Abstract
We previously showed that macrophages from macrophage-specific ATP-binding cassette transporter A1 (ABCA1) knockout (Abca1-M/-M) mice had an enhanced proinflammatory response to the Toll-like receptor (TLR) 4 agonist, lipopolysaccharide (LPS), compared with wild-type (WT) mice. In the present study, we demonstrate a direct association between free cholesterol (FC), lipid raft content, and hyper-responsiveness of macrophages to LPS in WT mice. Abca1-M/-M macrophages were also hyper-responsive to specific agonists to TLR2, TLR7, and TLR9, but not TLR3, compared with WT macrophages. We hypothesized that ABCA1 regulates macrophage responsiveness to TLR agonists by modulation of lipid raft cholesterol and TLR mobilization to lipid rafts. We demonstrated that Abca1-M/-M vs. WT macrophages contained 23% more FC in isolated lipid rafts. Further, mass spectrometric analysis suggested raft phospholipid composition was unchanged. Although cell surface expression of TLR4 was similar between Abca1-M/-M and WT macrophages, significantly more TLR4 was distributed in membrane lipid rafts in Abca1-M/-M macrophages. Abca1-M/-M macrophages also exhibited increased trafficking of the predominantly intracellular TLR9 into lipid rafts in response to TLR9-specific agonist (CpG). Collectively, our data suggest that macrophage ABCA1 dampens inflammation by reducing MyD88-dependent TLRs trafficking to lipid rafts by selective reduction of FC content in lipid rafts.
Keywords: free cholesterol, cytokines, immunology, lipid droplets, lymphocytes, retinoids
ATP-binding cassette transporter A1 (ABCA1) is a plasma membrane protein that functions to eliminate excess free cholesterol (FC) from tissues by effluxing cellular FC and phospholipid (PL) to lipid-free apolipoprotein AI, forming nascent HDL particles (1, 2). Therefore, ABCA1 plays a critical role in the movement of cholesterol from peripheral tissues to the liver in a process known as reverse cholesterol transport. Mutations that inactivate the human ABCA1 gene result in Tangier disease, which is characterized by extremely low plasma HDL cholesterol concentrations, mildly elevated plasma triglyceride levels, and accumulation of cholesterol in macrophages (3–5). ABCA1 protein is expressed to a variable extent in most cells in the body, and its expression is regulated by transcriptional activation and protein degradation (6, 7), making it difficult to determine its physiological role in individual tissues using global Abca1 knockout mice or cells in culture. However, generation of cell-specific Abca1 knockout mice has helped define the role of tissue-specific ABCA1 expression in whole body HDL biogenesis as well as several unanticipated roles for the transporter. For example, hepatocyte and intestinal epithelial cell ABCA1 contribute 70–80% and 20–30% of the plasma HDL pool, respectively (8, 9). Pancreatic β cell ABCA1 plays a role in insulin secretion (10) and brain ABCA1 regulates neuronal structure and function (11).
Macrophages, a primary cell type involved in innate immunity, have been implicated in chronic inflammatory diseases such as atherosclerosis and insulin resistance, where they accumulate in arteries (12, 13) and adipose tissue (14), respectively. Transplantation of bone marrow between wild-type (WT) and ABCA1 knockout mice has little effect on plasma HDL concentration (15, 16). Despite this, lipopolysaccharide (LPS)-induced sepsis was exacerbated in Abca1−/−/Ldlr−/− mice compared with Abca1−/− mice (17), suggesting a novel antiinflammatory role for macrophage ABCA1 in innate immunity. However, it was not clear from these studies whether the exacerbated proinflammatory response of Abca1−/−/Ldlr−/− mice to LPS was due to the massive cellular cholesteryl ester accumulation in macrophages, the absence of plasma HDL, or some other alteration of macrophages. In addition, ABCA1 expression decreases cellular plasma membrane rigidity by reducing formation of tightly packed lipid raft domains in the plasma membrane (18). Lipid rafts, enriched in FC and glycosphingolipid, play an important role in signal transduction by recruiting and concentrating signaling molecules in the plasma membrane (19). For instance, LPS activation of macrophage results in transient Toll-like receptor 4 (TLR4) trafficking to lipid rafts along with its cognate adaptor proteins (20–23) and subsequent secretion of inflammatory cytokines and chemokines. These studies suggest a link among ABCA1-mediated cellular lipid efflux, membrane lipid raft homeostasis, and activation of macrophages. Nevertheless, the molecular details regarding how ABCA1 expression affects macrophage inflammatory response are poorly understood.
To explore the specific role of ABCA1 in macrophages in vivo and in vitro, we generated macrophage-specific ABCA1 knockout (Abca1-M/-M) mice. Using this unique mouse model, we demonstrated that macrophages from Abca1-M/-M mice have a significant increase in FC and are more proinflammatory in vivo and in vitro in response to LPS via TLR4 compared with WT. This response was mediated through a myeloid differentiation primary-response protein 88 (MyD88)-dependent pathway and was independent of alterations in plasma lipid concentrations (24). We further showed that the hypersensitivity of Abca1-M/-M macrophages to LPS was most likely due to increased lipid raft content, presumably caused by increased intracellular FC accumulation. Recently, Yvan-Charvet et al. (25) observed a similar inflammatory phenotype in Abca1−/−Abcg1−/− macrophages compared with WT. Collectively, these studies suggest that ABC transporters suppress macrophage TLR4 activation, possibly through modulation of membrane lipid rafts.
However, several questions still remain unanswered. First, is there a direct relationship among macrophage FC content, lipid raft content, and the hyper-responsiveness to TLR4 agonist? Alternatively, is there a change of PL composition in lipid rafts that leads to increased lipid raft content in Abca1-M/-M macrophages? Third, do other TLR-specific agonists also induce increased inflammatory signaling in Abca1-M/-M macrophages? Finally, does the exaggerated TLR signaling result from increased trafficking of TLRs to lipid rafts? In the present study, we demonstrated: 1) a direct association between FC and lipid raft content that leads to hyper-responsiveness of macrophages to LPS in WT macrophages; 2) ABCA1 deletion in macrophages resulted in selective FC accumulation in lipid rafts without alternation of PL composition and enhanced activation of other MyD88-dependent TLRs compared with WT macrophages; and 3) ABCA1 deletion results in increased TLR4 and TLR9 localization in lipid rafts in both resting and stimulated states, suggesting a mechanistic explanation for the hyper-responsiveness of Abca1-M/-M macrophages to proinflammatory stimuli.
MATERIALS AND METHODS
Animals
Abca1+/+ (WT), Abca1+/−M (heterozygous), and Abca1-M/-M (homozygous) mice were generated as described previously (24). Mice were backcrossed to C57BL/6 background for six generations before use in the studies.
Cell culture
Peritoneal macrophages (PMs) were harvested from C57BL/6 backcrossed Abca1+/+ and Abca1-M/-M littermate mice 4 days after receiving an intraperitoneal injection of 1 ml 10% thioglycolate and plated in RPMI media containing 1% Nutridoma SP (NutSP) media (Roche Applied Science) as previously described (24). Bone marrow from Abca1+/+, Abca1+/−M, and Abca1-M/-M littermate mice was isolated and cultured in DMEM media containing 20% FBS and 30% L929 conditioned media for 5–7 days before being used in experiments as bone marrow-derived macrophages (BMDMs) (24).
FC, lipid raft content, surface TLR4, and inflammatory cytokine expression in WT macrophages
BMDMs from Abca1+/+ mice were plated in tissue culture dishes and cultured in RPMI media plus 1% NutSp overnight as described above. Cholesterol depletion and overloading of macrophages with methyl-β-cyclodextrin (MβCD) (Sigma-Aldrich) was performed as described previously (24). Briefly, BMDMs were incubated with or without prewarmed 10 mM MβCD at 37°C for 30 min to deplete cholesterol, or macrophages were incubated with MβCD-cholesterol (40 or 80 μg/ml) (Sigma-Aldrich) at 37°C for 60 min to overload macrophages with cholesterol. After three washes with PBS, macrophages were extracted with isopropanol to measure cholesterol content by gas-liquid chromatography (GLC) as described before (24) or incubated ± 100 ng/ml Kdo2-lipid A (Avanti Polar Lipids) for 6 h before quantification of cytokine expression by ELISA kits (BD Bioscience).
Flow cytometry
Elicited peritoneal cells from Abca1+/+ or Abca1-M/-M mice were stained directly or plated in tissue culture dishes and cultured in RPMI media plus 1% NutSp overnight as described above. To measure TLR4/MD2 surface expression, elicited peritoneal cells from Abca1+/+ or Abca1-M/-M mice were incubated in the presence or absence of 100 ng/ml LPS from Salmonella typhimurium (Sigma) for 1 h in suspension before staining with PE-F4/80 (BD Bioscience) and/or APC-TLR4/MD2 (MTS510) (eBioscience) or isotype control (eBioscience or BD Bioscience). To examine the content of lipid rafts or TLR4/MD2 complex in macrophages, after incubation with 10 mM MβCD or 80 μg/ml MβCD-cholesterol for 30 min or 1 h, macrophages were gently lifted using a cell scraper. Live PMs were then stained with 0.5 μg/ml cold Alexa fluor 488-cholera toxin B (CT-B) (Molecular Probes) for 15 min at 4°C or stained with APC-TLR4/MD2 (MTS510) for 30 min at 4°C or PMs were fixed with 2% paraformaldehyde and then stained with 10 μg/ml fPEG10000-chol (fluorescein ester of polyethylene glycol-derivatized cholesterol) (26) at room temperature for 10 min. Cell fluorescence was determined using a FACSCalibur flow cytometer (BD Bioscience) and data were analyzed using Flowjo software (Tree Star).
Inflammatory mediator expression in TLR agonist-treated macrophages
After overnight incubation, PMs were switched to serum-free RPMI-1640 media for 2 h before TLR agonist stimulation. BMDMs were incubated overnight in 1% NutSP media before stimulation with various TLR agonists. Macrophages were treated with 100 ng/ml Pam3SCK4 (TLR2), 10 μg/ml poly (I: C) (TLR3), 200 μM Loxoribine (TLR7), or 1 μM CpG (TLR9), or control CpG (agonists from InvivoGen) in serum-free RPMI-1640 media for 0–12 h, after which the culture supernatant was collected and stored at −80°C for cytokine ELISA (BD Bioscience).
Preparation of subcellular membrane fractions
BMDMs were used for all lipid raft isolation procedures to increase macrophage yield. The protein concentration in each isolated fraction was determined by the Bradford protein assay (Pierce).
Nondetergent method.
Lipid rafts were prepared according to the method of Smart et al. (27). Briefly, BMDMs were scraped into ice-cold 0.25 M sucrose, 1 mM EDTA, and 20 mM Tris (pH 7.8) (Buffer A) with protease inhibitors (1 mM PMSF, 1 mM NaF, 0.1 mM Na3VO4, 5 μg/ml leupeptin, 5 μg/ml aprotinin) and homogenized in 1 ml cold buffer A by 20 passages through a 22 G syringe needle. The nuclei and cell debris were pelleted by centrifugation for 10 min at 1,000 g. The postnuclear supernatant (PNS) was collected and stored on ice. The pellet was resuspended in Buffer A, homogenized, and centrifuged again. The two PNSs (2 ml) were combined and layered on top of 23 ml of 30% Percoll (Sigma-Aldrich) in Buffer A and centrifuged for 30 min at 84,000 g. The plasma membrane fraction was collected and sonicated on ice for a total of six bursts (50 J each time). The sonicated membrane fraction was made up to 23% Optiprep (Sigma-Aldrich) (final volume: 4 ml) by the addition of a 50% Optiprep stock in 20 mM Tris, 0.25 M sucrose, and 1 mM EDTA (pH 7.8) (Buffer C) and placed in the bottom of a centrifuge tube. An 8 ml gradient from 20% to 10% Optiprep was layered on top of the sample, which was then centrifuged for 90 min at 52,000 g in an SW41 swinging bucket rotor. The gradient was then fractioned into 12 or 13 1 ml fractions from top to bottom.
Simplified nondetergent method.
Lipid rafts were prepared according to the method of MacDonald et al. (28). Briefly, BMDMs were scraped into ice-cold 0.25 M sucrose, 1 mM CaCl2, 1 mM MgCl2, 20 mM Tris-HCl, pH 7.8 (Buffer B), and homogenized in 1 ml cold buffer B with protease inhibitors (1 mM PMSF, 1 mM NaF, 0.1 mM Na3VO4, 5 μg/ml leupeptin, 5 μg/ml aprotinin) by 20 passages through a 22 G syringe needle. The nuclei and cell debris were pelleted by centrifugation for 10 min at 1000 g. The PNS was collected and stored on ice. The pellet was resuspended in 1 ml of Buffer B, homogenized, and centrifuged again. The two PNSs (2 ml) were mixed with an equal volume (2 ml) of Solution D (50% Optiprep) and then centrifuged through 9 ml of 5–20% continuous Optiprep gradient using an SW41 rotor (90 min, 53,000 g, 4°C). Following centrifugation, thirteen 1 ml fractions were harvested from top to bottom.
Detergent extraction method.
Cold detergent resistance membranes (or lipid rafts) were prepared according to the method described by Fessler et al. (29). Briefly, BMDMs were washed three times with ice-cold PBS. Following the wash, cells were centrifuged (1,000 rpm, 10 min, 4°C) and resuspended in 1 ml of ice-cold lysis buffer (25 mM MES, pH 6.5, 150 mM NaCl, 1% Triton X-100, 1 mM PMSF, 1 mM NaF, 0.1 mM Na3VO4, 5 μg/ml leupeptin, 5 μg/ml aprotinin). Cells were vortexed for 15 s on ice and allowed to sit on ice for 10 min. This step was repeated three times and then the cell lysate was centrifuged (1,000 g, 10 min, 4°C). The supernatant (∼1 ml) was gently mixed with an equal volume of 80% sucrose in MBS (25 mM MES, pH 6.5, 150 mM NaCl) and then centrifuged through a 5–30% continuous sucrose gradient using an SW41 rotor (20 h, 37,000 rpm, 4°C). Following centrifugation, twelve 1 ml fractions were harvested from top to bottom.
To induce translocation of TLRs to lipid rafts, BMDMs from both WT and Abca1-M/-M mice were incubated in 1% NutSP-RPMI-1640 media overnight and then treated with ±100 ng/ml LPS from Salmonella typhimurium (Sigma-Aldrich) or ±1 μM CpG for 1 h, followed by preparation of lipid rafts and nonraft as described above. Macrophage lipid raft TLR content was calculated as a percentage of total membrane TLR (i.e., lipid rafts + nonraft fractions).
Immunoblotting and immunoprecipitation
Western blots were performed using specific antibodies against: caveolin-1 (Abcam), CD14 (Abcam) (as lipid raft marker), clathrin (Sigma-Aldrich) (as nonraft marker), TLR4 (cell signaling), or TLR9 (Abcam). TLR9 was immunoprecipitated with a TLR9 polyclonal antibody from pooled raft or nonraft fractions, followed by immunoblotting with antibody against TLR9. Blots were developed using HRP-linked secondary antibody. Immunoblots were visualized with the Supersignal substrate system (Pierce). Images were captured and quantified using the LSA-3000 imaging system and Multi Gauge software (Fujifilm Life Science).
Analysis of nuclear factor-κB pathway in the CpG-treated macrophages
BMDMs from both WT and Abca1-M/-M mice were incubated in 1% NutSP-RPMI-1640 media overnight before treated ± 1 μM CpG for 0–3 h, after which cells were processed for Western-blot analysis. Antibody to inhibitory κB protein α (IκBα) was purchased from Cell Signaling Technology, Inc. and antibody to β-actin from Sigma-Aldrich. Immunoblots were visualized as described above.
FC measurements
Raft and nonraft fractions isolated using Smart's nondetergent method were extracted using the Bligh-Dyer method (30), and cholesterol content was determined by GLC as previously described (24).
Lipidomic assay
The distribution of PL species in pooled raft or nonraft fractions or whole cells was measured using ESI/MS/MS as described in the supplementary data.
Statistics
Differences were compared with two-tailed Student's t-test or one-way ANOVA using GraphPad Prism software. P < 0.05 was considered statistically significant. Data are presented as the means ± SEM unless indicated otherwise.
RESULTS
Macrophage FC content is positively associated with lipid raft content and proinflammatory response
To directly examine the effect of FC on macrophage proinflammatory activation, we incubated WT macrophages with 10 mM MβCD (cholesterol depletion) or MβCD loaded with 40 or 80 μg/ml of cholesterol (cholesterol overloading) before cells were challenged with Kdo2-lipid A, a specific TLR4 agonist. Figure 1A shows that incubating macrophages with MβCD or MβCD-cholesterol resulted in a 2-fold variation in cellular FC content. Cholesteryl ester content in macrophages was negligible with all treatments (data not shown). Compared with no treatment, macrophages subjected to cholesterol depletion secreted less interleukin (IL)-6 and IL-12p40, whereas macrophages overloaded with cholesterol secreted more of these cytokines, suggesting that cytokine secretion in TLR4 activated macrophages has a significant positive association with cellular FC content (Fig. 1B). To determine whether rapid alteration of FC content changes lipid raft content or TLR4 surface expression, we measured lipid raft content of macrophages either depleted of or overloaded with cholesterol by flow cytometry. We used two types of raft markers: fPEG-chol (26, 31, 32), which partitions into membrane raft microdomains in direct relation to their cholesterol content, and Alexa fluor 488-CT-B, which binds to ganglioside GM1 located in lipid rafts. As shown in Fig. 1C, cellular fluorescent intensity of fPEG-chol increased with increasing macrophage FC content. Incubation of cells with MβCD also resulted in decreased CT-B binding, presumably due to the disruption of lipid raft structure by MβCD, but unlike fPEG-chol, cholesterol overloading did not result in an increase in CT-B binding on the cell surface, suggesting a rapid increase of FC on the cell surface does not alter the distribution of ganglioside GM1 (Fig. 1D). Furthermore, disrupting lipid rafts using MβCD resulted in a slight decrease of TLR4 surface expression compared with control, but cholesterol overloading did not significantly change the TLR4 surface expression (Fig. 1E).
Fig. 1.
Manipulation of cellular FC content alters proinflammatory status and cell surface lipid raft, but not TLR4, content. A: WT BMDMs were incubated at 37°C with MβCD for 0.5 h or with MβCD-chol for 1 h before FC measurement by GLC. B: WT BMDMs were incubated with Kdo2-lipid A for 6 h after incubation with MβCD or MβCD-chol, and cytokine expression was measured by ELISA. Cytokine concentration was plotted against FC content of macrophages. The line of best fit for IL-6 (r2 = 0.92) and IL-12p40 (r2 = 0.75) is shown. C–E: WT-elicited PMs were treated with MβCD or MβCD-chol before stained with fPEG-chol (C), Alexa fluor CT-B (D), or APC-TLR4/MD2 (MTS510) (E). Cells were analyzed using flow cytometry. Histogram of individual samples is shown in the left panel and mean fluorescence intensity is shown in the right panel. Values with unlike letters are significantly different (P < 0.05). Results represent mean ± SEM of 2 independent experiments; n = 4–5 dishes of cells/group. Chol: MβCD-cholesterol.
Abca1-M/-M macrophages are hypersensitive to stimulation by various TLR agonists
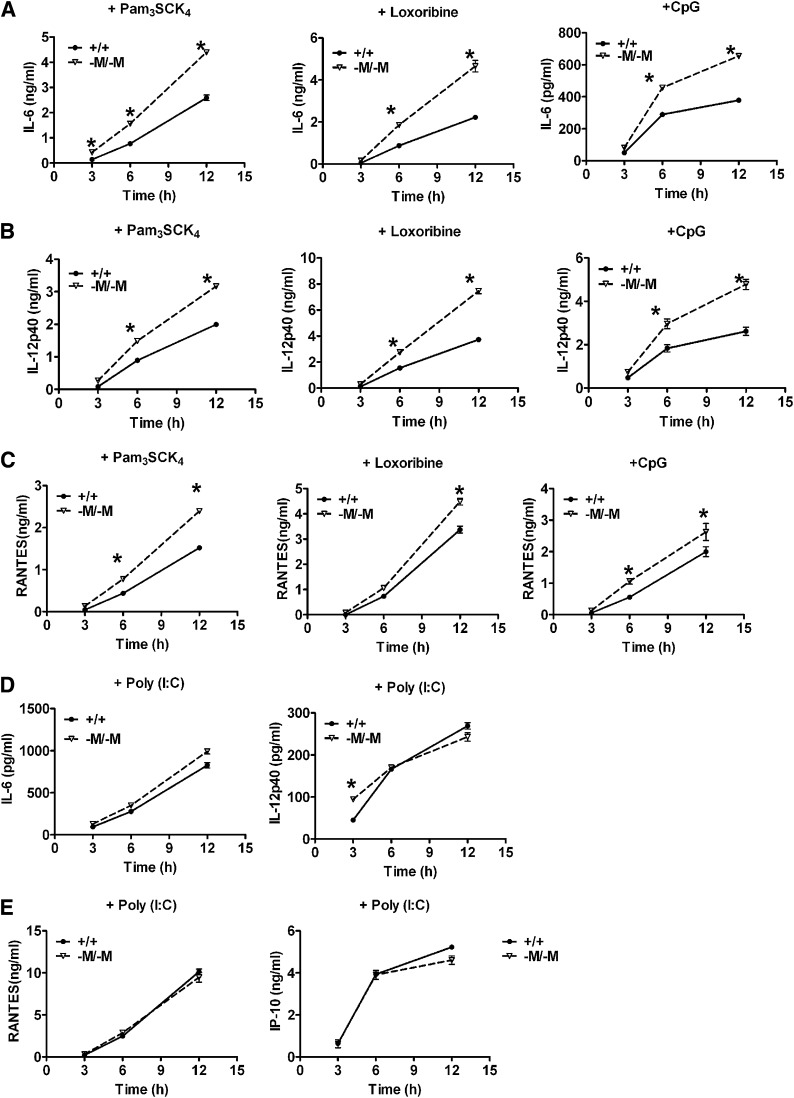
In addition to TLR4, we also investigated the effect of macrophage ABCA1 deficiency on the activation of other TLRs. PMs were treated with specific TLR agonists Pam3SCK4 (TLR2), poly (I:C) (TLR3), Loxoribine (TLR7), or CpG (TLR9) for 3–12 h and cytokine secretion from the stimulated cells was measured by ELISA. Compared with WT, Abca1-M/-M macrophages secreted more IL-6, IL-12p40, and RANTES over the time course after stimulation with Pam3SCK4, Loxoribine, or CpG (Fig. 2A–C). A similar pattern was observed for TNF-α secretion (data not shown). However, TLR3 stimulation by poly (I:C) resulted in similar secretion of inflammatory factors from WT and Abca1-M/-M macrophages (Fig. 2D, E). Similar results were obtained with BMDMs (data not shown). TLR3 is the only member of the TLR family whose signal transduction is independent of MyD88. The data in Fig. 2 suggest that ABCA1 deficiency mainly affects TLR pathways that signal through MyD88.
Fig. 2.
Abca1-M/-M macrophages are hypersensitive to TLR2, 7, and 9, but not to TLR3 stimulation. A–C: Elicited PMs were treated with Pam3SCK4 (TLR2), Loxoribine (TLR7), or TLR9 for 0–12 h. The secretion of inflammatory factors was measured by ELISA. D, E: PMs were treated with poly (I:C) (TLR3) for 0–12 h. Cytokine or chemokine secretion was measured by ELISA. Results represent mean ± SEM of at least two independent experiments; n = 3 dishes of cells/group. * P<0.05, compared with WT. +/+: WT; -M/-M: Abca1-M/-M.
Lipid rafts from Abca1-M/-M macrophages are specifically enriched in FC
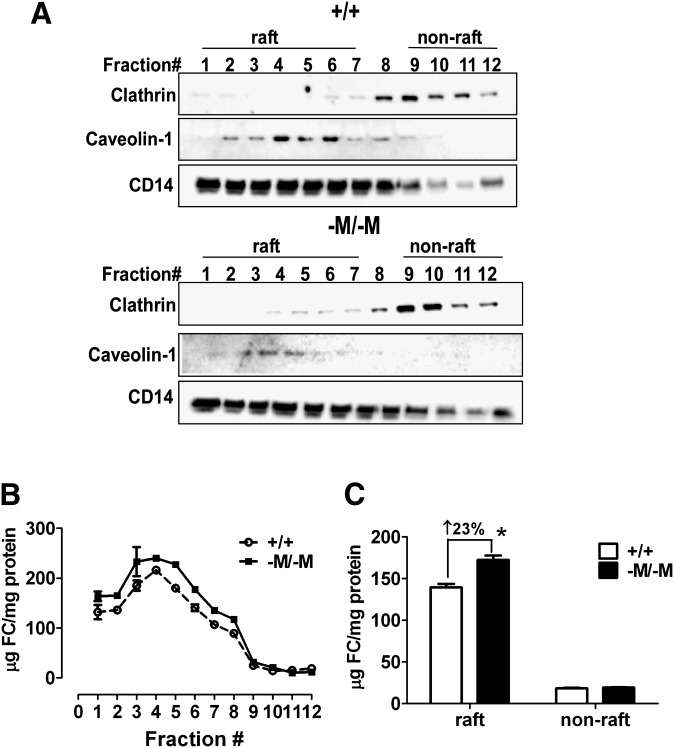
To determine whether the increase in lipid rafts previously described in Abca1-M/-M macrophages (24) is the direct result of increased FC accumulation in lipid rafts, we performed two experiments. First, we examined the intensity of FC-enriched microdomains by staining macrophages with fPEG-chol. As shown in Fig. 3A, D, compared with WT, Abca1-M/-M macrophages had a slight but significantly brighter staining of fPEG-chol, indicating more FC-enriched microdomains located on the cell surface. Furthermore, cholesterol depletion using MβCD normalized the difference in fPEG-chol staining between the two genotypes (Fig. 3B, D), whereas the difference of fPEG-chol staining was maintained when cells were overloaded with cholesterol (Fig. 3C, D). To determine whether the increased FC in Abca1-M/-M macrophages is located exclusively in lipid rafts, we isolated lipid rafts and nonrafts from macrophages using a nondetergent isolation method (27). Using raft (caveolin-1 and CD14) and nonraft (clathrin) markers, we designated the top eight fractions (nos. 1–7) as lipid rafts and the bottom fractions (nos. 9–12) as nonrafts (Fig. 4A). Interestingly, we found that in Abca1-M/-M cells, each lipid raft fraction contained more FC (Fig. 4B), resulting in an average 23.01 ± 2.12% greater FC content for the whole lipid raft fraction (i.e., fractions 1–7) compared with the same fractions from WT cells (Fig. 4C). On the other hand, FC content was similar in nonraft fractions of WT and Abca1-M/-M cells (Fig. 4B, C). Taken together, our data suggested that ABCA1 deficiency results in an increase in lipid raft content, likely due to a striking increase in FC content of this fraction. We also tested an alternative hypothesis that sphingomyelin (SM) content in lipid rafts may be increased in the absence of ABCA1, resulting in increased lipid raft content (supplementary Figs. II–IV). However, SM content and PL fatty acyl molecular species in raft and nonraft fractions were similar among the three genotypes of mice.
Fig. 3.
ABCA1 deficiency in macrophages resulted in increased membrane lipid raft content. Elicited PMs were incubated with or without MβCD for 0.5 h or MβCD-chol (80 µg/ml) for 1 h before staining with fPEG-chol. Cell fluorescence was analyzed using flow cytometry. A–C: Histograms show macrophage fPEG-chol staining in the absence (A) or presence (B) of MβCD or presence of MβCD-chol (C), respectively. D: Data from panels A–C are presented as mean ± SEM. Statistical significant differences between groups are indicated (*, P < 0.05; **, P < 0.01). Results are representative of two independent experiments with at least three mice per group. +/+: WT; -M/-M: Abca1-M/-M.
Fig. 4.
Macrophage ABCA1 deficiency results in an increase in lipid raft FC. A: Lipid raft and nonraft fractions were isolated using a nondetergent method. Each fraction was analyzed by Western blotting for the indicated proteins. B: FC and protein concentrations in each fraction in panel A were measured and the results were plotted as µg cholesterol/mg protein. Mean ± range (n = 2). Results are representative of two independent experiments. C: Data from panel B are presented as raft (fractions 1–7) and nonraft (fractions 9–12) cholesterol content for each genotype. * P < 0.05, compared with WT. +/+: WT; -M/-M: Abca1-M/-M.
Increased lipid raft TLR4 content in Abca1-M/-M macrophages
TLR4 signaling is initiated by ligand binding and transient trafficking of TLR4 to lipid rafts after macrophages are activated (20–23). Our data suggested that total cellular (24) and surface expression of TLR4 were indistinguishable between WT and Abca1-M/-M macrophages (supplementary Fig. V; see more details in supplementary Results). To investigate whether ABCA1 deficiency results in enhanced TLR4 recruitment to lipid rafts, we isolated rafts and nonrafts from BMDMs ± 100 ng/ml LPS and immunoblotted for TLR4. In resting macrophages, the majority of TLR4 was observed in nonraft fractions (Fig. 5A). However, a portion of TLR4 was also detected in raft fractions, colocalized with CD14. Interestingly, TLR4 was more diffusely distributed in the lipid raft fractions (fractions 1–7) of Abca1-M/-M BMDMs (Fig. 5B) compared with that in WT cells (Fig. 5A). To visualize the trafficking of TLR4 induced by LPS stimulation, we pooled raft and nonraft fractions isolated from BMDMs ± LPS treatment and performed Western-blot analysis. Lipid raft and nonraft fractions were identified based on the distribution of raft markers (caveolin-1, CD14, and flotillin-1) and the nonraft marker, clathrin (Fig. 5C). The majority of TLR4 was distributed in the nonraft membrane fraction in both WT and Abca1-M/-M BMDMs. However, we observed a significantly higher TLR4 content in lipid rafts from resting (i.e., nontreated) Abca1-M/-M BMDMs compared with WT cells (Fig. 5C, D). Upon LPS stimulation, a trend toward increased translocation of TLR4 into lipid rafts was observed in Abca1-M/-M relative to WT macrophages (Fig. 5C, D). As a control, another lipid raft protein, caveolin-1, was examined. Unlike TLR4, caveolin-1 content in lipid rafts was unaffected by LPS treatment of macrophages or by the genotype of macrophages (supplementary Fig. VI). Collectively, these data suggest that ABCA1 deficiency in macrophages results in a significant increase in FC-enriched lipid rafts, which concentrate and recruit more TLR4 in resting and potentially in LPS-activated macrophages.
Fig. 5.
Increased TLR4 in lipid rafts of Abca1-M/-M macrophages. A and B: Lipid raft and nonraft membrane regions were isolated using a nondetergent method and analyzed for TLR4, clathrin (non-raft marker), and CD14 (raft marker) distribution by Western blotting. C: BMDMs were treated with 100 ng/ml LPS for 1 h and then lipid raft (fractions 1–8) and nonraft (fractions 10–13) fractions were pooled, and the distribution of the indicated proteins was analyzed by Western blotting. Results are representative of two independent experiments. D: TLR4 content of macrophage lipid rafts was calculated as a percentage of total membrane TLR4 (lipid rafts + nonrafts). Data are presented as mean ± range (n = 2). Statistically significant differences between genotypes are indicated (* P < 0.05). +/+: WT, -M/-M: Abca1-M/-M.
Nuclear factor-κB pathway and lipid rafts are involved with the enhanced TLR9 signaling in Abca1-M/-M macrophages
TLR7 or TLR9 was shown to translocate from ER to the endosome/lysosome upon stimulation by specific agonists, resulting in activation of the nuclear factor (NF)-κB and mitogen-activated protein kinase (MAPK) pathways (33). To examine whether the NF-κB pathway is upregulated in TLR9 agonist (CpG)-treated Abca1-M/-M macrophages, we stimulated WT and Abca1-M/-M BMDMs with 1 μM CpG for 0–3 h before isolating cell lysates for immunoblotting. As shown in Fig. 6A, within 30 min in both genotypes of macrophages, CpG induced IκBα degradation, which is indicative of NFκB activation. Interestingly, compared with WT, Abca1-M/-M BMDMs showed a greater and prolonged IκBα degradation during 3 h of TLR9 stimulation. Because CpG stimulation was shown not only to induce translocation of TLR9 to endosomes but also to induce trafficking of TLR9 to plasma membrane lipid rafts (20, 34, 35), we next tested the hypothesis that CpG may induce more TLR9 translocation to lipid rafts in Abca1-M/-M versus WT macrophages. To test this hypothesis, we isolated lipid rafts and nonrafts from WT and Abca1-M/-M BMDMs using a simplified nondetergent isolation method (28). The majority of TLR9 was located in nonraft fractions in resting BMDMs (Fig. 6B). Next, we pooled raft (fractions 1–8) and nonraft (fractions 10–13) fractions isolated from BMDMs ± CpG treatment and performed Western-blot analysis (Fig. 6C) or immunoprecipitation (Fig. 6D) to visualize and quantify the trafficking of TLR9 to lipid rafts. As shown in Fig. 6C–E, we observed a trend of increased basal lipid raft TLR9 content in resting Abca1-M/-M cells (P = 0.08). More interestingly, CpG stimulation significantly triggered increased translocation of TLR9 from nonraft to lipid raft fractions in Abca1-M/-M vs. WT BMDMs (Fig. 6C–E). These combined results suggest that the increased lipid raft content in Abca1-M/-M cells increases MyD88-dependent activation of plasma membrane and intracellular TLRs through augmented translocation or trafficking to lipid rafts, resulting in hyper-activation of downstream signaling transduction and exaggerated proinflammatory cytokine expression.
Fig. 6.
NF-κB pathway and lipid rafts are involved with enhanced TLR9 signaling in Abca1-M/-M macrophages. A: BMDMs were incubated with 1 μM CpG for 0–3 h. Cell lysates were harvested and the proteins were analyzed by immunoblotting with the indicated antibodies. IκBα was quantified and normalized to β-actin. The degradation of IκBα induced by CpG stimulation is presented as fraction of basal protein level in control WT cells. B: BMDMs were fractioned into raft and nonraft fractions using a simplified nondetergent method. The protein distribution in each fraction was analyzed by Western blotting using the indicated antibodies. C: BMDMs were treated with 1 μM CpG for 1 h and then lipid rafts (fractions 1–8) and nonrafts (fractions 10–13) were pooled, and the distribution of the indicated proteins was analyzed by Western blotting. D: Lipid raft and nonraft fractions isolated as described in panel C were pooled and immunoprecipitated with anti-TLR9 polyclonal antibody, followed by immunoblotting with anti-TLR9 polyclonal antibody. Results are representative of two independent experiments. E: Lipid raft TLR9 content in macrophages was calculated as a percentage of total TLR9 (lipid rafts + nonrafts). Data are presented as mean ± SEM; n = 3. Statistically significant differences between groups are indicated (* P < 0.05). +/+: WT, -M/-M: Abca1-M/-M.
DISCUSSION
The well-known function of ABCA1 is to transport FC and PLs out of cells, protecting cells from excessive cholesterol accumulation. Interestingly, studies from our laboratory and others suggest an additional antiinflammatory role of macrophage ABCA1 (17, 24, 25, 36), but the mechanism still remains unclear. The present study provides direct evidence showing that ABCA1 deficiency in macrophages leads to selective FC accumulation in lipid rafts without alteration of PL composition. ABCA1 deficiency in macrophages also results in more TLR content in lipid rafts, consequently leading to enhanced TLR activation. To our knowledge, this is the first study to directly show that ABC transporters downregulate TLR (TLR2, 4, 7, and 9) signaling by reducing FC enrichment in lipid rafts and trafficking of TLRs into rafts.
Lipid rafts are liquid-ordered membrane microdomains enriched in FC and SM (19). ABCA1 expression results in redistribution of SM and FC from lipid raft to nonraft fractions in baby hamster kidney cells (18). Our previous study suggested that ABCA1 deficiency in macrophages was associated with an increase in lipid rafts, presumably due to the increase in FC accumulation (24). To test an alternative hypothesis that the increase in lipid rafts in Abca1-M/-M macrophages was the result of increased SM enrichment, we analyzed the PL profile of lipid rafts from macrophages using MS. Our data suggested that the relative distribution of SM and phosphatidylcholine fatty acyl species in lipid rafts was similar for WT and Abca1-M/-M macrophages (supplementary Fig. IV). Thus, the hypersensitivity of Abca1-M/-M macrophages to TLR activation was not due to alterations in SM or phosphatidylcholine content of lipid rafts. On the other hand, Abca1-M/-M macrophages contained more FC-enriched lipid rafts, demonstrated by fPEG-chol staining and cholesterol quantification in isolated lipid rafts.
Excessive FC in late endosomes or in the plasma membrane activates the p38 MAPK pathway through TLR3 or TLR4, respectively, without specific agonist stimulation (37), indicating that FC overloading alone is sufficient to activate TLRs in certain situations. In our study, when FC content of WT macrophages was varied over a 2-fold range, we observed a significant positive association among macrophage FC content, lipid raft content, and the inflammatory response to TLR4 agonist (Fig. 1). However, in Abca1-M/-M macrophages, FC content was only slightly increased (∼10%) compared with WT cells, and no significant difference in the activation of NF-κB and MAPK pathways or inflammatory gene expression was observed between WT and Abca1-M/-M macrophages in the absence of specific ligand stimulation (24, 25), suggesting that a subtle increase in FC content alone is sufficient to alter the plasma membrane fluidity but not sufficient to activate TLR signaling without specific agonist stimulation.
Of the 13 members in the TLR family discovered so far, TLR2 and TLR4 on the cell surface recognize bacterial lipoteichoic acid and LPS, respectively, whereas TLR3, 7, and 9, located in intracellular compartments, detect nucleic acids derived from pathogens (33). Beside their critical role in innate immunity, TLRs are also involved in the development of chronic inflammatory diseases, such as atherosclerosis (38, 39) and insulin resistance (40). In addition, TLR9 was shown to be involved in macrophage foam cell formation (41). We previously showed that ABCA1 deficiency resulted in an enhanced MyD88-dependent TLR4 signal (24). Here, we further demonstrated that ABCA1 deficiency also resulted in enhanced activation of TLR2, 7, and 9, but not TLR3. Despite having in common an intracellular localization, TLR7 and TLR9 employ MyD88, whereas TLR3 recruits TRIF to propagate downstream signal transduction. The mechanism by which ABCA1 deficiency favors MyD88-dependent TLR pathways remains unknown.
WT and Abca1−/−Abcg1−/− macrophages had similar cytokine gene expression when cells were treated with TLR2, TLR7, or TLR9 agonists (25). In contrast, when cholesterol loaded using MβCD-chol, Abca1−/−Abcg1−/− macrophages were hyper-sensitive to TLR4 stimulation compared with WT cells. In addition, Abca1−/−Abcg1−/− macrophages were also hyper-responsive to TLR3 stimulation with or without cholesterol loading (25). The difference between our study and the study of Yvan-Charvet et al. (25) is that Abca1−/−Abcg1−/− macrophages (without cholesterol overloading) were hyper-responsive to TLR3 and TLR4, but not to TLR2, 7, or 9 stimulation, whereas our Abca1-M/-M macrophages were hyper-responsive to TLR2, 4, 7, or 9 stimulation but not to TLR3 activation. Notably, Abcg1−/− Ldlr−/− macrophages had a 60% increase in FC compared with Ldlr−/− cells (25). Although Yvan-Charvet et al. (25) did not show evidence of FC enrichment in endosomes, the excess FC in Abca1−/−Abcg1−/− macrophages might accumulate in intracellular compartments, resulting in activation of TLR3 (37). In our study, FC content in Abca1-M/-M macrophages was only increased 10% compared with WT macrophages, and the FC enrichment was selective for lipid rafts. Therefore, the difference in FC content between Abca1−/−Abcg1−/− macrophages and Abca1-M/-M cells as well as the differential compartmental enrichment may account for the different responsiveness to TLRs stimulation.
TLR2 or TLR4 signaling is thought to be initiated after TLRs rapidly traffic to lipid rafts in response to specific ligands or saturated fatty acids (22, 23, 42). Based on these findings, we hypothesized that the enhanced TLR4 activation in Abca1-M/-M macrophages may be the result of an increased amount of TLR4 on the plasma membrane or increased TLR4 trafficking into lipid rafts. Our data suggested that total cellular and surface expression of TLR4 was indistinguishable between WT and Abca1-M/-M macrophages (supplementary Fig. V). Despite similar surface expression of TLR4, there was greater expression of TLR4 in lipid rafts of Abca1-M/-M macrophages in the resting state and potentially after LPS stimulation compared with WT macrophages (Fig. 5). Evidence suggests that recognition of CpG by TLR9 is likely to occur not only in endosomes, but also in plasma membrane lipid rafts (20, 34). By contrast, TLR3 activation does not occur in the rafts (43, 44). With these findings in mind, we hypothesized that the increased lipid raft content in Abca1-M/-M macrophages might also result in enhanced TLR9 trafficking into rafts and our results supported this hypothesis (Fig. 6). Because TLR2 and TLR4 (cell surface receptors) or TLR7 and TLR9 (intracellular receptors) share many properties and similar activation mechanisms, respectively (22, 45, 46), we assume that ABCA1 deficiency in macrophages results in increased translocation or recruitment of all MyD88-dependent TLRs (2, 4, 7 and 9) into lipid rafts, which leads to enhanced downstream signal transduction and augmented proinflammatory cytokine production in Abca1-M/-M macrophages.
It is intriguing to speculate that an important physiological and antiinflammatory role of macrophage ABCA1 is to suppress activation of TLRs by endogenous (saturated fatty acids) and exogenous (bacterial and viral products) stimuli by decreasing trafficking of TLRs into lipid rafts. If this speculation were true, specific agonists may be developed to treat chronic diseases that are characterized by chronic low grade inflammation by increasing expression of macrophage ABCA1, which has been shown to be highly inducible and regulated at several levels, including transcriptional and posttranslational (6, 7).
Supplementary Material
Footnotes
Abbreviations:
- Abca1+/+
- wild type mice at the Abca1 locus
- Abca1+/−M
- heterozygous macrophage-specific ABCA1 knockout
- Abca1-M/-M
- homozygous macrophage-specific knockout
- Abca1−/−
- total Abca1 knockout
- ABCG1
- ATP-binding cassette transporter G1
- BMDM
- bone marrow-derived macrophage
- CT-B
- cholera toxin B
- FC
- free cholesterol
- fPEG-chol
- fluorescein ester of polyethylene glycol-derivatized cholesterol
- GLC
- gas-liquid chromatography
- IκBα
- inhibitory κB protein α
- IL
- interleukin
- LPS
- lipopolysaccharide
- MAPK
- mitogen-activated protein kinase
- MβCD
- methyl-β-cyclodextrin
- MyD88
- myeloid differentiation primary-response protein 88
- NF
- nuclear factor
- NutSP
- Nutridoma SP
- PI
- phosphatidylinositol
- PL
- phospholipid
- PM
- peritoneal macrophage
- PNS
- postnuclear supernatant
- TLR
- Toll-like receptor
- WT
- wild type
This work was supported by National Institutes of Health Grants HL-49373, HL-094525, and AT-27820 (J.S.P.), American Heart Association fellowship 09POST2250225 (X.Z.), and the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences Z01 ES102005 (M.B.F.). The fPEG-cholesterol synthesis was supported by the National Institutes of Health Roadmap for Medical Research Initiative through its establishment of the Imaging Probe Development Center, administered by the National Heart, Lung, and Blood Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of Data, Results, and six figures.
REFERENCES
- 1.Oram J. F., Lawn R. M. 2001. ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J. Lipid Res. 42: 1173–1179. [PubMed] [Google Scholar]
- 2.Attie A. D., Kastelein J. P., Hayden M. R. 2001. Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J. Lipid Res. 42: 1717–1726. [PubMed] [Google Scholar]
- 3.Bodzioch M., Orso E., Klucken J., Langmann T., Bottcher A., Diederich W., Drobnik W., Barlage S., Buchler C., Porsch-Ozcurumez M., et al. 1999. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22: 347–351. [DOI] [PubMed] [Google Scholar]
- 4.Brooks-Wilson A., Marcil M., Clee S. M., Zhang L. H., Roomp K., van Dam M., Yu L., Brewer C., Collins J. A., Molhuizen H. O., et al. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22: 336–345. [DOI] [PubMed] [Google Scholar]
- 5.Rust S., Rosier M., Funke H., Real J., Amoura Z., Piette J. C., Deleuze J. F., Brewer H. B., Duverger N., Denefle P., et al. 1999. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22: 352–355. [DOI] [PubMed] [Google Scholar]
- 6.Oram J. F., Heinecke J. W. 2005. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 85: 1343–1372. [DOI] [PubMed] [Google Scholar]
- 7.Wang N., Chen W., Linsel-Nitschke P., Martinez L. O., Agerholm-Larsen B., Silver D. L., Tall A. R. 2003. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J. Clin. Invest. 111: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunham L. R., Kruit J. K., Iqbal J., Fievet C., Timmins J. M., Pape T. D., Coburn B. A., Bissada N., Staels B., Groen A. K., et al. 2006. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116: 1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timmins J. M., Lee J. Y., Boudyguina E., Kluckman K. D., Brunham L. R., Mulya A., Gebre A. K., Coutinho J. M., Colvin P. L., Smith T. L., et al. 2005. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115: 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunham L. R., Kruit J. K., Pape T. D., Timmins J. M., Reuwer A. Q., Vasanji Z., Marsh B. J., Rodrigues B., Johnson J. D., Parks J. S., et al. 2007. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 13: 340–347. [DOI] [PubMed] [Google Scholar]
- 11.Karasinska J. M., Rinninger F., Lutjohann D., Ruddle P., Franciosi S., Kruit J. K., Singaraja R. R., Hirsch-Reinshagen V., Fan J., Brunham L. R., et al. 2009. Specific loss of brain ABCA1 increases brain cholesterol uptake and influences neuronal structure and function. J. Neurosci. 29: 3579–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass C. K., Witztum J. L. 2001. Atherosclerosis. the road ahead. Cell. 104: 503–516. [DOI] [PubMed] [Google Scholar]
- 13.Lusis A. J. 2000. Atherosclerosis. Nature. 407: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112: 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haghpassand M., Bourassa P. A., Francone O. L., Aiello R. J. 2001. Monocyte/macrophage expression of ABCA1 has minimal contribution to plasma HDL levels. J. Clin. Invest. 108: 1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Eck M., Bos I. S., Kaminski W. E., Orsó E., Rothe G., Twisk J., Böttcher A., Van Amersfoort E. S., Christiansen-Weber T. A., Fung-Leung W. P., et al. 2002. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc. Natl. Acad. Sci. U S A. 99: 6298–6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francone O. L., Royer L., Boucher G., Haghpassand M., Freeman A., Brees D., Aiello R. J. 2005. Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler. Thromb. Vasc. Biol. 25: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 18.Landry Y. D., Denis M., Nandi S., Bell S., Vaughan A. M., Zha X. 2006. ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. J. Biol. Chem. 281: 36091–36101. [DOI] [PubMed] [Google Scholar]
- 19.Simons K., Toomre D. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1: 31–39. [DOI] [PubMed] [Google Scholar]
- 20.Nakahira K., Kim H. P., Geng X. H., Nakao A., Wang X., Murase N., Drain P. F., Wang X., Sasidhar M., Nabel E. G., et al. 2006. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J. Exp. Med. 203: 2377–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powers K. A., Szaszi K., Khadaroo R. G., Tawadros P. S., Marshall J. C., Kapus A., Rotstein O. D. 2006. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J. Exp. Med. 203: 1951–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Triantafilou M., Miyake K., Golenbock D. T., Triantafilou K. 2002. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 115: 2603–2611. [DOI] [PubMed] [Google Scholar]
- 23.Triantafilou M., Morath S., Mackie A., Hartung T., Triantafilou K. 2004. Lateral diffusion of Toll-like receptors reveals that they are transiently confined within lipid rafts on the plasma membrane. J. Cell Sci. 117: 4007–4014. [DOI] [PubMed] [Google Scholar]
- 24.Zhu X., Lee J. Y., Timmins J. M., Brown J. M., Boudyguina E., Mulya A., Gebre A. K., Willingham M. C., Hiltbold E. M., Mishra N., et al. 2008. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 283: 22930–22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yvan-Charvet L., Welch C., Pagler T. A., Ranalletta M., Lamkanfi M., Han S., Ishibashi M., Li R., Wang N., Tall A. R. 2008. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 118: 1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madenspacher J. H., Draper D. W., Smoak K. A., Li H., Griffiths G. L., Suratt B. T., Wilson M. D., Rudel L. L., Fessler M. B. 2010. Dyslipidemia induces opposing effects on intrapulmonary and extrapulmonary host defense through divergent TLR response phenotypes. J. Immunol. 185: 1660–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smart E. J., Ying Y. S., Mineo C., Anderson R. G. 1995. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc. Natl. Acad. Sci. USA. 92: 10104–10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macdonald J. L., Pike L. J. 2005. A simplified method for the preparation of detergent-free lipid rafts. J. Lipid Res. 46: 1061–1067. [DOI] [PubMed] [Google Scholar]
- 29.Fessler M. B., Arndt P. G., Frasch S. C., Lieber J. G., Johnson C. A., Murphy R. C., Nick J. A., Bratton D. L., Malcolm K. C., Worthen G. S. 2004. Lipid rafts regulate lipopolysaccharide-induced activation of Cdc42 and inflammatory functions of the human neutrophil. J. Biol. Chem. 279: 39989–39998. [DOI] [PubMed] [Google Scholar]
- 30.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 31.Ishitsuka R., Sato S. B., Kobayashi T. 2005. Imaging lipid rafts. J. Biochem. 137: 249–254. [DOI] [PubMed] [Google Scholar]
- 32.Sato S. B., Ishii K., Makino A., Iwabuchi K., Yamaji-Hasegawa A., Senoh Y., Nagaoka I., Sakuraba H., Kobayashi T. 2004. Distribution and transport of cholesterol-rich membrane domains monitored by a membrane-impermeant fluorescent polyethylene glycol-derivatized cholesterol. J. Biol. Chem. 279: 23790–23796. [DOI] [PubMed] [Google Scholar]
- 33.Kawai T., Akira S. 2008. Toll-like receptor and RIG-I-like receptor signaling. Ann. N.Y. Acad.Sci. 1143: 1–20. [DOI] [PubMed] [Google Scholar]
- 34.Latz E., Schoenemeyer A., Visintin A., Fitzgerald K. A., Monks B. G., Knetter C. F., Lien E., Nilsen N. J., Espevik T., Golenbock D. T. 2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5: 190–198. [DOI] [PubMed] [Google Scholar]
- 35.Leifer C. A., Kennedy M. N., Mazzoni A., Lee C., Kruhlak M. J., Segal D. M. 2004. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J. Immunol. 173: 1179–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koseki M., Hirano K., Masuda D., Ikegami C., Tanaka M., Ota A., Sandoval J. C., Nakagawa-Toyama Y., Sato S. B., Kobayashi T., et al. 2007. Increased lipid rafts and accelerated lipopolysaccharide-induced tumor necrosis factor-alpha secretion in Abca1-deficient macrophages. J. Lipid Res. 48: 299–306. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y., Ishibashi M., Seimon T., Lee M., Sharma S. M., Fitzgerald K. A., Samokhin A. O., Wang Y., Sayers S., Aikawa M., et al. 2009. Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circ. Res. 104: 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michelsen K. S., Wong M. H., Shah P. K., Zhang W., Yano J., Doherty T. M., Akira S., Rajavashisth T. B., Arditi M. 2004. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl. Acad. Sci. USA. 101: 10679–10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullick A. E., Tobias P. S., Curtiss L. K. 2005. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Invest. 115: 3149–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., Flier J. S. 2006. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116: 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J. G., Lim E. J., Park D. W., Lee S. H., Kim J. R., Baek S. H. 2008. A combination of Lox-1 and Nox1 regulates TLR9-mediated foam cell formation. Cell. Signal. 20: 2266–2275. [DOI] [PubMed] [Google Scholar]
- 42.Wong S. W., Kwon M. J., Choi A. M., Kim H. P., Nakahira K., Hwang D. 2009. Fatty acids modulate toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a ros-dependent manner. J. Biol. Chem. 284: 27384–27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexopoulou L., Holt A. C., Medzhitov R., Flavell R. A. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 413: 732–738. [DOI] [PubMed] [Google Scholar]
- 44.Kawai T., Akira S. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7: 131–137. [DOI] [PubMed] [Google Scholar]
- 45.Lee J., Chuang T. H., Redecke V., She L., Pitha P. M., Carson D. A., Raz E., Cottam H. B. 2003. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc. Natl. Acad. Sci. USA. 100: 6646–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R., Town T., Gokarn V., Flavell R. A., Chandawarkar R. Y. 2006. HSP70 enhances macrophage phagocytosis by interaction with lipid raft-associated TLR-7 and upregulating p38 MAPK and PI3K pathways. J. Surg. Res. 136: 58–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.