Abstract
Chronic hepatitis C virus (HCV) infection is associated with T-cell exhaustion that is mediated through upregulation of the PD-1 negative regulatory pathway. PD-1 expression is induced by HCV core protein, which also induces upregulation of SOCS-1, a key modulator that controls the Jak/STAT pathway regulating cytokine expression. To determine whether these two negative regulatory pathways are linked during T-cell signaling, SOCS-1 expression was examined by blocking the PD-1 pathway in T cells stimulated with anti-CD3/CD28 in the presence of HCV core protein. T cells isolated from healthy subjects or HCV-infected individuals were treated with anti-PD-1 or anti-PDL-1 antibodies in the presence or absence of HCV core protein, and SOCS-1 gene expression was detected by RT-PCR or immunoblotting, while T-cell functions were assayed by flow cytometric analyses. Both PD-1 and SOCS-1 gene expression were upregulated in healthy T cells exposed to HCV core protein, and blocking the PD-1 pathway downregulated SOCS-1 gene expression in these cells. Additionally, T cells isolated from chronically HCV-infected subjects exhibited increased PD-1 and SOCS-1 expression compared to healthy subjects, and SOCS-1 expression in T cells isolated from HCV-infected subjects was also inhibited by blocking PD-1 signaling; this in turn enhanced the phosphorylation of STAT-1, and improved the impaired T-cell proliferation observed in the setting of HCV infection. These data demonstrate that PD-1 and SOCS-1 are linked in dysregulating T-cell signaling during HCV infection, and their cross-talk may coordinately inhibit T-cell signaling pathways that lead to T-cell exhaustion during chronic viral infection.
Introduction
Hepatitis C virus (HCV) infects over 180 million people worldwide, and it exhibits a remarkable propensity to cause chronic hepatitis that may lead to the development of liver cirrhosis and hepatocellular carcinoma (1). T-cell exhaustion is one remarkable feature of chronic HCV infection, making it an excellent model to study the underlying mechanisms that impair T-cell receptor (TCR) signaling during persistent viral infection. In chronically HCV-infected individuals, the frequencies of cytotoxic T lymphocytes (CTL) are relatively low; similarly, the proliferative capacity as well as effector functions of HCV-specific CD4+/CD8+ T cells are impaired, and the production of Th-1-type cytokines (i.e., IL-2 and IFN-γ) is dramatically suppressed (2–5). While extensive observations describe immune disorders associated with HCV infection, less well understood are the molecular mechanisms underlying the T-cell dysfunction that occurs during HCV infection (6–7).
Our understanding of the mechanisms underlying T-cell dysfunction during persistent viral infection has been significantly advanced since the identification of programmed death-1 (PD-1) and suppressor of cytokine signaling-1 (SOCS-1), two important inhibitory molecules in regulation of TCR signaling (8–10). PD-1 is an immunoinhibitory receptor predominantly expressed on activated T and B lymphocytes. PD-1 ligand (PDL-1) is expressed on both hematopoietic and parenchymal cells, including T cells. PD-1/PDL-1 engagement induces immunoreceptor tyrosine phosphorylation, and delivers a negative signal to TCR pathways. Because rampant T- and B-cell signaling can have disastrous biological consequences, lymphocyte signaling pathways are tightly controlled at multiple levels to maintain an intricate balance between positive and negative intracellular signals in lymphocytes following antigenic encounter. SOCS-1 represents another level of inhibitory mechanism that is induced upon T-cell activation, leading to feedback inhibition of TCR signaling that includes Jak/STAT signaling.
Evidence is emerging of the involvement of PD-1 and SOCS-1 in immune disruption and viral persistence during chronic HCV infection, raising the possibility that therapeutic strategies targeting these inhibitory pathways might be of clinical benefit (11–15). One potential mediator of the effects of PD-1 and SOCS-1 is the nucleocapsid core antigen of HCV, an immunomodulatory protein that has been consistently shown to alter adaptive immune responses. HCV core protein is the first protein synthesized following viral infection, and is well conserved in different HCV genotypes. Notably, HCV core protein can be secreted by infected cells and detected circulating in the bloodstream of infected individuals. We have previously demonstrated that HCV core protein inhibits T-cell proliferation and promotes B-cell activation by interaction with a complement receptor, gC1qR, through differential regulation of PD-1 and SOCS-1 signaling in vitro (16–20). We have also shown that T-cell dysfunction in individuals with HCV infection is associated with increased expression of PD-1 (21), whereas aberrant B-cell activation during chronic HCV infection is associated with decreased expression of SOCS-1 (22). The relationship between PD-1 and SOCS-1 in regulating TCR signaling during viral infection, however, remains unknown. In this article we further demonstrate that PD-1 and SOCS-1, two inhibitory molecules that can be induced by HCV core protein, are actually linked in T cells, such that blocking PD-1 signaling downregulates SOCS-1 expression; as such, their crosstalk may coordinately inhibit the TCR pathway, leading to T-cell exhaustion during chronic viral infection.
Materials and Methods
Cell isolation and culture
An institutional review board (IRB)-approved protocol at James H. Quillen Veterans Administration Medical Center and East Tennessee State University (Johnson City, TN) has contributed to a database for the storage of blood samples from healthy subjects and HCV-infected individuals. Peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood of six HCV-infected patients as well as five healthy subjects by Ficoll density centrifugation with lympholyte-H (Cedarlane Labs, Burlington, Ontario, Canada). All HCV samples were acquired prior to antiviral therapy, and HCV RNA titers ranged from 23,000–50,000,000 copies/mL. CD4+ T cells were further purified from isolated PBMCs by incubation with a magnetic bead conjugated with anti-CD4 antibody, followed by positive selection per the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). The purified cells were washed two times and cultured with RPMI 1640 (Life Technologies, Gaithersburg, MD), containing 10% (vol:vol) fetal bovine serum (FBS, Life Technologies), penicillin-streptomycin (100 μg/mL for each drug; Life Technologies), L-glutamine (2 mM), and 2-mercaptoethanol (5.5 × 10−5 M, Life Technologies) at 37°C with 5% CO2 in a humidified atmosphere. Recombinant HCV core protein, NS3, and control β-galactosidase proteins were obtained from ViroGen (Watertown, MA), and used at 2 μg/mL as previously described (21). These proteins have been used extensively in immunological studies of HCV antigens, and have been documented to be free of LPS or RNA.
RT-PCR
For RT-PCR, 2 × 106 CD4+ T lymphocytes purified by magnetic beads from HCV patients or healthy donors were activated with anti-CD3/CD28 (1 μg/mL; BD Biosciences, San Jose, CA) in the presence or absence of HCV core protein (2 μg/mL; ViroGen) at 37°C in a 5% CO2 atmosphere for various periods (1 d, 3 d, or 5 d, as indicated in the results section). For blocking experiments, the purified T cells were pre-incubated for 6 h with 10 μg/mL of anti-PD-1, anti-PDL-1, or normal IgG control antibody (Santa Cruz Biotechnology, Santa Cruz, CA), followed by stimulation as described above. Anti-PDL-1 antibody was used as an effective blockade of PD-1 signaling, due to the ubiquitous expression of PDL-1 on hematopoietic cells that interferes with cell-cell PD-1/PDL-1 interactions. Total RNA was isolated from these cells with an RNA isolation kit (Qiagen Inc., Valencia, CA). A total of 1 μg of RNA was treated with DNase to digest genomic DNA, and then reverse transcribed using 1 μL oligo dT, 5 μL 5 × RT buffer, 4 μL dNTP mix, 1 μL RNase inhibitor, and 1 μL MuLV reverse transcriptase, in a total volume of 20 μL supplemented with deionized water under conditions of 60 min at 42°C, 5 min at 99°C, and 5 min at 4°C. Then 1 μL of cDNA generated in the RT reaction was added to the PCR master reaction with a final volume of 25 μL (Fisher Scientific, Pittsburgh, PA). The following PCR primer pairs were used for amplification of the genes: PD-1 sense: 5′-GCG GCC AGG ATG GTT CTT A-3’; antisense 5′-TAC TCC GTC TGC TCA GGG A-3′; SOCS-1 sense: 5′-ATG GTA GCA CAC AAC CAG GTG-3′; antisense 5′-TCA AAT CTG GAA GGG GAA GGA-3′; β-actin sense: 5′-GAG GAC TTG CGC TCA GGA GGA GC-3′; antisense 5′-TCA CCC ACA CTG TGC CCA TCT AC-3′; and hβ2M sense: 5′-ATG CCT GCC GTG TGA ACC AT-3′; antisense 5′-CAT CCA ATC CAA ATG CGG CAT CT-3′. PCR was carried out under conditions of 10 min at 95°C, followed by 35 cycles at 95°C for 45 sec, 58°C for 45 sec, and 72°C for 45 sec, followed by a single 10-min extension at 72°C. To control for genomic DNA contamination, equal amounts of cDNA from each sample were PCR amplified without RT. The resulting PCR products were separated on a 2% BioGel (Bio 101, Carlsbad, CA), and viewed by a multi-imager.
Quantitative real-time PCR was performed to confirm results from end-point PCR. The primers used were qSOCS1 F (5′-GAA CTG CTT TTT CGC CCT TA-3′’) and qSOCS1 R (5′-CTC GAA GAG GCA GTC GAA G-3′) or internal controls primers hB2M pair4-upQ (5′-ATG CCT GCC GTG TGA ACC AT-3′), and hβ2M pair4-lowQ (5′-CAT CCA ATC CAA ATG CGG CAT CT-3′). The following conditions were used for SOCS-1: 95°C for 10 min, 45 cycles of 95°C for 1 min, 56°C for 1 min, and 72°C for 1 min; 72°C for 10 min; and hold at 4°C. For hβ2M: 95°C for 2 min, 45 cycles of 95°C for 15 sec, 65°C for 15 sec, and 72°C for 17 sec; and hold at 4°C. The standard curve for Q-PCR was created using a qSOCS1 or hβ2M pair4 amplicon that was generated using end-point PCR (see the above conditions). The amplicon was quantified using a biophotometer and then diluted 10-fold. Data were analyzed from the average cycle threshold of three samples measured for SOCS-1 after normalizing the sample amplifications to the internal housekeeping control β2M gene.
Western blot
T cells were purified from PBMCs from HCV patients or healthy subjects using magnetic beads (Miltenyi Biotec) per the manufacturer's instructions. A total of 2 × 106 purified cells were activated with anti-CD3/CD28 (1 μg/mL; BD Biosciences) in the presence or absence of HCV core protein (2 μg/mL; ViroGen) at 37°C in a 5% CO2 atmosphere for various time points as described in the results section. For blocking experiments, the purified T cells were pre-incubated for 6 h with 10 μg/mL of anti-PD-1, anti-PDL-1, or normal IgG control antibody (Santa Cruz Biotechnology), followed by treatment as described above. Cell lysate was prepared for 30 min at 4°C with a lysis buffer (Fisher Scientific). Cell lysates were sonicated three times for 1 min each. Cellular debris was pelleted by centrifugation at 16,000 g, and supernatants were collected and frozen at −80°C if not loaded on the gel immediately. A total of 80 μg of protein was denatured with sample loading buffer at 100°C for 5 min and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by semi-dry transfer (GE Healthcare Biosciences, Piscataway, NJ) to a Hybond-P membrane (Amersham Biosciences, Arlington Heights, IL). The membrane was washed in 1× PBS Tween-20 for 20 min, and then blocked with 1.75% BSA TBS at room temperature for 2 h. The membranes were incubated with mouse monoclonal SOCS-1 antibody (Millipore, Billerica, MA), PD-1 (eBioscience, San Diego, CA), phospho-STAT-1 (BD Biosciences), or goat polyclonal β-actin antibody (Santa Cruz Biotechnology), mixed with the 1.75% BSA TBS at a dilution of 1:500–1000 each overnight at 4°C on a gentle shaker. After washing with 1× PBS Tween-20 for 20 min, the membranes were incubated with the secondary HRP-conjugated goat anti-mouse IgG (1:5000 dilution; Millipore, Billerica, Massachusetts) or HRP-conjugated rabbit anti-goat IgG antibody (1:20,000 dilution; Santa Cruz Biotechnology) at room temperature for 2 hours. The membrane was rinsed for 20 min in 1× PBS Tween-20 before Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL) was used for protein detection on X-OMAT-LS x-ray film (Eastman Kodak, Rochester, NY).
T-cell proliferation
PBMCs isolated from HCV patients were re-suspended in pre-warmed PBS/0.1% BSA at a final concentration of 1 × 106 cells/mL, incubated at 37°C for 10 min with 2 μL of 5 mM stock CFSE solution (CellTrace CFSE [carboxyfluorescein diacetate succinimidyl ester] Cell Proliferation Kit; Molecular Probes, Eugene, OR) per milliliter of cells for a final concentration of 10 μm. After quenching the staining by addition of five volumes of ice-cold culture media to the cells and standing on ice for 5 min, the cells were washed in fresh media three times by centrifugation. To determine the role of the PD-1 pathway in regulation of T-cell proliferation, the CFSE-labeled cells were incubated with anti-PDL-1 or a control isotype antibody (10 μg/mL; eBioscience) overnight, followed by stimulation of the cells with anti-CD3/CD28 antibodies (1 μg/mL each; BD Biosciences), HCV peptides (10 μg/mL; HLA-A-0201/NS3/1073–1081/CINGVCWTV, HLA-A-0201/NS3/1406–1415/KLVALGINAV, synthesized by GenScript Corp., Piscataway, NJ), or healthy lymphocytes (1:5 ratio by mixed lymphocyte culture), for 5 d. After double staining and gating on T lymphocytes, the cell proliferation, represented as CSFE dilution or left shift, was analyzed by flow cytometry with 488-nm excitation and emission filters appropriate for CFSE fluorescein. The percentage of cells as detected in the M1, M2, M3, and M4 gating was shown on the histogram of the gated T-cell populations.
Results
PD-1-dependent SOCS-1 induction in healthy T cells treated with HCV core protein
We have previously shown that chronic HCV infection as well as HCV core protein treatment inhibit T-cell activation and proliferation through interaction with a complement receptor, gC1qR (16–21). To determine the role of PD-1 and SOCS-1 in HCV core-mediated T-cell inhibition, we isolated CD4+ T cells from healthy subjects and treated them with HCV core protein in vitro, and PD-1 and SOCS-1 gene expression was detected by RT-PCR. As shown in Fig. 1A and 1B, healthy T cells exposed to HCV core protein with or without TCR stimulation exhibit increased gene expression of PD-1 and SOCS-1. As we have previously demonstrated, these T cells treated with HCV core protein exhibit an inhibited phenotype with impaired proliferation ability (16–21). Notably, this upregulation is similar to what we observed in T cells isolated in the setting of chronic viral infection, which is presumably a whole virus setting (21). To delineate the relationship between PD-1 and the SOCS-1 pathway in HCV core-mediated T-cell inhibition, we pre-treated healthy PBMCs or purified T cells with anti-PD-1, anti-PDL-1, or control antibody for 6 h. Then we incubated the cells with HCV core protein in the presence of anti-CD3 and anti-CD28 antibodies for 24 h. The cells were then subjected to SOCS-1 mRNA detection by RT-PCR. β-Actin served as a loading control. As shown in Fig. 1, HCV core-mediated SOCS-1 mRNA expression during PBMC (Fig. 1C) or purified T-cell (Fig. 1D) activation, could be abrogated by blocking the PD-1 pathway using either anti-PD-1 or anti-PDL-1 antibody compared with the control antibody. Notably, SOCS-1 upregulation (Fig. 1D), in response to HCV core or even TCR stimulation alone, was diminished upon PD-1 blockade, suggesting that a baseline relationship between SOCS-1 and PD-1 may exist.
FIG. 1.
Blocking the PD-1 pathway inhibits SOCS-1 mRNA expression in healthy T cells exposed to HCV core protein. (A) PD-1 upregulation in healthy T cells exposed to HCV core protein. CD4+ T lymphocytes isolated from healthy donors using antibody-conjugated magnetic beads, as described in the text, were stimulated with (top) or without (middle) anti-CD3/CD28 in the presence or absence of HCV core protein for 24 h. Total RNA was isolated from the treated cells, and PD-1 gene expression was detected by RT-PCR. β-Actin served as a control. The bar graph at the bottom shows the relative PD-1 expression in untreated and HCV core-treated T cells in the absence of TCR stimulation. (B) SOCS-1 upregulation in healthy T cells exposed to HCV core protein. CD4+ T lymphocytes isolated from healthy donors as above were stimulated with (top) or without (middle) anti-CD3/CD28 in the presence or absence of HCV core protein for 24 h. SOCS-1 gene expression was detected by RT-PCR, and β-actin served as a control. The bar graph at the bottom shows relative SOCS-1 expression in untreated and HCV core-treated T cells in the absence of TCR stimulation. (C) PD-1 blockade inhibits HCV core-induced SOCS-1 expression in PBMCs. PBMCs isolated from healthy donors were treated with anti-PD-1, anti-PDL-1, or control antibody for 6 h, followed by stimulation with HCV core protein and anti-CD3/CD28 for 24 h. SOCS-1 gene expression was detected by RT-PCR, and β-actin served as a control. (D) PD-1 blockade inhibits HCV core-induced SOCS-1 expression in purified lymphocytes. CD4+ T lymphocytes isolated from healthy donors as above were treated with anti-PD-1, anti-PDL-1, or control antibody for 6 h, followed by stimulation with HCV core protein and anti-CD3/CD28 (top) or anti-CD3/CD28 alone (middle) for 24 h. SOCS-1 gene expression was detected by RT-PCR, and β-actin served as a control. A graphic representation of the CD3/CD28-stimulated SOCS-1 expression is shown at bottom. (E) PD-1 blockade regulates SOCS-1 gene expression as detected by real-time RT-PCR. Healthy PBMCs were treated with or without anti-PD-1 or anti-PDL-1 antibody for 6 h, followed by stimulation with anti-CD3/CD28 in the presence or absence of HCV core protein for 24 h. SOCS-1 gene expression was detected by quantitative real-time RT-PCR, and β2M gene served as an internal control. The average cycle threshold of three samples measured for SOCS-1 after normalizing to β2M gene is shown with error bars.
To confirm the results, we subjected PBMCs to TCR and HCV core stimulation as described above, followed by quantitative detection of SOCS-1 gene expression with real-time RT-PCR. Fig. 1E shows the average cycle threshold of three samples measured for SOCS-1 after normalizing the sample amplifications to the internal housekeeping control β2M gene. Similarly to the data demonstrated by end-point RT-PCR, HCV core treatment induced an increased amount of SOCS-1 expression, and therefore the detection of the SOCS-1 amplification cycle occurred at an earlier threshold compared to PBMCs stimulated with anti-CD3/CD28 alone. As we have shown by end-point RT-PCR, blocking the PD-1 pathway leads to a downregulation of SOCS-1 expression in TCR-stimulated and HCV core-treated cells, and is represented as an increase in amplification cycles before threshold detection by real-time RT-PCR.
Because SOCS-1 mRNA expression may not exhibit a linear relationship with its protein expression in T cells induced by HCV core protein, we employed immunoblotting to detect SOCS-1 protein expression in purified T cells treated as described above. As shown in Fig. 2A, we found upregulation of SOCS-1 protein expression in T cells exposed to HCV core protein. We next demonstrated that blocking the PD-1 pathway by anti-PD-1 or anti-PDL-1, but not control antibody, can downregulate HCV core-induced SOCS-1 expression in healthy T cells during TCR signaling (Fig. 2B).
FIG. 2.
Blocking the PD-1 pathway inhibits SOCS-1 protein expression in healthy T cells exposed to HCV core protein. (A) SOCS-1 upregulation in healthy T cells exposed to HCV core protein. CD4+ T cells isolated from healthy donors using antibody-conjugated magnetic beads were stimulated with anti-CD3/CD28 in the presence or absence of HCV core protein for 24 h. SOCS-1 protein expression was detected by Western blot, and β-actin served as a control. (B) PD-1 blocking inhibits HCV core-induced SOCS-1 expression in activated T cells. T cells isolated from healthy donors as above were treated with anti-PD-1, anti-PDL-1, or control antibody for 6 h, followed by stimulation with anti-CD3/CD28 and HCV core protein for 24 h. SOCS-1 protein expression was detected by Western blot, and β-actin served as a control.
Blocking the PD-1 pathway downregulates SOCS-1 expression in T cells from chronically HCV-infected individuals
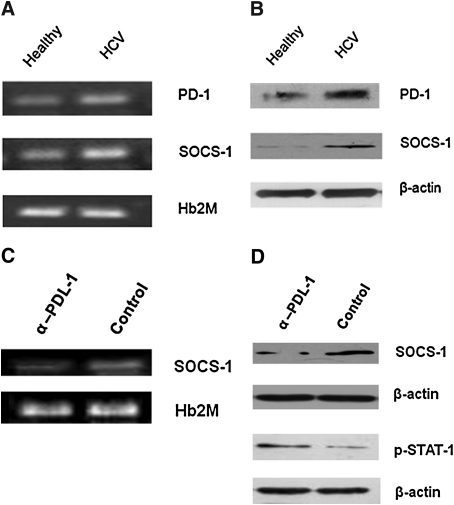
Blocking the PD-1 pathway to inhibit SOCS-1 expression in healthy T cells that have been briefly exposed to HCV core protein in vitro only partially mimics an acute phase of viral infection. It would be of greater clinical benefit to investigate whether blocking this pathway could regulate SOCS-1 expression in T cells that had been persistently exposed to the viral proteins during chronic HCV infection. To this end, we first determined the expression levels of PD-1 and SOCS-1 in T cells isolated from chronically HCV-infected individuals, and compared them with those from healthy subjects. As shown in Fig. 3A, both PD-1 and SOCS-1 mRNA expression were detected at higher levels in T cells from HCV patients than those of healthy subjects by RT-PCR. This was confirmed by immunoblotting to detect PD-1 and SOCS-1 proteins in T cells from HCV patients and healthy subjects (Fig. 3B). The data were reproducible in repeated experiments using T cells isolated from different HCV patients and healthy donors.
FIG. 3.
Blocking the PD-1 pathway inhibits SOCS-1 expression in T lymphocytes from chronically HCV-infected patients. (A) PD-1 and SOCS-1 mRNA are upregulated in T cells from chronically HCV-infected patients. T cells were isolated from HCV-infected patients as well as healthy subjects using antibody-conjugated magnetic beads as described in the text, and stimulated with anti-CD3/CD28 for 24 h. PD-1 and SOCS-1 were detected by RT-PCR, and human β2M served as a loading control. (B) PD-1 and SOCS-1 protein expression is upregulated in T cells from chronically HCV-infected patients. T lymphocytes were isolated from HCV-infected patients as well as healthy subjects as above, and stimulated with anti-CD3/CD28 for 24 h. PD-1 and SOCS-1 were detected by Western blot, and β-actin served as a loading control. (C) PD-1 blockade inhibits SOCS-1 gene expression in T cells from HCV-infected subjects. T lymphocytes isolated from HCV patients were treated with anti-PDL-1 or control antibody overnight, followed by stimulation with anti-CD3/CD28 for 5 d. SOCS-1 gene expression was detected by RT-PCR, and β-actin served as a control. (D) PD-1 blockade inhibits SOCS-1 protein expression and increases STAT-1 phosphorylation in T cells from HCV-infected patients. T lymphocytes isolated from HCV patients were treated with anti-PDL-1 or control antibody overnight, followed by stimulation with anti-CD3/CD28 for 5 d. SOCS-1 protein expression and STAT-1 phosphorylation were detected by Western blot. β-Actin served as a control. Data were reproducible in independent experiments using T cells isolated from at least three different subjects.
We thus further determined whether blocking the PD-1 pathway could affect SOCS-1 expression in the setting of chronic HCV infection. To this end, T cells isolated from chronically HCV-infected patients were pre-incubated with anti-PDL-1 or control antibody overnight, then stimulated with anti-CD3/CD28 antibodies for 1, 3, or 5 d, followed by RT-PCR and immunoblotting to detect SOCS-1 mRNA and protein expression. Since both PD-1 and SOCS-1 molecules had been upregulated in the setting of chronic HCV infection, changes in SOCS-1 expression could only be detected after several cycles of cell replication while blocking the PD-1 pathway in vitro. In fact, we did not detect any changes in SOCS-1 gene expression at 24 h of treatment in repeated experiments using T cells isolated from HCV patients, which differs from the HCV core-treated healthy T-cell model. SOCS-1 reduction upon PD-1 blockade can only be observed 72 h following TCR stimulation (data not shown), and more significantly at 5 d of treatment. As shown in Fig. 3C and D, blocking PD-1 signaling and TCR activation for 5 d resulted in a significant reduction of SOCS-1 expression compared with control antibody treatment. This result was reproducible in independent experiments using T cells isolated from different HCV patients.
It is well-known that SOCS-1 protein regulates TCR signaling through inhibition of the Jak/STAT pathway, a pathway that has been reported to be inhibited in the setting of chronic HCV infection (10,15,23–24). We thus further examined the phosphorylation status of signal transducer and activator of transcription protein (STAT)-1 while blocking PD-1 signaling in T cells isolated from HCV patients (Fig. 3D). In contrast to the reduced amount of SOCS-1 expression, STAT-1 phosphorylation was increased in T cells treated with anti-PDL-1 versus control antibody, while β-actin was not affected by the treatment. These data suggest that blocking the PD-1 pathway can inhibit SOCS-1 expression, and in turn can activate the Jak/STAT pathway.
Blocking the PD-1 pathway rescues the function of T cells from chronically HCV-infected individuals
We have previously shown that blocking PD-1 signaling can abrogate HCV core-induced T-cell inhibition and apoptosis in healthy subjects (21). To determine whether blocking PD-1 signaling can improve the impaired T-cell function in cells from chronically HCV-infected individuals, we examined T-cell proliferation by CFSE assay (Fig. 4). We incubated PBMCs isolated from HCV patients with anti-PDL-1 or control antibody overnight, followed by stimulation of the cells with either anti-CD3/CD28 antibodies (1 μg/mL; Fig. 4A), HCV peptides (10 μg/mL; Fig. 4B), or healthy lymphocytes (1:5 ratio; Fig. 4C) for 5 d. CFSE dilutions (left shift) on gated T-cell populations were observed more significantly in the setting of PD-1 blockade versus control treatment, although the improvement of their proliferative ability varied under different stimulating conditions.
FIG. 4.
Blocking the PD-1 pathway can improve the proliferative capability of T cells from chronically HCV-infected individuals. (A) Improved T-cell proliferation by blocking the PD-1 pathway in T lymphocytes stimulated by anti-CD3/CD28. PBMCs isolated from HCV patients were CFSE-labeled, followed by anti-PDL-1 or control antibody treatment overnight, and anti-CD3/CD28 stimulation for 5 d. After double staining, the T lymphocytes were gated and CFSE dilution, which represents T-cell proliferation, as well as the percentage of cells left shifted on each gating, are shown in the histograms. (B) Improved T-cell proliferation by blocking the PD-1 pathway in T lymphocytes stimulated by HCV peptides. PBMCs isolated from HCV-infected subjects were CFSE-labeled, followed by anti-PDL-1 or control antibody treatment overnight, and HCV NS3 peptide stimulation for 5 d. T-cell proliferation was analyzed as described above. (C) Improved T-cell proliferation by blocking the PD-1 pathway in T lymphocytes stimulated by healthy T cells. PBMCs isolated from three individual HCV-infected subjects were CFSE-labeled, and treated with anti-PDL-1 or control antibody overnight, followed by stimulation with healthy lymphocytes for 5 d. T-cell proliferation was analyzed as above.
Discussion
In this study, we demonstrate that PD-1 and SOCS-1 are upregulated in healthy T cells exposed to HCV core protein, and that blocking PD-1 pathway signaling can downregulate SOCS-1 gene expression in HCV core-treated healthy T cells. Additionally, T cells isolated from chronically HCV-infected individuals exhibited increased PD-1 and SOCS-1 expression compared to healthy subjects. SOCS-1 gene expression in T cells isolated from HCV patients is also inhibited by blocking PD-1 signaling, in turn improving the phosphorylation of STAT-1, and the impaired T-cell proliferation seen in the setting of chronic HCV infection. To our knowledge, this is the first report demonstrating that PD-1 and SOCS-1, two inhibitory molecules that can be induced by HCV core protein, are mechanistically linked in T cells, and that their cross-talk might coordinately inhibit the T-cell signaling pathway, leading to T-cell exhaustion during chronic viral infection.
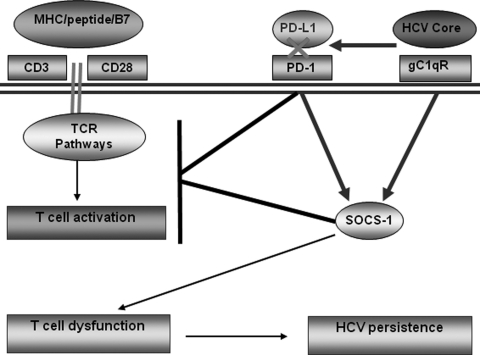
Upon antigenic encounter with pathogens, T cells are primed and activated in order to clear the pathogen and control the infection. In chronically HCV-infected patients, T-cell responses are, however, dampened by viral proteins that lead to viral persistence. The mechanisms leading to T-cell dysfunction during HCV infection are not fully understood. Recently, PD-1 and SOCS-1 have been shown to play important roles in regulating TCR signaling pathways, and appear to contribute to the establishment of chronic HCV infection (12–15,20–24). The exact relationship between these two negative immune modulatory pathways and whether they can coordinately regulate T-cell signaling during HCV infection have been unclear. This study suggests a possible shared mechanism or cross-linkage between these two immunomodulatory pathways during chronic HCV infection. It seems possible based on our studies of TCR-stimulated T cells that a relationship between PD-1 and SOCS-1 exists at baseline, and that HCV manipulates these pathways to facilitate persistence. We propose a model, shown in Fig. 5, for the pathogenesis of HCV persistence based on this and our other published studies. In this model, HCV core antigen secreted during viral infection can bind to gC1qR expressed on the surface of T cells. This interaction can lead to the upregulation of PD-1 and SOCS-1 expression, which will deliver negative signaling to MHC/peptide-induced activation of TCR pathways, leading to T-cell dysfunction and contributing to HCV persistence. Upon blockade of PD-1 signaling, HCV core-induced SOCS-1 expression is downregulated and the observed T-cell dysfunctions are recovered. Understanding the mechanisms and the relationships between PD-1 and SOCS-1 during the immune disruption and viral persistence that occurs with chronic HCV infection raises the possibility that therapeutic strategies targeting these inhibitory pathways might be of clinical benefit.
FIG. 5.
Proposed model for immunodysregulation via PD-1/SOCS-1 during chronic HCV infection. This model is based on multiple studies involving these molecules (7,16–21).
Our data from healthy T cells exposed to HCV core protein again support an immunomodulatory role for this nucleocapsid protein, which is amply expressed during HCV infection and secreted into the bloodstream. While both PD-1 and SOCS-1 are upregulated upon antigenic exposure, blocking PD-1 can clearly impair the upregulation of SOCS-1. Alterations in both PD-1 and SOCS-1 signaling have been described in the setting of HCV infection, but a dependent relationship has not been heretofore noted in this or any other viral infection. Upregulation of these key proteins has, however, been observed in multiple viral infections. Findings of very recent studies in HSV (25), RSV (26), and HIV infection (27), for example, all support a role for SOCS-1 upregulation as a mechanism for immune evasion. Since PD-1 upregulation has also been described during chronic viral infections, including HSV and HIV, it seems possible if not likely that explorations into a link between PD-1 and SOCS-1 during these infections will also demonstrate a dependent relationship.
It is worth noting that different T-cell subsets may display differential expression of PD-1 and SOCS-1. While we have previously shown that PD-1 is upregulated on both CD4+ and CD8+ T cells in the setting of chronic infection (21), we do not yet know if SOCS-1 is acting similarly, as these studies focused on CD4+ T cells or bulk PBMCs. We have, however, observed an increase in the CD45RO memory T-cell subset in the setting of chronic HCV infection, and increased PD-1 expression on this CD454RO subset compared to CD45RA populations (unpublished data). Since our data would suggest a relationship between PD-1 and SOCS-1 signaling pathways, it would be worth examining these subpopulations further in future studies.
It is possible that the relationship between PD-1 and SOCS-1 is more complex than a simple and direct linear pathway. As part of a multifunctional negative feedback system, SOCS-1 could, for example, be altering PD-1 expression or that of its cognate ligand, PDL-1, in a more two-way signaling process. Using a SOCS-1 antagonist, as recently described (25), or silencing studies in which SOCS protein expression is blocked, would be useful approaches to determining if this is occurring, and these studies are ongoing.
Acknowledgments
This work was supported by grants to Z.Q.Y./J.P.M. (National Institutes of Health [NIH] National Institute of Allergy and Infectious Diseases [NIAID] AI084057) and J.P.M./Z.Q.Y. (NIH NIAID AI072750). L. Ni, a joint Ph.D. student, was supported in part by the China Scholarship Council (CSC 2008655005). C.L. Zhang, a visiting scholar, holds a grant for viral hepatitis research from Guangzhou Municipal Health Bureau, China. The authors appreciate the technical assistance of Ms. Mary Howell. This publication is the result of work supported with resources and the use of facilities at the James H. Quillen Veterans Affairs Medical Center. The contents of this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.King E. Trabue C. Yin D. Yao ZQ. Moorman JP. Hepatitis C: the complications of immune dysfunction. Exp Rev Clin Immunol. 2007;3:145–157. doi: 10.1586/1744666X.3.2.145. [DOI] [PubMed] [Google Scholar]
- 2.Rehermann B. Chang KM. McHutchison JG. Kokka R. Houghton M. Chisari FV. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in subjects with chronic hepatitis C virus infection. J Clin Invest. 1996;98:1432–1440. doi: 10.1172/JCI118931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang KM. Rehermann B. McHutchison JG. Pasquinelli C. Southwood S. Sette A. Chisari FV. Immunological significance of cytotoxic T lymphocyte epitope variants in subjects chronically infected by the hepatitis C virus. J Clin Invest. 1997;100:2376–2385. doi: 10.1172/JCI119778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wedemeyer H. He XS. Nascimbeni M, et al. Impaired effector function of hepatitis C virus specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 5.Lechmann M. Woitas RP. Langhans B, et al. Decreased frequency of HCV core-specific peripheral blood mononuclear cells with type 1 cytokine secretion in chronic hepatitis. Can J Hepatol. 1999;31:971–978. doi: 10.1016/s0168-8278(99)80307-9. [DOI] [PubMed] [Google Scholar]
- 6.Eisen-Vandervelde A. Yao ZQ. Hahn YS. The molecular basis of HCV-mediated immune dysregulation. Clin Immunol. 2004;111:16–21. doi: 10.1016/j.clim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Yao ZQ. Ray S. Eisen-Vandervelde A. Waggoner S. Hahn YS. Hepatitis C virus: Immunosuppression by complement regulatory pathway. Viral Immunol. 2001;14:277–295. doi: 10.1089/08828240152716547. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura H. Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001;22:265–268. doi: 10.1016/s1471-4906(01)01888-9. [DOI] [PubMed] [Google Scholar]
- 9.Okazaki T. Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 10.Alexander WS. Suppressors of cytokine signaling (SOCS) in the immune system. Nat Rev Immunol. 2002;2:410–416. doi: 10.1038/nri818. [DOI] [PubMed] [Google Scholar]
- 11.Penna A. Pilli M. Zerbini A, et al. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 12.Golden-Mason L. Palmer B. Klarguist J. Mengshol JA. Gastelblanco N. Rosen HR. Upregulation of PD-1 expression on circulating and hepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden-Mason L. Klarguist J. Wahed AS. Rosen HR. Cutting edge: programmed death-1 expression is increased on immunocytes in chronic hepatitis C virus and predicts failure of response to antiviral therapy, race-dependent differences. J Immunol. 2008;180:3637–3641. doi: 10.4049/jimmunol.180.6.3637. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi H. Fujie H. Shintani Y, et al. Hepatitis C virus core protein exerts an inhibitory effect on suppressor of cytokine signaling (SOCS)-1 gene expression. J Hepatol. 2005;43:757–763. doi: 10.1016/j.jhep.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Vlotides G. Sörensen AS. Kopp F, et al. SOCS-1 and SOCS-3 inhibit IFN-alpha-induced expression of the antiviral proteins 2,5-OAS and MxA. Biochem Biophys Res Commun. 2004;320:1007–1014. doi: 10.1016/j.bbrc.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 16.Kittlesen DJ. Chianese-Bullick KA. Yao ZQ. Braciale TJ. Hahn YS. Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T lymphocyte proliferation. J Clin Invest. 2000;106:1239–1249. doi: 10.1172/JCI10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao ZQ. Nguyen DT. Hiotellis AI. Hahn YS. Hepatitis C virus core protein inhibits human T lymphocyte responses by a complement-dependent regulatory pathway. J Immunol. 2001;167:5264–5272. doi: 10.4049/jimmunol.167.9.5264. [DOI] [PubMed] [Google Scholar]
- 18.Yao ZQ. Eisen-Vandervelde A. Ray S. Hahn YH. HCV core/gC1qR interaction arrests T cell cycle progression through stabilization of the cell cycle inhibitor p27kip1. Virology. 2003;314:271–282. doi: 10.1016/s0042-6822(03)00419-7. [DOI] [PubMed] [Google Scholar]
- 19.Yao ZQ. Eisen-Vandervelde A. Waggoner SN. Cale EM. Hahn YS. Direct binding of hepatitis C virus core to gC1qR on CD4+ and CD8+ T cells leads to impaired activation of Lck and Akt. J Virol. 2004;78:6409–6419. doi: 10.1128/JVI.78.12.6409-6419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao ZQ Prayther D. Trabu C. Dong ZP. Moorman J. Differential regulation of SOCS-1 signaling in T and B lymphocytes by HCV core protein. Immunology. 2008;125:197–207. doi: 10.1111/j.1365-2567.2008.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao ZQ. King E. Prayther D. Yin D. Zhang Y. Moorman JP. T cell dysfunction by hepatitis C virus core protein involves PD-1/PDL-1 signaling. Viral Immunol. 2007;20:276–287. doi: 10.1089/vim.2006.0096. [DOI] [PubMed] [Google Scholar]
- 22.Moorman JP. Dong ZP. Ni L. Zhang CL. Borthwick T. Yao ZQ. Abnormal B lymphocyte activation associated with TALL-1 overexpression and SOCS-1 deregulation in chronic HCV infection. Immunology. 2009;128:227–235. doi: 10.1111/j.1365-2567.2009.03106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helbig KJ. Yip E. McCartney EM. Eyre NS. Beard MR. A screening method for identifying disruptions in interferon signaling reveals HCV NS3/4a disrupts Stat-1 phosphorylation. Antiviral Res. 2008;77:169–176. doi: 10.1016/j.antiviral.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Kondo Y. Sung VM. Machida K. Liu M. Lai MM. Hepatitis C virus infects T cells and affects interferon-gamma signaling in T cell lines. Virology. 2007;361:161–173. doi: 10.1016/j.virol.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Frey KG. Ahmed CMI. Dabelic R, et al. HSV1-induced SOCS-1 expression in keratinocytes: use of a SOCS-1 antagonist to block a novel mechanism of viral immune evasion. J Immunology. 2009;183:1253–1262. doi: 10.4049/jimmunol.0900570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto K. Ishibashi K. Ishioka K, et al. RSV replication is attenuated by counteracting expression of the suppressor of cytokine signaling (SOCS) molecules. Virology. 2009;391:162–170. doi: 10.1016/j.virol.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 27.Yadav A. Fitzgerald P. Sajadi MM. Gilliam B. Lafferty MK. Redfield R. Reid W. Increased expression of suppressor of cytokine signaling-1 (SOCS-1): a mechanism for dysregulated T helper-1 responses in HIV disease. Virology. 2003;385:126–133. doi: 10.1016/j.virol.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]