These papers provide insight into the mechanism(s) employed by leukocytes to increased monolayer permeability and mediate their passage from the blood to the tissue.
- 32.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. Embo J. 2002;21:4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fachinger G, Deutsch U, Risau W. Functional interaction of vascular endothelial-protein-tyrosine phosphatase with the angiopoietin receptor Tie-2. Oncogene. 1999;18:5948–5953. doi: 10.1038/sj.onc.1202992. [DOI] [PubMed] [Google Scholar]
- 34.Baumer S, Keller L, Holtmann A, Funke R, August B, Gamp A, Wolburg H, Wolburg-Buchholz K, Deutsch U, Vestweber D. Vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) activity is required for blood vessel development. Blood. 2006;107:4754–4762. doi: 10.1182/blood-2006-01-0141. [DOI] [PubMed] [Google Scholar]
- 35.Dominguez MG, Hughes VC, Pan L, Simmons M, Daly C, Anderson K, Noguera-Troise I, Murphy AJ, Valenzuela DM, Davis S, et al. Vascular endothelial tyrosine phosphatase (VE-PTP)-null mice undergo vasculogenesis but die embryonically because of defects in angiogenesis. Proc Natl Acad Sci U S A. 2007;104:3243–3248. doi: 10.1073/pnas.0611510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nottebaum AF, Cagna G, Winderlich M, Gamp AC, Linnepe R, Polaschegg C, Filippova K, Lyck R, Engelhardt B, Kamenyeva O, et al. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J Exp Med. 2008;205:2929–2945. doi: 10.1084/jem.20080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. •• These authors dissect a VEGF-induced signaling cascade that leads to phosphorylation of serine 665 on VE-cadherin. This serine phosphorylation promotes β-arrestin binding to VE-cadherin and internalization into clathrin-coated vesicles.
- 38.Gavard J, Hou X, Qu Y, Masedunskas A, Martin D, Weigert R, Li X, Gutkind JS. A role for a CXCR2/phosphatidylinositol 3-kinase gamma signaling axis in acute and chronic vascular permeability. Mol Cell Biol. 2009;29:2469–2480. doi: 10.1128/MCB.01304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol Biol Cell. 2005;16:5141–5151. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iyer S, Ferreri DM, DeCocco NC, Minnear FL, Vincent PA. VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1143–L1153. doi: 10.1152/ajplung.00305.2003. [DOI] [PubMed] [Google Scholar]
- 42.Murakami M, Nguyen LT, Zhuang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118:3355–3366. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alcaide P, Newton G, Auerbach S, Sehrawat S, Mayadas TN, Golan DE, Yacono P, Vincent P, Kowalczyk A, Luscinskas FW. p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood. 2008;112:2770–2779. doi: 10.1182/blood-2008-03-147181. • These authors demonstrate that p120-catenin prevents ICAM-1-mediated phosphorylation of VE-cadherin on tyrosine 658, inhibiting leukocyte transmigration.
- 44.Fukuhara S, Sako K, Noda K, Zhang J, Minami M, Mochizuki N. Angiopoietin-1/Tie2 receptor signaling in vascular quiescence and angiogenesis. Histol Histopathol. 2010;25:387–396. doi: 10.14670/HH-25.387. [DOI] [PubMed] [Google Scholar]
- 45. Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. • These authors demonstrate that through inhibition of Src activity, Ang1 prevents VEGF-induced phosphorylation and internalization of VE-cadherin, thereby providing one mechanism for how Ang1 exerts its protective effects on the vasculature.
- 46.Regan-Klapisz E, Krouwer V, Langelaar-Makkinje M, Nallan L, Gelb M, Gerritsen H, Verkleij AJ, Post JA. Golgi-associated cPLA2alpha regulates endothelial cell-cell junction integrity by controlling the trafficking of transmembrane junction proteins. Mol Biol Cell. 2009;20:4225–4234. doi: 10.1091/mbc.E08-02-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Almagro S, Durmort C, Chervin-Petinot A, Heyraud S, Dubois M, Lambert O, Maillefaud C, Hewat E, Schaal JP, Huber P, et al. The motor protein myosin-X transports VE-cadherin along filopodia to allow the formation of early endothelial cell-cell contacts. Mol Cell Biol. 2010;30:1703–1717. doi: 10.1128/MCB.01226-09. • These authors demonstrate that VE-cadherin is transported along filopodial actin filaments by Myosin-X. This leads to accumulation of VE-cadherin at filopodia tips, which can then engage neighboring cells to promote cell-cell adhesion.
- 48.Gory S, Dalmon J, Prandini MH, Kortulewski T, de Launoit Y, Huber P. Requirement of a GT box (Sp1 site) and two Ets binding sites for vascular endothelial cadherin gene transcription. J Biol Chem. 1998;273:6750–6755. doi: 10.1074/jbc.273.12.6750. [DOI] [PubMed] [Google Scholar]
- 49.Lelievre E, Mattot V, Huber P, Vandenbunder B, Soncin F. ETS1 lowers capillary endothelial cell density at confluence and induces the expression of VE-cadherin. Oncogene. 2000;19:2438–2446. doi: 10.1038/sj.onc.1203563. [DOI] [PubMed] [Google Scholar]
- 50.Birdsey GM, Dryden NH, Amsellem V, Gebhardt F, Sahnan K, Haskard DO, Dejana E, Mason JC, Randi AM. Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood. 2008;111:3498–3506. doi: 10.1182/blood-2007-08-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Visvader JE, Fujiwara Y, Orkin SH. Unsuspected role for the T-cell leukemia protein SCL/tal-1 in vascular development. Genes Dev. 1998;12:473–479. doi: 10.1101/gad.12.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deleuze V, Chalhoub E, El-Hajj R, Dohet C, Le Clech M, Couraud PO, Huber P, Mathieu D. TAL-1/SCL and its partners E47 and LMO2 up-regulate VE-cadherin expression in endothelial cells. Mol Cell Biol. 2007;27:2687–2697. doi: 10.1128/MCB.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carter WB, Niu G, Ward MD, Small G, Hahn JE, Muffly BJ. Mechanisms of HER2-induced endothelial cell retraction. Ann Surg Oncol. 2007;14:2971–2978. doi: 10.1245/s10434-007-9442-4. [DOI] [PubMed] [Google Scholar]
- 54.Lopez D, Niu G, Huber P, Carter WB. Tumor-induced upregulation of Twist, Snail, and Slug represses the activity of the human VE-cadherin promoter. Arch Biochem Biophys. 2009;482:77–82. doi: 10.1016/j.abb.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 55.Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze'ev A. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J Cell Biol. 2003;163:847–857. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zerlin M, Julius MA, Kitajewski J. Wnt/Frizzled signaling in angiogenesis. Angiogenesis. 2008;11:63–69. doi: 10.1007/s10456-008-9095-3. [DOI] [PubMed] [Google Scholar]
- 57.Grazia Lampugnani M, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, et al. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rudini N, Felici A, Giampietro C, Lampugnani M, Corada M, Swirsding K, Garre M, Liebner S, Letarte M, ten Dijke P, et al. VE-cadherin is a critical endothelial regulator of TGF-beta signalling. Embo J. 2008;27:993–1004. doi: 10.1038/emboj.2008.46. •• These authors demonstrate that VE-cadherin associates with all members of the TGF-β receptor complex, and positively regulates its signaling to Smad transcription factors. This work provides insights into how VE-cadherin clustering promotes anti-proliferative and anti-migratory effects on endothelial cells.
- 60.Abraham S, Yeo M, Montero-Balaguer M, Paterson H, Dejana E, Marshall CJ, Mavria G. VE-Cadherin-mediated cell-cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol. 2009;19:668–674. doi: 10.1016/j.cub.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 61.Nelson CM, Pirone DM, Tan JL, Chen CS. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA. Mol Biol Cell. 2004;15:2943–2953. doi: 10.1091/mbc.E03-10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nelson CM, Chen CS. VE-cadherin simultaneously stimulates and inhibits cell proliferation by altering cytoskeletal structure and tension. J Cell Sci. 2003;116:3571–3581. doi: 10.1242/jcs.00680. [DOI] [PubMed] [Google Scholar]
- 63.Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell. 2002;13:1175–1189. doi: 10.1091/mbc.01-07-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fukuhra S, Sakurai A, Yamagishi A, Sako K, Mochizuki N. Vascular endothelial cadherin-mediated cell-cell adhesion regulated by a small GTPase, Rap1. J Biochem Mol Biol. 2006;39:132–139. doi: 10.5483/bmbrep.2006.39.2.132. [DOI] [PubMed] [Google Scholar]
- 65.Glading A, Han J, Stockton RA, Ginsberg MH. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol. 2007;179:247–254. doi: 10.1083/jcb.200705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lampugnani MG, Orsenigo F, Rudini N, Maddaluno L, Boulday G, Chapon F, Dejana E. CCM1 regulates vascular-lumen organization by inducing endothelial polarity. J Cell Sci. 2010;123:1073–1080. doi: 10.1242/jcs.059329. [DOI] [PubMed] [Google Scholar]
- 67.Beckers CM, Garcia-Vallejo JJ, van Hinsbergh VW, van Nieuw Amerongen GP. Nuclear targeting of beta-catenin and p120ctn during thrombin-induced endothelial barrier dysfunction. Cardiovasc Res. 2008;79:679–688. doi: 10.1093/cvr/cvn127. [DOI] [PubMed] [Google Scholar]
- 68.Harris ES, Nelson WJ. APC Regulates Endothelial Cell Migration Independent of Roles in {beta}-catenin Signaling and Cell-Cell Adhesion. Mol Biol Cell. 2010 doi: 10.1091/mbc.E10-03-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang J, O'Donnell JJ, 3rd, Holian O, Vincent PA, Kim KS, Lum H. P120 catenin represses transcriptional activity through Kaiso in endothelial cells. Microvasc Res. 2010 doi: 10.1016/j.mvr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daniel JM. Dancing in and out of the nucleus: p120(ctn) and the transcription factor Kaiso. Biochim Biophys Acta. 2007;1773:59–68. doi: 10.1016/j.bbamcr.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 71.Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. • see annotation below
- 73. Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. • These two papers are the first to demonstrate Wnt/β-catenin signaling is important for proper development and maintenance of the blood brain barrier.
- 74.Skurk C, Maatz H, Rocnik E, Bialik A, Force T, Walsh K. Glycogen-Synthase Kinase3beta/beta-catenin axis promotes angiogenesis through activation of vascular endothelial growth factor signaling in endothelial cells. Circ Res. 2005;96:308–318. doi: 10.1161/01.RES.0000156273.30274.f7. [DOI] [PubMed] [Google Scholar]