Abstract
Sleep disturbance is increasingly recognized as an important, but understudied, mechanism in the complex and multi-factorial causation of the symptoms and functional disability associated with psychiatric disorders. This review proposes that it is biologically plausible for sleep disturbance to be mechanistically transdiagnostic. More specifically, we propose that sleep disturbance is aetiologically linked to various forms of psychopathology through: its reciprocal relationship with emotion regulation and its shared/interacting neurobiological substrates in (a) genetics - genes known to be important in the generation and regulation of circadian rhythms have been linked to a range of disorders and (b) dopaminergic and serotonergic function - we review evidence for the interplay between these systems and sleep/circadian biology. The clinical implications include potentially powerful and inexpensive interventions including interventions targeting light exposure, dark exposure, the regulation of social rhythms and the reduction of anxiety. We also consider the possibility of developing a ‘transdiagnostic’ treatment; one treatment that would reduce sleep disturbance across psychiatric disorders.
Keywords: sleep, circadian, monoamines, genes, psychiatric disorder, transdiagnostic
1. High comorbidity between sleep and psychiatric disorders
Sleep disturbance is highly comorbid with many, if not most, psychiatric disorders (Benca, Obermeyer, Thisted, & Gillin, 1992; NIH, 2005). Moreover, sleep disturbance is increasingly recognized as an important, but understudied, mechanism in the complex and multi-factorial causation of the symptoms and functional disability associated with psychiatric disorders (A. G. Harvey, 2008). As such, sleep disturbance may qualify for status as a transdiagnostic process.
Sleep disturbance in psychiatric disorders can present as objectively measured alterations in sleep architecture, as well as insomnia, hypersomnia, delayed sleep phase, reduced sleep need, nightmares and nocturnal panic attacks. In the classic meta-analysis by Benca, Obermeyer, Thisted and Gillin (1992), polysomnographic data from 177 studies provided strong evidence for a link between objective sleep disturbance and the presence of psychiatric disorder. For example, compared to controls, patients with affective disorders, anxiety disorders, dementia, schizophrenia and primary insomnia showed decreased total sleep times, reduced sleep efficiency (percentage of bedtime asleep) and increased sleep latency (time to fall asleep). Total sleep time was also reduced in alcoholism, and sleep efficiency was reduced in eating disorder patients. Across several disorders a decrement in non-Rapid Eye Movement (NREM) sleep was observed as well as a longer latency to the first REM episode. Also, increased percent of REM sleep was observed in the affective disorders. On most measures, patients with affective disorders diverged markedly from controls. Importantly for the present argument, however, patients diagnosed with an affective disorder did not differ significantly from members of other disorder classes on any measure, suggesting that sleep disturbance is not unique to the affective spectrum.
The Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2000) lists sleep disturbance as a symptom of many psychiatric disorders. There are many other psychiatric disorders for which sleep disturbance is not a listed symptom but is recognized as part of the clinical presentation. For example, patients diagnosed with panic disorder often report sleep-onset insomnia and avoidance behavior relating to going to sleep due to the fear of having a panic attack during the night (Michelle G. Craske & Rowe, 1997; Lepola, Koponen, & Leinonen, 1994). Further, social phobia has been associated with significantly poorer sleep quality, longer sleep onset latency, more frequent sleep disturbance, and more severe daytime dysfunction compared to sex and age matched controls (Stein, Kroft, & Walker, 1993). Finally, patients with a diagnosis of schizophrenia have shown increased sleep disturbance and insomnia prior to relapsing into a psychotic episode (Chemerinski, et al., 2002).
2. Advantages of identifying transdiagnostic processes
As discussed elsewhere (Harvey, Watkins, Mansell, & Shafran, 2004), processes may be descriptively transdiagnostic by virtue of simple co-occurrence across a range of psychiatric disorders. More interesting, of course, are processes that are mechanistically transdiagnostic, i.e. co-occurrence demonstrably arises from some form of casual inter-relationship between the process and psychiatric disorders.
Confirmation that a process is mechanistically transdiagnostic has clinical implications. First, it provides a new perspective on issues of diagnostic overlap across psychiatric disorders. Results of the National Comorbidity Survey make a strong case for the relative rarity of ‘pure’ cases: Indeed, the vast majority of the lifetime disorders are comorbid disorders (Krueger & Markon, 2006). A transdiagnostic perspective raises the possibility that disorders co-occur because they share common mechanisms (sleep disturbance being one candidate). Second, if some psychiatric disorders are similar with respect to the processes that maintain them, advances made in the context of one disorder will be more rapidly tested for their application to other disorders. This already happens to some extent. However, transfer is often alarmingly slow. For example, see Table 1 for a demonstration of the time lag (for two decades!) for the transfer of knowledge of cognitive behaviour therapy for insomnia to other psychiatric disorders. The advantage of the transdiagnostic perspective is that it could lead to more rapid transfer of advances to a broader range of disorders. Third, a transdiagnostic approach might lead to the specification of a single treatment module or principal that is effective across a wide range of disorders (McHugh, Murray, & Barlow, 2009). For example, a fascinating possibility with significant public health implications would be the potential for developing and testing a transdiagnostic sleep intervention (A.G. Harvey, 2008; 2009b).
Table 1.
Transfer of Advances is Slow!
| CBT for Insomnia (e.g., Bootzin, 1972; Spielman, Saskin, & Thorpy, 1987) | CBT for Insomnia that is comorbid with: |
| Alcohol dependence (Arnedt et al., 2007) | |
| Depression (Manber et al., 2008) | |
| Chronic pain (Currie et al., 2000) | |
| Substance use disorder (Currie et al., 2004) | |
| Fibromyalgia (Edinger et al., 2005) | |
| Medical and psychiatric comorbidity (Lichstein et al., 2000; Perlis et al., 2001) |
3. Aim
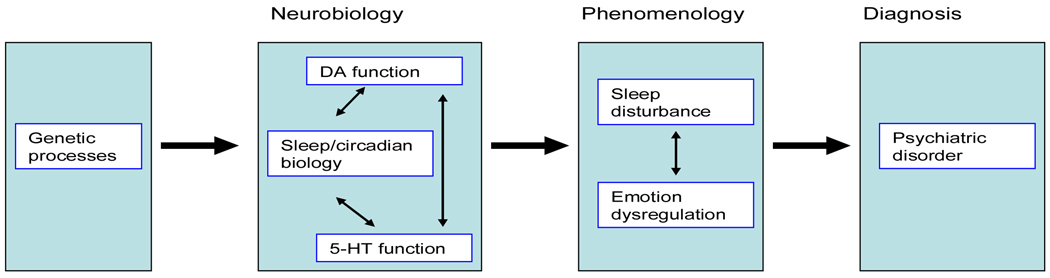
The aim of this paper is to continue the examination of sleep disturbance as a transdiagnostic process. Specifically, we will consider if it is biologically plausible for sleep disturbance to be mechanistically transdiagnostic1. As summarised above, phenomenological overlap between sleep disturbance and various psychiatric disorders is well recognised, but neurobiological characterisation of this association would further support a mechanistic role for sleep disturbance, and encourage modular interventions targeting the sleep disturbance component of psychiatric disorders. The argument developed herein can be represented schematically as in Figure 1 below.
Figure 1.
The Biological Plausibility of Sleep Disturbance as a Transdiagnostic Process in the Multifactorial Cause and/or Maintenance of Psychiatric Disorder
Before delving into the details and evidence, a number of features of Figure 1 should be noted. First, the endpoint of the model is psychiatric disorder generally (box 4). There is evidence that the transdiagnostic significance of sleep disturbance extends to other pathologies including schizophrenia (see Kantrowitz, Citrome, & Javitt, 2009; Takao, Tachikawa, Kawanishi, Mizukami, & Asada, 2007), childhood onset disorders (see Matsuura, Tateno, & Aou, 2008; Nicholas, et al., 2007) and personality disorders (see Guile, et al., 2009; Hornung, et al., 2008). However, the existing literature provides strongest support for the mood and anxiety disorders. Second, we limit our neurobiological attention to molecular genetics (box 1) and the two most researched neurotransmitters (dopamine [DA] and serotonin [5-HT], box 2). Other biological processes that are shared by sleep disturbance and psychiatric disorder include functions of the amygdala, HPA axis and noradrenergic systems (Stunkard, Allison, & Lundgren, 2008). A detailed review of these processes is beyond the scope of this paper. Third, we have construed sleep disturbance and emotion regulation as linked phenomena (box 3) in the pathway to psychiatric disorder (box 4), but the causal dynamics between these three processes is likely multifaceted. Fourth, important feedback loops have not been included in the model: neurobiological states, for example, are affected by the behavioural consequences of circadian output and emotion regulation strategies (see below and Murray, in press). Finally, the single pathway in Figure 1 adequately captures the topic of this review, but is patently not a comprehensive model of the aetiology of psychiatric disorder.
The core of this paper is a review of evidence that the neurobiology of the sleep/circadian systems interact with pathways known to be important in psychiatric disorders (box 2 of Figure 1). Prior to considering this literature in Section 5, the nature and regulation of human sleep is briefly outlined (Section 4). Our characterisation highlights the fact that circadian and sleep systems are adapted to be open to environmental information (not shown in Figure 1), with implications for transdiagnostic non-pharmacological interventions, the topic of the paper’s final section (Section 6).
4. Human sleep: the sleep vs. circadian distinction
Human sleep can be divided into rapid eye movement (‘REM’) sleep and non-rapid eye movement (‘NREM’) sleep, which is in turn divided into four stages (imaginatively called Stages 1, 2, 3 and 4). Sleep in adults follows an organized pattern starting with Stage 1 NREM sleep, deepening to Stage 4 NREM sleep, and then moving into REM, with each NREM-REM cycle taking between 70 and 120 minutes (Hirshkowitz, Moore, O'Connor, Bellamy, & Cunningham, 1997; Shneerson, 2000).
The sleep/wake cycle is regulated by an interaction between two processes. The first is circadian (also known as Process C), arising from the endogenous pacemaker in the hypothalamic suprachiasmatic nuclei (SCN) (Reppert & Weaver, 2002). The SCN receives photic information gathered by photoreceptor cells in the retina. The retina does not only contain the classical photoreceptors (rods and cones) but also photoresponsive retinal ganglion cells containing the pigment melanopsin, which follow a pathway called the retinohypothalamic tract, terminating in the SCN (Moore, 2007). Light received at the retina is then transformed into a neural signal and sent to the brain (Reme, Wirz-Justice, & Terman, 1991). Light is also associated with the increased release or suppression of cortisol, melatonin and thyroid stimulating hormone (Leproult, Colecchia, L'Hermite-Baleriaux, & Van Cauter, 2001). The output of the SCN cannot be directly measured in humans, but it can be discerned in the 24-hour rhythms of processes it regulates (including core body temperature, secretion of melatonin and cortisol) (Redfern, Waterhouse, & Minors, 1991). At the molecular level, intrinsically rhythmic cells of the SCN generate self-sustained rhythmicity via an autoregulatory transcription-translation feedback loop regulating expression of the Period (Per1, Per2, Per3), cryptochrome (Cry1, Cry2), TIM, DEC1 and DEC2 genes (Takahashi, Hong, Ko, & McDearmon, 2008). One important downstream projection of the SCN is the pineal gland, which secretes melatonin. Secretion of melatonin peaks at night and is practically non-existent during the day. Melatonin is a crucial factor in the initiation of sleep.
The endogenous period generated in the SCN is close to, but generally not equal to 24 h. The process by which the pacemaker is both set to a 24 h period and kept in appropriate phase with seasonally shifting astronomical daylength is called entrainment. An important adaptation of the circadian system, therefore, is its fundamentally open nature (Mrosovsky, 1999). Entrainment occurs via zeitgebers which are environmental events that can affect the phase and period of the clock. The primary zeitgeber in most species is the daily alteration of light and dark caused by the planet’s rotation (Roennebert & Foster, 1997). This is because light reception on the retina is transformed into a neural signal and sent to the brain (Reme, et al., 1991). The SCN is also responsive to non-photic cues such as arousal/locomotor activity, social cues, feeding, sleep deprivation and temperature (Mistlberger, Antle, Glass, & Miller, 2000). For example, higher daily regularity in social rhythms (the timing of social interactions, mealtimes, etc.) has been found to be associated with a stronger endogenous temperature rhythm, suggesting stronger overall circadian functioning (Monk, Petrie, Hayes, & Kupfer, 1994). Exogenous factors have a powerful impact on both the circadian and sleep systems. Indeed, stronger social rhythms are associated with better subjective sleep quality (Monk, et al., 1994). As these exogenous factors are relatively easy to modify, they have become the target of powerful and affordable interventions, an issue to which we will return.
The second process regulating sleep-wake is homeostatic (also known as Process S). Process S regulates the duration and structure of sleep on the basis of the history of sleep and wakefulness: sleep pressure increases during wake and dissipates during sleep. Circadian and homoeostatic processes act in concert to maintain wakefulness during the day and to promote a consolidated sleep period at night (Borbely, 1982; Dijk & Franken, 2005).
Under natural conditions, Process C and Process S operate in synchrony, and their independent actions can only be discerned with laboratory-based manipulations. In the gold-standard forced desynchrony (FD) protocol (Czeisler, et al., 1999), participants live on an imposed non-24 hour (typically 28 hr) sleep schedule. Under these conditions, the circadian oscillator continues to cycle at approximately 24 hours and desynchronises from the sleep/wake cycle which adopts the enforced 28-hour period, thereby enabling the separate estimate of circadian and sleep-homeostatic components (Czeisler, et al., 1999). The FD protocol is emotionally and physically challenging, raising ethical and clinical concerns for participants with a psychiatric diagnosis. Consequently, very few diagnosed patients have undergone FD protocols, limiting our understanding of circadian*sleep interactions in psychiatric disorder. Because of the difficulty separating circadian and sleep-related drivers, the less specific term “biological rhythms” will be used below when the data does not support a clear distinction.
5. Sleep disturbance and psychiatric disorder: Evidence for shared/interacting neurobiology
The notable co-occurrence of sleep disturbance and psychiatric disorders has been discussed elsewhere (Benca, et al., 1992; A.G. Harvey, 2008). Here, we take the argument for transdiagnostic significance a step further. We propose that sleep disturbance is aetiologically linked to various forms of psychopathology through: i) its reciprocal relationship with emotion regulation (box 3 of Figure 1), ii) shared/interacting neurobiological substrates in genetics (box 1) and iii) dopaminergic and serotonergic function (box 2).
5.1 Association between sleep disturbance and emotion dysregulation
Behavioural data support the common lay observation that sleep disturbance strongly increases negative mood (e.g., Dinges, et al., 1997; Drake, et al., 2001; El-Sheikh, Buckhalt, Cummings, & Keller, 2007; Franzen, Siegle, & Buysse, 2008; Hamilton, Catley, & Karlson, 2007; Novati, et al., 2008). Sleep loss has been shown to not only increase negative emotional response to goal-thwarting events, but also decrease positive emotional responses to goal-enhancing events (Zohar, Tzischinsky, Epsten, & Lavie, 2005). High levels of emotional arousal can also disturb sleep, raising the possibility of vicious cycles between sleep disturbance and emotion dysregulation (Dahl & Lewin, 2002). At the neural systems level, sleep deprivation has been linked to decreased medial-prefrontal cortical activity and increased amygdala activation, a distribution of activation consistent with impaired top-down regulation of emotional responses (Yoo, Gujar, Hu, Jolesz, & Walker, 2007). Conversely, emotion circuits affect homeostatic and circadian drives for sleep (Saper, Cano, & Scammell, 2005; Yoshida, McCormack, Espana, Crocker, & Scammell, 2006)2.
The bidirectional relationship between sleep disturbance and emotion regulation is consistent with the former as a transdiagnostic process. An area for future research is the question of which part of the dynamic affords greatest therapeutic leverage.
5.2 Association between circadian genes and psychiatric disorder
Genes known to be important in the generation and regulation of circadian rhythms have been linked to a range of disorders (Lamont, Legault-Coutu, Cermakian, & Boivin, 2007). The most-replicated findings pertain to mood disorders. Bipolar disorder has been associated with Timeless, Clock (311 T to C) and BMal1 (Murray & Harvey, in press), polymorphisms in the CLOCK protein (homozygous for the C alleles) are related to sleep abnormalities in major depressive disorder (Serretti, et al., 2003) and PERIOD2, NPAS2, and BMAL1 are associated with seasonal affective disorder (Partonen, et al., 2007).
Associations between circadian genes and non-mood disorders have also been reported. For example, Per1 and BDNF have been associated with ADHD (Lasky-Su, et al., 2008), PER3 and TIMELESS have been found to be associated with schizophrenia/schizoaffective disorder (Mansour, et al., 2006) and a haplotype of the hPer2 gene was associated with high versus low alcohol intake in a sample of 215 alcohol-dependent patients (Schumann, 2007; Spanagel, et al., 2005).
Importantly, consistent with the proposal that psychiatric disorders are likely to be associated with multiple genes of small effect (Mansour, Monk, & Nimgaonkar, 2005), most of the associations reported above are modest and failures to replicate are common (Monteleone, et al., 2008; Tortorella, Monteleone, Martiadis, Perris, & Maj, 2007).
Compared with our understanding of circadian genes in psychiatric disorders, much less is known about the genes involved in sleep and their potential involvement in psychopathology (Bamne, Mansour, Monk, Buysee, & Nimgaonkar, submitted). Specific criteria for the constituents of a ‘sleep gene’ are currently under discussion (Andretic, Franken, & Tafti, 2008), and the possible association of sleep genes with psychiatric disorders is a priority for future research.
5.3 Serotonin and dopamine systems in psychiatric disorders
Alterations in the brain monoamines serotonin (5-HT) and dopamine (DA) have been implicated in the aetiology and pharmacotherapy of a range of psychiatric and medical disorders. Both monoamines are associated with cognitive, emotional and bodily functioning; serotonin plays a role in attention, cognition, and information processing (Richtand & McNamara, 2008; Richtand, et al., 2008), while dopamine is important for motivation, psychomotor movement, reward processing and the ability to experience pleasure (Bressan & Crippa, 2005; Dunlop & Nemeroff, 2007).
Disturbed serotonergic function is a recognised pathway to mood disorders (Carver, Johnson, & Joormann, 2008), anxiety disorders, eating disorders (Kaye, 2008; Vaswani, Linda, & Ramesh, 2003), schizophrenia, addiction and non-psychiatric conditions including epilepsy, migraines and pain (Hedlund, 2009). At the genetic level, polymorphisms of serotonin transporter genes have been linked to both bipolar disorder and unipolar depression (Lotrich & Pollock, 2004; Luddington, Mandadapu, Husk, & El-Mallakh, 2009). The literature also recognises serotonergic function in a diathesis-stress framework: for example, an interaction between environmental stress and polymorphisms of the transporter genes has been linked to susceptibility to depressed mood (Khan, Jacobson, Gardner, Prescott, & Kendler, 2005).
Disturbed dopaminergic function is a replicated correlate of psychotic disorders, most notably schizophrenia where high levels of dopamine release are associated with hallucinations (Lyon, et al., 2009). Dopamine circuitry is also strongly involved in addiction (Carlezon & Thomas, 2009), mood disorders (Berk, et al., 2007; Malhi & Berk, 2007), eating disorders (Bergen, et al., 2005), pathological gambling and hypersexuality (Goodman, 2008). Changes to the mesolimbic dopaminergic system may be a factor in the faulty reward processing of anhedonic depressed patients (Martin-Soelch, 2009). As with serotonin, a diathesis-stress model of dopamine’s involvement in psychopathology has received support (Moghaddam, 2002; E. Walker, Mittal, & Tessner, 2008).
The DA and 5-HT systems do not operate independently, and their interaction is probably important in psychopathology (Esposito, Di Matteo, & Di Giovanni, 2008). For example, animal models suggest that 5-HT2 receptor antagonism modulates DA neuron firing activity due to antipsychotic medication (Olijslagers, Werkman, McCreary, Kruse, & Wadman, 2006). Similarly, 5-HT2C receptor agonists decrease, while 5-HT2C receptor antagonists enhance mesocorticolimbic DA function (Esposito, 2006).
In summary, there is ample evidence that the separate and interacting effects of DA and 5-HT are etiologically important in a range of psychiatric disorders. Next, we review literature showing the interplay between these systems and sleep/circadian biology.
5.4 Links between the sleep/circadian systems and the serotonin system
The SCN contains one of the densest serotonergic plexes in the brain (L. P. Morin, 1999). Electrical stimulation of the serotonergic medial (MRN) and dorsal raphe nuclei (DRN) increases 5-HT content in the SCN, confirming raphe nuclei involvement in the serotonergic activity of the SCN. Gross disruption of serotonergic function (lesioning the raphe nuclei or chemically disrupting 5-HT neurons) does not obliterate circadian rhythms but produces a diminution of overall amplitude, as well as a tendency towards phase advance and increased period (Prosser, 2000). Functionally, therefore, 5-HT seems to enhance the overall stability of circadian rhythmicity via its efferent from the MRN (Hannibal & Fahrenkrug, 2006). Serotonin is also specifically involved in the regulation of the SCN by non-photic zeitgebers (Hannibal & Fahrenkrug, 2006; Jiang, Teshima, Yang, Yoshioka, & Allen, 2000), and the stabilising effect of 5-HT may arise from its part in the reciprocal interplay between photic and non-photic zeitgebers (Hannibal & Fahrenkrug, 2006).
There is also evidence that circadian function modulates the serotonin system (Miller, Morin, Schwartz, & Moore, 1996; Mistlberger, et al., 2000). The marked diurnal rhythm in serotonergic activity in the brain, including the SCN (Glass, Grossman, Farnbauch, & DiNardo, 2003) and the raphe nuclei (Cagampang, Yamazaki, Otori, & Inouye, 1993) has an endogenous circadian component, as does the rhythm of 5-HT receptor activity (see Nagayama, 1999 for a review). Furthermore, 5-HT activity is influenced by light exposure and season (Lambert, Reid, Kaye, Jennings, & Esler, 2002).
Based on a series of investigations in drosophila, Yuan and colleagues (2005) identified a molecular connection between serotonin signaling and the central clock component TIM (viz., the serotonin receptor d5-HT1B inhibits molecular and behavioural responses of the clock to light) and showed that serotonin signaling is in turn modulated by light and circadian components (viz., d5-HT1B levels are altered in fly circadian mutants). The authors conclude that “Mutual regulation of the circadian and serotonin systems may be necessary to maintain the normal physiological functions of both systems” (p. 125).
The circadian*serotonergic interaction in fact appears in some existing models of psychiatric disorder. For example, a bidirectional relationship between circadian and serotonergic function is a prominent feature of Mistlberger et al’s conceptual model of depression (Mistlberger, et al., 2000). Specifically, it is hypothesised that serotonin mediates phase-shifting effects of behavioural stimuli on circadian rhythms. It has also been proposed that the serotonergic projection from the MRN to the SCN is the anatomical interface between circadian function and mood disorder (Reghunandanan & Reghunandanan, 2006; J. Sprouse, 2004; see also J. Sprouse, Braselton, & Reynolds, 2006).
The relationship between serotonin and sleep per se is complex, and advances in technology have led to overturning of earlier conclusions (see Ursin, 2002). Serotonergic neurons in the MRN and DRN are one component of the ascending arousal system (Fuller, Gooley, & Saper, 2006). The firing of these neurones correlates with wakefulness, and they are virtually inactive during REM sleep (Adrien, 2002). Moreover, sleep deprivation induces neurochemical changes similar to depression, including decreased sensitivity of the 5-HT1A receptor system (Novati, et al., 2008; Roman, Walstra, Luiten, & Meerlo, 2005).
A range of evidence therefore suggests that the sleep/circadian systems are intimately involved in serotonergic function and vice versa. One final vivid example of the link: the circadian hormone melatonin is a derivative of serotonin, and the SCN modulates serotonin’s metabolising into melatonin (Snyder, Borjigin, & Sassone-Corsi, 2006).
5.5 Links between the sleep/circadian systems and the dopamine system
Extensive research into the psychiatric implications of a circadian*dopaminergic interaction has emphasised dopamine’s role in reward activation. The brain’s reward pathways can be parsed into appetitive/dopaminergic and consummatory components, with the former being most clearly associated with positive affects and reward activation (Ashby, Isen, & Turken, 1999; Bressan & Crippa, 2005).
The involvement of the circadian system in the reward activation of animals is well understood (e.g., Abarca, Albrecht, & Spanagel, 2002; Andretic, Chaney, & Hirsh, 1999; Cain, Ko, Chalmers, & Ralph, 2004; Dudley, et al., 2003; Garcia, et al., 2000; McClung, et al., 2005; Ralph, et al., 2002; Reick, Garcia, Dudley, & McKnight, 2001; Sleipness, Sorg, & Jansen, 2005, 2007). We have recently shown that in humans, positive moods and other measures of reward activation are moderated by the circadian system (Murray, et al., 2009), and hypothesised that disturbance in an interacting circadian*reward circuit is causally important in mood disorders. Consistent with this hypothesis, Roybal and colleagues (2007) have shown that Clock mutant mice display increased dopaminergic activity in the ventral tegmental area (VTA), as well as behaviours homologous to mania (hyperactivity, decreased sleep, increase in the reward value of cocaine, etc.). Significantly, these behaviours are normalised by administration of lithium, and by expression of a functional CLOCK protein specifically in the VTA.
The dopaminergic system is also prominent in the neurobiology of sleep per se. Dopamine has been called a key substance in the regulation of sleep-wake (Lima, et al., 2008), and dopaminergic neurons of the ventral tegmental area and substantia nigra pars compacta are implicated in this process (Monti & Monti, 2007). The dopaminergic D2 receptor, in particular, seems to play a specific role in REM sleep (Dahan, et al., 2007; Lima, et al., 2008). Accordingly, sedation is an unwanted side-effect of antipsychotic medications, which operate by blocking dopaminergic receptors (Ongini, Bonizzoni, Ferri, Milani, & Trampus, 1993), and the wake-promoting effects of modafinil, amphetamine and caffeine have been shown to depend on the dopamine transporter gene (Wisor, et al., 2001).
Finally, as noted above, DA and 5-HT systems are interacting players in the pathway to psychiatric disorder. It is interesting that this DA*5-HT interaction can be traced into circadian function. For example, dopaminergic and serotonergic pathways play functional roles in the activity of the endogenous circadian pacemaker. Photic input is conveyed from the eye to the SCN via the retinohypothalamic tract (RHT) and indirectly by the geniculohypothalamic tract (GHT) from the intergeniculate leaflet (Moore, 1996). Both dopamine (Yan, Bobula, Svenningsson, Greengard, & Silver, 2006) and serotonin (Moyer & Kennaway, 2000) participate in these entrainment pathways.
One important domain for future research is to focus on the architecture of sleep across disorders. Since the classic meta-analysis by Benca et al. (1992), the sleep architecture findings have become more complicated with findings emerging specific to gender (Armitage, 2007), to the second versus the first half of the night (Cartwright, Young, Mercer, & Bears, 1998) and influence by medications (Eidelman, Talbot, Gruber, Hairston, & Harvey, in press). Developments in knowledge about the role of specific stages of sleep in non-patient groups (M. P. Walker & Stickgold, 2006), along with more sophisticated scoring methods (Armitage, 1995), will breathe new life into this aspect of transdiagnostic sleep research.
5.7 Summary and provisional conclusion
The serotonergic and dopaminergic systems are strongly implicated in a range of diagnoses through their impact on fundamental cognitive, emotional, motivational and motor processes. The preceding review provides a range of converging evidence that the sleep/circadian systems are intricately connected with these two prominent aetiological factors.
It seems reasonable to provisionally conclude that the transdiagnostic significance of sleep disturbance is not limited to its phenotypic association, but extends to its participation in the mechanisms underpinning psychopathology. Specifically, one of the ways that sleep disturbance is causally involved in psychiatric disorder is a shared substrate – sleep disturbance and psychiatric disorder are neurobiologically intertwined.
6. Clinical implications of sleep disturbance as a transdiagnostic process
A number of clinical hypotheses arise from the provisional conclusion that sleep disturbance is mechanistically transdiagnostic. First, if sleep disturbance and the symptoms/processes of psychiatric disorders are jointly maintained, then interventions for sleep/circadian functioning may also reduce the symptoms associated with the comorbid psychiatric disorder. Second, as flagged earlier, if sleep disturbance is truly transdiagnostic in this mechanistic sense, practitioners could be trained in the application of a sleep intervention with broad application across disorders and profound public health implications (A.G. Harvey, 2008). Third, although not reviewed here, there is a well-known association between poor sleep and impaired quality of life (Roth & Ancoli-Israel, 1999). It seems likely that improving sleep/circadian functioning will have impacts beyond symptom reduction across a range of psychiatric disorders (for implications for physical health, quality of life and the development of other psychiatric disorders see (Harvey, 2009a).
Finally, the preceding arguments hold particular relevance for interventions offered by clinical psychologists. As noted above, the sleep/circadian systems are adapted to be responsive to unconditioned environmental stimuli, particularly light, eating and social activity. Therefore, unlike serotonergic and dopaminergic function, the neurobiology of sleep/circadian function can be modulated non-pharmacologically. Behavioural and light manipulations for sleep and circadian function therefore constitute transdiagnostic interventions for the clinical psychologist’s armamentarium.
Two types of therapeutic intervention will be briefly described - modulation of biological rhythms by timed light exposure and modulation of biological rhythms by stabilisation of social/behavioural rhythms. The majority of work into these strategies has been in the context of mood disorders (see Wirz-Justice, et al., 2005; Wirz-Justice, Benedetti, & Terman, 2009; Wirz-Justice, et al., 2004), but the review above gives confidence that they have broader potential application. Like other behavioural interventions, light and social rhythm manipulations are best presented within a collaborative therapeutic framework (Berk, Berk, & Castle, 2004; Corsini & Wedding, 2005). More specific detail about chronotherapeutic interventions can be found in several excellent clinical texts (e.g., E. Frank, 2005; Wirz-Justice, et al., 2009).
6.1 Therapeutic modulation of light exposure
Bright light exposure is the first line treatment for depressions with a seasonal pattern, and has also proven effective (to a lesser extent) in purely nonseasonal depression (Even, Schröder, Friedman, & Rouillon, 2008; Golden, et al., 2005; Tuunainen, Kripke, & Endo, 2006). Limited trials of light therapy have also been conducted in schizoaffective disorder (Oren, Cubells, & Litsch, 2001), ante-partum depression (Oren, et al., 2002) and chronic fatigue syndrome (M. Terman, Levine, Terman, & Doherty, 1998).
Light treatment involves the patient sitting in front of a light therapy unit at a distance yielding a benchmark intensity of light, usually ranging from 2,500–10,000 lux (J. S. Terman, et al., 1990). The distance will vary somewhat with different light sources but roughly 16–24 inches is optimal. The placement should be such that the area around the eyes is illuminated. It is not necessary for the patient to look directly at the unit, and most patients read or eat while undergoing light exposure. As with all behavioural interventions, clinicians must pre-empt problems adhering to light treatment (Michalak, Murray, Wilkinson, Dowrick, & Lam, 2007).
Light restriction may also be effective for the hyperarousal component of some psychiatric disorders. For example, Barbini, Benedetti, Colombo, Dotoli, Bernasconi, Cigala-Fulgosi et al. (2005) randomly allocated bipolar patients in a manic episode to 14 hours of darkness over three 3 consecutive days, or treatment as usual. Those who received dark therapy exhibited a startling and rapid decrease in manic symptoms relative to the treatment as usual group.
An important caveat on manipulation of light is that some individuals may be hypersensitive to light’s mood and arousal effects. For example, Sit and colleagues (2007) attempted to treat bipolar depression by exploring 3 durations at 2 times of day using 7,000 lux white light. Comparing exposures of 15, 30, or 45 min/d across 2-wk epochs and measuring overall levels of depression, 1 of 4 women treated in the morning entered a sustained period of remission whereas 3 developed mixed mood states (including irritability, elevated energy and racing thoughts). The optimal titration and timing of light in light sensitive individuals is a matter of ongoing interest (Anderson, Glod, Dai, Cao, & Lockley, 2009).
Recent discoveries as to the neurobiological effects of light are likely to have a major impact on light treatment research and practice. Novel photoreceptors have been identified in the mammalian eye that are most sensitive to short-wavelength blue light (G. Vandewalle, et al., 2007; Gilles Vandewalle, et al., 2007). In humans, it appears that blue light is the most effective wavelength for suppressing pineal melatonin production during the night. As compared to an equal photon density of green (555 nm), blue light (460 nm) is twice as effective at phase-shifting the timing of the circadian clock and preferentially improves alertness, as measured by subjective ratings, changes in activation state in the brain, and auditory performance (Lockley, Brainard, & Czeisler, 2003). A small number of studies have investigated the functional and clinical implications of the importance of wavelength in quantifying the ‘dose’ of light (Anderson, et al., 2009; Phelps, 2008; G. Vandewalle, et al., 2007), but the implications of these findings are far-reaching (Brainard & Hanifin, 2005).
The recent discovery that light’s neurobiological effects are blue-shifted raises the possibility for more sophisticated research into type of light exposure and dosage. We also emphasize that behavioral interventions focused on the management of natural sources of light are an even less expensive way of taking advantage of this powerful input to the circadian system. Behavioral activation and behavioral scheduling protocols can include an emphasis on exposure to sunlight, especially for patients who have trouble waking in the morning (e.g., depressed adolescents with a delayed phase or hypersomnic patients). Similarly, completing a functional analysis of exposure to light in the evening can help patients downregulate sufficiently and allow the biology governing sleep onset to take over. For example, turning off television, computers and turning down lights an hour before bedtime can be a simple but powerful intervention for some patients. We note the intriguing case report of two severely manic patients who were asked to enter a darkened room for a daytime nap. Van Sweden (1986) reported that both ‘showed Stage 2 NREM sleep, the usually objective marker of “true” sleep, within seconds following eye closure’ (p. 312). This study raises the provocative idea that the reduced sleep need evident patients with bipolar disorder during hypomania and mania may be a problem of insufficient opportunity to sleep rather than a disorder of lowered sleep need per se. This is a possibility that chimes well with Johnson’s (2005) research documenting the propensity toward excessive goal seeking among bipolar patients. Preoccupation with goal pursuit may contribute to the perception of a decreased need for sleep in manic patients.
6.2 Social rhythm therapy
As noted above, the circadian system is primarily entrained by light, but is also sensitive to activity and social cues. Ehlers, Frank and others (Ehlers, Frank, & Kupfer, 1988; E. Frank, 2007; Healy & Waterhouse, 1995) have argued that rhythmic features of the social environment (such as the timing of sleep, eating and exercise) are significant components of human circadian entrainment. Consequently, the social zeitgeber hypothesis of depression proposes that major life events (such as loss of a significant relationship) not only have psychological meaning but also weaken zeitgeber information through destabilization of daily activities and light exposure (see Grandin, Alloy, & Abramson, 2006 for an overview). Consistent with the social zeitgeber hypothesis, two studies have found that onset of manic episodes is associated with life events that involve disruption of social rhythms (Malkoff-Schwartz, et al., 1998; Malkoff-Schwartz, et al., 2000).
The clinical application of the social zeitgeber hypothesis is Social Rhythm Therapy , a largely behavioural psychotherapy designed to maintain stability in social rhythms. In the treatment of bipolar disorder, Social Rhythm Therapy is typically integrated with principles from interpersonal psychotherapy in a treatment known as Interpersonal and Social Rhythm Therapy (IPSRT, E. Frank, et al., 1994). IPSRT has proven effective for bipolar disorder in two large studies (Ellen Frank, et al., 2005; Miklowitz, et al., 2007). Indeed, stabilising of social rhythms is a core component of all psychosocial treatments for bipolar disorder (Miklowitz, Goodwin, Bauer, & Geddes, 2008), and is a prominent wellness tool amongst high functioning patients (Suto, Murray, Hale, Amari, & Michalak, in press).
To our knowledge, IPSRT is yet to be tested outside bipolar disorder, but the literature reviewed in Section 4 above suggests that behavioural support for biological rhythm regularity potentially has beneficial effects on various symptoms associated with a range of disorders.
6.3 Sleep focused versus anxiety focused interventions
As suggested above, there is strong evidence for a reciprocal relationship between sleep disturbance and problems with emotion regulation. An intriguing clinical question is whether psychological interventions should focus on improving sleep/circadian function or whether the focus of intervention should be on improving emotion regulation, particularly elevated levels of anxiety. One of the leading treatments (in terms of the compelling evidence base), cognitive behaviour therapy for insomnia (C. M. Morin, et al., 2006), focuses mainly on improving sleep and circadian functioning. As described in Table 2, the stimulus control and sleep restriction interventions, for which the most evidence has accrued, emphasize regularity in the sleep-wake schedule.
Table 2.
Sleep restriction (Spielman et al., 1987) and stimulus control (Bootzin & Epstein, 2000) instructions
| Method | Instructions |
|---|---|
| Sleep Restriction | Keep a sleep diary for 7–14 days |
| Calculate total sleep time (TST) and time in bed (TIB) for each night | |
| Compute sleep efficiency (SE) = TST / TIB × 100 | |
| Next week: Cut bedtime to actual amount patient reports sleeping, but not <5 hours/night | |
| Prohibit sleep outside of these hours (if too sleepy then allow 30 minute nap before 3pm) | |
| Based on average of 5 nights, when SE is >85%, increase bedtime by 15 minutes | |
| With the elderly, SE increase bedtime when SE reaches 80%. | |
| Stimulus Control | Go to bed only when sleepy |
| Use the bed only for sleeping – do not read, watch TV, or eat in bed | |
| If unable to sleep (in 20 mins), move to another room. Stay up until really sleepy. The goal is to associate the bed with falling asleep quickly | |
| Repeat tactic immediately above as often as necessary | |
| Awaken at the same time every morning regardless of total sleep time | |
| Do not nap |
The alternate or additional possibility, that intervention should focus on reducing anxiety, is an implication of Saper et al.’s (Cano, Mochizuki, & Saper, 2008; Saper, Chou, & Scammell, 2001) model of sleep which states that sleep is controlled by a ‘flip-flop switch’ between the ventrolateral preoptic nucleus (VLPO; neurons in the VLPO are activated during sleep) and the arousal system. Saper and colleagues propose that sleep disturbance occurs when the VLPO is fully activated but not able to flip off the arousal system because it’s being ‘excited intensely’ by the limbic system (Cano, et al., 2008). A hypothesis arising from this model is that intervention should target reducing limbic activation. The latter may well be possible with the cognitive therapy (Harvey, Sharpley, Ree, Stinson, & Clark, 2007) and mindfulness interventions (Heidenreich, Tuin, Pflug, Michal, & Michalak, 2006; Ong, Shapiro, & Manber, 2008) that have been developed in recent years for sleep problems. These interventions focus on developing new skills for more effectively managing worry, rumination and intrusive thoughts as well as other cognitive processes so as to reduce the associated limbic system activation and sleep-interfering arousal.
6.4 Transdiagnostic versus disorder specific sleep treatments
Will it be possible to develop a ‘transdiagnostic’ treatment, one treatment that will reduce sleep disturbance across patients with psychiatric disorders? If it were possible, a transdiagnostic treatment would have the great advantage of easy dissemination. This is important as developing and disseminating transdiagnostic treatment protocols (as opposed to disorder specific treatment protocols) would reduce the heavy burden on clinicians, who must already learn multiple treatment protocols that often share many common theoretical underpinnings and interventions.
However, we also emphasize that the type of sleep disturbance across psychiatric disorders is complex which reduces the likelihood that there will be a ‘one size fits all approach’. There are various types of sleep disturbance, all different, that can present as comorbid with psychiatric disorders. These include insomnia, hypersomnia, delayed sleep phase, reduced sleep need, nightmares and nocturnal panic attacks. As such, perhaps it will be necessary to develop sleep specific treatments for the various psychiatric disorders.
One possible resolution may be to devise a sleep–focused treatment that includes ‘core’ modules that would be delivered to all patients, regardless of diagnosis, and ‘optional’ modules to cover the disorder-specific aspects of the sleep disturbance. One such approach is briefly summarized in Table 3 (Harvey, 2009b).
Table 3.
Core and Optional Modules of a Transdiagnostic Sleep Intervention (Harvey, 2009b)
| Case Formulation | Case formulation involves a detailed functional analysis of a typical recent night and a recent day:
|
| Sleep Education (core module) | Provide education about the endogenous (e.g., the circadian clock) and exogenous (e.g., light, social rhythms) processes that govern sleep. |
| Motivational Interviewing (core module) | Assist the patient to honestly review the pros and cons of change. |
| Regularizing the circadian phase (core module) | The goal is to gradually move toward a regular sleep schedule 7 days a week (with no more than a 2 hour difference between weekdays and weekends). Approaches:
|
| Daytime Functioning (core module) | Interventions that assist the patient to generate energy (Ree & Harvey, 2004). |
| Treating Insomnia (optional module) | Stimulus control and sleep restriction (see Table 2). |
| Treating Hypersomnia (optional module) | Collaborate to set sleep goals (reduce to approximately 8 hours per night) and set life goals (so there is something to get up for). Assist the patient to identify one small step toward these goals for the coming week. Engage in a detailed discussion of how to achieve these goals and identify possible obstacles. |
| Nightmares (optional module) | Imagery rehearsal therapy is a two-tier process wherein:
|
| Nocturnal panic (optional module) | Provide accurate information about panic attacks, with an emphasis on the harmless nature of physiological fluctuations during sleep. Exposure therapy to induce physical sensations associated with panic (e.g., heart increase, hyperventilzation, dizziness) in order to help the patient weaken associations between internal physiological cues and the typical panic response (Craske & Tsao, 2005). |
| Reduced sleep need (optional module) | Characteristic of mania and hypomania within the bipolar spectrum disorders. A preliminary trial of ‘dark therapy’ has been published by Barbini et al. (Harvey, 2009b). Bipolar patients in a manic episode were given 14 hours of enforced darkness for 3 consecutive days or treatment as usual. Those who received dark therapy exhibited a decrease in manic symptoms relative to the treatment as usual group. This finding raises the possibility that a behavioural intervention that controls light and dark may be helpful for reduced sleep need. |
7. Conclusions
We have reviewed evidence suggesting that it is biologically plausible for sleep disturbance to be an important transdiagnostic process. We have shown that sleep disturbance is aetiologically linked to various forms of psychopathology through (a) its reciprocal relationship with emotion regulation, (b) genes known to be important in the generation and regulation of circadian rhythms that have been linked to a range of disorders and (c) the interplay between systems known to be important across a range of psychiatric disorders, namely the dopamine and serotonin systems, as well as sleep/circadian biology. We are excited about the simple but powerful interventions that arise from this review. Interventions targeting light exposure, dark exposure, the regulation of social rhythms and/or the reduction of anxiety may be helpful in improving sleep across a range of disorders. Although we recognize the complexity of the sleep disturbance that can be characteristic of psychiatric disorders, research into a ‘transdiagnostic’ sleep treatment is now warranted.
Acknowledgments
This project was supported by National Institute of Mental Health Grant No. R34 MH080958 and R01MH079188.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We do not imply that biological plausibility is more significant than cognitive or behavioural plausibility, but these are issues for future papers.
We prioritise emotion regulation as a process that interacts with sleep disturbance in the predisposition to psychiatric disorder solely because of the weight of data in this area. However, we recognize that the domains of arousal and neurocognitive function are also abnormal in many psychiatric disorders and modulated by sleep/circadian systems. Attention to these features of disorder would have demanded attention to additional neurotransmitters, particularly noradrenaline.
References
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrien J. Neurobiological bases for the relation between sleep and depression. Sleep Medicine Reviews. 2002;6:341–351. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th, text revision ed. Washington, D.C: APA; 2000. [Google Scholar]
- Anderson JL, Glod CA, Dai J, Cao Y, Lockley SW. Lux vs. wavelength in light treatment of Seasonal Affective Disorder. Acta Psychiatrica Scandinavica. 2009;120:203–212. doi: 10.1111/j.1600-0447.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285:1066–1068. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- Andretic R, Franken P, Tafti M. Genetics of sleep. Annual Review of Genetics. 2008;42:361–388. doi: 10.1146/annurev.genet.42.110807.091541. [DOI] [PubMed] [Google Scholar]
- Armitage R. Microarchitectural findings in sleep EEG in depression: diagnostic implications. Biol Psychiatry. 1995;37(2):72–84. doi: 10.1016/0006-3223(94)00082-E. [DOI] [PubMed] [Google Scholar]
- Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand Suppl. 2007;(433):104–115. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- Arnedt JT, Conroy D, Rutt J, Aloia MS, Brower KJ, Armitage R. An open trial of cognitive-behavioral treatment for insomnia comorbid with alcohol dependence. Sleep Medicine. 2007;8:176–180. doi: 10.1016/j.sleep.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Isen AM, Turken AU. A neuropsychological theory of positive affect and its influence on cognition. Psychological Review. 1999;106:529–550. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Bamne MN, Mansour H, Monk TH, Buysee DJ, Nimgaonkar VL. Approaches to unravel the genetics of sleep. doi: 10.1016/j.smrv.2010.01.001. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbini B, Benedetti F, Colombo C, Dotoli D, Bernasconi A, Cigala-Fulgosi M, et al. Dark therapy for mania: a pilot study. Bipolar Disorders. 2005;7:98–101. doi: 10.1111/j.1399-5618.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Archives of General Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. discussion 669–670. [DOI] [PubMed] [Google Scholar]
- Bergen AW, Yeager M, Welch RA, Haque K, Ganjei JK, van den Bree MB, et al. Association of multiple DRD2 polymorphisms with anorexia nervosa. Neuropsychopharmacology. 2005;30:1703–1710. doi: 10.1038/sj.npp.1300719. [DOI] [PubMed] [Google Scholar]
- Berk M, Berk L, Castle D. A collaborative approach to the treatment alliance in bipolar disorder. Bipolar Disorders. 2004;6:504–518. doi: 10.1111/j.1399-5618.2004.00154.x. [DOI] [PubMed] [Google Scholar]
- Berk M, Dodd S, Kauer-Sant'anna M, Malhi GS, Bourin M, Kapczinski F, et al. Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatrica Scandinavica. 2007;116 Suppl. 434:41–49. doi: 10.1111/j.1600-0447.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- Bootzin RR. Stimulus control treatment for insomnia. Proceedings of the American Psychological Association. 1972;7:395–396. [Google Scholar]
- Bootzin RR, Epstein DR. Stimulus control. Lichstein, Kenneth L. 2000 [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Human Neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP. Photons, clocks, and consciousness. Journal of Biological Rhythms. 2005;20:314–325. doi: 10.1177/0748730405278951. [DOI] [PubMed] [Google Scholar]
- Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behaviour--review of data from preclinical research. Acta psychiatrica Scandinavica. 2005;111 Suppl. 427:14–21. doi: 10.1111/j.1600-0447.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- Cagampang FR, Yamazaki S, Otori Y, Inouye SI. Serotonin in the raphe nuclei: regulation by light and an endogenous pacemaker. Neuroreport. 1993;5:49–52. doi: 10.1097/00001756-199310000-00012. [DOI] [PubMed] [Google Scholar]
- Cain SW, Ko CH, Chalmers JA, Ralph MR. Time of day modulation of conditioned place preference in rats depends on the strain of rat used. Neurobiology of Learning and Memory. 2004;81:217–220. doi: 10.1016/j.nlm.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. The Journal of Neuroscience. 2008;28:10167–10184. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56 Suppl. 1:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright R, Young MA, Mercer P, Bears M. Role of REM sleep and dream variables in the prediction of remission from depression. Psychiatry Research. 1998;80:249–255. doi: 10.1016/s0165-1781(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychological Bulletin. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemerinski E, Ho BC, Flaum M, Arndt S, Fleming F, Andreasen NC. Insomnia as a predictor for symptom worsening following antipsychotic withdrawal in schizophrenia. Comprehensive Psychiatry. 2002;43:393–396. doi: 10.1053/comp.2002.34627. [DOI] [PubMed] [Google Scholar]
- Corsini RJ, Wedding D. Current psychotherapies. 7th ed. Itasca, Ill: F.E. Peacock; 2005. [Google Scholar]
- Craske MG, Rowe MK. Nocturnal panic. Clinical Psychology: Science and Practice. 1997;4:153–174. [Google Scholar]
- Craske MG, Tsao JC. Assessment and treatment of nocturnal panic attacks. Sleep Medicine Reviews. 2005;9:173–184. doi: 10.1016/j.smrv.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Currie SR, Clark S, Hodgins DC, El-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction. 2004;99:1121–1132. doi: 10.1111/j.1360-0443.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- Currie SR, Wilson KG, Pontefract AJ, deLaplante L. Cognitive-behavioral treatment of insomnia secondary to chronic pain. Journal of Consulting and Clinical Psychology. 2000;68:407–416. doi: 10.1037//0022-006x.68.3.407. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. Journal of Adolescent Health. 2002;31 Suppl. 6:175–184. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- Dijk D, Franken P. Interaction of sleep homeostasis and circadian rhythmicity: dependent or independent systems? In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: Saunders; 2005. pp. 418–434. [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–267. [PubMed] [Google Scholar]
- Drake CL, Roehrs TA, Burduvali E, Bonahoom A, Rosekind M, Roth T. Effects of rapid versus slow accumulation of eight hours of sleep loss. Psychophysiology. 2001;38:979–987. doi: 10.1111/1469-8986.3860979. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, et al. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Archives of General Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Archives of Internal Medicine. 2005;165:2527–2535. doi: 10.1001/archinte.165.21.2527. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms. A unified approach to understanding the etiology of depression. Archives of General Psychiatry. 1988;45:948–952. doi: 10.1001/archpsyc.1988.01800340076012. [DOI] [PubMed] [Google Scholar]
- Eidelman P, Talbot LS, Gruber J, Hairston I, Harvey AG. Sleep architecture as a correlate and predictor of symptoms and impairment in inter-episode bipolar disorder: Taking on the challenge of medications. Journal of Sleep Research. doi: 10.1111/j.1365-2869.2010.00826.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Cummings EM, Keller P. Sleep disruptions and emotional insecurity are pathways of risk for children. The Journal of Child Psychology and Psychiatry. 2007;48:88–96. doi: 10.1111/j.1469-7610.2006.01604.x. [DOI] [PubMed] [Google Scholar]
- Esposito E. Serotonin-dopamine interaction as a focus of novel antidepressant drugs. Current Drug Targets. 2006;7:177–185. doi: 10.2174/138945006775515455. [DOI] [PubMed] [Google Scholar]
- Esposito E, Di Matteo V, Di Giovanni G. Serotonin-dopamine interaction: an overview. Progress in Brain Research. 2008;172:3–6. doi: 10.1016/S0079-6123(08)00901-1. [DOI] [PubMed] [Google Scholar]
- Even C, Schröder CM, Friedman S, Rouillon F. Efficacy of light therapy in nonseasonal depression: A systematic review. Journal of Affective Disorders. 2008;108:11–23. doi: 10.1016/j.jad.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Frank E. Treating bipolar disorder: a clinician's guide to interpersonal and social rhythm therapy. NY: Guilford Press; 2005. [Google Scholar]
- Frank E. Interpersonal and social rhythm therapy: a means of improving depression and preventing relapse in bipolar disorder. Journal of Clinical Psychology: In Session. 2007;63:463–473. doi: 10.1002/jclp.20371. [DOI] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Ehlers CL, Monk TH, Cornes C, Carter S, et al. Interpersonal and social rhythm therapy for bipolar disorder: integrating interpersonal and behavioral approaches. Behavior Therapist. 1994;17:143–149. [Google Scholar]
- Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Eagiolini AM, et al. Two-Year Outcomes for Interpersonal and Social Rhythm Therapy in Individuals With Bipolar I Disorder. Archives of General Psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. Journal of Sleep Research. 2008;17:34–41. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. Journal of Biological Rhythms. 2006;21:482–493. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- Garcia JA, Zhang D, Estill SJ, Michnoff C, Rutter J, Reick M, et al. Impaired cued and contextual memory in NPAS2-deficient mice. Science. 2000;288:2226–2230. doi: 10.1126/science.288.5474.2226. [DOI] [PubMed] [Google Scholar]
- Glass JD, Grossman GH, Farnbauch L, DiNardo L. Midbrain raphe modulation of nonphotic circadian clock resetting and 5-HT release in the mammalian suprachiasmatic nucleus. The Journal of Neuroscience. 2003;23:7451–7460. doi: 10.1523/JNEUROSCI.23-20-07451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. The American Journal of Psychiatry. 2005;162:656–662. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- Goodman A. Neurobiology of addiction. An integrative review. Biochemical Pharmacology. 2008;75:266–322. doi: 10.1016/j.bcp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Grandin LD, Alloy LB, Abramson LY. The social zeitgeber theory, circadian rhythms, and mood disorders: Review and evaluation. Clinical Psychology Review. 2006;26:679–694. doi: 10.1016/j.cpr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Guile JM, Huynh C, Desrosiers L, Bouvier H, MacKay J, Chevrier E, et al. Exploring sleep disturbances in adolescent borderline personality disorder using actigraphy: a case report. International Journal of Adolescent Medicine and Health. 2009;21:123–126. doi: 10.1515/ijamh.2009.21.1.123. [DOI] [PubMed] [Google Scholar]
- Hamilton NA, Catley D, Karlson C. Sleep and the affective response to stress and pain. Health Psychology. 2007;26:288–295. doi: 10.1037/0278-6133.26.3.288. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Fahrenkrug J. Neuronal input pathways to the brain's biological clock and their functional significance. Vol. 182. Berlin: Springer; 2006. [PubMed] [Google Scholar]
- Harvey AG. Insomnia, Psychiatric Disorders, and the Transdiagnostic Perspective. Current Directions in Psychological Science. 2008;17:299–303. [Google Scholar]
- Harvey AG. Sleep and circadian rhythms in bipolar disorder: Seeking synchrony, harmony, and regulation. The American Journal of Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- Harvey AG. The Adverse Consequences of Sleep Disturbance in Pediatric Bipolar Disorder: Implications for Intervention. Child and Adolescent Psychiatry Clinics of North America. 2009a;18:321–338. doi: 10.1016/j.chc.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Harvey AG. A Transdiagnostic Approach to Treating Sleep Disturbance in Psychiatric Disorders. Cognitive Behaviour Therapy. 2009b;38 Suppl. 1:35–42. doi: 10.1080/16506070903033825. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Sharpley AL, Ree MJ, Stinson K, Clark DM. An open trial of cognitive therapy for chronic insomnia. Behaviour Research and Therapy. 2007;45:2491–2501. doi: 10.1016/j.brat.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Watkins E, Mansell W, Shafran R. Cognitive behavioural processes across psychological disorders : A transdiagnostic approach to research and treatment. Oxford: Oxford University Press; 2004. [Google Scholar]
- Healy D, Waterhouse JM. The circadian system and the therapeutics of the affective disorders. Pharmacology & Therapeutics. 1995;65:241–263. doi: 10.1016/0163-7258(94)00077-g. [DOI] [PubMed] [Google Scholar]
- Hedlund PB. The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology (Berl) 2009;206:345–354. doi: 10.1007/s00213-009-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich T, Tuin I, Pflug B, Michal M, Michalak J. Mindfulness-based cognitive therapy for persistent insomnia: a pilot study. Psychotherapy and Psychosomatics. 2006;75:188–189. doi: 10.1159/000091778. [DOI] [PubMed] [Google Scholar]
- Hirshkowitz M, Moore CA, O'Connor S, Bellamy M, Cunningham GR. Androgen and sleep-related erections. Journal of Psychosomatic Research. 1997;42:541–546. doi: 10.1016/s0022-3999(97)00006-8. [DOI] [PubMed] [Google Scholar]
- Hornung OP, Regen F, Warnstedt C, Anghelescu I, Danker-Hopfe H, Heuser I, et al. Declarative and procedural memory consolidation during sleep in patients with borderline personality disorder. Journal of Psychiatric Research. 2008;42:653–658. doi: 10.1016/j.jpsychires.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Teshima K, Yang Y, Yoshioka T, Allen CN. Pre- and postsynaptic actions of serotonin on rat suprachiasmatic nucleus neurons. Brain Research. 2000;866:247–256. doi: 10.1016/s0006-8993(00)02294-0. [DOI] [PubMed] [Google Scholar]
- Johnson SL. Mania and dysregulation in goal pursuit: a review. Clinical Psychology Review. 2005;25:241–262. doi: 10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz J, Citrome L, Javitt D. GABA(B) receptors, schizophrenia and sleep dysfunction: a review of the relationship and its potential clinical and therapeutic implications. CNS Drugs. 2009;23:681–691. doi: 10.2165/00023210-200923080-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiology and Behavior. 2008;94:121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Jacobson KC, Gardner CO, Prescott CA, Kendler KS. Personality and comorbidity of common psychiatric disorders. The British Journal of Psychiatry. 2005;186:190–196. doi: 10.1192/bjp.186.3.190. [DOI] [PubMed] [Google Scholar]
- Krakow B, Zadra A. Clinical management of chronic nightmares: Imagery rehearsal therapy. Behavioral Sleep Medicine. 2006;4:45–70. doi: 10.1207/s15402010bsm0401_4. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Reinterpreting comorbidity: a model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert GW, Reid C, Kaye DM, Jennings GL, Esler MD. Effect of sunlight and season on serotonin turnover in the brain. The Lancet. 2002;360:1840–1842. doi: 10.1016/s0140-6736(02)11737-5. [DOI] [PubMed] [Google Scholar]
- Lamont EW, Legault-Coutu D, Cermakian N, Boivin DB. The role of circadian clock genes in mental disorders. Dialogues in Clinical Neuroscience. 2007;9:333–342. doi: 10.31887/DCNS.2007.9.3/elamont. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky-Su J, Neale BM, Franke B, Anney RJL, Zhou K, Maller JB, et al. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B:1345–1354. doi: 10.1002/ajmg.b.30867. [DOI] [PubMed] [Google Scholar]
- Lepola U, Koponen H, Leinonen E. Sleep in panic disorders. Journal of Psychosomatic Research. 1994;38 Suppl. 1:105–111. doi: 10.1016/0022-3999(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Leproult R, Colecchia EF, L'Hermite-Baleriaux M, Van Cauter E. Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. Journal of Clinical Endocrinology and Metabolism. 2001;86:151–157. doi: 10.1210/jcem.86.1.7102. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Wilson NM, Johnson CT. Psychological treatment of secondary insomnia. Psychology and Aging. 2000;15:232–240. doi: 10.1037//0882-7974.15.2.232. [DOI] [PubMed] [Google Scholar]
- Lima MMS, Andersen ML, Reksidler AB, Silva A, Zager A, Zanata SM, et al. Blockage of dopaminergic D2 receptors produces decrease of REM but not of slow wave sleep in rats after REM sleep deprivation. Behavioural Brain Research. 2008;188:406–411. doi: 10.1016/j.bbr.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. The Journal of Clinical Endocrinology and Metabolism. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Pollock BG. Meta-analysis of serotonin transporter polymorphisms and affective disorders. Psychiatric Genetics. 2004;14:121–129. doi: 10.1097/00041444-200409000-00001. [DOI] [PubMed] [Google Scholar]
- Luddington NS, Mandadapu A, Husk M, El-Mallakh RS. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Primary Care Companion to The Journal of Clinical Psychiatry. 2009;11:93–102. doi: 10.4088/pcc.08r00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon GJ, Abi-Dargham A, Moore H, Lieberman JA, Javitch JA, Sulzer D. Presynaptic Regulation of Dopamine Transmission in Schizophrenia. Schizophrenia Bulletin. 2009 doi: 10.1093/schbul/sbp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi GS, Berk M. Does dopamine dysfunction drive depression? Acta Psychiatrica Scandinavica. 2007;115 Suppl. 433:116–124. doi: 10.1111/j.1600-0447.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- Malkoff-Schwartz S, Frank E, Anderson B, Sherrill JT, Siegel L, Patterson D, et al. Stressful life events and social rhythm disruption in the onset of manic and depressive bipolar episodes. Archives of General Psychiatry. 1998;55:702–707. doi: 10.1001/archpsyc.55.8.702. [DOI] [PubMed] [Google Scholar]
- Malkoff-Schwartz S, Frank E, Anderson BP, Hlastala SA, Luther JF, Sherrill JT. Social rhythm disruption and stressful life events in the onset of bipolar and unipolar episodes. Psychological Medicine. 2000;30:1005–1016. doi: 10.1017/s0033291799002706. [DOI] [PubMed] [Google Scholar]
- Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour HA, Monk TH, Nimgaonkar VL. Circadian genes and bipolar disorder. Annals of Medicine. 2005;37:196–205. doi: 10.1080/07853890510007377. [DOI] [PubMed] [Google Scholar]
- Mansour HA, Wood J, Logue T, Chowdari KV, Dayal M, Kupfer DJ, et al. Association study of eight circadian genes with bipolar I disorder, schizoaffective disorder and schizophrenia. Genes, Brain and Behavior. 2006;5:150–157. doi: 10.1111/j.1601-183X.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C. Is depression associated with dysfunction of the central reward system? Biochemical Society Transactions. 2009;37:313–317. doi: 10.1042/BST0370313. [DOI] [PubMed] [Google Scholar]
- Matsuura H, Tateno K, Aou S. Dynamical properties of the two-process model for sleep-wake cycles in infantile autism. Cognitive Neurodynamics. 2008;2:221–228. doi: 10.1007/s11571-008-9051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Murray HW, Barlow DH. Balancing fidelity and adaptation in the dissemination of empirically-supported treatments: The promise of transdiagnostic interventions. Behaviour Research and Therapy. 2009 doi: 10.1016/j.brat.2009.07.005. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak EE, Murray G, Wilkinson C, Dowrick C, Lam RW. A pilot study of adherence with light treatment for seasonal affective disorder. Psychiatry Research. 2007;149:315–320. doi: 10.1016/j.psychres.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ, Goodwin GM, Bauer MS, Geddes JR. Common and specific elements of psychosocial treatments for bipolar disorder: a survey of clinicians participating in randomized trials. Journal of Psychiatric Practice. 2008;14:77–85. doi: 10.1097/01.pra.0000314314.94791.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Wisniewski SR, Kogan JN, et al. Psychosocial treatments for bipolar depression: a 1- year randomized trial from the systematic treatment enhancement program. Archives of General Psychiatry. 2007;64:419–426. doi: 10.1001/archpsyc.64.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Morin LP, Schwartz WJ, Moore RY. New insights into the mammalian circadian clock. Sleep. 1996;19:641–667. doi: 10.1093/sleep/19.8.641. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Antle MC, Glass JD, Miller JD. Behavioral and Serotonergic Regulation of Circadian Rhythms. Biological Rhythm Research. 2000;31:240–283. [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biological Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Monk TH, Petrie SR, Hayes AJ, Kupfer DJ. Regularity of daily life in relation to personality, age, gender, sleep quality and circadian rhythms. Journal of Sleep Research. 1994;3:196–205. doi: 10.1111/j.1365-2869.1994.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Tortorella A, Docimo L, Maldonato MN, Canestrelli B, De Luca L, et al. Investigation of 3111T/C polymorphism of the CLOCK gene in obese individuals with or without binge eating disorder: Association with higher body mass index. Neuroscience Letters. 2008;435:30–33. doi: 10.1016/j.neulet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Medicine Reviews. 2007;11:113–133. doi: 10.1016/j.smrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Moore RY. Entrainment pathways and the functional organization of the circadian system. Progress in Brain Research. 1996;111:103–119. doi: 10.1016/s0079-6123(08)60403-3. [DOI] [PubMed] [Google Scholar]
- Moore RY. Suprachiasmatic nucleus in sleep-wake regulation. Sleep Medicine. 2007;8 Suppl. 3:27–33. doi: 10.1016/j.sleep.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: An update of recent evidence (1998–2004) Sleep. 2006;29:1396–1406. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- Morin LP. Serotonin and the regulation of mammalian circadian rhythmicity. Annals of Medicine. 1999;31:12–33. doi: 10.3109/07853899909019259. [DOI] [PubMed] [Google Scholar]
- Moyer RW, Kennaway DJ. Serotonin depletion decreases light induced c-fos in the rat suprachiasmatic nucleus. Neuroreport. 2000;11:1021–1024. doi: 10.1097/00001756-200004070-00025. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Critical Assessment of Methods and Concepts in Nonphotic Phase Shifting. Biological Rhythm Research. 1999;30:135–148. [Google Scholar]
- Murray G. Circadian and sleep/wake considerations in the practical management of bipolar disorder. In: Young A, Ferrier N, Michalak EE, editors. Practical management of bipolar disorder. Cambridge: Cambridge University Press; (in press) [Google Scholar]
- Murray G, Harvey A. Circadian rhythms and sleep in bipolar disorder. In: Yatham LN, Maj M, editors. Bipolar Disorder: Clinical and Neurobiological Foundations. NY: John Wiley & Sons; (in press) [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, et al. Nature's clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009;9:705–716. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- Nagayama H. Influences of biological rhythms on the effects of psychotropic drugs. Psychosomatic Medicine. 1999;61:618–629. doi: 10.1097/00006842-199909000-00006. [DOI] [PubMed] [Google Scholar]
- Nicholas B, Rudrasingham V, Nash S, Kirov G, Owen MJ, Wimpory DC. Association of Per1 and Npas2 with autistic disorder: support for the clock genes/social timing hypothesis. Molecular Psychiatry. 2007;12:581–592. doi: 10.1038/sj.mp.4001953. [DOI] [PubMed] [Google Scholar]
- NIH. National Institutes of Health State of the Science Conference Statement: Manifestations and management of chronic insomnia in adults. [June 13–15, 2005];Sleep. 2005 28:1049–1057. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- Novati A, Roman V, Cetin T, Hagewoud R, den Boer JA, Luiten PG, et al. Chronically restricted sleep leads to depression-like changes in neurotransmitter receptor sensitivity and neuroendocrine stress reactivity in rats. Sleep. 2008;31:1579–1585. doi: 10.1093/sleep/31.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olijslagers J, Werkman T, McCreary A, Kruse C, Wadman W. Modulation of midbrain dopamine neurotransmission by serotonin, a versatile interaction between neurotransmitters and significance for antipsychotic drug action. Current Neuropharmacology. 2006;4:59–68. doi: 10.2174/157015906775203020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong JC, Shapiro SL, Manber R. Combining mindfulness meditation with cognitive-behavior therapy for insomnia: a treatment-development study. Behavior Therapy. 2008;39:171–182. doi: 10.1016/j.beth.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongini E, Bonizzoni E, Ferri N, Milani S, Trampus M. Differential effects of dopamine D-1 and D-2 receptor antagonist antipsychotics on sleepwake patterns in the rat. Journal of Pharmacology and Experimental Therapeutics. 1993;266:726–731. [PubMed] [Google Scholar]
- Oren DA, Cubells JF, Litsch S. Bright light therapy for schizoaffective disorder. The American Journal of Psychiatry. 2001;158:2086–2087. doi: 10.1176/appi.ajp.158.12.2086-a. [DOI] [PubMed] [Google Scholar]
- Oren DA, Wisner KL, Spinelli M, Epperson CN, Peindl KS, Terman JS, et al. An open trial of morning light therapy for treatment of antepartum depression. The American Journal of Psychiatry. 2002;159:666–669. doi: 10.1176/appi.ajp.159.4.666. [DOI] [PubMed] [Google Scholar]
- Partonen T, Treutlein J, Alpman A, Frank J, Johansson C, Depner M, et al. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Annals of Medicine. 2007;39:229–238. doi: 10.1080/07853890701278795. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Sharpe M, Smith MT, Greenblatt D, Giles D. Behavioral treatment of insomnia: treatment outcome and the relevance of medical and psychiatric morbidity. Journal of Behavioral Medicine. 2001;24:281–296. doi: 10.1023/a:1010770807823. [DOI] [PubMed] [Google Scholar]
- Phelps J. Dark therapy for bipolar disorder using amber lenses for blue light blockade. Medical Hypotheses. 2008;70:224–229. doi: 10.1016/j.mehy.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Prosser RA. Serotonergic Actions and Interactions on the SCN Circadian Pacemaker: In Vitro Investigations. Biological Rhythm Research. 2000;31:315–339. [Google Scholar]
- Ralph MR, Ko CH, Antoniadis EA, Seco P, Irani F, Presta C, et al. The significance of circadian phase for performance on a reward-based learning task in hamsters. Behavioural Brain Research. 2002;136:179–184. doi: 10.1016/s0166-4328(02)00131-6. [DOI] [PubMed] [Google Scholar]
- Redfern PH, Waterhouse JM, Minors DS. Circadian rhythms: principles and measurement. Pharmacology & Therapeutics. 1991;49:311–327. doi: 10.1016/0163-7258(91)90061-p. [DOI] [PubMed] [Google Scholar]
- Ree M, Harvey AG. Investigating Safety Behaviours in Insomnia: The Development of the Sleep-related Behaviours Questionnaire (SRBQ) Behaviour Change. 2004;21:26–36. [Google Scholar]
- Reghunandanan V, Reghunandanan R. Neurotransmitters of the suprachiasmatic nuclei. Journal of Circadian Rhythms. 2006;4:2. doi: 10.1186/1740-3391-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reick M, Garcia JA, Dudley C, McKnight SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science. 2001;293:506–509. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- Reme CE, Wirz-Justice A, Terman M. The visual input stage of the mammalian circadian pacemaking system: I. Is there a clock in the mammalian eye? Journal of Biological Rhythms. 1991;6:5–29. doi: 10.1177/074873049100600104. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Richtand NM, McNamara RK. Serotonin and dopamine interactions in psychosis prevention. Progress in Brain Research. 2008;172:141–153. doi: 10.1016/S0079-6123(08)00907-2. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Welge JA, Logue AD, Keck PE, Jr, Strakowski SM, McNamara RK. Role of serotonin and dopamine receptor binding in antipsychotic efficacy. Progress in Brain Research. 2008;172:155–175. doi: 10.1016/S0079-6123(08)00908-4. [DOI] [PubMed] [Google Scholar]
- Roennebert T, Foster RG. Twilight times: light and the circadian system. Photochemistry and Photobiology. 1997;66:549–561. doi: 10.1111/j.1751-1097.1997.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Roman V, Walstra I, Luiten PG, Meerlo P. Too little sleep gradually desensitizes the serotonin 1A receptor system. Sleep. 2005;28:1505–1510. [PubMed] [Google Scholar]
- Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: Results of the 1991 National Sleep Foundation Survey. II. Sleep. 1999;22 Suppl. 2:S354–S358. [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Cano G, Scammell TE. Homeostatic, circadian, and emotional regulation of sleep. Journal of Comprehensive Neurology. 2005;493:92–98. doi: 10.1002/cne.20770. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends in Neurosciences. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Schumann G. Okey Lecture 2006: identifying the neurobiological mechanisms of addictive behaviour. Addiction. 2007;102:1689–1695. doi: 10.1111/j.1360-0443.2007.01942.x. [DOI] [PubMed] [Google Scholar]
- Serretti A, Benedetti F, Mandelli L, Lorenzi C, Pirovano A, Colombo C, et al. Genetic dissection of psychopathological symptoms: insomnia in mood disorders and CLOCK gene polymorphism. American Journal Medical Genetics. 2003;121B:35–38. doi: 10.1002/ajmg.b.20053. [DOI] [PubMed] [Google Scholar]
- Shneerson JM. Handbook of sleep medicine. Oxford: Blackwell Science; 2000. [Google Scholar]
- Sit D, Wisner KL, Hanusa BH, Stull S, Terman M. Light therapy for bipolar disorder: a case series in women. Bipolar Disorders. 2007;9:918–927. doi: 10.1111/j.1399-5618.2007.00451.x. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Time of day alters long-term sensitization to cocaine in rats. Brain Research. 2005;1065:132–137. doi: 10.1016/j.brainres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Research. 2007;1129:34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Borjigin J, Sassone-Corsi P. Discovering light effects on the brain. The American Journal of Psychiatry. 2006;163:771. doi: 10.1176/ajp.2006.163.5.771. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nature Medicine. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45–56. [PubMed] [Google Scholar]
- Sprouse J. Pharmacological modulation of circadian rhythms: a new drug target in psychotherapeutics. Expert Opinion on Therapeutic Targets. 2004;8:25–38. doi: 10.1517/14728222.8.1.25. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Braselton J, Reynolds L. Fluoxetine modulates the circadian biological clock via phase advances of suprachiasmatic nucleus neuronal firing. Biological Psychiatry. 2006;60:896–899. doi: 10.1016/j.biopsych.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Stein MB, Kroft CD, Walker JR. Sleep impairment in patients with social phobia. Psychiatry Research. 1993;49:251–256. doi: 10.1016/0165-1781(93)90065-o. [DOI] [PubMed] [Google Scholar]
- Stunkard A, Allison K, Lundgren J. Issues for DSM-V: night eating syndrome. The American Journal of Psychiatry. 2008;165:424. doi: 10.1176/appi.ajp.2007.07081351. [DOI] [PubMed] [Google Scholar]
- Suto M, Murray G, Hale S, Amari E, Michalak E. What Works for People with Bipolar Disorder? Tips from the Experts. Journal of Affective Disorders. doi: 10.1016/j.jad.2009.11.004. (in press) [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nature Reviews. Genetics. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao T, Tachikawa H, Kawanishi Y, Mizukami K, Asada T. CLOCK gene T3111C polymorphism is associated with Japanese schizophrenics: a preliminary study. European Neuropsychopharmacology. 2007;17:273–276. doi: 10.1016/j.euroneuro.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Terman JS, Terman M, Schlager D, Rafferty B, Rosofsky M, Link MJ, et al. Efficacy of brief, intense light exposure for treatment of winter depression. Psychopharmacology Bulletin. 1990;26:3–11. [PubMed] [Google Scholar]
- Terman M, Levine SM, Terman JS, Doherty S. Chronic fatigue syndrome and seasonal affective disorder: comorbidity, diagnostic overlap, and implications for treatment. The American Journal of Medicine. 1998;105:115S–124S. doi: 10.1016/s0002-9343(98)00172-7. [DOI] [PubMed] [Google Scholar]
- Tortorella A, Monteleone P, Martiadis V, Perris F, Maj M. The 3111T/C polymorphism of the CLOCK gene confers a predisposition to a lifetime lower body weight in patients with anorexia nervosa and bulimia nervosa: a preliminary study. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144B:992–995. doi: 10.1002/ajmg.b.30508. [DOI] [PubMed] [Google Scholar]
- Tuunainen A, Kripke DF, Endo T. Light therapy for non-seasonal depression (Cochrane Review) The Cochrane Library. 2006;2 doi: 10.1002/14651858.CD004050.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin R. Serotonin and sleep. Sleep Medicine Reviews. 2002;6:55–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- Van Sweden B. Disturbed vigilance in mania. Biological Psychiatry. 1986;21:311–313. doi: 10.1016/0006-3223(86)90052-1. [DOI] [PubMed] [Google Scholar]
- Vandewalle G, Gais S, Schabus M, Balteau E, Carrier J, Darsaud A, et al. Wavelength-Dependent Modulation of Brain Responses to a Working Memory Task by Daytime Light Exposure. Cerebral Cortex. 2007;17:2788–2795. doi: 10.1093/cercor/bhm007. [DOI] [PubMed] [Google Scholar]
- Vandewalle G, Schmidt C, Albouy Gv, Sterpenich V, Darsaud A, Rauchs Gr, et al. Brain Responses to Violet, Blue, and Green Monochromatic Light Exposures in Humans: Prominent Role of Blue Light and the Brainstem. PLoS ONE. 2007;2:e1247. doi: 10.1371/journal.pone.0001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annual Review of Clinical Psychology. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep, Memory, And Plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Benedetti F, Berger M, Lam RW, Martiny K, Terman M, et al. Chronotherapeutics (light and wake therapy) in affective disorders. Psychological Medicine. 2005;35:939–944. doi: 10.1017/s003329170500437x. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Benedetti F, Terman M. Chronotherapeutics for Affective Disorders: A Clinician's Manual for Light & Wake Therapy. 1 ed. Basel: Karger; 2009. [Google Scholar]
- Wirz-Justice A, Terman M, Oren DA, Goodwin FK, Kripke DF, Whybrow PC, et al. Brightening depression. Science. 2004;303:467–469. doi: 10.1126/science.303.5657.467c. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. The Journal of Neuroscience. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Bobula JM, Svenningsson P, Greengard P, Silver R. DARPP-32 involvement in the photic pathway of the circadian system. The Journal of Neuroscience. 2006;26:9434–9438. doi: 10.1523/JNEUROSCI.2538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Current Biology. 2007;17:R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. The Journal of Comparative Neurology. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;47:115–127. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Zohar D, Tzischinsky O, Epsten R, Lavie P. The effects of sleep loss on medical residents' emotional reactions to work events: A cognitive-energy model. Sleep. 2005;28:47–54. doi: 10.1093/sleep/28.1.47. [DOI] [PubMed] [Google Scholar]