Abstract
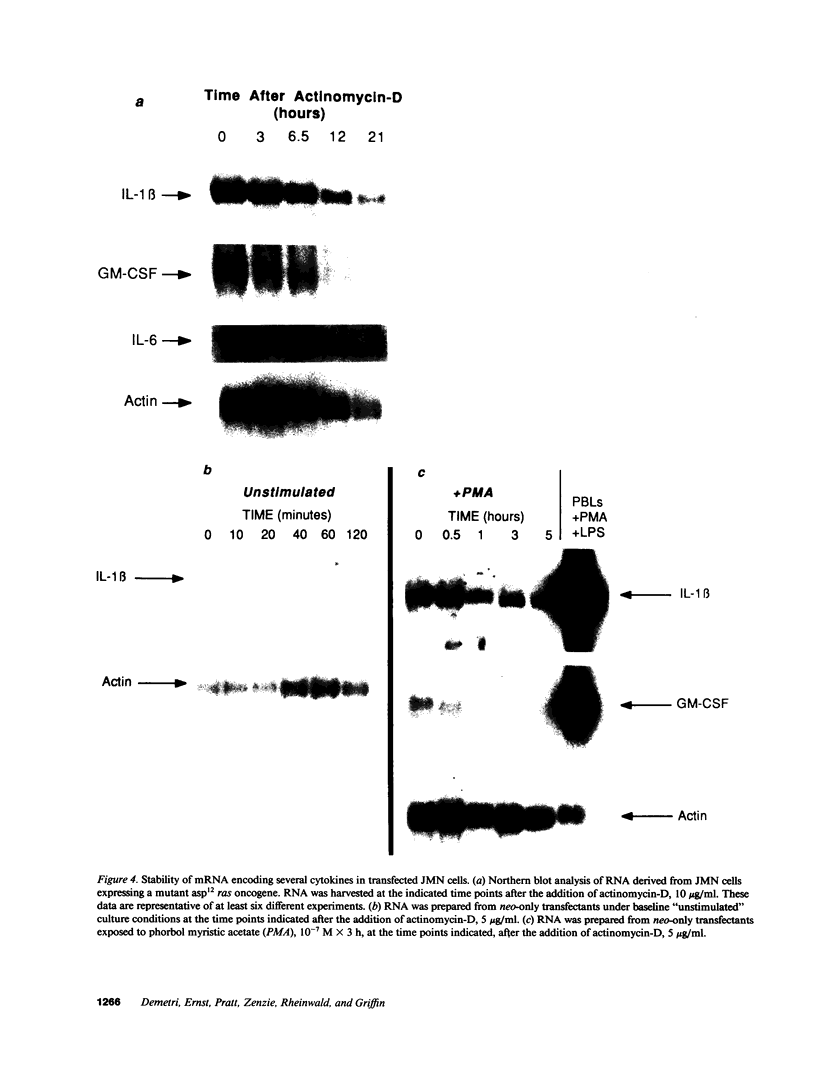
Autonomous production of cytokines such as the hematopoietic colony-stimulating factors (CSFs), IL-1, or IL-6 has been demonstrated in numerous human and murine neoplasms, and may be involved in the pathogenesis of several paraneoplastic syndromes such as leukocytosis, fever, and hypercalcemia. Because of the high frequency with which mutations in ras protooncogenes have been detected in human tumors, as well as evidence linking ras gene products to activation of certain cellular functions, we investigated whether ras mutations might influence the regulation of cytokine genes. Normal human fibroblasts transfected with a mutant val12 H-ras oncogene expressed increased levels of mRNA transcripts encoding granulocyte-CSF (G-CSF), granulocyte-macrophage-CSF (GM-CSF), and IL-1 beta compared with controls. Human mesothelioma cells transfected with a mutant asp12 N-ras oncogene exhibited similar alterations in cytokine gene expression. Estimates of transcriptional activity by nuclear run-on analysis revealed a selective increase in transcription only for the IL-1 gene. Analysis of mRNA half-life demonstrated a marked increase in the stability of numerous cytokine transcripts, including G-CSF, GM-CSF, IL-1, and IL-6. The addition of anti-IL-1 neutralizing antibody to cultures of cells expressing ras mutants did not block the expression of any of the cytokines examined, suggesting that the baseline expression of GM-CSF, G-CSF, and IL-6 was not a secondary event due to the increased transcription of IL-1. These results indicate that mutations in ras genes may alter expression of several cytokine genes through both transcriptional and posttranscriptional mechanisms.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Gordon D. F., Wood W. M., Janson R. W., Joslin F. G., Jameel S. IL-1 beta production in cultured human monocytes is regulated at multiple levels. J Immunol. 1989 Jul 1;143(1):118–126. [PubMed] [Google Scholar]
- Bagby G. C., Jr, Dinarello C. A., Wallace P., Wagner C., Hefeneider S., McCall E. Interleukin 1 stimulates granulocyte macrophage colony-stimulating activity release by vascular endothelial cells. J Clin Invest. 1986 Nov;78(5):1316–1323. doi: 10.1172/JCI112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Behbehani A. M., Hunter W. J., Chapman A. L., Lin F. Studies of a human mesothelioma. Hum Pathol. 1982 Sep;13(9):862–866. doi: 10.1016/s0046-8177(82)80083-x. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Broudy V. C., Kaushansky K., Segal G. M., Harlan J. M., Adamson J. W. Tumor necrosis factor type alpha stimulates human endothelial cells to produce granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7467–7471. doi: 10.1073/pnas.83.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistra S. A., Griffin J. D. Regulation of the production and function of granulocytes and monocytes. Semin Hematol. 1988 Jul;25(3):173–188. [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. Y., Slamon D. J., Nimer S. D., Golde D. W., Gasson J. C. Regulation of expression of human granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8669–8673. doi: 10.1073/pnas.83.22.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Goustin A. S., Soderquist A. M., Shipley G. D., Wolfshohl J., Carpenter G., Moses H. L. Transforming growth factor alpha and beta expression in human colon cancer lines: implications for an autocrine model. Cancer Res. 1987 Sep 1;47(17):4590–4594. [PubMed] [Google Scholar]
- Connell N. D., Rheinwald J. G. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell. 1983 Aug;34(1):245–253. doi: 10.1016/0092-8674(83)90155-1. [DOI] [PubMed] [Google Scholar]
- Demetri G. D., Zenzie B. W., Rheinwald J. G., Griffin J. D. Expression of colony-stimulating factor genes by normal human mesothelial cells and human malignant mesothelioma cells lines in vitro. Blood. 1989 Aug 15;74(3):940–946. [PubMed] [Google Scholar]
- Di Persio J. F., Brennan J. K., Lichtman M. A., Speiser B. L. Human cell lines that elaborate colon-stimulating activity for the marrow cells of man and other species. Blood. 1978 Mar;51(3):507–519. [PubMed] [Google Scholar]
- Ernst T. J., Ritchie A. R., Demetri G. D., Griffin J. D. Regulation of granulocyte- and monocyte-colony stimulating factor mRNA levels in human blood monocytes is mediated primarily at a post-transcriptional level. J Biol Chem. 1989 Apr 5;264(10):5700–5703. [PubMed] [Google Scholar]
- Farr C. J., Saiki R. K., Erlich H. A., McCormick F., Marshall C. J. Analysis of RAS gene mutations in acute myeloid leukemia by polymerase chain reaction and oligonucleotide probes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1629–1633. doi: 10.1073/pnas.85.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fleming T. P., Matsui T., Molloy C. J., Robbins K. C., Aaronson S. A. Autocrine mechanism for v-sis transformation requires cell surface localization of internally activated growth factor receptors. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8063–8067. doi: 10.1073/pnas.86.20.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Fujita J., Srivastava S. K., Kraus M. H., Rhim J. S., Tronick S. R., Aaronson S. A. Frequency of molecular alterations affecting ras protooncogenes in human urinary tract tumors. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3849–3853. doi: 10.1073/pnas.82.11.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto Y., Kaibuchi K., Oku N., Hori Y., Takai Y. Activation of the c-fos serum-response element by the activated c-Ha-ras protein in a manner independent of protein kinase C and cAMP-dependent protein kinase. J Biol Chem. 1990 Jan 15;265(2):774–780. [PubMed] [Google Scholar]
- Furth M. E., Aldrich T. H., Cordon-Cardo C. Expression of ras proto-oncogene proteins in normal human tissues. Oncogene. 1987 Mar;1(1):47–58. [PubMed] [Google Scholar]
- Furukawa Y., DeCaprio J. A., Freedman A., Kanakura Y., Nakamura M., Ernst T. J., Livingston D. M., Griffin J. D. Expression and state of phosphorylation of the retinoblastoma susceptibility gene product in cycling and noncycling human hematopoietic cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2770–2774. doi: 10.1073/pnas.87.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Imler J. L., Schatz C., Wasylyk C., Chatton B., Wasylyk B. A Harvey-ras responsive transcription element is also responsive to a tumour-promoter and to serum. Nature. 1988 Mar 17;332(6161):275–278. doi: 10.1038/332275a0. [DOI] [PubMed] [Google Scholar]
- Jat P. S., Cepko C. L., Mulligan R. C., Sharp P. A. Recombinant retroviruses encoding simian virus 40 large T antigen and polyomavirus large and middle T antigens. Mol Cell Biol. 1986 Apr;6(4):1204–1217. doi: 10.1128/mcb.6.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K., Lin N., Adamson J. W. Interleukin 1 stimulates fibroblasts to synthesize granulocyte-macrophage and granulocyte colony-stimulating factors. Mechanism for the hematopoietic response to inflammation. J Clin Invest. 1988 Jan;81(1):92–97. doi: 10.1172/JCI113316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Keating M. T., Williams L. T. Autocrine stimulation of intracellular PDGF receptors in v-sis-transformed cells. Science. 1988 Feb 19;239(4842):914–916. doi: 10.1126/science.2829358. [DOI] [PubMed] [Google Scholar]
- Kern J. A., Lamb R. J., Reed J. C., Daniele R. P., Nowell P. C. Dexamethasone inhibition of interleukin 1 beta production by human monocytes. Posttranscriptional mechanisms. J Clin Invest. 1988 Jan;81(1):237–244. doi: 10.1172/JCI113301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen P. J., Dinarello C. A., Strom T. B. Glucocorticoids inhibit transcriptional and post-transcriptional expression of interleukin 1 in U937 cells. J Immunol. 1987 Dec 15;139(12):4129–4134. [PubMed] [Google Scholar]
- Koeffler H. P., Gasson J., Ranyard J., Souza L., Shepard M., Munker R. Recombinant human TNF alpha stimulates production of granulocyte colony-stimulating factor. Blood. 1987 Jul;70(1):55–59. [PubMed] [Google Scholar]
- Koeffler H. P., Gasson J., Tobler A. Transcriptional and posttranscriptional modulation of myeloid colony-stimulating factor expression by tumor necrosis factor and other agents. Mol Cell Biol. 1988 Aug;8(8):3432–3438. doi: 10.1128/mcb.8.8.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J. M., Weinstein D., Gubler U., Vilcek J. Induction of membrane-associated interleukin 1 by tumor necrosis factor in human fibroblasts. J Immunol. 1987 Apr 1;138(7):2137–2142. [PubMed] [Google Scholar]
- Lee S. W., Tsou A. P., Chan H., Thomas J., Petrie K., Eugui E. M., Allison A. C. Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gene and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1204–1208. doi: 10.1073/pnas.85.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morstyn G., Burgess A. W. Hemopoietic growth factors: a review. Cancer Res. 1988 Oct 15;48(20):5624–5637. [PubMed] [Google Scholar]
- Munker R., Gasson J., Ogawa M., Koeffler H. P. Recombinant human TNF induces production of granulocyte-monocyte colony-stimulating factor. Nature. 1986 Sep 4;323(6083):79–82. doi: 10.1038/323079a0. [DOI] [PubMed] [Google Scholar]
- Muraguchi A., Hirano T., Tang B., Matsuda T., Horii Y., Nakajima K., Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988 Feb 1;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. W., Kraus M. H., Srivastava S. K., Levine P. H., Aaronson S. A. High frequency of N-ras activation in acute myelogenous leukemia. Blood. 1986 Mar;67(3):753–757. [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Rubin C. M., Houck C. M., Deininger P. L., Friedmann T., Schmid C. W. Partial nucleotide sequence of the 300-nucleotide interspersed repeated human DNA sequences. Nature. 1980 Mar 27;284(5754):372–374. doi: 10.1038/284372a0. [DOI] [PubMed] [Google Scholar]
- Sager R., Tanaka K., Lau C. C., Ebina Y., Anisowicz A. Resistance of human cells to tumorigenesis induced by cloned transforming genes. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7601–7605. doi: 10.1073/pnas.80.24.7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Hattori T., Matsuoka M., Asou N., Yamamoto S., Sagawa K., Takatsuki K. Autocrine stimulation of interleukin 1 beta in acute myelogenous leukemia cells. J Exp Med. 1987 Nov 1;166(5):1597–1602. doi: 10.1084/jem.166.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariban E., Luebbers R., Kufe D. Transcriptional and posttranscriptional control of c-fos gene expression in human monocytes. Mol Cell Biol. 1988 Jan;8(1):340–346. doi: 10.1128/mcb.8.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Fujii Y., Ono M., Nomura H., Shizume K. Production of interleukin 1 alpha-like factor and colony-stimulating factor by a squamous cell carcinoma of the thyroid (T3M-5) derived from a patient with hypercalcemia and leukocytosis. Cancer Res. 1987 Dec 15;47(24 Pt 1):6474–6480. [PubMed] [Google Scholar]
- Schuler G. D., Cole M. D. GM-CSF and oncogene mRNA stabilities are independently regulated in trans in a mouse monocytic tumor. Cell. 1988 Dec 23;55(6):1115–1122. doi: 10.1016/0092-8674(88)90256-5. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Sieff C. A., Niemeyer C. M., Mentzer S. J., Faller D. V. Interleukin-1, tumor necrosis factor, and the production of colony-stimulating factors by cultured mesenchymal cells. Blood. 1988 Oct;72(4):1316–1323. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Stanley E., Metcalf D., Sobieszczuk P., Gough N. M., Dunn A. R. The structure and expression of the murine gene encoding granulocyte-macrophage colony stimulating factor: evidence for utilisation of alternative promoters. EMBO J. 1985 Oct;4(10):2569–2573. doi: 10.1002/j.1460-2075.1985.tb03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Tainsky M. A., Cooper C. S., Giovanella B. C., Vande Woude G. F. An activated rasN gene: detected in late but not early passage human PA1 teratocarcinoma cells. Science. 1984 Aug 10;225(4662):643–645. doi: 10.1126/science.6740333. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. Regulation of cytokine gene expression. Annu Rev Immunol. 1988;6:439–464. doi: 10.1146/annurev.iy.06.040188.002255. [DOI] [PubMed] [Google Scholar]
- Thorens B., Mermod J. J., Vassalli P. Phagocytosis and inflammatory stimuli induce GM-CSF mRNA in macrophages through posttranscriptional regulation. Cell. 1987 Feb 27;48(4):671–679. doi: 10.1016/0092-8674(87)90245-5. [DOI] [PubMed] [Google Scholar]
- Tubo R. A., Rheinwald J. G. Normal human mesothelial cells and fibroblasts transfected with the EJras oncogene become EGF-independent, but are not malignantly transformed. Oncogene Res. 1987 Sep-Oct;1(4):407–421. [PubMed] [Google Scholar]
- Ucker D. S., Yamamoto K. R. Early events in the stimulation of mammary tumor virus RNA synthesis by glucocorticoids. Novel assays of transcription rates. J Biol Chem. 1984 Jun 25;259(12):7416–7420. [PubMed] [Google Scholar]
- Van Damme J., Opdenakker G., Simpson R. J., Rubira M. R., Cayphas S., Vink A., Billiau A., Van Snick J. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin 1 and tumor necrosis factor. J Exp Med. 1987 Mar 1;165(3):914–919. doi: 10.1084/jem.165.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellenga E., Rambaldi A., Ernst T. J., Ostapovicz D., Griffin J. D. Independent regulation of M-CSF and G-CSF gene expression in human monocytes. Blood. 1988 Jun;71(6):1529–1532. [PubMed] [Google Scholar]
- Wano Y., Hattori T., Matsuoka M., Takatsuki K., Chua A. O., Gubler U., Greene W. C. Interleukin 1 gene expression in adult T cell leukemia. J Clin Invest. 1987 Sep;80(3):911–916. doi: 10.1172/JCI113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Interleukin 1 induces interleukin 1. II. Recombinant human interleukin 1 induces interleukin 1 production by adult human vascular endothelial cells. J Immunol. 1987 Sep 15;139(6):1911–1917. [PubMed] [Google Scholar]
- Yang Y. C., Tsai S., Wong G. G., Clark S. C. Interleukin-1 regulation of hematopoietic growth factor production by human stromal fibroblasts. J Cell Physiol. 1988 Feb;134(2):292–296. doi: 10.1002/jcp.1041340217. [DOI] [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Sanderson C. J., Hapel A. J., Campbell H. D., Young I. G. Constitutive synthesis of interleukin-3 by leukaemia cell line WEHI-3B is due to retroviral insertion near the gene. Nature. 1985 Sep 19;317(6034):255–258. doi: 10.1038/317255a0. [DOI] [PubMed] [Google Scholar]
- Young D. C., Demetri G. D., Ernst T. J., Cannistra S. A., Griffin J. D. In vitro expression of colony-stimulating factor genes by human acute myeloblastic leukemia cells. Exp Hematol. 1988 Jun;16(5):378–382. [PubMed] [Google Scholar]
- Young D. C., Wagner K., Griffin J. D. Constitutive expression of the granulocyte-macrophage colony-stimulating factor gene in acute myeloblastic leukemia. J Clin Invest. 1987 Jan;79(1):100–106. doi: 10.1172/JCI112769. [DOI] [PMC free article] [PubMed] [Google Scholar]