Abstract
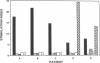
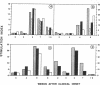
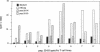
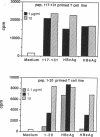
Several lines of experimental evidence suggest that inclusion of core sequences in the hepatitis B vaccine may represent a feasible strategy to increase the efficacy of the vaccination. In order to identify immunodominant core epitopes, peripheral blood T cells purified from 23 patients with acute hepatitis B and different HLA haplotypes were tested with a panel of 18 short synthetic peptides (15 to 20 amino acids [AA]) covering the entire core region. All patients except one showed a strong T cell proliferative response to a single immunodominant 20 amino acid sequence located within the aminoterminal half of the core molecule. Two additional important sequences were also identified at the aminoterminal end and within the carboxyterminal half of the core molecule. These sequences were able to induce significant levels of T cell proliferation in 69 and 73% of the patients studied, respectively. T cell response to these epitopes was HLA class II restricted. The observations that (a) polyclonal T cell lines produced by PBMC stimulation with native HBcAg were specifically reactive with the relevant peptides and that (b) polyclonal T cell lines produced with synthetic peptides could be restimulated with native HBcAg, provide evidence that AA sequences contained within the synthetic peptides represent real products of the intracellular processing of the native core molecule. In conclusion, the identification of immunodominant T cell epitopes within the core molecule provides the molecular basis for the design of alternative and hopefully more immunogenic vaccines.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accolla R. S. Analysis of the structural heterogeneity and polymorphism of human Ia antigens. Four distinct subsets of molecules are coexpressed in the Ia pool of both DR1,1 homozygous and DR3,W6 heterozygous B cell lines. J Exp Med. 1984 Feb 1;159(2):378–393. doi: 10.1084/jem.159.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accolla R. S., Gross N., Carrel S., Corte G. Distinct forms of both alpha and beta subunits are present in the human Ia molecular pool. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4549–4551. doi: 10.1073/pnas.78.7.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y., Stevens C. E., Lin C. C., Hsieh F. J., Wang K. Y., Sun T. S., Szmuness W. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double-blind, placebo-controlled trial. Hepatology. 1983 Mar-Apr;3(2):135–141. doi: 10.1002/hep.1840030201. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P. Monomorphic anti-HLA-A,B,C monoclonal antibodies detecting molecular subunits and combinatorial determinants. J Immunol. 1982 Jan;128(1):129–135. [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Clarke B. E., Newton S. E., Carroll A. R., Francis M. J., Appleyard G., Syred A. D., Highfield P. E., Rowlands D. J., Brown F. Improved immunogenicity of a peptide epitope after fusion to hepatitis B core protein. 1987 Nov 26-Dec 2Nature. 330(6146):381–384. doi: 10.1038/330381a0. [DOI] [PubMed] [Google Scholar]
- Collier A. C., Corey L., Murphy V. L., Handsfield H. H. Antibody to human immunodeficiency virus (HIV) and suboptimal response to hepatitis B vaccination. Ann Intern Med. 1988 Jul 15;109(2):101–105. doi: 10.7326/0003-4819-109-2-101. [DOI] [PubMed] [Google Scholar]
- Corte G., Calabi F., Damiani G., Bargellesi A., Tosi R., Sorrentino R. Human Ia molecules carrying DC1 determinants differ in both alpha- and beta-subunits from Ia molecules carrying DR determinants. Nature. 1981 Jul 23;292(5821):357–360. doi: 10.1038/292357a0. [DOI] [PubMed] [Google Scholar]
- DeLisi C., Berzofsky J. A. T-cell antigenic sites tend to be amphipathic structures. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7048–7052. doi: 10.1073/pnas.82.20.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari C., Mondelli M. U., Penna A., Fiaccadori F., Chisari F. V. Functional characterization of cloned intrahepatic, hepatitis B virus nucleoprotein-specific helper T cell lines. J Immunol. 1987 Jul 15;139(2):539–544. [PubMed] [Google Scholar]
- Ferrari C., Penna A., Bertoletti A., Valli A., Antoni A. D., Giuberti T., Cavalli A., Petit M. A., Fiaccadori F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990 Nov 15;145(10):3442–3449. [PubMed] [Google Scholar]
- Ferrari C., Penna A., Giuberti T., Tong M. J., Ribera E., Fiaccadori F., Chisari F. V. Intrahepatic, nucleocapsid antigen-specific T cells in chronic active hepatitis B. J Immunol. 1987 Sep 15;139(6):2050–2058. [PubMed] [Google Scholar]
- Itoh Y., Takai E., Ohnuma H., Kitajima K., Tsuda F., Machida A., Mishiro S., Nakamura T., Miyakawa Y., Mayumi M. A synthetic peptide vaccine involving the product of the pre-S(2) region of hepatitis B virus DNA: protective efficacy in chimpanzees. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9174–9178. doi: 10.1073/pnas.83.23.9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwarson S., Tabor E., Thomas H. C., Snoy P., Gerety R. J. Protection against hepatitis B virus infection by immunization with hepatitis B core antigen. Gastroenterology. 1985 Mar;88(3):763–767. doi: 10.1016/0016-5085(85)90148-9. [DOI] [PubMed] [Google Scholar]
- McLachlan A., Milich D. R., Raney A. K., Riggs M. G., Hughes J. L., Sorge J., Chisari F. V. Expression of hepatitis B virus surface and core antigens: influences of pre-S and precore sequences. J Virol. 1987 Mar;61(3):683–692. doi: 10.1128/jvi.61.3.683-692.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Chisari F. V., Kent S. B., Thorton G. B. Immune response to the pre-S(1) region of the hepatitis B surface antigen (HBsAg): a pre-S(1)-specific T cell response can bypass nonresponsiveness to the pre-S(2) and S regions of HBsAg. J Immunol. 1986 Jul 1;137(1):315–322. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Moriarty A., Thornton G. B. Immune response to hepatitis B virus core antigen (HBcAg): localization of T cell recognition sites within HBcAg/HBeAg. J Immunol. 1987 Aug 15;139(4):1223–1231. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Stahl S., Wingfield P., Thornton G. B., Hughes J. L., Jones J. E. Comparative immunogenicity of hepatitis B virus core and E antigens. J Immunol. 1988 Nov 15;141(10):3617–3624. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science. 1986 Dec 12;234(4782):1398–1401. doi: 10.1126/science.3491425. [DOI] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Thornton G. B., Hughes J. L. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature. 1987 Oct 8;329(6139):547–549. doi: 10.1038/329547a0. [DOI] [PubMed] [Google Scholar]
- Milich D. R., Thornton G. B., Neurath A. R., Kent S. B., Michel M. L., Tiollais P., Chisari F. V. Enhanced immunogenicity of the pre-S region of hepatitis B surface antigen. Science. 1985 Jun 7;228(4704):1195–1199. doi: 10.1126/science.2408336. [DOI] [PubMed] [Google Scholar]
- Mondelli M., Vergani G. M., Alberti A., Vergani D., Portmann B., Eddleston A. L., Williams R. Specificity of T lymphocyte cytotoxicity to autologous hepatocytes in chronic hepatitis B virus infection: evidence that T cells are directed against HBV core antigen expressed on hepatocytes. J Immunol. 1982 Dec;129(6):2773–2778. [PubMed] [Google Scholar]
- Murray K., Bruce S. A., Hinnen A., Wingfield P., van Erd P. M., de Reus A., Schellekens H. Hepatitis B virus antigens made in microbial cells immunise against viral infection. EMBO J. 1984 Mar;3(3):645–650. doi: 10.1002/j.1460-2075.1984.tb01861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Bruce S. A., Wingfield P., van Eerd P., de Reus A., Schellekens H. Protective immunisation against hepatitis B with an internal antigen of the virus. J Med Virol. 1987 Oct;23(2):101–107. doi: 10.1002/jmv.1890230202. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Strick N., Stark D., Sproul P. Genetic restriction of immune responsiveness to synthetic peptides corresponding to sequences in the pre-S region of the hepatitis B virus (HBV) envelope gene. J Med Virol. 1985 Oct;17(2):119–125. doi: 10.1002/jmv.1890170204. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P., Tan A., Termijtelen A., Demotz S., Corradin G., Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989 Dec;19(12):2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- Pasek M., Goto T., Gilbert W., Zink B., Schaller H., MacKay P., Leadbetter G., Murray K. Hepatitis B virus genes and their expression in E. coli. Nature. 1979 Dec 6;282(5739):575–579. doi: 10.1038/282575a0. [DOI] [PubMed] [Google Scholar]
- Petit M. A., Pillot J. HBc and HBe antigenicity and DNA-binding activity of major core protein P22 in hepatitis B virus core particles isolated from the cytoplasm of human liver cells. J Virol. 1985 Feb;53(2):543–551. doi: 10.1128/jvi.53.2.543-551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos S., Fuchs K., Roggendorf M. Protection of woodchucks from infection with woodchuck hepatitis virus by immunization with recombinant core protein. J Gen Virol. 1989 Aug;70(Pt 8):2087–2095. doi: 10.1099/0022-1317-70-8-2087. [DOI] [PubMed] [Google Scholar]
- Roossinck M. J., Jameel S., Loukin S. H., Siddiqui A. Expression of hepatitis B viral core region in mammalian cells. Mol Cell Biol. 1986 May;6(5):1393–1400. doi: 10.1128/mcb.6.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon A., Feldhaus J., Robins R. A. Single step separation of human T and B cells using AET treated srbc rosettes. J Immunol Methods. 1976;12(3-4):285–288. doi: 10.1016/0022-1759(76)90050-8. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Kilgus J., Doran D. M., Matile H., Etlinger H., Trzeciak A., Gillessen D., Pink J. R. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988 Dec 22;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- Spits H., Borst J., Giphart M., Coligan J., Terhorst C., De Vries J. E. HLA-DC antigens can serve as recognition elements for human cytotoxic T lymphocytes. Eur J Immunol. 1984 Apr;14(4):299–304. doi: 10.1002/eji.1830140404. [DOI] [PubMed] [Google Scholar]
- Stahl S. J., Murray K. Immunogenicity of peptide fusions to hepatitis B virus core antigen. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6283–6287. doi: 10.1073/pnas.86.16.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring D. N., Ou J. H., Masiarz F. R., Rutter W. J. A signal peptide encoded within the precore region of hepatitis B virus directs the secretion of a heterogeneous population of e antigens in Xenopus oocytes. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8405–8409. doi: 10.1073/pnas.85.22.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. E., Alter H. J., Taylor P. E., Zang E. A., Harley E. J., Szmuness W. Hepatitis B vaccine in patients receiving hemodialysis. Immunogenicity and efficacy. N Engl J Med. 1984 Aug 23;311(8):496–501. doi: 10.1056/NEJM198408233110803. [DOI] [PubMed] [Google Scholar]
- Szmuness W., Stevens C. E., Harley E. J., Zang E. A., Oleszko W. R., William D. C., Sadovsky R., Morrison J. M., Kellner A. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980 Oct 9;303(15):833–841. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- Szmuness W., Stevens C. E., Zang E. A., Harley E. J., Kellner A. A controlled clinical trial of the efficacy of the hepatitis B vaccine (Heptavax B): a final report. Hepatology. 1981 Sep-Oct;1(5):377–385. doi: 10.1002/hep.1840010502. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Trucco M. M., Garotta G., Stocker J. W., Ceppellini R. Murine monoclonal antibodies against HLA structures. Immunol Rev. 1979;47:219–252. doi: 10.1111/j.1600-065x.1979.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Uy A., Bruss V., Gerlich W. H., Köchel H. G., Thomssen R. Precore sequence of hepatitis B virus inducing e antigen and membrane association of the viral core protein. Virology. 1986 Nov;155(1):89–96. doi: 10.1016/0042-6822(86)90170-4. [DOI] [PubMed] [Google Scholar]
- Watson A. J., DeMars R., Trowbridge I. S., Bach F. H. Detection of a novel human class II HLA antigen. 1983 Jul 28-Aug 3Nature. 304(5924):358–361. doi: 10.1038/304358a0. [DOI] [PubMed] [Google Scholar]