Abstract
Ca2+ functions as an important signaling messenger right from beginning of the life to final moment of the end of the life. Ca2+ is needed at several steps of the cell cycle such as early G1, at the G1/S, and G2/M transitions. The Ca2+ signals in the form of time-dependent changes in intracellular Ca2+ concentrations, [Ca2+]i, are presented as brief spikes organized into regenerative Ca2+ waves. Ca2+-mediated signaling pathways have also been shown to play important roles in carcinogenesis such as transformation of normal cells to cancerous cells, tumor formation and growth, invasion, angiogenesis and metastasis. Since the global Ca2+ oscillations arise from Ca2+ waves initiated locally, it results in stochastic oscillations because although each cell has many IP3Rs and Ca2+ ions, the law of large numbers does not apply to the initiating event which is restricted to very few IP3Rs due to steep Ca2+ concentration gradients. The specific Ca2+ signaling information is likely to be encoded in a calcium code as the amplitude, duration, frequency, waveform or timing of Ca2+ oscillations and decoded again at a later stage. Since Ca2+ channels or pumps involved in regulating Ca2+ signaling pathways show altered expression in cancer, one can target these Ca2+ channels and pumps as therapeutic options to decrease proliferation of cancer cells and to promote their apoptosis. These studies can provide novel insights into alterations in Ca2+ wave patterns in carcinogenesis and lead to development of newer technologies based on Ca2+ waves for the diagnosis and therapy of cancer.
Keywords: Apoptosis, Calcium, Cancer, Cell cycle, Metastasis, Oscillations, Signaling, Stochastic, Wave
Introduction
At the beginning of life, Ca2+ mediates the process of fertilization as well as regulates the cell cycle events during the early developmental processes. Once the cells differentiate to perform specific functions, Ca2+ regulates several processes as diverse as energy transduction, secretions, apoptosis, muscle contraction, chemotaxis and neuronal synaptic plasticity in learning and memory (Berridge 2009; Berridge et al. 2003; Clapham 2007; Mikoshiba 2007). Since Ca2+ ions are toxic to the cells, the intracellular concentration of Ca2+, [Ca2+]i, in resting cells is usually maintained very low at ~100 nM. While in normal salt solution, the hydrated Ca2+ can diffuse 40 μm in 1 sec (Einstein 1905); in cells, due to the presence of several charged molecules, the Ca2+ diffusion rates are slower. In order to utilize Ca2+ as a second messenger, cells have devised an ingenious mechanism of signaling that has overcome the inherent problems associated with lower diffusion rates and cytotoxicity of Ca2+, by presenting changes in Ca2+ concentration as brief spikes which are often organized as regenerative waves (Berridge 1993; Clapham 2007; Rey et al. 2010).
To provide for a very fast and effective Ca2+-signaling, the cells use a great amount of energy to maintain almost 20 000-fold Ca2+-gradient between their intracellular (~100 nM free) and extracellular (~ 1.2 mM) Ca2+ concentrations. To maintain this 20 000-fold Ca2+ gradient, the cells chelate, compartmentalize, or remove Ca2+ from the cytoplasm (Clapham 2007). Various cellular proteins with Ca2+- binding affinities ranging between nM to mM are utilized by the cells to buffer the cellular Ca2+ as well as to regulate cellular processes via Ca2+-signaling (see Fig. 1). The binding of Ca2+ to proteins can change protein conformations in terms of shape and charge, and thus modify its functions (Westheimer 1987). Ca2+-mediated signaling pathways have been shown to play important roles in cancer initiation, tumor formation, tumor progression, metastasis, invasion and angiogenesis (Block et al. 2010; Rizzuto and Pozzan 2006; Saidak et al. 2009). Some of the Ca2+-regulated pathways involved in cancers are briefly summarized in BOX 1.
Fig. 1.
Ca2+ signaling in cells. In resting cells the intracellular Ca2+ concentration, [Ca2+]i, is maintained at ~100 nM by Ca2+ removal via plasma membrane Ca2+- ATPase (PMCA) and Ca2+ uptake into ER by SR Ca2+- ATPase (SERCA) transporters. The Na/Ca exchanger (NCX), a major secondary regulator of [Ca2+]i, is electrogenic, exchanging three Na ions for one Ca2+. Intracellular Ca2+ hyperpolarizes many cells by activating K+ channels, and in some cells, Cl− channels. This decreases CaV channel activity but increases the driving force across active Ca2+-permeant channels. In excitatory Ca2+ signaling, plasma membrane ion channels are triggered to open by changes in voltage, or extra- or intracellular ligand binding. When open, ~1 million Ca2+ ions/s/channel flow down the 20000 fold Ca2+ gradient (ECa ~ +150 mV). Initial increase in [Ca2+]i triggers more release, primarily from ER via Ca2+-sensitive RyR. GPCR or receptor tyrosine kinase-mediated activation of PLC cleaves PIP2 into IP3 and DAG. IP3 is a ligand for the intracellular IP3R channel spanning the membrane of the ER. GPCRs catalyze the exchange of GDP for GTP on Gα subunits, releasing active Gα and Gβγ subunits that in turn activate PLCβ.
Both the genetic and epigenetic mechanisms have been proposed for the specific roles of Ca2+ signaling in cancer (Hanahan and Weinberg 2000; Jaffe 2005). As a result of mutations, the normal cells can be transformed to cancerous cells by acquiring cancer-specific properties such as uncontrollable proliferation, immortality, and self-sufficiency in growth signals (Futreal et al. 2005; Hanahan and Weinberg 2000). Since Ca2+-gradients and waves have been shown to be involved in normal developmental processes, the epigenetic theory proposes that altered Ca2+-gradients and waves participate in carcinogenesis (Jaffe 2005). The intracellular Ca2+ waves in concert with various other signal-transduction cascades, regulate a variety of processes including gene expression (De Koninck and Schulman 1998; Dolmetsch 2003; Dolmetsch et al. 1998). The intracellular Ca2+-induced activation of protein kinase C (PKC) can lead to phosphorylation of methyltransferase that is involved in DNA methylation (DePaoli-Roach et al. 1986). In normal cells, Ca2+- signaling is required for cell proliferation, whereas some transformed cells and tumor cell lines show altered dependency on Ca2+ to maintain proliferation (Cook and Lockyear 2006; Whitfield 1992). Ca2+- signaling pathways are remodeled or deregulated in cancer that result in changes in their physiology and distinguish them from non-malignant cells (Hanahan and Weinberg 2000; Wang et al. 2010). Remodeling or deregulation of Ca2+- signaling pathways can provide means by which cancer cells can overcome systemic anticancer defense mechanisms. The altered Ca2+- signaling pathways can also lead to genetic diversity found in cancerous tissues thereby providing effective cellular strategies to the selection pressure to acquire specific traits.
In this review, we first describe our present understanding of Ca2+- signaling in cells including Ca2+ fluxes across the plasma membrane, Ca2+ translocation across the ER, wave nature of Ca2+ signals, and the role of various Ca2+ binding proteins followed by extensive discussion on the role of Ca2+ signaling in cancer cells proliferation and apoptosis (Berridge 2005; Carafoli 2004; Petersen 2005; Rizzuto and Pozzan 2006).
Box 1: Ca2+ and cancer.
The cells maintain a 20 000-fold gradient of Ca2+ between extracellular free Ca2+ (~ 1.2 mM) and resting cytoplasmic free Ca2+ (~ 100 nM). Depending upon the stimulus, [Ca2+]i can exceed more than 1 μM (Rizzuto and Pozzan 2006; Rizzuto et al. 2003). The spatio-temporal nature of changes in the free Ca2+ can regulate many cellular processes including cancer initiation, progression, angiogenesis, and metastasis as described below (Rizzuto and Pozzan 2006; Rizzuto et al. 2003; Saidak et al. 2009):
Transcription
The changes in Ca2+ wave oscillation frequency can activate transcription factors such as nuclear factor of activated T cells (NFAT) resulting in modulation of cellular transcription (Carafoli 2002; Dolmetsch 2003; Rizzuto and Pozzan 2006). Experiments using the “calcium clamp” have shown that the sensitivity of different transcription factors to [Ca2+]i oscillations is highly frequency dependent (Dolmetsch et al. 1997; Lewis 2003).
Cell cycle
Since Ca2+ regulates the cell cycle at several stages, it is involved in cell proliferation through various signaling pathways (Cullen and Lockyer 2002; Ding et al. 2010; Minaguchi et al. 2006; Rey et al. 2010; Zhong et al. 2010). The Ca2+-induced activation of the transcription of early genes is involved in the transition from G0 to G1. The subcellular localization of key proteins associated with cell cycle and carcinogenesis is influenced by Ca2+ (Cook and Lockyer 2006). Ca2+ has been shown to regulate the phosphorylation of retinoblastoma protein in late G1 phase (Cook and Lockyer 2006).
Genotoxicity
Ca2+ can modulate poly-(ADP-ribose) polymerase-1 (PARP1) as well as DNA damage response pathways which in turn can influence genomic stability and cell survival (Bentle et al. 2006).
Differentiation
Cancer cells can become dedifferentiated into cancer stem cells in the tumorigenic process, and Ca2+ signaling is implicated in the differentiation process either through the extracellular Ca2+-sensing receptor and/or through alterations in intracellular Ca2+ (Bikle et al. 2004).
Angiogenesis
By mobilizing release of Ca2+ from ER, angiogenic factors such as vascular endothelial growth factor can increase [Ca2+]i and therefore induce Ca2+ signaling that in turn plays critical roles in angiogenesis (Munaron and Fiorio 2009; Patton et al. 2003).
Telomerase
Cancer cells upregulate telomerase expression in order to maintain their telomeres for cellular immortality. The Ca2+ binding protein S100A8 can inhibit the activity of telomerase (Rosenberger et al. 2007).
Motility
Ca2+ has been associated with motility for a long time since Sidney Ringer found that it was the Ca2+ in London's “hard” tap water that was required for heart contraction (Ringer 1883). The intracellular Ca2+ signaling plays important role in cellular motility such as during tumor invasion and metastasis, the extracellular Ca2+ signaling has been shown to be associated with bone metastasis (Amuthan et al. 2002; Berridge et al. 2003; Huang et al. 2004; Zhong et al. 2010).
Apoptosis
The increase in [Ca2+]i and [Ca2+]mito and the activation of mitochondrial membrane permeabilization can lead to apoptosis and necrosis (Rizzuto and Pozzan 2006; Rizzuto et al. 2003). Similarly, a decrease in the Ca2+ content of the lumen of the ER is associated with resistance to apoptosis (Rizzuto et al. 2003; Pinton and Rizzuto 2006).
Ca2+ signaling initiation
In order to maintain low [Ca2+]i, the ATPase pump such as sarcoendoplasmic reticular Ca2+ ATPase (SERCA) pumps in Ca2+- into ER by exchanging protons for two Ca2+ per ATP hydrolyzed whereas plasma membrane Ca2+-ATPase (PMCA) removes Ca2+ by exchanging protons for 1 Ca2+ per ATP hydrolyzed (Figure 1). In addition, the Na+/Ca2+ exchangers (NCX), and the Na+/Ca2+-K+ exchangers (NCKX) exchange one Ca2+ ion for three Na+ ions or cotransport one K+ ion with one Ca2+ ion in exchange for four Na+ ions, respectively. In its “forward” mode, inward (depolarizing) Na+ current drives Ca2+ extrusion from the cell. While the PMCAs are effective in maintaining low [Ca2+]i over longer durations, NCX and NCKX function at a much faster rate to maintain low [Ca2+]i (Hilgemann et al. 2006). Transient receptor potential (TRP) ion channels formed by tetrameric assembly around a pore, are weakly voltage-sensitive, nonselective ion channels (Goswami et al. 2010; Ramsey et al. 2006). TRP channels depolarize cells and increase intracellular Na+ and Ca2+. TRP channels are greatly potentiated by phospholipase C (PLC) activation by G protein-coupled receptors (GPCRs) or tyrosine-kinase receptors (TKR).
The voltage dependent Ca2+ channels (CaVs) are the fastest Ca2+ signaling proteins and each CaV channel conducts approximately one million Ca2+ ions per second down the 20 000-fold gradient therefore a few thousand CaV channels/cell can increase [Ca2+]i by more than 10 fold within milliseconds (Fig.1). A change in membrane potential opens the CaV “gate” and allows Ca2+ to move into the cytoplasm. In the presence of normal extracellular [Ca2+]o, the binding of Ca2+ to CaV provides ion selectivity to enter the pore (Gouaux and Mackinnon 2005; Long et al. 2005). However, when external Ca2+ is removed, these Ca2+ channels become nonselective and allow the entry of Na+ and K+ across the cell membrane. The increases in [Ca2+]i can initiate translocation of several proteins, e.g., protein kinase C family proteins to specific regions of membranes. Another Ca2+-dependent membrane targeting scheme is used by annexins, where phosphoryl moieties of the membrane replace charge from carbonyl oxygens and water in a unique Ca2+-binding fold (Gerke et al. 2005).
The highly negatively charged phosphatidylinositol 4, 5-bisphosphate (PIP2) is bound to inner leaflets of plasma membranes by its acyl chains (see Fig.1). The positively charged residues on many peripheral and integral membrane proteins can attract negatively charged PIP2, leading to increase in the local concentration of PIP2 that attracts the positively charged proteins cluster close to the membrane. The negatively charged calmodulin present in the cytoplasm competes with PIP2 and may pull the positively charged protein segment off the membrane (McLaughlin and Murray 2005).
A universal mechanism for Ca2+ signaling is the release of Ca2+ from intracellular Ca2+ stores such as ER or SR (Fig.1). G-protein coupled receptors (GPCRs), primarily Gq/11 subtypes can activate phospholipase Cβ (PLCβ) and tyrosine kinase receptors (TKR) can activate PLCγ which then cleave PIP2 into 1,4,5-inositol trisphosphate (IP3) and diacylglycerol (DAG). IP3 binding to the IP3 receptors (IP3R) that are present in the ER causes efflux of Ca2+ from the ER to the cytoplasm resulting in increase in [Ca2+]i from ~100 nM to ~1 μM for several seconds (Berridge 2009; Mikoshiba 2007). The binding of Ca2+ to the C2 domain of PKC α, β1, β2, and γ subtypes initiates translocation to the membrane, where coincident DAG binding activates it. Ca2+- sensitive DAG kinase phosphorylates DAG to produce phosphatidic acid, while DAG lipase converts DAG to arachidonic acid. (Fig.1). IP3R mediated efflux of Ca2+ from the ER in response receptor activation empties the ER as PMCAs pump Ca2+ out of the cell faster than it can be repleted. Slowly over seconds after such store depletion, a Ca2+ entry mechanism is activated. This mechanism is called store-operated Ca2+ entry (Putney 2005). A slow and tiny but highly selective Ca2+ conductance is activated when ER [Ca2+] drops and it is called Ca2+-release activated current (ICRAC) (Parekh and Penner 1997).
IP3Rs are nonselective cationic channels that conduct Ca2+. The IP3R complex is a massive tetrameric channel with its pore formed by a homotetramers of ~3,000 amino acids. The six transmembrane-spanning domain is at the very C-terminal end of each subunit, that provides for a large number of cytoplasmic regulatory sites and protein-binding domains. Regulated by an intrinsic suppressor domain, a hinged clamshell-like structure clasps IP3. Many proteins such as IP3R -binding protein (IRBIT) released with IP3 are proposed to interact with the IP3R whereas binding of ERp44 confers redox sensing (Mikoshiba 2007). Like the IP3R, ryanodine receptors (RyRs) are also massive tetrameric (2.2 mDa) channels permeant to Ca2+ that span the ER or SR. The primary natural agonist of RyR is Ca2+, low [Ca2+]i opens the channel to allow Ca2+ to flow out of the ER/SR whereas higher [Ca2+]i near the openings of channel inhibits Ca2+ gating and thus prevents [Ca2+]i overload. Approximately 80% of the RyR's mass is located towards cytoplasm, where it interacts with several proteins such as Ca/CaM/CaMKII, FK-506-binding proteins, mAKAP/PKA, PR130/calcineurin, spinophilin, and sorcin. Several other proteins such as triadin, junctin, and calsequestrin also regulate ER Ca2+ availability to the pore (Bers 2004). IP3Rs and/or RyRs are also sensitive to the redox status of cells, to nitric oxide/Snitrosylation, and to quinones/reactive oxygen species (Waring 2005).
Calcium waves in signaling
The release of Ca2+ from the ER is a nonlinear, cooperative process wherein IP3 binds to four receptor sites on the IP3R, one on each subunit of the tetramer (Mikoshiba 2007). In both ER and SR, IP3Rs and RyRs are at first potentiated, then inhibited by Ca2+. Small perturbations in conditions, such as ambient [Ca2+]i, [IP3], and various regulators can cause uncoordinated bursts of local release across a cell (called “sparks” for their appearance in Ca2+ imaging fluorescence microscopy (see Fig. 2; Guatimosim et al. 2002). The brief opening of these channels gives rise to localized pulses (Fig. 2) such as the sparks or blips and puffs (Cheng et al. 1993; Parker and Yao 1996; Yao et al. 1995). The appearance of sparks or blips and puffs results in increase in the [Ca2+]i (Bootman and Berridge 1996; Parker and Yao 1996). At higher levels of IP3, the blips grow into larger puffs that can act as initiation sites for the onset of Ca2+ waves (Fig. 2). If the IP3R are sufficiently sensitized, they will respond to the Ca2+ diffusing away from a puff site and thereby propagate the signal through a process of calcium-induced calcium release (CICR) as depicted in Fig. 2. These waves are the mechanisms that coordinate the release of Ca2+ by all the IP3R. Therefore, increasing spark frequency can cascade and become regenerative and is seen as two- or three dimensional waves of changes in [Ca2+]i that propagate within cells (Fig.2). These Ca2+ waves can interact with each other and create spirals or annihilate each other. The information transmitted by these Ca2+ waves can arrive as a stimulus at the plasma membrane and is translated into intracellular Ca2+ oscillations (Falcke 2004).
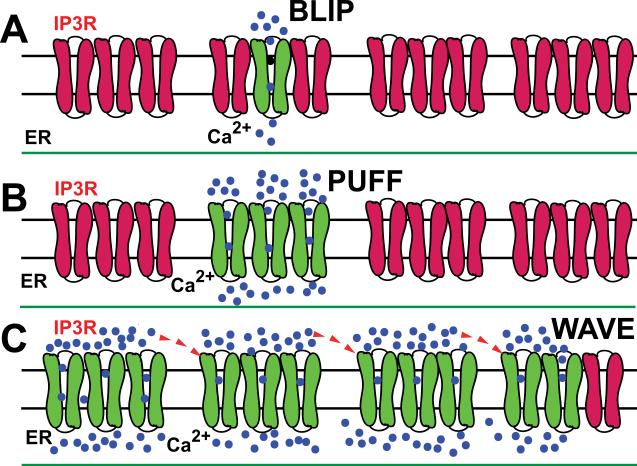
FIG. 2.
Elementary and global events of calcium signaling by IP3 in a dose-dependent manner. Inositol trisphosphate receptors (IP3R) are shown arranged in clusters that form Ca2+ release sites in the ER. A: at low IP3 concentration, a small number of IP3R (shown in green) release Ca2+ (shown in blue) into the cytoplasm as elementary event called “blip” whereas others IP3R (shown in dark red) are not bound with IP3 and therefore are not active. B: at intermediate concentration of IP3, a group of IP3R opens to release Ca2+ to form “puff” which remains local because the neighboring IP3Rs are not active. C: at higher concentrations of IP3, the activation of a large number of IP3R leads to formation and global propagation of Ca2+ waves. Ca2+ released at one cluster can trigger Ca2+ release at adjacent clusters by calcium-induced calcium release (CICR) that leads to the formation of Ca2+ waves which propagate by successive cycles of Ca2+ release, diffusion, and CICR.
The smallest Ca2+ release events, blips (Parker et al. 1996), probably reflect random openings of single IP3R (Fig. 2). Larger events, puffs, lasting tens of milliseconds and restricted to a volume of ~ 0.5 fl, reflect the almost simultaneous opening of a few IP3Rs within a cluster (Parker et al. 1996; Suhara et al. 2006). Several coordinated puffs can form oscillations and waves as shown in Fig. 2 (Falcke 2004). Ca2+ oscillations, therefore, depend upon both the spatial organization of IP3Rs and their regulation by Ca2+ although the links between IP3R activities and Ca2+ oscillations are not fully understood (Taylor and Laude 2002).
How are random molecular events such as blips and puffs orchestrated into global intracellular Ca2+ oscillations? It is generally assumed if many molecules are involved in these processes, the cell behaves like a continuously stirred reactor and the law of large numbers can be used to predict such Ca2+ oscillations (van Kampen 2001). Though most models of the dynamics of intracellular Ca2+ release use the law of large numbers yet it is not consistent with experimental analyses showing that global oscillations arise from Ca2+ waves initiated locally (Falcke 2004; Marchant and Parker 2001). Therefore, such a local initiation of Ca2+ waves is predicted to lead to stochastic oscillations because although each cell has many IP3Rs and Ca2+ ions, the law of large numbers does not apply to the initiating event which is restricted to very few IP3Rs due to steep Ca2+ concentration gradients (Falcke 2003, 2004).
The stochastic nature of the release of Ca2+ from internal calcium stores such as ER into the cytoplasm have been studied by several investigators (Cheng et al. 1993). All ion channels have certain properties such as open or closed, conducting or non-conducting, with opening and closing describable as Markov processes. The stochastic nature of Ca2+ release is nicely shown by the observed onset of whole-cell Ca2+ oscillations as [IP3] is increased in Xenopus oocytes (Marchant and Parker 2001). While at low [IP3], only puffs are observed because there is not enough Ca2+ released from a cluster to stimulate Ca2+ release from neighboring sites, therefore the Ca2+ transient is purely local (see Fig. 2). At moderately higher [IP3], the amount of Ca2+ released from each IP3R increases, leading to formation of Ca2+ waves that emerge from a nucleation site. However, at moderate levels of [IP3], global events are rare and in many cases there are waves that progress only a short distance before dying out. However, at very high [IP3], global Ca2+ waves are seen to occur regularly with a well-defined period.
The changes in the cytoplasmic Ca2+ can be defined as follows (Falcke 2004):
| (eq. 1) |
where [Ca2+]i is the cytoplasmic concentration of Ca2+, Jrel is the Ca2+ flux (in units of concentration per unit time) into the cytoplasm through release from calcium stores and Juptake is the flux of Ca2+ via uptake into the calcium stores. The Ca2+ release flux Jrel can be described by the following equation:
| (eq. 2) |
where Po is the open probability, g is the maximal conductance, and the Ca2+ channel driving force [Ca2+]ER − [Ca2+]i, is difference between ER calcium [Ca2+]ER and cytoplasmic calcium [Ca2+]i. To account for the stochastic and spatial nature of the receptors, a more accurate model can be described by the following equation:
| (eq. 3) |
where Jkrel represents the release of calcium from the kth release unit and xk represents the spatial location of single channels or small clusters of channels. Jkrel can be described by the following equation:
| (eq. 4) |
where pk is a random variable with values 0 or 1 to indicate whether the release unit is closed or open. In presence of a very large number of Ca2+ release sites, pk can be replaced by the average open probability Po and thus obtain the whole-cell model in the limit that diffusion is large compared to the total size of the cell. However, these assumptions are not valid in many physiological situations, such as in oocytes and pancreatic beta cells (Sherman and Rinzel 1991). Although it is possible to use Monte Carlo simulations to describe the random behavior of Ca2+ release events, such computations may not provide correct answers in multicellular settings (Bugrim et al. 1997; Falcke 2003).
Cell-to-Cell Signaling
The Ca2+ signaling among cells can occur in two ways. In the first process, the cells can use gap junction (connexin) channels to communicate with one another (Rosselleo et al. 2009). In the second process, the cell-to-cell signaling is performed by transmitter-gated Ca2+ permeant ion channels such as NMDA, nicotinic, purinergic ionotropic channels. The opening of CaV can rapidly increase the local periplasmic [Ca2+] resulting in triggering of protein-fusion complexes such as synaptotagmins and SNARE complexes, and fusion of vesicles containing transmitter molecules with the plasma membrane. These transmitter molecules such as ATP, acetylcholine, or glutamate that are released outside the cell could gate Ca2+-permeant channels namely P2X, nicotinic receptors, and NMDA receptors on adjacent cell membranes (Block et al. 2010; Jahn and Scheller 2006; Rosselleo et al. 2009; Sudhof 2004).
Remodeling of Ca2+ signaling in cancer cell proliferation
Ca2+ plays important role throughout the mammalian cell cycle such as in early G1, at the G1/S, and G2/M transitions (Fig. 3). The expression of immediate-early genes in G1, such as FOS, JUN and MYC, and the retinoblastoma (RB1) phosphorylation at the G1/S boundary are regulated by Ca2+ (Pande et al. 1996; Takuwa et al. 1996). Similarly, the Ca2+ binding protein calmodulin (CaM) plays crucial roles in cell cycle progression through G1 and mitosis (Kahl and Means 2003; Rasmussen and Means 1989). It has been shown that the cells can be arrested in G1 stage by inhibition of CaM kinase (CaMK) leading to loss of cyclin D1 (CCND1) expression, and inhibition of cyclin-dependent kinase 4 (CDK4) and CDK2 (Fig. 3). Another target of CaM, calcineurin also plays a major role in the cell cycle progression through G1 and S phases by regulating cAMP-responsive element binding protein 1 (CREB1) and NFAT (Kahl and Means 2004). In its inactive phosphorylated state, NFAT localizes in the cytoplasm, but following an increase in [Ca2+]i, the activated calcineurin dephosphorylates NFAT, which then translocates to the nucleus and regulates expression of its target genes (see Fig. 4). The influx of Ca2+ through plasma membrane channels such as the store-operated channel ORAI1 as well as efflux of Ca2+ from the ER can activate calcineurin and NFAT signaling pathway for inducing changes in cell cycle gene expression (Fig. 4; Gwack et al. 2007).
Fig. 3.
Regulation of cell cycle by Ca2+. Ca2+ signaling at various key stages of the cell cycle plays crucial roles such as the activation and expression of transcription factors FOS and JUN, CREB) and NFAT as the cells enter the G1 phase. These transcription factors in turn regulate the D-type cyclins, required for activation of cyclin D-CDK4 complexes (D-K4). Later in G1 phase of cell cycle, Ca2+ plays an important role in the activity of D-K4 and E-K2 complexes required for phosphorylation and consequently inactivation of retinoblastoma (RB) that causes cells to progress to S phase. During the G1/S and G2/M transitory phases of cell cycle, the Ca2+ oscillations play important roles in centrosome duplication, maturation, and separation in cytokinesis.
Fig. 4.
Ca2+ waves activate transcription factor NFAT that in turn controls cell cycle. The changes in Ca2+ waves oscillation frequency can activate transcription factors such as transcription factor NFAT resulting in regulation of cellular transcription. Calcineurin plays a major role in the cell cycle progression through G1 and S phases by regulating CREB1 and the nuclear NFAT. In its inactive phosphorylated state, NFATs localizes in the cytoplasm, but following an increase in [Ca2+]i, the activated calcineurin dephosphorylates NFAT which then translocates to the nucleus and regulates expression of its target genes. The influx of Ca2+ through plasma membrane channels such as the store-operated channel ORAI1 as well as efflux of Ca2+ from the ER can activate calcineurin and NFAT signaling pathway for inducing changes in cell cycle gene expression. Experiments using the “calcium clamp” have shown that the sensitivity of different transcription factors to [Ca2+]i oscillations is highly frequency dependent.
In prostate cancer, it has been shown that the increased expression of TRPV6, a constitutively active Ca2+ channel, resulted in increased Ca2+ entry and therefore enhanced NFAT activation (Lehen'kyi et al. 2007). TRPV6 expression in high-grade prostate cancer can therefore act as biomarker of prognosis (Fixemer et al. 2003). Similarly, the overexpression of a constitutively active NFATC1 mutant in 3T3-L1 fibroblasts induced the expression of MYC, cyclins D1 and D2 (CCND2) resulting in a transformed phenotype (Buchholz et al. 2006). Since cyclin E and E2F are transcriptional targets of MYC, the NFAT-MYC signaling pathway connecting calcineurin and Ca2+ is crucial in cell cycle and provides for a very important role of Ca2+ (see Fig. 4).
Although the Ca2+-dependent signaling mechanisms are frequently remodeled or deregulated in cancer cells, the lack of studies showing mutations in genes associated with the Ca2+ regulating proteins may indicate that remodeling of Ca2+ signaling could arise due to epigenetic changes in gene expression and/or post-translational changes (Table 1) in the properties of existing signaling components (Monteith et al. 2007; Saidak et al. 2009).
Table 1.
The changes in the expression of Ca2+ channels and pumps in cancer.
| Ca2+ Channel/pump | Type of Cancer | mRNA level | Protein level | Reference |
|---|---|---|---|---|
| A. SERCA Pumps | ||||
| 1. SERCA2 | Colon and Lung cancer | ↓ | ND | Korosec et al. 2006 |
| Thyroid cancer | ↓ | ↓ | Pacifico et al., 2003 | |
| Oral cancer; squamous cell carcinoma | ↓ | ↓ | Endo et al., 2004 | |
| Colorectal cancer | ↑ | ND | Chung et al., 2006 | |
| 2. SERCA3 | Colon cancer | ND | ↓ | Brouland et al.,2005 |
| B. PMCA Pumps | ||||
| 1. PMCA | Skin and lung cancer | ND | ↓ | Reisner et al., 1997 |
| 2. PMCA1 | Oral cancer; squamous cell carcinoma | ↓ | ↓ | Saito et al., 2006 |
| Breast cancer | ↑ | ND | Lee et al., 2002 | |
| Skin cancer | ↓ | ND | Reisner et al., 1997 | |
| 3. PMCA2 | Breast cancer | ↑ | ND | Lee et al., 2005 |
| 4. PMCA4 | Skin cancer | ↓ | ND | Reisner et al., 1997 |
| C. IP3R/RyRs | ||||
| IP3R2 | Non-small-cell lung cancer | ↑ | ND | Heighway et al., 1996 |
| IP3R3 | Gastric cancer | ↑ | ↑ | Sakakura et al.,2003 |
| RyR1 | Thyoma | ↓ | ND | Kusner et al., 1998 |
| D. Voltage-gated Channels | ||||
| CaV1.2 | Colon cancer | ↑ | ND | Wang et al., 2000 |
| CaV1.1 | Colorectal cancer | ↑ | ND | Zhang et al., 1997 |
| CaV3.1 | Glioma | ↑ | ND | Latour et al., 2004 |
| Colorectal cancer | ↓ | ND | Toyota et al., 1999 | |
| CaV3.3 | Colon cancer | ↓ | ND | Paz et al., 2003 |
| E. TRP Channels | ||||
| TRPV1 | Bladder cancer | ND | ↓ | Lazzeri et al., 2005 |
| Glioma | ↑ | ↑ | Contassot et al., 2004 | |
| TRPV6 | Prostate cancer | ↑ | ND | Fixemer et al., 2003 |
| Breast, ovary, thyroid, and colon cancers | ↑ | ND | Zhuang et al., 2002 | |
| TRPM1 | Melanoma | ↓ | ND | Deeds et al., 2000 |
| TRPM8 | Prostate cancer | ↑ | ND | Schmidt et al., 2006 |
| Colorectal cancer | ↑ | ND | Tsavler et al., 2001 | |
Remodeling of Ca2+ signaling in cancer cell survival and death
Several studies have shown higher levels of [Ca2+]i in activation and execution of cell death (Szado et al. 2008). However, through increased expression of pro-survival signaling proteins such as the anti-apoptotic BCL2 family members and suppression of death signaling pathways, the cancer cell can survive in presence of death-inducing stimuli (Edinger and Thompson 2004; Hanahan and Weiberg 2000).
By virtue of regulating the levels of [Ca2+]i, the two organelles namely ER and mitochondria play crucial roles in Ca2+ signaling and deciding the ultimate fate of the cell. The same Ca2+ efflux from ER that is responsible for regulating processes for maintaining life, could act as a death-inducing signal at higher efflux of Ca2+ (Scorrano et al. 2003). It has been shown that in cells in which IP3R expression was either reduced or completely silenced, showed significantly less apoptosis (Jayaraman and Marks 1997). Similarly, the decrease in the level of basal IP3 also prevented cell death (Szado et al. 2008). The higher levels of [Ca2+]i resulting due to increased efflux of Ca2+ from the IP3R activity, in turn can cause cell death through cytochrome c binding to IP3R or due to the activation of caspases (Aseefa et al. 2003). Similarly the activation of RyRs can generate increased levels of cytoplasmic Ca2+ that can participate in cell apoptosis (Hajnoczky et al., 2000). The targeting of mitochondrial networks by the proximity of Ca2+ signals arising from the ER can play important functions in cell death (Csordas et al., 2006).
In order for the cancer cells to proliferate at higher rates and still protect themselves from apoptosis, the cancer cells need to remodel the Ca2+ signaling machinery. Since ER and mitochondria play significant roles in the regulation of cell proliferation and apoptosis, the remodeling of Ca2+ signaling machinery in ER and mitochondria in cancer cells seems imminent during oncogenic transformation. In addition, in the cancer cells the increased expression of anti-apoptotic members of the BCL2 family of proteins, or decreased expression of the pro-apoptotic BH3-only proteins or BAX or BAK can protect these cells from apoptosis by modulating intracellular Ca2+ signals (Rong and Distelhorst 2008; Scorrano et al. 2003). It has been shown that BCL2 family of proteins can decrease the level of Ca2+ fluxes from the ER either by binding to IP3R or by decreasing the Ca2+ content in the lumen of ER through inhibition of SERCA2 (Chen et al. 2004). In addition BCL2 can decrease the sensitivity of the mitochondrial Ca2+ uptake process as well as increasing their capacity to accumulate more Ca2+ (Danial and Korsmeyer 2004). Scorrano et al. (2003) have shown decreased level of ER Ca2+ and Ca2+ signals in apoptosis-resistant Bax- and Bak-knockout mouse embryonic fibroblasts.
Alterations in Ca2+ channels and pumps in cancer
Since Ca2+ channels or pumps involved in regulating Ca2+ signaling pathways show altered expression in cancer, one can target these Ca2+ channels and pumps as therapeutic options to downregulate proliferation of cancer cells and enhance their apoptosis. Table 1 shows a comprehensive list of Ca2+ transport proteins such as specific isoforms of active transport Ca2+ pumps such as the PMCAs and the SERCAs and Ca2+ channels, including voltage-gated and those of the TRP class and ATP-activated P2X ion channel family that are altered in cancer. The altered expression or activity of Ca2+ channels and pumps could either cause or promote cancers through modulation of Ca2+ concentrations in cytoplasm, ER/SR, and mitochondria as well as spatio-temporal nature of Ca2+ signaling (Berridge et al. 2003; Berridge 2009; Mikoshiba 2007). The overexpression of proteins such as IP3R Ca2+ release channels that can cause an increase in Ca2+ leakage from the ER, thereby reducing the Ca2+ content of the ER Ca2+ stores or reduced sequestration of Ca2+ as a consequence of lower levels of SERCA2, could lead to decrease in the apoptotic rates (Rizzuto et al. 2003).
In addition, the Ca2+ channels and pumps can directly modify the transcription of genes and their products, e.g., the proteolytically cleaved 75 kDa C-terminal fragment of CaV1.2, an L-type voltage gated Ca2+ channel termed calcium channel associated transcriptional regulator or CCAT translocates to the nucleus and alters the transcription of several genes such as Myc, Bcl-associated death promoter (Bad) and artemin (Gomez-Ospina et al. 2006). Nuclear CCAT protein levels increase or decrease in response to low and high intracellular Ca2+, respectively (Chung and Jan 2006; Gomez-Ospina et al. 2006). The isoform-specific upregulation of the mRNA and protein of the plasma membrane Ca2+ pump, PMCA4 leads to differentiation of a human colon cancer cell line (Aung et al. 2007). In colon cancer, SERCA3 protein expression is either reduced or completely silenced compared with normal tissue resulting in a loss of differentiation in tumor cells (Gelebart et al., 2002). Similarly, as a result of decrease in SERCA2b expression, androgen-sensitive prostate cancer epithelial cells become androgen insensitive as a result of neuroendocrine differentiation process (Vanoverberghe et al. 2004).
Therapeutic targeting of Ca2+ signaling in cancer
In normal cells, the Ca2+ signaling is highly regulated spatially such as between ER and mitochondria, between PMCA and ER, including the spatial role played by the cytoskeletal elements and temporally in terms of Ca2+ oscillations and Ca2+ wave frequencies, amplitudes, and durations (Berridge et al. 2003; Rizzuto and Pozzan 2006; Berridge 1997). However, in cancer cells, such a spatio-temporal regulation of Ca2+ signaling is significantly modulated in terms of frequencies, amplitudes and duration of Ca2+ signals. Therefore, specific targeting of Ca2+ channels or pumps with restricted tissue distribution, altered expression in cancer and/or a role in the regulation of tumorigenic pathways, could specifically disrupt Ca2+ homeostasis in cancer cells. Treating both normal and cancer cells with an agent that disrupts these pathways may kill the cancer cell, owing to the loss of redundancy (Ding et al. 2010). One of the approaches in this direction involves selectively altering the activity of the Ca2+ channel or pump so as to inhibit Ca2+-dependent tumorigenic pathways such as in cell proliferation. It is also possible to exploit the altered expression of the Ca2+ channel or pump in cancer cells such as by using an activator of a PMCA that is overexpressed in a cancer cell, Ca2+ influx into the cell can then be increased that can lead to activation of cell death pathways and/or disruption of cell-cycle progression. Such a strategy has been adopted in the control of LNCaP prostate cancer cells by use of menthol as an activator of TRPM8 (Mahieu et al. 2007). In addition, by using inhibitors of pumps that either sequester Ca2+ into intracellular stores or increase Ca2+ efflux across the plasma membrane can lead to excessive [Ca2+]i as well as excessive mitochondrial Ca2+ uptake resulting in cell death. An improved knowledge of the functioning of Ca2+ channels, pumps, and exchangers will help in treating cancer (Berridge 1997, 2003; Rizzuto and Pozzan 2006; Wang et al. 2010; Zhong et al. 2010).
Conclusions
The intracellular Ca2+ waves generated as a result of concerted activities of several cellular Ca2+ channels and pumps provides for a very effective and fast signaling that is needed at every step of the way in the day-to-day life of a living organism. The living cells have evolved to encode these Ca2+ waves as calcium code for information transfer in terms of the amplitude, duration, frequency, waveform or timing of Ca2+ oscillations that are then decoded at a later stage of signal transduction. In cancer cells, the altered expression of these Ca2+ channels and pumps cause alterations in Ca2+ wave characteristics resulting in a modified calcium code. Therefore, one can target these Ca2+ channels and pumps as therapeutic options to decrease cancer cell proliferation and increase cancer cell apoptosis. Such novel and highly innovative strategies can provide rationale and approaches for the design and development of novel technologies based on Ca2+ waves for the diagnosis and treatment of cancer.
Supplementary Material
Acknowledgements
The authors acknowledge the Florida International University Foundation's Faculty Research Award to J.P., and the Research Conference Awards from the National Institutes of Health, Flight Attendant Medical Research Institute, Society for Free Radical Research International and the Oxygen Club of California to K.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amuthan G, et al. Mitochondrial stress-induced calcium signaling, phenotypic changes and invasive behavior in human lung carcinoma A549 cells. Oncogene. 2002;21:7839–7849. doi: 10.1038/sj.onc.1205983. [DOI] [PubMed] [Google Scholar]
- Assefa Z, et al. Caspase 3induced truncation of type 1 inositol trisphosphate receptor accelerates apoptotic cell death and induces inositol trisphosphate independent calcium release during apoptosis. Journal of Biological Chemistry. 2004;279:43227–43236. doi: 10.1074/jbc.M403872200. [DOI] [PubMed] [Google Scholar]
- Aung CS, Kruger WA, Poronnick P, Roberts-Thomson SJ, Monteith GR. Plasma membrane Ca2+-ATPase expression during colon cancer cell line differentiation. Biochemical Biophysical Research Communication. 2007;355:932–936. doi: 10.1016/j.bbrc.2007.02.050. [DOI] [PubMed] [Google Scholar]
- Bentle MS, Reinicke KE, Bey EA, Spitz DR, Boothman DA. Calcium-dependent modulation of poly(ADP-ribose) polymerase-1 alters cellular metabolism and DNA repair. Journal of Biological Chemistry. 2006;281:33684–33696. doi: 10.1074/jbc.M603678200. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and Ca2+ signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Unlocking the secrets of cell signaling. Annual Review of Physiology. 2005;67:1–21. doi: 10.1146/annurev.physiol.67.040103.152647. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signaling mechanisms. Biochimica et Biophysica Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature Reviews on Molecular and Cell Biology. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. Journal of Molecular Cell Cardiology. 2004;37:417–429. doi: 10.1016/j.yjmcc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Oda Y, Xie Z. Calcium and 1, 25(OH)2D: interacting drivers of epidermal differentiation. Journal of Steroid Biochemistry and Molecular Biology. 2004;89–90:355–360. doi: 10.1016/j.jsbmb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Block GJ, DiMattia GD, Prockop DJ. Stanniocalcin-1 regulates extracellular ATP-induced calcium waves in human epithelial cancer cells by stimulating ATP release from bystander cells. PLos One. 2010;5(4):e10237. doi: 10.1371/journal.pone.0010237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Berridge MJ. Sub-cellular Ca2+ signals underlying waves and graded responses in HeLa cells. Current Biology. 1996;6:855–865. doi: 10.1016/s0960-9822(02)00609-7. [DOI] [PubMed] [Google Scholar]
- Brouland JP, et al. The loss of sarco/endoplasmic reticulum calcium transport ATPase 3 expression is an early event during the multistep process of colon carcinogenesis. American Journal of Surgical Pathology. 2005;167:233–242. doi: 10.1016/S0002-9440(10)62968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz M, et al. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO Journal. 2006;25:3714–3724. doi: 10.1038/sj.emboj.7601246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugrim AE, Zhabotinsky AM, Epstein IR. Calcium waves in a model with a randomly spatially discrete distribution of Ca2+ release sites. Biophysical Journal. 1997;73:2897–2906. doi: 10.1016/S0006-3495(97)78318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. Special issue: calcium signaling and disease. Biochemical Biophysical Research Communication. 2004;322:1097. [Google Scholar]
- Carafoli E. Calcium signaling: a tale for all seasons. Proceedings of National Academy of Sciences USA. 2002;99:1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, et al. Bcl-2 functionally interacts with inositol 1, 4, 5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1, 4, 5- trisphosphate. Journal of Cell Biology. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Chung FY, et al. Sarco/endoplasmic reticulum calcium- ATPase 2 expression as a tumor marker in colorectal cancer. American Journal of Surgical Pathology. 2006;30:969–974. doi: 10.1097/00000478-200608000-00006. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Jan LY. Channeling to the nucleus. Neuron. 2006;52:937–940. doi: 10.1016/j.neuron.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Clapham D. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Contassot E, et al. Arachidonylethanolamide induces apoptosis of human glioma cells through vanilloid receptor-1. Journal of Neuropathology and Experimantal Neurology. 2004;63:956–963. doi: 10.1093/jnen/63.9.956. [DOI] [PubMed] [Google Scholar]
- Cook SJ, Lockyer PJ. Recent advances in Ca2+- dependent Ras regulation and cell proliferation. Cell Calcium. 2006;39:101–112. doi: 10.1016/j.ceca.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Csordas G, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. Journal of Cell Biology. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ, Lockyer PJ. Integration of calcium and Ras signalling. Nature Reviews on Molecular and Cell Biology. 2002;3:339–348. doi: 10.1038/nrm808. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Deeds J, Cronin F, Duncan LM. Patterns of melastatin mRNA expression in melanocytic tumors. Human Pathology. 2000;31:1346–1356. [PubMed] [Google Scholar]
- De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- DePaoli-Roach A, Roach PJ, Zucker KE, Smith SS. Selective phosphorylation of human DNA methyltransferase by protein kinase C. FEBS Letters. 1986;197:149–153. doi: 10.1016/0014-5793(86)80316-7. [DOI] [PubMed] [Google Scholar]
- Ding X, He Z, Zhou K, Cheng J, Yao H, Lu D, Cai R, Jin Y, Dong B, Xu Y, Wang Y. Essential role of TRPC6 channels in G2/M phase transition and development of human glioma. Journal of National Cancer Institute. 2010;102:1052–1068. doi: 10.1093/jnci/djq217. [DOI] [PubMed] [Google Scholar]
- Dolmetsch R. Excitation-transcription coupling: signaling by ion channels to the nucleus. Science STKE 2003, PE4. 2003 doi: 10.1126/stke.2003.166.pe4. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Current Opinion on Cell Biology. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Einstein A. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Annalen der Physik. 1905;17:549–560. [Google Scholar]
- Endo Y, et al. Sarcoendoplasmic reticulum Ca2+-ATPase type 2 downregulated in human oral squamous cell carcinoma. International Journal of Cancer. 2004;110:225–231. doi: 10.1002/ijc.20118. [DOI] [PubMed] [Google Scholar]
- Falcke M. On the role of stochastic channel behavior in intracellular Ca2+ dynamics. Biophysical Journal. 2003;84:42–56. doi: 10.1016/S0006-3495(03)74831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcke M. Reading the patterns in living cells- the physics of Ca2+ signalling. Advances in Physics. 2004;53:255–440. [Google Scholar]
- Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H. Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene. 2003;22:7858–7861. doi: 10.1038/sj.onc.1206895. [DOI] [PubMed] [Google Scholar]
- Futreal PA, Wooster R, Stratton MR. Somatic mutations in human cancer: insights from resequencing the protein kinase gene family. Cold Spring Harbor Symposium on Quantitative Biology. 2005;70:43–49. doi: 10.1101/sqb.2005.70.015. [DOI] [PubMed] [Google Scholar]
- Gelebart P, et al. Expression of endomembrane calcium pumps in colon and gastric cancer cells. Induction of SERCA3 expression during differentiation. Journal of Biological Chemistry. 2002;277:26310–26320. doi: 10.1074/jbc.M201747200. [DOI] [PubMed] [Google Scholar]
- Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nature Reviews on Molecular and Cell Biology. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage gated calcium channel Ca(V)1. 2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami C, Kuhn J, Heppenstall PA, Hucho T. Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PLoS One 19. 2010;5(7):e11654. doi: 10.1371/journal.pone.0011654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouaux E, Mackinnon R. Principles of selective ion transport in channels and pumps. Science. 2005;310:1461–1465. doi: 10.1126/science.1113666. [DOI] [PubMed] [Google Scholar]
- Guatimosim S, Dilly K, Santana LF, Saleet Jafri M, Sobie EA, Lederer WJ. Local Ca2+ signaling and EC coupling in heart: Ca2+ sparks and the regulation of the [Ca2+]i transient. Journal of Molecular Cell Cardioliology. 2002;34:941–950. doi: 10.1006/jmcc.2002.2032. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Csordas G, Madesh M, Pacher P. Control of apoptosis by IP3 and ryanodine receptor driven calcium signals. Cell Calcium. 2000;28:349–363. doi: 10.1054/ceca.2000.0169. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Heighway J, Betticher DC, Hoban PR, Altermatt HJ, Cowen R. Coamplification in tumors of KRAS2, type 2 inositol 1, 4, 5 triphosphate receptor gene, and a novel human gene, KRAG. Genomics. 1996;35:207–214. doi: 10.1006/geno.1996.0340. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Yaradanakul A, Wang Y, Fuster D. Molecular control of cardiac sodium homeostasis in health and disease. Journal of Cardiovascular Electrophysiology. 2006;17(Suppl 1):S47–S56. doi: 10.1111/j.1540-8167.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- Huang JB, Kindzelskii AL, Clark AJ, Petty HR. Identification of channels promoting calcium spikes and waves in HT1080 tumor cells: their apparent roles in cell motility and invasion. Cancer Research. 2004;64:2482–2489. doi: 10.1158/0008-5472.can-03-3501. [DOI] [PubMed] [Google Scholar]
- Jaffe LF. A calcium based theory of carcinogenesis. Advances in Cancer Research. 2005;94:231–263. doi: 10.1016/S0065-230X(05)94006-2. [DOI] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs–engines for membrane fusion. Nature Reviews on Molecular and Cell Biology. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Jayaraman T, Marks AR. T cells deficient in inositol 1, 4, 5-trisphosphate receptor are resistant to apoptosis. Molecular Cell Biology. 1997;17:3005–3012. doi: 10.1128/mcb.17.6.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl CR, Means AR. Calcineurin regulates cyclin D1 accumulation in growth-stimulated fibroblasts. Molecular Biology of the Cell. 2004;15:1833–1842. doi: 10.1091/mbc.E03-10-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Korosec B, Glavac D, Rott T, Ravnik-Glavac M. Alterations in the ATP2A2 gene in correlation with colon and lung cancer. Cancer Genetics and Cytogenetics. 2006;171:105–111. doi: 10.1016/j.cancergencyto.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Kusner LL, Mygland A, Kaminski HJ. Ryanodine receptor gene expression thymomas. Muscle Nerve. 1998;21:1299–1303. doi: 10.1002/(sici)1097-4598(199810)21:10<1299::aid-mus8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Latour I, et al. Expression of T-type calcium channel splice variants in human glioma. Glia. 2004;48:112–119. doi: 10.1002/glia.20063. [DOI] [PubMed] [Google Scholar]
- Lazzeri M, et al. Transient receptor potential vanilloid type 1 (TRPV1) expression changes from normal urothelium to transitional cell carcinoma of human bladder. European Urology. 2005;48:691–698. doi: 10.1016/j.eururo.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Lee WJ, et al. Expression of plasma membrane calcium pump isoform mRNAs in breast cancer cell lines. Cell Signal. 2002;14:1015–1022. doi: 10.1016/s0898-6568(02)00049-9. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Roberts-Thomson SJ, Monteith GR. Plasma membrane calcium-ATPase 2 and 4 in human breast cancer cell lines. Biochememical Biophysical Research Communication. 2005;337:779–783. doi: 10.1016/j.bbrc.2005.09.119. [DOI] [PubMed] [Google Scholar]
- Lehen'kyi V, Flourakis M, Skryma R, Prevarskaya N. TRPV6 channel controls prostate cancer cell proliferation via Ca2+/NFAT-dependent pathways. Oncogene. 2007;26:7380–7385. doi: 10.1038/sj.onc.1210545. [DOI] [PubMed] [Google Scholar]
- Lewis RS. Calcium oscillations in T-cells: mechanisms and consequences for gene expression. Biochemical Society Transaction. 2003;31:925–929. doi: 10.1042/bst0310925. [DOI] [PubMed] [Google Scholar]
- Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- Long SB, Campbell EB, MacKinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- Mahieu F, et al. TRPM8-independent mentholinduced Ca2+ release from endoplasmic reticulum and golgi. Journal of Biological Chemistry. 2007;282:3325–3336. doi: 10.1074/jbc.M605213200. [DOI] [PubMed] [Google Scholar]
- Marchant J, Callamaras N, Parker I. Initiation of IP3-mediated Ca2+ waves in Xenopus oocytes. EMBO Journal. 1999;18:5285–5299. doi: 10.1093/emboj/18.19.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant J, Parker I. Role of elementary Ca2+ puffs in generating repetitive Ca2+ oscillations. EMBO Journal. 2001;20:65–76. doi: 10.1093/emboj/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- Mikoshiba K. IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. Journal of Neurochemistry. 2007;102:1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- Minaguchi T, Waite KA, Eng C. Nuclear localization of PTEN is regulated by Ca2+ through a tyrosil phosphorylation-independent conformational modification in major vault protein. Cancer Research. 2006;66:11677–11682. doi: 10.1158/0008-5472.CAN-06-2240. [DOI] [PubMed] [Google Scholar]
- Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ. Calcium and cancer: targeting Ca2+ transport. Nature Review on Cancer. 2007:519–530. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- Munaron L, Fiorio PA. Endothelial calcium machinery and angiogenesis: understanding physiology to interfere with pathology. Current Medicinal Chemistry. 2009;16:4691–4703. doi: 10.2174/092986709789878210. [DOI] [PubMed] [Google Scholar]
- Pacifico F, et al. The expression of the sarco/ endoplasmic reticulum Ca2+-ATPases in thyroid and its down-regulation following neoplastic transformation. Journal of Molecular Endocrinology. 2003;30:399–409. doi: 10.1677/jme.0.0300399. [DOI] [PubMed] [Google Scholar]
- Pande G, Kumar NA, Manogaran PS. Flow cytometric study of changes in the intracellular free calcium during the cell cycle. Cytometry. 1996;24:55–63. doi: 10.1002/(SICI)1097-0320(19960501)24:1<55::AID-CYTO7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiological Reviews. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Parker I, Choi J, Yao Y. Elementary events of InsP3- induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell Calcium. 1996;20:105–121. doi: 10.1016/s0143-4160(96)90100-1. [DOI] [PubMed] [Google Scholar]
- Parker I, Yao Y. Ca2+ transients associated with openings of inositol trisphosphate-gated channels in Xenopus oocytes. Journal of Physiology (London) 1996;491:663–668. doi: 10.1113/jphysiol.1996.sp021247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton AM, Kassis J, Doong H, Kohn EC. Calcium as a molecular target in angiogenesis. Current Pharmaceutical Design. 2003;9:543–551. doi: 10.2174/1381612033391559. [DOI] [PubMed] [Google Scholar]
- Paz MF, et al. Genetic unmasking of epigenetically silenced tumor suppressor genes in colon cancer cells deficient in DNA methyltransferases. Human Molecular Genetetics. 2003;12:2209–2219. doi: 10.1093/hmg/ddg226. [DOI] [PubMed] [Google Scholar]
- Petersen OH. Ca2+ signalling and Ca2+-activated ion channels in exocrine acinar cells. Cell Calcium. 2005;38:171–200. doi: 10.1016/j.ceca.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Pinton P, Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differentiation. 2006;13:1409–1418. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Regulation of CRAC channel activity by recruitment of silent channels to a high open-probability gating mode. Journal of General Physiology. 2006;128:373–386. doi: 10.1085/jgp.200609588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr Capacitative calcium entry: sensing the calcium stores. Journal of Cell Biology. 2005;169:381–382. doi: 10.1083/jcb.200503161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annual Reviews of Physiology. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Rasmussen CD, Means AR. Calmodulin is required for cell-cycle progression during G1 and mitosis. EMBO Journal. 1989;8:73–82. doi: 10.1002/j.1460-2075.1989.tb03350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner PD, Brandt PC, Vanaman TC. Analysis of plasma membrane Ca2+-ATPase expression in control and SV40-transformed human fibroblasts. Cell Calcium. 1997;21:53–62. doi: 10.1016/s0143-4160(97)90096-8. [DOI] [PubMed] [Google Scholar]
- Rey O, Young SH, Jacamo R, Moyer MP, Rozengurt E. Extracellular calcium sensing receptor stimulation in human colonic epithelial cells induces intracellular calcium oscillations and proliferation inhibition. Journal of Cell Physiology. 2010;225:73–83. doi: 10.1002/jcp.22198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribiczey P, et al. Isoform-specific up-regulation of plasma membrane Ca2+-ATPase expression during colon and gastric cancer cell differentiation. Cell Calcium. 2007;42:590–605. doi: 10.1016/j.ceca.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringer S. A further contribution regarding the influence of the different constituents of the blood on the contraction of the heart. Journal of Physiology. 1883;4:29–42.3. doi: 10.1113/jphysiol.1883.sp000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiological Reviews. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, et al. Calcium and apoptosis: facts and hypotheses. Oncogene. 2003;22:8619–8627. doi: 10.1038/sj.onc.1207105. [DOI] [PubMed] [Google Scholar]
- Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annual Review of Physiology. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- Rosenberger S, Thorey IS, Werner S, Boukamp P. A novel regulator of telomerase: S100A8 mediates differentiation-dependent and calcium-induced inhibition of telomerase activity in the human epidermal keratinocyte line HaCaT. Journal of Biological Chemistry. 2007;282:6126–6135. doi: 10.1074/jbc.M610529200. [DOI] [PubMed] [Google Scholar]
- Rossello RA, Wang Z, Kizana E, Krebsbach PH, Kohn DH. Connexin 43 as a signaling platform for increasing the volume and spatial distribution of regenerated tissue. Proceedings of National Academy of Sciences USA. 2009;106:13219–13224. doi: 10.1073/pnas.0902622106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidak Z, Mentaverri R, Brown EM. The role of the calcium-sensing receptor in the development and progression of cancer. Endocrinology Review. 2009;30:178–195. doi: 10.1210/er.2008-0041. [DOI] [PubMed] [Google Scholar]
- Saito K, et al. Plasma membrane Ca2+-ATPase isoform 1 down-regulated in human oral cancer. Oncology Report. 2006;15:49–55. [PubMed] [Google Scholar]
- Sakakura C, et al. Possible involvement of inositol-1,4,5-trisphosphate receptor type 3 (IP3R3) in the peritoneal dissemination of gastric cancers. Anticancer Research. 2003;23:3691–3697. [PubMed] [Google Scholar]
- Schmidt U, et al. Quantitative multi-gene expression profiling of primary prostate cancer. Prostate. 2006;66:1521–1534. doi: 10.1002/pros.20490. [DOI] [PubMed] [Google Scholar]
- Scorrano L, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Sherman A, Rinzel J. Model for synchronization of pancreatic β-cells by gap junction coupling. Biophysical Journal. 1991;59:547–559. doi: 10.1016/S0006-3495(91)82271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G, Dai L, Miura R, Sherman A. Asymptotic analysis of buffered calcium diffusion near a point source. SIAM Journal of Applied Mathematics. 2001;61:1816–1838. [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annual Reviews on Neurosciences. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Suhara W, Kobayashi M, Sagara H, Hamadad K, Goto T, Fujimoto I, Torimitsu K, Mikoshiba K. Visualization of inositol 1,4, 5-trisphosphate receptor by atomic force microscopy. Neuroscience Letters. 2006;391:102–107. doi: 10.1016/j.neulet.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Szado T, et al. Phosphorylation of inositol 1, 4, 5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proceedings of National Academy of Sciences USA. 2008;105:2427–2432. doi: 10.1073/pnas.0711324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa N, Zhou W, Kumada M, Takuwa Y. Ca2+-dependent stimulation of retinoblastoma gene product phosphorylation and p34cdc2 kinase activation in serum-stimulated human fibroblasts. Journal of Biological Chemistry. 1993;268:138–145. [PubMed] [Google Scholar]
- Taylor CW, Laude A. IP3 receptors and their regulation by calmodulin and cytosolic Ca2+ Cell Calcium. 2002;32:321–334. doi: 10.1016/s0143416002001859. [DOI] [PubMed] [Google Scholar]
- Toyota M, Ho C, Ohe-Toyota M, Baylin SB, Issa JP. Inactivation of CACNA1G, a T-type calcium channel gene, by aberrant methylation of its 5' CpG island in human tumors. Cancer Research. 1999;59:4535–4541. [PubMed] [Google Scholar]
- Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Research. 2001;61:3760–3769. [PubMed] [Google Scholar]
- van Kampen NG. Stochastic Processes in Physics and Chemistry. North-Holland, Amsterdam, The Netherlands: 2001. [Google Scholar]
- Vanoverberghe K, et al. Ca2+ homeostasis and apoptotic resistance of neuroendocrine-differentiated prostate cancer cells. Cell Death and Differentiation. 2004;11:321–330. doi: 10.1038/sj.cdd.4401375. [DOI] [PubMed] [Google Scholar]
- Wang Q, Symes AJ, Kane CA, Freeman A, Nariculam J, Munson P, Thrasivoulou C, Masters JR, Ahmed A. A novel role for wnt/ca signaling in actin cytoskeleton remodeling and cell motility in prostate cancer. PLOS One. 2010;5(5):e10456. doi: 10.1371/journal.pone.0010456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XT, et al. The mRNA of L-type calcium channel elevated in colon cancer: protein distribution in normal and cancerous colon. American Journal of Pathology. 2000;157:1549–1562. doi: 10.1016/S0002-9440(10)64792-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring P. Redox active calcium ion channels and cell death. Archives of Biochemistry and Biophysics. 2005;434:33–42. doi: 10.1016/j.abb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Weinstein IB. Cancer. Addiction to oncogenes — the Achilles heal of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- Westheimer FH. Why nature chose phosphates. Science. 1987;235:1173–1178. doi: 10.1126/science.2434996. [DOI] [PubMed] [Google Scholar]
- Whitfield JF. Calcium signals and cancer. Critical Reviews on Oncology. 1992;3:55–90. [PubMed] [Google Scholar]
- Yao Y, Choi J, Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. Journal of Physiology (London) 1995;482:533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Yeow WS, Zou C, Wassell R, Wang C, Pestell RG, Quong JN, Quong AA. Cyclin D1/cyclin-dependent kinase 4 interacts with filamin A and affects the migration and invasion potential of breast cancer cells. Cancer Research. 2010;70:2105–2114. doi: 10.1158/0008-5472.CAN-08-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L, et al. Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Laboratory Investigations. 2002;82:1755–1764. doi: 10.1097/01.lab.0000043910.41414.e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.