Abstract
Mesenchymal stem cells (MSCs) possess a set of several fairly unique properties which make them ideally suited both for cellular therapies/regenerative medicine, and as vehicles for gene and drug delivery. These include: 1) relative ease of isolation; 2) the ability to differentiate into a wide variety of seemingly functional cell types of both mesenchymal and non-mesenchymal origin; 3) the ability to be extensively expanded in culture without a loss of differentiative capacity; 4) they are not only hypoimmunogenic, but they produce immunosuppression upon transplantation; 5) their pronounced anti-inflammatory properties; and 6) their ability to home to damaged tissues, tumors, and metastases following in vivo administration. In this review, we summarize the latest research in the use of mesenchymal stem cells in regenerative medicine, as immunomodulatory/anti-inflammatory agents, and as vehicles for transferring both therapeutic genes in genetic disease and genes designed to destroy malignant cells.
Isolation and Characterization of MSC
In pioneering studies [1, 2] performed over 30 years ago, Friedenstein demonstrated that fibroblastoid cells obtained from the bone marrow were capable of transferring the hematopoietic microenvironment to ectopic sites, thus establishing the concept that the marrow microenvironment resided within the so-called stromal cells of the marrow. Years later, scientists began to explore the full potential of these microenvironmental cells, and results of these studies led to the realization that this population harbored cells with properties of true stem cells. These cells were officially termed mesenchymal stem cells (MSC) [3]. MSC are now known to make up a key part of the stromal microenvironment that supports the hematopoietic stem cell and drives the process of hematopoiesis. Despite their essential role within the bone marrow, MSC only comprise roughly 0.001–0.01% of cells within the marrow [4]. The most straightforward method for obtaining MSC is to simply rely on MSC’s plastic adherence and their ability to be passaged with trypsin to obtain a relatively morphologically homogeneous population of fibroblastic cells from a bulk mononuclear cell preparation within only two to three passages in culture [5–7]. While this method is certainly straightforward, true MSC account for only a small percentage of this highly heterogeneous population, making results obtained with cells prepared in this fashion difficult to interpret. To avoid this problem, numerous groups have worked to identify antigens that are unique to MSC. While there are currently no markers that specifically identify MSC, several markers have proven useful for obtaining highly enriched MSC populations. The first of these markers to be identified was Stro-1, an antibody that reacts with non-hematopoietic bone marrow stromal precursor cells [8]. Although the antigen recognized by this antibody has not yet been identified, we and others have found that by tri-labeling bone marrow cells with Stro-1, anti-CD45, and anti-GlyA, and selecting the Stro-1+CD45-GlyA- cells, it is possible to consistently obtain a homogeneous population that is highly enriched for MSC [9–15]. In addition to Stro-1, Table 1 provides a summary of some of the markers and characteristics which have been used to isolate MSC to date. As can be seen, human MSC do not express markers which have been associated with other stem cell populations (like hematopoietic stem cells) such as CD34, CD133, or c-kit. However, antibodies such as SB-10, SH2, SH3, and SH4 have been developed over the years and numerous surface antigens such CD13, CD29, CD44, CD63, CD73, CD90, CD166 have been used to attempt to isolate MSC [16–18]. Unfortunately, all of these antigens appear to be expressed on a wide range of cell types within the body in addition to MSC. This lack of a unique marker suggests that to obtain a pure population of MSC that are functionally homogeneous, investigators will likely either have to await the development of novel antibodies that recognize as yet unidentified antigens that are unique to primitive MSC, or employ strategies in which multiple antibodies are combined to allow for positive selection of MSC and depletion of cells of other lineages that share expression of the antigens recognized by the MSC antibody in question, as we have done with Stro-1, CD45, and GlyA.
Table 1.
Properties/Markers for Isolating/Identifying MSC
| Exhibited/Expressed by MSC |
|
|---|---|
| Plastic Adherence | Yes |
| Passagable with trypsin | Yes |
| Stro-1 | Yes |
| form CFU-F colonies | Yes |
| SB-10 | Yes |
| SH2 | Yes |
| SH3 | Yes |
| SH4 | Yes |
| CD13 | Yes |
| CD29 | Yes |
| CD44 | Yes |
| CD63 | Yes |
| CD73 | Yes |
| CD90 | Yes |
| CD166 | Yes |
| CD45 | No |
| Gly-A | No |
| CD34 | No |
| CD133 | No |
| c-kit | No |
Sources of MSC
Although much of the work to date has focused on MSC isolated from adult bone marrow, it is important to realize that cells that appear phenotypically and functionally to be MSC have now been isolated by our group and others from numerous tissues, including brain, liver, lung, fetal blood, umbilical cord blood, kidney, and even liposuction material [19–26]. The broad distribution of MSC throughout the body leads one to postulate that MSC are likely to play a critical role in organ homeostasis, perhaps providing supportive factors like in the bone marrow, and/or mediating maintenance/repair within their respective tissue. Importantly, although MSC from each of these tissues appear similar with respect to phenotype and overall differentiative potential, studies at the RNA and protein level have now revealed that subtle differences exist between MSC from these various tissues, with MSC from each tissue possessing a molecular fingerprint indicative of their tissue of origin [21, 22, 27–31]. Using a noninjury fetal sheep transplantation model, we showed that these differences result in a bias for human MSC to home to and give rise to cells of their tissue of origin in vivo [32, 33]. This suggests that the ideal source of MSC may differ depending on the specific disease to be treated and the desired target organ.
Despite the apparent presence of MSC within many of the major organs of the body, the relatively non-invasive fashion with which adipose tissue or bone marrow can be obtained, and the fact that both these tissues could readily be obtained from each patient to be treated, combine to suggest that these two tissues will likely be the predominant source of MSC employed in clinical applications in the foreseeable future. However, additional in vitro and in vivo experiments will likely have to be performed to rigorously assess the inherent safety of adipose tissue-derived MSC before these cells will see widespread clinical use, since at least one recent study has suggested that they may be inherently less genetically stable than MSC isolated from bone marrow [34]. Indeed, two other studies showed that, upon prolonged in vitro expansion, adipose tissue-derived MSC can exhibit aneuploidy [35, 36] and have now been shown to undergo transformation [37, 38], raising the possibility that these cells could prove tumorigenic when used in vivo. In stark contrast, however, are results of a recent study investigating the inherent safety of adipose-derived MSC. This new study has now shown that, even if genomic instability is intentionally induced with genotoxic agents, adipose tissue-derived MSC respond to this insult by undergoing terminal adipogenic differentiation rather than transformation [39]. While the reasons for the conflicting nature of the results from these different studies are not currently known, one can speculate that differing methods employed for isolating and culturing MSC, differing levels of contaminating non-MSC cells in the cultures, as well as the duration of the culture (i.e., the number of times the cells have been passaged) are likely to be contributing factors. Bearing this possible instability in mind, at this point it seems prudent, regardless of the source of MSC, to only make clinical use of cells that have been passaged fewer than 25 times in culture [40].
MSC in Regenerative Medicine
The in vitro and in vivo differentiation of MSC into the various mesenchymal cell types found within the bone marrow, i.e. bone, cartilage, and fat, has now been described by numerous laboratories, and the conditions to bring about each of these differentiative pathways have been delineated in detail [7]. Recent studies employing microarrays [30, 41–43] have now begun to shed light on the molecular mechanisms responsible for commitment to and progression along each of these lineages, providing investigators with information that is vital for developing more efficient means of differentiating MSC along specific desired pathways. These studies have also begun to reveal some of the genes and signaling pathways that are important for maintaining MSC in an undifferentiated state, helping to better define this somewhat elusive stem cell population. Promising results from preclinical studies examining the use of MSC for bone and cartilage repair [44, 45] quickly led to clinical trials using MSC to treat large bone defects [46], articular cartilage defects [47], and osteogenesis imperfecta [48–51], and these trials have thus far confirmed the exciting results from animal studies, highlighting the therapeutic potential of MSC.
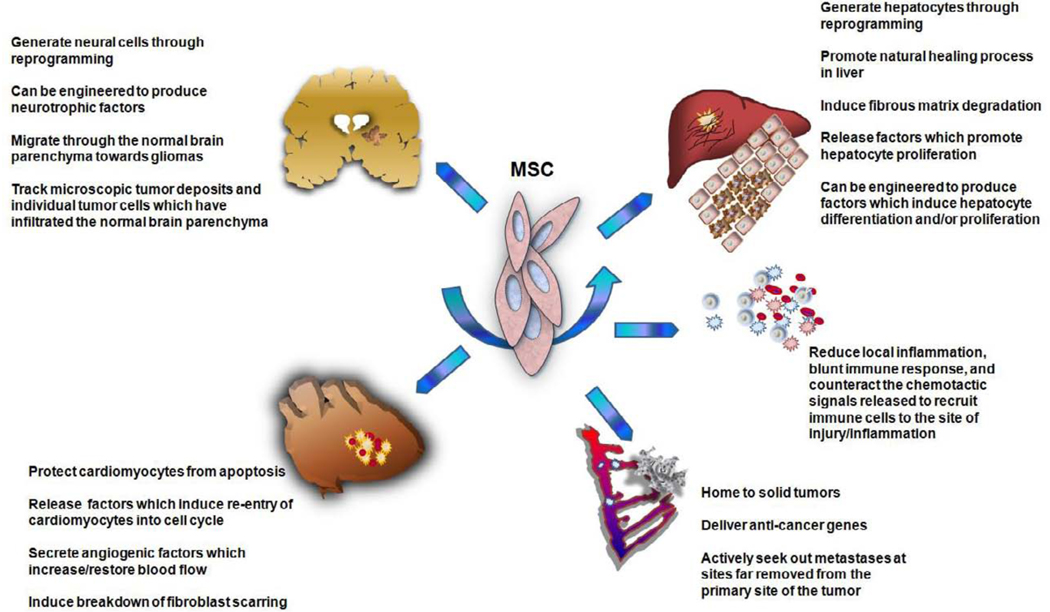
Although the ability of MSC to produce cartilage and bone quickly established MSC as a promising therapeutic modality to treat a wide range of injuries and diseases of the skeletal system, further study into MSC biology revealed that these cells have far broader differentiative capabilities than was initially anticipated. For example, MSC also have the ability to generate what appear to be functional skeletal muscle cells in vitro [52] and in vivo [53, 54], raising the possibility that MSC could one day be used to treat diseases like Duchenne Muscular Dystrophy [54]. Following the demonstration that MSC could give rise to all cells of the musculoskeletal system subsequent studies showed, quite remarkably, that MSC had the ability to give rise not only to cells of mesenchymal derivation, but in fact, to cells of all three germinal layers. The first of the studies to demonstrate that MSC had the ability to cross developmental boundaries and reprogram to differentiate into cells of another germ layer convincingly demonstrated that MSC could give rise to both neural and glial (ectoderm) cell types in vitro and in vivo [55–58]. These findings raised the exciting possibility that MSC could serve as a readily available source of cells for treating injuries or degenerative conditions within the central nervous system; clinical situations for which there are currently very limited, if any, options, other than simply masking the symptoms or slowing disease progression. Indeed, numerous in vivo transplantation studies have now confirmed these exciting in vitro results by showing that MSC have the ability to mediate repair following spinal cord injury [59–61], ischemic injury and stroke [62–72], demyelinating conditions [73–77], experimentally induced Parkinson’s disease [78–80] and potentially treat a host of other injuries and degenerative diseases of the CNS [81–84]. Although questions have been raised as to the mechanism by which this apparent “trans-differentiation” occurs [85], the demonstration by numerous groups that MSC can indeed give rise to neural cells in various stages of differentiation and mediate functional improvement for a variety of injuries/diseases within the CNS provides the impetus for further studies attempting to increase the efficiency and predictability of this process and harness this potential for therapy.
Having now established that MSC were able to interconvert from cells of one germinal layer to those of another, numerous groups began rapidly testing the ability of MSC to differentiate into yet other tissue-specific cells types. Studies were published showing that MSC could give rise in vitro to cells which appear, phenotypically and morphologically, and behave like cardiomyocytes [86–88] and to endothelium [89], engraft within the injured myocardium [90–106], and form Purkinje fibers in vivo [9, 15, 107]. These preclinical studies generated a tremendous amount of excitement in the field of regenerative medicine, since they strongly suggested that MSC could be used as therapy to mediate cardiac repair post-infarct, diseases within the conduction pathways of the heart, or even, perhaps, to treat chronic progressive cardiac diseases such as congestive heart failure [91, 101, 108–110]. These exciting results, coupled with preclinical animal studies showing functional cardiac improvement following MSC infusion (discussed in detail in the later section entitled “MSC as Trophic Factories”), were the driving force for launching several clinical trials to investigate whether MSC are able to mediate repair in the post-infarct heart [97, 111–116].
As a last major example of MSC in regenerative medicine, we will address their use in liver repair. Liver failure is a life-threatening condition for which the only curative treatment is whole or partial organ transplantation. Given the crippling shortage of donor livers available for transplant and the high mortality and morbidity associated with the need for lifelong immunosuppression following liver transplantation, a major focus of regenerative medicine over the past several years has been to identify cells which could be used to repopulate and/or repair the damaged, failing liver. We and others have devoted a great deal of energy to demonstrating the ability of MSC from various sources to serve as therapeutics for liver disease [11, 13, 14, 33, 117–143]. It is now clear that, not only do MSC have the ability to generate, in vitro and in vivo, cells which are indistinguishable from native hepatocytes, but transplantation of MSC in a range of model systems can result in fairly robust formation of hepatocytes which repair a variety of inborn genetic defects, toxin-induced injuries, and even fibrosis. This wealth of supportive data in several different species has led to the utilization of MSC for liver disease in 3 clinical trials thus far [144–146]. While the patient numbers were low and the approaches employed were not curative, the patients’ clinical parameters have shown a trend of improvement, supporting further studies into the use of MSC in this context.
MSC as Trophic “Factories”
One issue which has complicated interpretation of the data generated from the afore-mentioned studies in liver as well as those conducted looking at the potential of MSC to mediate repair in the heart and other organ systems, is the fact that a therapeutic benefit is often observed in the absence of any evidence of sustained engraftment of the transplanted MSC within the damaged organ. Instead, it appears that the transplantation of MSC somehow stimulates the damaged host organ to repair itself without the donor cells actually having to persist long-term within the recipient. These findings initially led to a great deal of debate as to whether MSC can actually generate many of the cell types they appeared to produce in vivo or if, perhaps, all the effects they produce are simply mediated through release of soluble factors.
A variety of evidence from animal studies has now indicated that both MSC’s direct differentiation and their indirect effects through secretion of factors which stimulate the regeneration of endogenous cells are likely to play important roles in promoting tissue recovery [126, 147–164]. A diagrammatic summary of these findings appears in Figure 1. For example, studies have now shown that MSC protein extracts or conditioned medium collected from MSC cultures [153, 154, 162] can produce many of the same beneficial effects of MSC on the post-infarct heart and in the damaged/diseased liver [148, 165]. Moreover, studies from other groups have now shown that MSC release paracrine factors which protect cardiomyocytes from apoptosis in a mitochondrial-dependent fashion [163]. Furthermore, MSC were recently shown to produce and release insulin-like growth factor I (IGF-I), which can then act upon neonatal cardiomyocytes to induce upregulation of c-kit and re-entry of these cells into cell cycle [164]. MSC further activated expression of hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and insulin-like growth factor II (IGF-II) within the myocardium [161]. Further complicating matters, however, are other studies which have provided evidence that many of the beneficial effects of MSC on the injured heart may have little to do with the repair/regeneration of the damaged myocardium, but with the ability of MSC to promote angiogenesis [155]. Other studies have now provided clues as to how MSC may mediate this effect. MSC appear to secrete VEGF and basic fibroblast growth factor (bFGF) upon contacting the injured myocardium, which stimulates the formation of new vessels and increases capillary density to increase/restore blood flow to the infracted region [158]. Recent evidence now suggests that many of these paracrine actions of MSC are mediated by their release of small 50–100nm exosomes [157], which contain not only potentially beneficial proteins, but also pre-microRNA’s [152], the function of which still remains to be determined. It would thus seem that MSC may mediate therapeutic benefit not only by engrafting within the damaged tissue and producing tissue-specific cells to correct for the existing deficit, but also by acting as a sort of protein factory, churning out factors which act upon the endogenous cells within the damaged myocardium and encourage the injured heart to assume the task of mediating its own repair.
Figure 1.
MSC as Trophic Factories
In addition to these paracrine and trophic activities, in the case of both the liver and the heart, it would seem that MSC have additional properties which help to not only reduce existing damage, but promote the healing process. In the case of the liver, recent studies have shown that MSC have the ability to enhance fibrous matrix degradation, likely through the induction of matrix metalloproteinases (MMP’s), suggesting that MSC may be ideally suited for treatment of liver diseases involving fibrosis [130, 131, 138, 142, 166–169]. Likewise, in the heart, MSC release paracrine factors which attenuate fibroblast proliferation and inhibit collagen synthesis/deposition [159], apparently by stimulating cardiac fibroblasts to secrete MMP’s 2 and 9 and express membrane type I MMP on their surface. These results in these two organ systems are very exciting, because they imply that MSC could potentially mediate beneficial effects even at more advanced stages of disease, once fibrosis had set in. The ability to apply therapy at later points in the disease would enable much larger numbers of patients to benefit from this treatment. However, these results must be interpreted carefully and with tempered enthusiasm, because other studies have suggested that under different conditions, transplanted MSC may actually contribute to the myofibroblast pool and thus enhance the fibrotic process, at least within the liver [128, 169–173]. This has led to the current feeling within the field that the effect of MSC will probably vary with the nature of the injury/disease that is being treated, the specific experimental model in which the therapy is being tested, and perhaps even the time frame of MSC application, such that MSC could be beneficial if administered at certain stages of disease progression and harmful if administered at other stages.
MSC as Vehicles for Delivering Therapeutic Genes
While MSC possess tremendous therapeutic potential by virtue of their ability to lodge/engraft within multiple tissues in the body and both give rise to tissue-specific cells and release trophic factors that trigger the tissue’s own endogenous repair pathways, gene therapists have realized that these properties are just the beginning of the therapeutic applications for MSC [24, 174, 175]. By using gene therapy to engineer MSC to either augment their own natural production of specific desired proteins or to enable them to express proteins outside of their native repertoire, it is possible to greatly broaden the spectrum of diseases for which MSC could provide therapeutic benefit. Unlike hematopoietic stem cells which are notoriously difficult to modify with most viral vectors while preserving their in vivo potential, MSC can be readily transduced with all of the major clinically prevalent viral vector systems including those based upon adenovirus [176–178], the murine retroviruses [178–182], lentiviruses [183–188], and AAV [189, 190], and efficiently produce a wide range of cytoplasmic, membrane-bound, and secreted protein products. This ease of transduction coupled with the ability to subsequently select and expand only the gene-modified cells in vitro to generate adequate numbers for transplantation combine to make MSC one of the most promising stem cell populations for use in gene therapy studies and trials.
To date, the majority of studies using gene-modified MSC have been undertaken with the purpose of enhancing the natural abilities of MSC to mediate repair within various tissues. Using the heart as an example, once investigators discovered the identity of some of the key trophic factors responsible for MSC’s beneficial effect on the injured myocardium, they undertook studies using MSC engineered to overexpress a number of these factors [93, 183, 191–196]. As anticipated, the “gene-enhanced” MSC were substantially more effective than their unmodified counterparts. Studies of this nature have not been limited to the heart. On the contrary, similar studies have also been performed to repair the damaged CNS using MSC engineered to produce neurotrophic factors [64, 69, 70, 197–199], and to repair the injured liver using MSC expressing proteins involved in hepatocyte differentiation and/or proliferation [132, 200]. In each case thus far, MSC engineered to express higher levels of proteins known to be beneficial for the tissue in question have produced even better results than unmodified MSC.
Despite the many advantages of using MSC as gene delivery vehicles, few studies have thus far explored this potential for the treatment of genetic diseases. One disease for which MSC are being actively pursued for delivery of a therapeutic gene is hemophilia A. Both hemophilia A and B are rather unique among the genetic diseases because expression of the missing coagulation factor (FVIII or FIX, respectively) does not need to be expressed in either a cell- or tissue-specific fashion to mediate correction. Although the liver is thought to be the primary natural site of synthesis of FVIII and FIX, expression of these factors in other tissues exerts no deleterious effects. As long as the proteins are expressed in cells which have ready access to the circulation, they can be secreted into the bloodstream and exert their appropriate clotting activity. The hemophilias are also unique in that very low levels of gene correction/expression are actually required to exert a pronounced therapeutic benefit. To convert a patient from a severe, life-threatening phenotype to a moderate phenotype and thus greatly improve their quality of life, levels of FVIII or IX of only 2–3% of normal are required. From the standpoint of feasibility, studies have already shown that MSC can be transduced with FVIII-expressing viral vectors and secrete high levels of FVIII protein. Importantly, FVIII purified from the conditioned medium of the transduced MSC was proven to have a specific activity, relative electrophoretic mobility, and proteolytic activation pattern that was virtually identical to that of FVIII produced by other commercial cell lines [201]. Given the widespread distribution and engraftment of MSC following their systemic infusion and their ability to efficiently process and secrete high amounts of biologically active FVIII, they are, not surprisingly, being viewed as ideal vehicles for delivering a FVIII transgene throughout the body and thus providing long-term/permanent correction of hemophilia A [201–203]. Extrapolating the work thus far on using MSC to deliver therapeutic genes for the hemophilias, and combining this with the large amount of evidence we have summarized herein demonstrating the abilities of MSC to give rise to cells of numerous tissue types from all three germinal layers, one can envision MSC soon being used as vehicles to deliver gene therapy vectors to numerous tissues in the body, thus promising to provide a permanent cure for a diverse range of diseases.
Use of MSC as Immunomodulatory/Anti-Inflammatory Agents
In addition to their broad differentiative capabilities and their potential utility as gene delivery vehicles, MSC represent a rather unique cell from an immunological standpoint, since MSC are known to be relatively hypoimmunogenic. They do not normally express MHC class II or the co-stimulatory molecules CD80 and CD82, unless they are stimulated with IFN-γ. As such, they do not seem to serve as very good targets for lysis by cytotoxic T cells or NK cells, and do not really stimulate the proliferation of allogeneic lymphocytes when used as stimulators in a traditional mixed lymphocyte reaction. Given these properties, it is perhaps not surprising that a large body of evidence is now accumulating that MSC can be readily transplanted across allogeneic barriers without eliciting an immune response [204, 205]. Thus, if one wished to use MSC to treat an inherited genetic defect within a given tissue, MSC from an unrelated donor could be used for transplantation, greatly increasing the feasibility of using these stem cells for therapy. Perhaps even more important from the standpoint of their potential use as therapeutics, more recent studies have provided evidence that MSC are not only relatively non-immunogenic, but they also appear to have the ability to exert both immunosuppressive and anti-inflammatory properties both in vitro and in vivo. These properties appear to result from MSC’s ability to intervene, at multiple levels, with the generation and propagation of an immune response. To name just a few examples, MSC have been demonstrated to interfere with the generation and maturation of cytotoxic T cells and helper T cells [206–215], dendritic cells (and thus antigen presentation) [216–219], and B cells [220], effectively crippling each arm of the adaptive immune system. In addition to actively shutting down the generation of immune effector cells, MSC also appear to indirectly suppress the generation of an immune response by inducing the formation of potent Tregs, although the mechanism by which this comes about is still the subject of active research [40, 221–223]. MSC are also known to express an arsenal of factors [40] such as transforming growth factor-β1 [210, 211], prostaglandin-E2 [221], nitric oxide [224], IL-10 [209, 225], HLA-G [226] , hepatocyte growth factor [210], and, following stimulation with IFN-γ, indoleamine 2,3-dioxygenase [227, 228]. The combined effects of these various factors serve to reduce local inflammation, blunt immune response, and counteract the chemotactic signals released to recruit immune cells to the site of injury/inflammation. Scientists and clinicians have already begun exploring whether it is possible to exploit these properties and use MSC as an adjunct in HSC or solid tissue transplantation to prevent GVHD or graft rejection in the event that the cells/tissue to be transplanted are not ideally matched [40, 229–232]. Proof of this principle has come from recent clinical trials in which it was demonstrated that co-transplanting MSC with the HSC graft led to a significant reduction in both acute and chronic GVHD [233–236]. Collectively considering all of these immunomodulatory properties, MSC can be viewed as one of the few universal donor cell types that could likely be transplanted to treat numerous injuries/diseases without the necessity of exquisite HLA-matching between the donor and the recipient. Moreover, their use appears to promote the acceptance and survival of mismatched cells and tissues following transplantation, further extending their therapeutic utility.
In addition to their numerous differentiative, trophic, and immunomodulatory properties, a large number of preclinical animal studies examining the potential of MSC isolated from adult tissues have also highlighted another interesting and clinically valuable characteristic of MSC; their ability to selectively navigate to sites of injury and/or inflammation within the body. Once reaching these specific sites, the MSC then mediate repair both by engrafting and generating tissue-specific cells within the injured tissue (but contributing very little if at all to other tissues that are functionally normal [94–96]), and by releasing trophic factors that blunt the inflammatory response and often promote healing by activating the tissue’s own endogenous repair mechanisms. While the mechanisms responsible for this trafficking to sites of injury are currently not well understood, this observation has raised the exciting prospect of using MSC to treat a wide array of diseases in which inflammation plays a key role such as stroke [62–72], rheumatoid arthritis [237], asthma [238–240] and allergic rhinitis [241], and both acute and chronic lung injury [242]. Recently, these valuable anti-inflammatory properties at sites of injury have been explored as a possible adjunct or first line treatment for muscular dystrophy [54], since the constant damage to weakened muscle fibers creates a hostile environment which frequently induced apoptosis of transplanted muscle progenitors or stem cells, precluding their ability to repair the damaged muscle. It is hoped that transplanting MSC systemically will allow their migration to sites of active muscle degeneration, thereby rendering the local microenvironment more receptive to cells transplanted to regenerate the defective muscle fibers. Studies exploring this possible use of MSC are still relatively early in development, so the true clinical utility of this approach will not likely be defined for some time.
Based on the widespread distribution of MSC and their demonstrated ability to mediate repair in a wide range of injuries/diseases, it is intriguing to speculate that MSC may in fact represent a latent pool of pluripotent stem cells, distributed ubiquitously throughout the body [10], potentially capable of migrating to sites of injury/inflammation and generating tissue-specific cells and/or releasing trophic/immunomodulatory factors to repair the damage in question. The issue that needs to be resolved to tap this reservoir of potentially therapeutic cells is the delineation of methods for mobilizing the MSC from their places of residence into the circulation, from which they could theoretically traffic wherever they were needed. Such methods could one day allow the patient’s own MSC to repair the injury, obviating the need for transplantation.
MSC as Anti-Cancer Agents
Cancer represents another condition in which there is a selective need for new cells created by the forming tumor. Studies over the last several years have now revealed that MSC have the ability to “sense” this need, migrate to the forming tumor following intravenous administration, and contribute to the newly forming tumor “stroma”. While this may not seem ideal, since the MSC could, in fact, provide support to the growing tumor, this property has now been realized to present a very powerful and unique means of selectively delivering anti-cancer gene products to tumor cells in vivo [243, 244]. Three of the gene products which have thus far received the most attention are IL-2 [245, 246], IFN-β [243, 244], and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [247–250]. Unfortunately, the utility of these and many other biological agents that could be used for cancer therapy is often limited by both their short half-life in vivo and their pronounced toxicity due to effects on normal, non-malignant cells within the body. Using MSC to deliver these therapeutics promises to solve both of these problems, since the MSC can selectively migrate to the tumor site and release their therapeutic payload locally. This greatly increases the agent’s concentration within the tumor and significantly lowers its systemic toxicity. In addition, by genetically modifying the MSC with viral vectors, the engrafted MSC will steadily release the therapeutic agent, allowing a single administration to result in long-lasting effects. Other studies have now provided evidence that MSC have the ability to not only selectively home to solid tumors [243, 244, 247], but also to actively seek out metastases at sites far removed from the primary site of the tumor [244, 248, 250, 251]. This ability has recently been proven to be of great therapeutic value in the treatment of lung metastases arising from both breast cancer and melanoma in a murine xenograft model [244]. Given the difficulty and frequent lack of success using traditional approaches such as surgery, radiotherapy, and chemotherapeutic agents to treat tumors which are either highly invasive or prone to metastasis, this property of MSC will likely prove to be of great clinical value in the near future. One form of cancer for which the use of MSC is receiving a great deal of attention is glioblastoma multiforme (GBM). GBM represents the most common form of malignant glioma. Despite decades of research and many advances in the treatment of this disease with conventional surgery, radiotherapy, and chemotherapy, there is no cure, and the current prognosis is dismal, with a median survival of only 6–18 months. The failure of current therapies to cure this disease arises predominantly from the highly invasive nature of this cancer and the inability of these agents to effectively target tumor cells which have disseminated into the normal parenchyma of the brain, at sites distant from the main tumor mass. Given the ability of MSC to home to tumors and their ability to track to metastases, a number of studies have been performed evaluating the ability of gene-modified MSC to treat GBM. These studies have now shown that MSC migrate through the normal brain parenchyma towards gliomas [246, 249, 250, 252] and to track microscopic tumor deposits and individual tumor cells which have infiltrated the normal brain parenchyma [246, 249–252]. While these migratory properties are certainly interesting, even more exciting are the dramatic therapeutic benefits these same studies have shown, with reduction in tumor size, and pronounced improvements in survival. It is important to note that these studies used MSC as the sole therapy, and definite benefits were observed. In the clinical setting, the current plan is to use gene-modified MSC as an adjunct after surgical resection. In this scenario, the majority of the tumor mass would be surgically removed, and the MSC would then be transplanted to remove the residual malignant cells at the site of the tumor and to hunt down any invasive tumor cells that have migrated away from the site of the primary tumor. In this context, one would imagine that the therapeutic benefit of the MSC will likely be even more pronounced, since their anti-tumor effects could be focused only on the small number of residual tumor cells that evaded removal during surgery. Thus, the remarkable success seen in studies aimed at treating GBM, one of the most devastating forms of cancer, highlight the tremendous potential MSC harbor as gene delivery vehicles for the treatment of many forms of cancer for which current therapeutic strategies are ineffective.
Conclusions
With numerous investigators around the globe having established and verified that MSC harbor the ability to cross embryonic germ layers and give rise to a wide range of what developmental biology had taught were tissue-specific cells, it is now clear that the differentiative capacity of MSC is far broader than anyone would have foreseen at the time Friedenstein originally described his bone marrow-derived CFU-F. In addition to this tremendous differentiative potential, the relative ease with which MSC can be isolated, propagated in culture, and modified with a variety of viral-based vectors, and their intrinsic ability to seek out sites of injury/inflammation within the body argues that MSC may be ideally suited as cellular therapeutics and gene delivery vehicles for numerous diseases/injuries affecting each of the major organ systems of the body. Figure 2 provides a diagrammatic summary of some of the key properties of MSC which make them one of the ideal cell types for use as therapeutics and vehicles for gene and drug delivery.
Figure 2.
Overview of Key Properties of MSC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedenstein AJ. Osteogenic stem cells in the bone marrow. Bone and Mineral. 1991;7:243–272. [Google Scholar]
- 2.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 4.Galotto M, Berisso G, Delfino L, Podesta M, Ottaggio L, Dallorso S, Dufour C, Ferrara GB, Abbondandolo A, Dini G, Bacigalupo A, Cancedda R, Quarto R. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol. 1999;27(9):1460–1466. doi: 10.1016/s0301-472x(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 5.Kassem M. Mesenchymal stem cells: biological characteristics and potential clinical applications. Cloning Stem Cells. 2004;6(4):369–374. doi: 10.1089/clo.2004.6.369. [DOI] [PubMed] [Google Scholar]
- 6.Luria EA, Panasyuk AF, Friedenstein AY. Fibroblast colony formation from monolayer cultures of blood cells. Transfusion. 1971;11(6):345–349. doi: 10.1111/j.1537-2995.1971.tb04426.x. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78(1):55–62. [PubMed] [Google Scholar]
- 9.Airey JA, Almeida-Porada G, Colletti EJ, Porada CD, Chamberlain J, Movsesian M, Sutko JL, Zanjani ED. Human mesenchymal stem cells form Purkinje fibers in fetal sheep heart. Circulation. 2004;109(11):1401–1407. doi: 10.1161/01.CIR.0000124222.16321.26. [DOI] [PubMed] [Google Scholar]
- 10.Almeida-Porada MG, Porada C, ElShabrawy D, Simmons PJ, Zanjani ED. Human marrow stromal cells (MSC) represent a latent pool of stem cells capable of generating long-term hematopoietic cells. Blood. 2001;98(Part 1):713a. [Google Scholar]
- 11.Chamberlain J, Yamagami T, Colletti E, Theise ND, Desai J, Frias A, Pixley J, Zanjani ED, Porada CD, Almeida-Porada G. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology. 2007;46(6):1935–1945. doi: 10.1002/hep.21899. [DOI] [PubMed] [Google Scholar]
- 12.Colletti E, Zanjani ED, Porada CD, Almeida-Porada MG. Tales from the Crypt: Mesenchymal Stem Cells for Replenishing the Intestinal Stem Cell Pool. Blood. 2008;112 Abstract 390. [Google Scholar]
- 13.Colletti EJ, AJA, Zanjani ED, Porada CD, Almeida-Porada G. Human Mesenchymal Stem Cells differentiate promptly into tissue-specific cell types without cell fusion, mitochondrial or membrane vesicular transfer in fetal sheep. Blood. 2007;110(11):135a. [Google Scholar]
- 14.Colletti EJ, Airey JA, Liu W, Simmons PJ, Zanjani ED, Porada CD, Almeida-Porada G. Generation of tissue-specific cells from MSC does not require fusion or donor-to-host mitochondrial/membrane transfer. Stem Cell Res. 2009;2(2):125–138. doi: 10.1016/j.scr.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colletti EJ, Almeida-Porada G, Chamberlain J, Zanjani ED, Airey JA. The time course of engraftment of human mesenchymal stem cells in fetal heart demonstrates that Purkinje fiber aggregates derive from a single cell and not multi-cell homing. Exp Hematol. 2006;34(7):926–933. doi: 10.1016/j.exphem.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Bruder SP, Ricalton NS, Boynton RE, Connolly TJ, Jaiswal N, Zaia J, Barry FP. Mesenchymal stem cell surface antigen SB-10 corresponds to activated leukocyte cell adhesion molecule and is involved in osteogenic differentiation. J Bone Miner Res. 1998;13(4):655–663. doi: 10.1359/jbmr.1998.13.4.655. [DOI] [PubMed] [Google Scholar]
- 17.Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13(1):69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24(2):376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 19.Almeida-Porada G, El Shabrawy D, Porada C, Zanjani ED. Differentiative potential of human metanephric mesenchymal cells. Exp Hematol. 2002;30(12):1454–1462. doi: 10.1016/s0301-472x(02)00967-0. [DOI] [PubMed] [Google Scholar]
- 20.Fan CG, Tang FW, Zhang QJ, Lu SH, Liu HY, Zhao ZM, Liu B, Han ZB, Han ZC. Characterization and neural differentiation of fetal lung mesenchymal stem cells. Cell Transplant. 2005;14(5):311–321. doi: 10.3727/000000005783983070. [DOI] [PubMed] [Google Scholar]
- 21.Gotherstrom C, West A, Liden J, Uzunel M, Lahesmaa R, Le Blanc K. Difference in gene expression between human fetal liver and adult bone marrow mesenchymal stem cells. Haematologica. 2005;90(8):1017–1026. [PubMed] [Google Scholar]
- 22.in ‘t Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88(8):845–852. [PubMed] [Google Scholar]
- 23.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 24.Morizono K, De Ugarte DA, Zhu M, Zuk P, Elbarbary A, Ashjian P, Benhaim P, Chen IS, Hedrick MH. Multilineage cells from adipose tissue as gene delivery vehicles. Hum Gene Ther. 2003;14(1):59–66. doi: 10.1089/10430340360464714. [DOI] [PubMed] [Google Scholar]
- 25.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 27.Garol NJ, Yamagami T, Osborne C, Porada CD, Zanjani ED, Almeida-Porada G. Tissue-specific molecular signature may explain differentiative bias of human MSC from different tissues. Blood. 2007;110(11):570a. [Google Scholar]
- 28.Mazhari S, Desai J, Chamberlain J, Porada C, Zanjani ED, Almeida-Porada G. Proteomic Analysis Reveals Intrinsic Differences between Phenotypically Identical Mesenchymal Stem Cells. Blood. 2005;106(11) [Google Scholar]
- 29.Mazhari SM, Porada CD, Chamberlain J, Zanjani ED, Almeida-Porada G. Characterization of membrane proteins of mesenchymal stem cells from human liver. Experimental Hematology. 2006;34(9, Suppl. 1):80. [Google Scholar]
- 30.Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, Bae YC, Jung JS. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14(4–6):311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 31.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 32.Chamberlain J, Frias A, Porada C, Zanjani ED, Almeida-Porada G. Neural Generation in vivo differs with route of administration and source of mesenchymal stem cells. Experimental Hematology. 2005;33(7):47a. [Google Scholar]
- 33.Almeida-Porada MG, Chamberlain J, Frias A, Porada CD, Zanjani ED. Tissue of Origin Influences In Vivo Differentiative Potential of Mesenchymal Stem Cells. Blood. 2003;102(11) abstract #1304. [Google Scholar]
- 34.Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, Zuffardi O, Locatelli F. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67(19):9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 35.Bochkov NP, Voronina ES, Kosyakova NV, Liehr T, Rzhaninova AA, Katosova LD, Platonova VI, Gol'dshtein DV. Chromosome variability of human multipotent mesenchymal stromal cells. Bull Exp Biol Med. 2007;143(1):122–126. doi: 10.1007/s10517-007-0031-0. [DOI] [PubMed] [Google Scholar]
- 36.Buyanovskaya OA, Kuleshov NP, Nikitina VA, Voronina ES, Katosova LD, Bochkov NP. Spontaneous aneuploidy and clone formation in adipose tissue stem cells during different periods of culturing. Bull Exp Biol Med. 2009;148(1):109–112. doi: 10.1007/s10517-009-0647-3. [DOI] [PubMed] [Google Scholar]
- 37.Rubio D, Garcia S, De la Cueva T, Paz MF, Lloyd AC, Bernad A, Garcia-Castro J. Human mesenchymal stem cell transformation is associated with a mesenchymal-epithelial transition. Exp Cell Res. 2008;314(4):691–698. doi: 10.1016/j.yexcr.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65(8):3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 39.Altanerova V, Horvathova E, Matuskova M, Kucerova L, Altaner C. Genotoxic damage of human adipose-tissue derived mesenchymal stem cells triggers their terminal differentiation. Neoplasma. 2009;56(6):542–547. doi: 10.4149/neo_2009_06_542. [DOI] [PubMed] [Google Scholar]
- 40.Crop M, Baan C, Weimar W, Hoogduijn M. Potential of mesenchymal stem cells as immune therapy in solid-organ transplantation. Transpl Int. 2009;22(4):365–376. doi: 10.1111/j.1432-2277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 41.de Jong DS, Vaes BL, Dechering KJ, Feijen A, Hendriks JM, Wehrens R, Mummery CL, van Zoelen EJ, Olijve W, Steegenga WT. Identification of novel regulators associated with early-phase osteoblast differentiation. J Bone Miner Res. 2004;19(6):947–958. doi: 10.1359/JBMR.040216. [DOI] [PubMed] [Google Scholar]
- 42.Hishikawa K, Miura S, Marumo T, Yoshioka H, Mori Y, Takato T, Fujita T. Gene expression profile of human mesenchymal stem cells during osteogenesis in three-dimensional thermoreversible gelation polymer. Biochem Biophys Res Commun. 2004;317(4):1103–1107. doi: 10.1016/j.bbrc.2004.03.165. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura T, Shiojima S, Hirai Y, Iwama T, Tsuruzoe N, Hirasawa A, Katsuma S, Tsujimoto G. Temporal gene expression changes during adipogenesis in human mesenchymal stem cells. Biochem Biophys Res Commun. 2003;303(1):306–312. doi: 10.1016/s0006-291x(03)00325-5. [DOI] [PubMed] [Google Scholar]
- 44.Ando W, Tateishi K, Hart DA, Katakai D, Tanaka Y, Nakata K, Hashimoto J, Fujie H, Shino K, Yoshikawa H, Nakamura N. Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials. 2007;28(36):5462–5470. doi: 10.1016/j.biomaterials.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 45.Cancedda R, Mastrogiacomo M, Bianchi G, Derubeis A, Muraglia A, Quarto R. Bone marrow stromal cells and their use in regenerating bone. Novartis Found Symp. 2003;249:133–143. discussion 143-7, 170-4, 239-41. [PubMed] [Google Scholar]
- 46.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344(5):385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 47.Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13(5):595–600. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- 48.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99(13):8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 50.Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97(5):1227–1231. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- 51.Le Blanc K, Gotherstrom C, Ringden O, Hassan M, McMahon R, Horwitz E, Anneren G, Axelsson O, Nunn J, Ewald U, Norden-Lindeberg S, Jansson M, Dalton A, Astrom E, Westgren M. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79(11):1607–1614. doi: 10.1097/01.tp.0000159029.48678.93. [DOI] [PubMed] [Google Scholar]
- 52.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18(12):1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 53.Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309(5732):314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- 54.Ichim TE, Alexandrescu DT, Solano F, Lara F, Campion Rde N, Paris E, Woods EJ, Murphy MP, Dasanu CA, Patel AN, Marleau AM, Leal A, Riordan NH. Mesenchymal stem cells as anti-inflammatories: implications for treatment of Duchenne muscular dystrophy. Cell Immunol. 2010;260(2):75–82. doi: 10.1016/j.cellimm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Bossolasco P, Cova L, Calzarossa C, Rimoldi SG, Borsotti C, Deliliers GL, Silani V, Soligo D, Polli E. Neuro-glial differentiation of human bone marrow stem cells in vitro. Exp Neurol. 2005;193(2):312–325. doi: 10.1016/j.expneurol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290(5497):1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 57.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96(19):10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290(5497):1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 59.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99(4):2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neuhuber B, Timothy Himes B, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035(1):73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 61.Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine (Phila Pa 1976) 2004;29(18):1971–1979. doi: 10.1097/01.brs.0000138273.02820.0a. [DOI] [PubMed] [Google Scholar]
- 62.Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol. 2006;199(1):56–66. doi: 10.1016/j.expneurol.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol. 2003;183(2):355–366. doi: 10.1016/s0014-4886(03)00089-x. [DOI] [PubMed] [Google Scholar]
- 64.Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience. 2005;136(1):161–169. doi: 10.1016/j.neuroscience.2005.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73(6):778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 66.Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189(1–2):49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 67.Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92(6):692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 68.Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007(1–2):1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 69.Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11(1):96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 70.Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9(2):189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59(4):514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 72.Zheng W, Honmou O, Miyata K, Harada K, Suzuki J, Liu H, Houkin K, Hamada H, Kocsis JD. Therapeutic benefits of human mesenchymal stem cells derived from bone marrow after global cerebral ischemia. Brain Res. 2010;1310:8–16. doi: 10.1016/j.brainres.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 73.Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J Neurosci. 2002;22(15):6623–6630. doi: 10.1523/JNEUROSCI.22-15-06623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akiyama Y, Radtke C, Honmou O, Kocsis JD. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002;39(3):229–236. doi: 10.1002/glia.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Mancardi G, Pedotti R, Uccelli A. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61(3):219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- 76.Jin HK, Carter JE, Huntley GW, Schuchman EH. Intracerebral transplantation of mesenchymal stem cells into acid sphingomyelinase-deficient mice delays the onset of neurological abnormalities and extends their life span. J Clin Invest. 2002;109(9):1183–1191. doi: 10.1172/JCI14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5):1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 78.Blandini F, Cova L, Armentero MT, Zennaro E, Levandis G, Bossolasco P, Calzarossa C, Mellone M, Giuseppe B, Deliliers GL, Polli E, Nappi G, Silani V. Transplantation of Undifferentiated Human Mesenchymal Stem Cells Protects against 6-Hydroxydopamine Neurotoxicity in the Rat. Cell Transplant. 2009 doi: 10.3727/096368909X479839. [DOI] [PubMed] [Google Scholar]
- 79.Cova L, Armentero MT, Zennaro E, Calzarossa C, Bossolasco P, Busca G, Lambertenghi Deliliers G, Polli E, Nappi G, Silani V, Blandini F. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson's disease. Brain Res. 2010;1311:12–27. doi: 10.1016/j.brainres.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Chen J, Wang L, Zhang L, Lu M, Chopp M. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Neurosci Lett. 2001;316(2):67–70. doi: 10.1016/s0304-3940(01)02384-9. [DOI] [PubMed] [Google Scholar]
- 81.Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53(3):697–702. doi: 10.1227/01.neu.0000079333.61863.aa. discussion 702-3. [DOI] [PubMed] [Google Scholar]
- 82.Phinney DG, Isakova I. Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr Pharm Des. 2005;11(10):1255–1265. doi: 10.2174/1381612053507495. [DOI] [PubMed] [Google Scholar]
- 83.Pisati F, Bossolasco P, Meregalli M, Cova L, Belicchi M, Gavina M, Marchesi C, Calzarossa C, Soligo D, Lambertenghi-Deliliers G, Bresolin N, Silani V, Torrente Y, Polli E. Induction of neurotrophin expression via human adult mesenchymal stem cells: implication for cell therapy in neurodegenerative diseases. Cell Transplant. 2007;16(1):41–55. doi: 10.3727/000000007783464443. [DOI] [PubMed] [Google Scholar]
- 84.Prockop DJ, Azizi SA, Phinney DG, Kopen GC, Schwarz EJ. Potential use of marrow stromal cells as therapeutic vectors for diseases of the central nervous system. Prog Brain Res. 2000;128:293–297. doi: 10.1016/s0079-6123(00)28026-6. [DOI] [PubMed] [Google Scholar]
- 85.Tondreau T, Lagneaux L, Dejeneffe M, Massy M, Mortier C, Delforge A, Bron D. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72(7):319–326. doi: 10.1111/j.1432-0436.2004.07207003.x. [DOI] [PubMed] [Google Scholar]
- 86.Fukuda K. Reprogramming of bone marrow mesenchymal stem cells into cardiomyocytes. C R Biol. 2002;325(10):1027–1038. doi: 10.1016/s1631-0691(02)01524-x. [DOI] [PubMed] [Google Scholar]
- 87.Shim WS, Jiang S, Wong P, Tan J, Chua YL, Tan YS, Sin YK, Lim CH, Chua T, Teh M, Liu TC, Sim E. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun. 2004;324(2):481–488. doi: 10.1016/j.bbrc.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 88.Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, Zhou H, Chen Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004;229(7):623–631. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 89.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332(2):370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 90.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 91.Tomita S, Mickle DA, Weisel RD, Jia ZQ, Tumiati LC, Allidina Y, Liu P, Li RK. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg. 2002;123(6):1132–1140. doi: 10.1067/mtc.2002.120716. [DOI] [PubMed] [Google Scholar]
- 92.Yoon J, Min BG, Kim YH, Shim WJ, Ro YM, Lim DS. Differentiation, engraftment and functional effects of pre-treated mesenchymal stem cells in a rat myocardial infarct model. Acta Cardiol. 2005;60(3):277–284. doi: 10.2143/AC.60.3.2005005. [DOI] [PubMed] [Google Scholar]
- 93.Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L, Mirotsou M, Pratt RE, Dzau VJ. Genetic Modification of Mesenchymal Stem Cells Overexpressing CCR1 Increases Cell Viability, Migration, Engraftment, and Capillary Density in the Injured Myocardium. Circ Res. 2010 Apr 8; doi: 10.1161/CIRCRESAHA.109.196030. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang W, Ma A, Wang T, Han K, Liu Y, Zhang Y, Dong A, Du Y, Huang X, Wang J, Lei X, Zheng X. Homing and differentiation of mesenchymal stem cells delivered intravenously to ischemic myocardium in vivo: a time-series study. Pflugers Arch. 2006;453(1):43–52. doi: 10.1007/s00424-006-0117-y. [DOI] [PubMed] [Google Scholar]
- 95.Jiang W, Ma A, Wang T, Han K, Liu Y, Zhang Y, Zhao X, Dong A, Du Y, Huang X, Wang J, Lei X, Zheng X. Intravenous transplantation of mesenchymal stem cells improves cardiac performance after acute myocardial ischemia in female rats. Transpl Int. 2006;19(7):570–580. doi: 10.1111/j.1432-2277.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- 96.Jiang WH, Ma AQ, Zhang YM, Han K, Liu Y, Zhang ZT, Wang TZ, Huang X, Zheng XP. Migration of intravenously grafted mesenchymal stem cells to injured heart in rats. Sheng Li Xue Bao. 2005;57(5):566–572. [PubMed] [Google Scholar]
- 97.Katritsis DG, Sotiropoulou P, Giazitzoglou E, Karvouni E, Papamichail M. Electrophysiological effects of intracoronary transplantation of autologous mesenchymal and endothelial progenitor cells. Europace. 2007;9(3):167–171. doi: 10.1093/europace/eul184. [DOI] [PubMed] [Google Scholar]
- 98.Li Q, Turdi S, Thomas DP, Zhou T, Ren J. Intra-myocardial delivery of mesenchymal stem cells ameliorates left ventricular and cardiomyocyte contractile dysfunction following myocardial infarction. Toxicol Lett. 2010 March 23; doi: 10.1016/j.toxlet.2010.03.009. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohnishi S, Yanagawa B, Tanaka K, Miyahara Y, Obata H, Kataoka M, Kodama M, Ishibashi-Ueda H, Kangawa K, Kitamura S, Nagaya N. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol. 2007;42(1):88–97. doi: 10.1016/j.yjmcc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 100.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98(18):10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Song H, Song BW, Cha MJ, Choi IG, Hwang KC. Modification of mesenchymal stem cells for cardiac regeneration. Expert Opin Biol Ther. 2010;10(3):309–319. doi: 10.1517/14712590903455997. [DOI] [PubMed] [Google Scholar]
- 102.Tang J, Wang J, Guo L, Kong X, Yang J, Zheng F, Zhang L, Huang Y. Mesenchymal stem cells modified with stromal cell-derived factor 1 alpha improve cardiac remodeling via paracrine activation of hepatocyte growth factor in a rat model of myocardial infarction. Mol Cells. 2010;29(1):9–19. doi: 10.1007/s10059-010-0001-7. [DOI] [PubMed] [Google Scholar]
- 103.Westrich J, Yaeger P, He C, Stewart J, Chen R, Seleznik G, Larson S, Wentworth B, O'Callaghan M, Wadsworth S, Akita G, Molnar G. Factors Affecting Residence Time of Mesenchymal Stromal Cells (MSC) Injected into the Myocardium. Cell Transplant. 2010 March 31; doi: 10.3727/096368910X494911. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 104.Xu J, Chen Q, Shi C, Yin Z. Overexpression of CXCR1/CXCR2 on mesenchymal stromal cells may be an effective treatment for acute myocardial infarction. Cytotherapy. 2009;11(8):990–991. doi: 10.3109/14653240903233099. [DOI] [PubMed] [Google Scholar]
- 105.Yau TM, Tomita S, Weisel RD, Jia ZQ, Tumiati LC, Mickle DA, Li RK. Beneficial effect of autologous cell transplantation on infarcted heart function: comparison between bone marrow stromal cells and heart cells. Ann Thorac Surg. 2003;75(1):169–176. doi: 10.1016/s0003-4975(02)04290-x. discussion 176-7. [DOI] [PubMed] [Google Scholar]
- 106.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106(33):14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, Greschat S, Knipper A, Bender J, Degistirici O, Gao J, Caplan AI, Colletti EJ, Almeida-Porada G, Muller HW, Zanjani E, Wernet P. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200(2):123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Noort WA, Feye D, Van Den Akker F, Stecher D, Chamuleau SA, Sluijter JP, Doevendans PA. Mesenchymal stromal cells to treat cardiovascular disease: strategies to improve survival and therapeutic results. Panminerva Med. 2010;52(1):27–40. [PubMed] [Google Scholar]
- 109.Rameshwar P, Qiu H, Vatner SF. Stem cells in cardiac repair in an inflammatory microenvironment. Minerva Cardioangiol. 2010;58(1):127–146. [PubMed] [Google Scholar]
- 110.Kocher AA, Schlechta B, Gasparovicova A, Wolner E, Bonaros N, Laufer G. Stem cells and cardiac regeneration. Transpl Int. 2007;20(9):731–746. doi: 10.1111/j.1432-2277.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- 111.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mohyeddin-Bonab M, Mohamad-Hassani MR, Alimoghaddam K, Sanatkar M, Gasemi M, Mirkhani H, Radmehr H, Salehi M, Eslami M, Farhig-Parsa A, Emami-Razavi H, Alemohammad MG, Solimani AA, Ghavamzadeh A, Nikbin B. Autologous in vitro expanded mesenchymal stem cell therapy for human old myocardial infarction. Arch Iran Med. 2007;10(4):467–473. [PubMed] [Google Scholar]
- 113.Ripa RS, Haack-Sorensen M, Wang Y, Jorgensen E, Mortensen S, Bindslev L, Friis T, Kastrup J. Bone marrow derived mesenchymal cell mobilization by granulocyte-colony stimulating factor after acute myocardial infarction: results from the Stem Cells in Myocardial Infarction (STEMMI) trial. Circulation. 2007;116(11 Suppl):I24–I30. doi: 10.1161/CIRCULATIONAHA.106.678649. [DOI] [PubMed] [Google Scholar]
- 114.Hoogduijn MJ, Crop MJ, Peeters AM, Korevaar SS, Eijken M, Drabbels JJ, Roelen DL, Maat AP, Balk AH, Weimar W, Baan CC. Donor-derived mesenchymal stem cells remain present and functional in the transplanted human heart. Am J Transplant. 2009;9(1):222–230. doi: 10.1111/j.1600-6143.2008.02450.x. [DOI] [PubMed] [Google Scholar]
- 115.Chen SL, Fang WW, Qian J, Ye F, Liu YH, Shan SJ, Zhang JJ, Lin S, Liao LM, Zhao RC. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J (Engl) 2004;117(10):1443–1448. [PubMed] [Google Scholar]
- 116.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94(1):92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 117.Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40(6):1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- 118.Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, Takimoto R, Iyama S, Matsunaga T, Ohtani S, Matsuura A, Hamada H, Niitsu Y. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106(2):756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 119.Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109(10):1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Almeida-Porada G, Porada C, Zanjani ED. Adult stem cell plasticity and methods of detection. Rev Clin Exp Hematol. 2001;5(1):26–41. doi: 10.1046/j.1468-0734.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 121.Almeida-Porada G, Porada C, Zanjani ED. Plasticity of human stem cells in the fetal sheep model of human stem cell transplantation. Int J Hematol. 2004;79(1):1–6. doi: 10.1007/BF02983526. [DOI] [PubMed] [Google Scholar]
- 122.Almeida-Porada G, Zanjani ED. A large animal noninjury model for study of human stem cell plasticity. Blood Cells Mol Dis. 2004;32(1):77–81. doi: 10.1016/j.bcmd.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 123.Aurich H, Sgodda M, Kaltwasser P, Vetter M, Weise A, Liehr T, Brulport M, Hengstler JG, Dollinger MM, Fleig WE, Christ B. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 2008 doi: 10.1136/gut.2008.154880. [DOI] [PubMed] [Google Scholar]
- 124.Aurich I, Mueller LP, Aurich H, Luetzkendorf J, Tisljar K, Dollinger MM, Schormann W, Walldorf J, Hengstler JG, Fleig WE, Christ B. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007;56(3):405–415. doi: 10.1136/gut.2005.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, Kato T, Okochi H, Ochiya T. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2008 doi: 10.1111/j.1440-1746.2008.05496.x. [DOI] [PubMed] [Google Scholar]
- 126.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, Kawamata M, Kato T, Okochi H, Ochiya T. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells. 2008;26(10):2705–2712. doi: 10.1634/stemcells.2008-0034. [DOI] [PubMed] [Google Scholar]
- 127.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, Okochi H, Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46(1):219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 128.di Bonzo LV, Ferrero I, Cravanzola C, Mareschi K, Rustichell D, Novo E, Sanavio F, Cannito S, Zamara E, Bertero M, Davit A, Francica S, Novelli F, Colombatto S, Fagioli F, Parola M. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut. 2008;57(2):223–231. doi: 10.1136/gut.2006.111617. [DOI] [PubMed] [Google Scholar]
- 129.Enns GM, Millan MT. Cell-based therapies for metabolic liver disease. Mol Genet Metab. 2008;95(1–2):3–10. doi: 10.1016/j.ymgme.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 130.Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78(1):83–88. doi: 10.1097/01.tp.0000128326.95294.14. [DOI] [PubMed] [Google Scholar]
- 131.Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S, Niioka M, Watanabe T, Okano H, Matsuzaki Y, Shiota G, Okazaki I. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45(1):213–222. doi: 10.1002/hep.21477. [DOI] [PubMed] [Google Scholar]
- 132.Ishikawa T, Terai S, Urata Y, Marumoto Y, Aoyama K, Sakaida I, Murata T, Nishina H, Shinoda K, Uchimura S, Hamamoto Y, Okita K. Fibroblast growth factor 2 facilitates the differentiation of transplanted bone marrow cells into hepatocytes. Cell Tissue Res. 2006;323(2):221–231. doi: 10.1007/s00441-005-0077-0. [DOI] [PubMed] [Google Scholar]
- 133.Luk JM, Wang PP, Lee CK, Wang JH, Fan ST. Hepatic potential of bone marrow stromal cells: development of in vitro co-culture and intra-portal transplantation models. J Immunol Methods. 2005;305(1):39–47. doi: 10.1016/j.jim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 134.Lysy PA, Campard D, Smets F, Malaise J, Mourad M, Najimi M, Sokal EM. Persistence of a chimerical phenotype after hepatocyte differentiation of human bone marrow mesenchymal stem cells. Cell Prolif. 2008;41(1):36–58. doi: 10.1111/j.1365-2184.2007.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Muraca M, Ferraresso C, Vilei MT, Granato A, Quarta M, Cozzi E, Rugge M, Pauwelyn KA, Caruso M, Avital I, Inderbitzin D, Demetriou AA, Forbes SJ, Realdi G. Liver repopulation with bone marrow derived cells improves the metabolic disorder in the Gunn rat. Gut. 2007;56(12):1725–1735. doi: 10.1136/gut.2007.127969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006;44(4):742–748. doi: 10.1016/j.jhep.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 137.Popp FC, Piso P, Schlitt HJ, Dahlke MH. Therapeutic potential of bone marrow stem cells for liver diseases. Curr Stem Cell Res Ther. 2006;1(3):411–418. doi: 10.2174/157488806778226759. [DOI] [PubMed] [Google Scholar]
- 138.Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40(6):1304–1311. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- 139.Sgodda M, Aurich H, Kleist S, Aurich I, Konig S, Dollinger MM, Fleig WE, Christ B. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp Cell Res. 2007;313(13):2875–2886. doi: 10.1016/j.yexcr.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 140.Talens-Visconti R, Bonora A, Jover R, Mirabet V, Carbonell F, Castell JV, Gomez-Lechon MJ. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J Gastroenterol. 2006;12(36):5834–5845. doi: 10.3748/wjg.v12.i36.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Theise ND, Krause DS. Bone marrow to liver: the blood of Prometheus. Semin Cell Dev Biol. 2002;13(6):411–417. doi: 10.1016/s1084952102001283. [DOI] [PubMed] [Google Scholar]
- 142.Zhao DC, Lei JX, Chen R, Yu WH, Zhang XM, Li SN, Xiang P. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11(22):3431–3440. doi: 10.3748/wjg.v11.i22.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zheng JF, Liang LJ. Intra-portal transplantation of bone marrow stromal cells ameliorates liver fibrosis in mice. Hepatobiliary Pancreat Dis Int. 2008;7(3):264–270. [PubMed] [Google Scholar]
- 144.Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, Baharvand H, Ghavamzadeh A, Malekzadeh R. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10(4):459–466. [PubMed] [Google Scholar]
- 145.Mohamadnejad M, Namiri M, Bagheri M, Hashemi SM, Ghanaati H, Zare Mehrjardi N, Kazemi Ashtiani S, Malekzadeh R, Baharvand H. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol. 2007;13(24):3359–3363. doi: 10.3748/wjg.v13.i24.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y, Okita K, Sakaida I. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24(10):2292–2298. doi: 10.1634/stemcells.2005-0542. [DOI] [PubMed] [Google Scholar]
- 147.Parekkadan B, van Poll D, Megeed Z, Kobayashi N, Tilles AW, Berthiaume F, Yarmush ML. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;363(2):247–252. doi: 10.1016/j.bbrc.2007.05.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2(9):e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kuo TK, Hung SP, Chuang CH, Chen CT, Shih YR, Fang SC, Yang VW, Lee OK. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134(7) doi: 10.1053/j.gastro.2008.03.015. 2111-21, 2121 e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 151.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166(3):585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 152.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38(1):215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dai W, Hale SL, Kloner RA. Role of a paracrine action of mesenchymal stem cells in the improvement of left ventricular function after coronary artery occlusion in rats. Regen Med. 2007;2(1):63–68. doi: 10.2217/17460751.2.1.63. [DOI] [PubMed] [Google Scholar]
- 154.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11(4):367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 155.Huang NF, Lam A, Fang Q, Sievers RE, Li S, Lee RJ. Bone marrow-derived mesenchymal stem cells in fibrin augment angiogenesis in the chronically infarcted myocardium. Regen Med. 2009;4(4):527–538. doi: 10.2217/rme.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ladage D, Brixius K, Steingen C, Mehlhorn U, Schwinger RH, Bloch W, Schmidt A. Mesenchymal stem cells induce endothelial activation via paracine mechanisms. Endothelium. 2007;14(2):53–63. doi: 10.1080/10623320701343319. [DOI] [PubMed] [Google Scholar]
- 157.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 158.Li Z, Guo J, Chang Q, Zhang A. Paracrine role for mesenchymal stem cells in acute myocardial infarction. Biol Pharm Bull. 2009;32(8):1343–1346. doi: 10.1248/bpb.32.1343. [DOI] [PubMed] [Google Scholar]
- 159.Mias C, Lairez O, Trouche E, Roncalli J, Calise D, Seguelas MH, Ordener C, Piercecchi-Marti MD, Auge N, Salvayre AN, Bourin P, Parini A, Cussac D. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27(11):2734–2743. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 160.Ohnishi S, Sumiyoshi H, Kitamura S, Nagaya N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett. 2007;581(21):3961–3966. doi: 10.1016/j.febslet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 161.Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296(6):H1888–H1897. doi: 10.1152/ajpheart.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DP. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1(2):129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 163.Xiang MX, He AN, Wang JA, Gui C. Protective paracrine effect of mesenchymal stem cells on cardiomyocytes. J Zhejiang Univ Sci B. 2009;10(8):619–624. doi: 10.1631/jzus.B0920153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yu XY, Geng YJ, Li XH, Lin QX, Shan ZX, Lin SG, Song YH, Li Y. The effects of mesenchymal stem cells on c-kit up-regulation and cell-cycle re-entry of neonatal cardiomyocytes are mediated by activation of insulin-like growth factor 1 receptor. Mol Cell Biochem. 2009;332(1–2):25–32. doi: 10.1007/s11010-009-0170-x. [DOI] [PubMed] [Google Scholar]
- 165.van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47(5):1634–1643. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 166.Abdel Aziz MT, Atta HM, Mahfouz S, Fouad HH, Roshdy NK, Ahmed HH, Rashed LA, Sabry D, Hassouna AA, Hasan NM. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin Biochem. 2007;40(12):893–899. doi: 10.1016/j.clinbiochem.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 167.Li JT, Liao ZX, Ping J, Xu D, Wang H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. J Gastroenterol. 2008;43(6):419–428. doi: 10.1007/s00535-008-2180-y. [DOI] [PubMed] [Google Scholar]
- 168.Zhao ZH, Xin SJ, Zhao JM, Wang SS, Liu P, Yin TY, Zhou GD. [Dynamic expression of matrix metalloproteinase-2, membrane type-matrix metalloproteinase-2 in experimental hepatic fibrosis and its reversal in rat] Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2004;18(4):328–331. [PubMed] [Google Scholar]
- 169.Alison M, Islam S, Lim S. Stem cells in liver regeneration, fibrosis and cancer: the good, the bad and the ugly. J Pathol. 2008 doi: 10.1002/path.2453. [DOI] [PubMed] [Google Scholar]
- 170.Asawa S, Saito T, Satoh A, Ohtake K, Tsuchiya T, Okada H, Neilson EG, Gotoh M. Participation of bone marrow cells in biliary fibrosis after bile duct ligation. J Gastroenterol Hepatol. 2007;22(11):2001–2008. doi: 10.1111/j.1440-1746.2006.04708.x. [DOI] [PubMed] [Google Scholar]
- 171.Baba S, Fujii H, Hirose T, Yasuchika K, Azuma H, Hoppo T, Naito M, Machimoto T, Ikai I. Commitment of bone marrow cells to hepatic stellate cells in mouse. J Hepatol. 2004;40(2):255–260. doi: 10.1016/j.jhep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 172.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45(3):429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 173.Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou-Gharios G, Jeffery R, Iredale JP, Forbes SJ. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130(6):1807–1821. doi: 10.1053/j.gastro.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 174.Ozawa K, Sato K, Oh I, Ozaki K, Uchibori R, Obara Y, Kikuchi Y, Ito T, Okada T, Urabe M, Mizukami H, Kume A. Cell and gene therapy using mesenchymal stem cells (MSCs) J Autoimmun. 2008;30(3):121–127. doi: 10.1016/j.jaut.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 175.Reiser J, Zhang XY, Hemenway CS, Mondal D, Pradhan L, La Russa VF. Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases. Expert Opin Biol Ther. 2005;5(12):1571–1584. doi: 10.1517/14712598.5.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bosch P, Fouletier-Dilling C, Olmsted-Davis EA, Davis AR, Stice SL. Efficient adenoviral-mediated gene delivery into porcine mesenchymal stem cells. Mol Reprod Dev. 2006;73(11):1393–1403. doi: 10.1002/mrd.20593. [DOI] [PubMed] [Google Scholar]
- 177.Bosch P, Stice SL. Adenoviral transduction of mesenchymal stem cells. Methods Mol Biol. 2007;407:265–274. doi: 10.1007/978-1-59745-536-7_18. [DOI] [PubMed] [Google Scholar]
- 178.Roelants V, Labar D, de Meester C, Havaux X, Tabilio A, Gambhir SS, Di Ianni M, Bol A, Bertrand L, Vanoverschelde JL. Comparison between adenoviral and retroviral vectors for the transduction of the thymidine kinase PET reporter gene in rat mesenchymal stem cells. J Nucl Med. 2008;49(11):1836–1844. doi: 10.2967/jnumed.108.052175. [DOI] [PubMed] [Google Scholar]
- 179.Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation expansion characterization viral transduction, and production of conditioned medium. Methods Mol Biol. 2009;482:281–294. doi: 10.1007/978-1-59745-060-7_18. [DOI] [PubMed] [Google Scholar]
- 180.Meyerrose TE, De Ugarte DA, Hofling AA, Herrbrich PE, Cordonnier TD, Shultz LD, Eagon JC, Wirthlin L, Sands MS, Hedrick MA, Nolta JA. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 2007;25(1):220–227. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Piccoli C, Scrima R, Ripoli M, Di Ianni M, Del Papa B, D'Aprile A, Quarato G, Martelli MP, Servillo G, Ligas C, Boffoli D, Tabilio A, Capitanio N. Transformation by retroviral vectors of bone marrow-derived mesenchymal cells induces mitochondria-dependent cAMP-sensitive reactive oxygen species production. Stem Cells. 2008;26(11):2843–2854. doi: 10.1634/stemcells.2007-0885. [DOI] [PubMed] [Google Scholar]
- 182.Sales VL, Mettler BA, Lopez-Ilasaca M, Johnson JA, Jr, Mayer JE., Jr Endothelial progenitor and mesenchymal stem cell-derived cells persist in tissue-engineered patch in vivo: application of green and red fluorescent protein-expressing retroviral vector. Tissue Eng. 2007;13(3):525–535. doi: 10.1089/ten.2006.0128. [DOI] [PubMed] [Google Scholar]
- 183.Fan L, Lin C, Zhuo S, Chen L, Liu N, Luo Y, Fang J, Huang Z, Lin Y, Chen J. Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur J Heart Fail. 2009;11(11):1023–1030. doi: 10.1093/eurjhf/hfp135. [DOI] [PubMed] [Google Scholar]
- 184.Meyerrose TE, Roberts M, Ohlemiller KK, Vogler CA, Wirthlin L, Nolta JA, Sands MS. Lentiviral-transduced human mesenchymal stem cells persistently express therapeutic levels of enzyme in a xenotransplantation model of human disease. Stem Cells. 2008;26(7):1713–1722. doi: 10.1634/stemcells.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang F, Dennis JE, Awadallah A, Solchaga LA, Molter J, Kuang Y, Salem N, Lin Y, Tian H, Kolthammer JA, Kim Y, Love ZB, Gerson SL, Lee Z. Transcriptional profiling of human mesenchymal stem cells transduced with reporter genes for imaging. Physiol Genomics. 2009;37(1):23–34. doi: 10.1152/physiolgenomics.00300.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xiang J, Tang J, Song C, Yang Z, Hirst DG, Zheng QJ, Li G. Mesenchymal stem cells as a gene therapy carrier for treatment of fibrosarcoma. Cytotherapy. 2009;11(5):516–526. doi: 10.1080/14653240902960429. [DOI] [PubMed] [Google Scholar]
- 187.Zhang XY, La Russa VF, Reiser J. Transduction of bone-marrow-derived mesenchymal stem cells by using lentivirus vectors pseudotyped with modified RD114 envelope glycoproteins. J Virol. 2004;78(3):1219–1229. doi: 10.1128/JVI.78.3.1219-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang XY, La Russa VF, Bao L, Kolls J, Schwarzenberger P, Reiser J. Lentiviral vectors for sustained transgene expression in human bone marrow-derived stromal cells. Mol Ther. 2002;5(5 Pt 1):555–565. doi: 10.1006/mthe.2002.0585. [DOI] [PubMed] [Google Scholar]
- 189.Kumar S, Mahendra G, Nagy TR, Ponnazhagan S. Osteogenic differentiation of recombinant adeno-associated virus 2-transduced murine mesenchymal stem cells and development of an immunocompetent mouse model for ex vivo osteoporosis gene therapy. Hum Gene Ther. 2004;15(12):1197–1206. doi: 10.1089/hum.2004.15.1197. [DOI] [PubMed] [Google Scholar]
- 190.Stender S, Murphy M, O'Brien T, Stengaard C, Ulrich-Vinther M, Soballe K, Barry F. Adeno-associated viral vector transduction of human mesenchymal stem cells. Eur Cell Mater. 2007;13:93–99. doi: 10.22203/ecm.v013a10. discussion 99. [DOI] [PubMed] [Google Scholar]
- 191.Deuse T, Peter C, Fedak PW, Doyle T, Reichenspurner H, Zimmermann WH, Eschenhagen T, Stein W, Wu JC, Robbins RC, Schrepfer S. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation. 2009;120(11 Suppl):S247–S254. doi: 10.1161/CIRCULATIONAHA.108.843680. [DOI] [PubMed] [Google Scholar]
- 192.Guo YH, He JG, Wu JL, Yang L, Zhang DS, Tan XY, Qi RD. Hepatocyte growth factor and granulocyte colony-stimulating factor form a combined neovasculogenic therapy for ischemic cardiomyopathy. Cytotherapy. 2008;10(8):857–867. doi: 10.1080/14653240802419278. [DOI] [PubMed] [Google Scholar]
- 193.Liu XH, Bai CG, Xu ZY, Huang SD, Yuan Y, Gong DJ, Zhang JR. Therapeutic potential of angiogenin modified mesenchymal stem cells: angiogenin improves mesenchymal stem cells survival under hypoxia and enhances vasculogenesis in myocardial infarction. Microvasc Res. 2008;76(1):23–30. doi: 10.1016/j.mvr.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 194.Tang J, Wang J, Yang J, Kong X, Zheng F, Guo L, Zhang L, Huang Y. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg. 2009;36(4):644–650. doi: 10.1016/j.ejcts.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 195.Wang Y, Feng C, Xue J, Sun A, Li J, Wu J. Adenovirus-mediated hypoxia-inducible factor 1alpha double-mutant promotes differentiation of bone marrow stem cells to cardiomyocytes. J Physiol Sci. 2009;59(6):413–420. doi: 10.1007/s12576-009-0050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang Y, Sun A, Xue J, Feng C, Li J, Wu J. Bone marrow derived stromal cells modified by adenovirus-mediated HIF-1alpha double mutant protect cardiac myocytes against CoCl2-induced apoptosis. Toxicol In Vitro. 2009;23(6):1069–1075. doi: 10.1016/j.tiv.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 197.Lu Z, Hu X, Zhu C, Wang D, Zheng X, Liu Q. Overexpression of CNTF in Mesenchymal Stem Cells reduces demyelination and induces clinical recovery in experimental autoimmune encephalomyelitis mice. J Neuroimmunol. 2009;206(1–2):58–69. doi: 10.1016/j.jneuroim.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 198.Lu ZQ, Hu XQ, Zhu CS, Zheng XP, Wan DJ, Liu RY, Huang BJ, Huang WL. [Bone marrow stromal cells transfected with ciliary neurotrophic factor gene ameliorates the symptoms and inflammation in C57BL/6 mice with experimental allergic encephalomyelitis] Nan Fang Yi Ke Da Xue Xue Bao. 2009;29(12):2355–2361. [PubMed] [Google Scholar]
- 199.Zhao MZ, Nonoguchi N, Ikeda N, Watanabe T, Furutama D, Miyazawa D, Funakoshi H, Kajimoto Y, Nakamura T, Dezawa M, Shibata MA, Otsuki Y, Coffin RS, Liu WD, Kuroiwa T, Miyatake S. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J Cereb Blood Flow Metab. 2006;26(9):1176–1188. doi: 10.1038/sj.jcbfm.9600273. [DOI] [PubMed] [Google Scholar]
- 200.Aquino JB, Bolontrade MF, Garcia MG, Podhajcer OL, Mazzolini G. Mesenchymal stem cells as therapeutic tools and gene carriers in liver fibrosis and hepatocellular carcinoma. Gene Ther. 2010 March 12; doi: 10.1038/gt.2010.10. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 201.Doering CB. Retroviral modification of mesenchymal stem cells for gene therapy of hemophilia. Methods Mol Biol. 2008;433:203–212. doi: 10.1007/978-1-59745-237-3_12. [DOI] [PubMed] [Google Scholar]
- 202.Pipe SW, High KA, Ohashi K, Ural AU, Lillicrap D. Progress in the molecular biology of inherited bleeding disorders. Haemophilia. 2008;14 Suppl 3:130–137. doi: 10.1111/j.1365-2516.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 203.Van Damme A, Chuah MK, Dell'accio F, De Bari C, Luyten F, Collen D, VandenDriessche T. Bone marrow mesenchymal cells for haemophilia A gene therapy using retroviral vectors with modified long-terminal repeats. Haemophilia. 2003;9(1):94–103. doi: 10.1046/j.1365-2516.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- 204.Bartholomew A, Patil S, Mackay A, Nelson M, Buyaner D, Hardy W, Mosca J, Sturgeon C, Siatskas M, Mahmud N, Ferrer K, Deans R, Moseley A, Hoffman R, Devine SM. Baboon mesenchymal stem cells can be genetically modified to secrete human erythropoietin in vivo. Hum Gene Ther. 2001;12(12):1527–1541. doi: 10.1089/10430340152480258. [DOI] [PubMed] [Google Scholar]
- 205.Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, Sturgeon C, Hewett T, Chung T, Stock W, Sher D, Weissman S, Ferrer K, Mosca J, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29(2):244–255. doi: 10.1016/s0301-472x(00)00635-4. [DOI] [PubMed] [Google Scholar]
- 206.Le Blanc K, Ringden O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(5):321–334. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 207.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57(1):11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 208.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129(1):118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 209.Batten P, Sarathchandra P, Antoniw JW, Tay SS, Lowdell MW, Taylor PM, Yacoub MH. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: relevance to tissue engineering human heart valves. Tissue Eng. 2006;12(8):2263–2273. doi: 10.1089/ten.2006.12.2263. [DOI] [PubMed] [Google Scholar]
- 210.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 211.Groh ME, Maitra B, Szekely E, Koc ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005;33(8):928–934. doi: 10.1016/j.exphem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 212.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 213.Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol. 2007;179(5):2824–2831. doi: 10.4049/jimmunol.179.5.2824. [DOI] [PubMed] [Google Scholar]
- 214.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity tolerance, and suppression. J Biomed Sci. 2005;12(1):47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 215.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 216.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noel D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25(8):2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 217.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 218.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177(4):2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 219.Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13(3):263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 220.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 221.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 222.Maccario R, Podesta M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S, Frassoni F, Locatelli F. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90(4):516–525. [PubMed] [Google Scholar]
- 223.Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92(7):881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 224.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 225.Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, Mathieu N, Sensebe L, Zhang Y, Gorin NC, Thierry D, Fouillard L. Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2007;13(4–5):217–226. doi: 10.3727/000000006780666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nasef A, Mathieu N, Chapel A, Frick J, Francois S, Mazurier C, Boutarfa A, Bouchet S, Gorin NC, Thierry D, Fouillard L. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation. 2007;84(2):231–237. doi: 10.1097/01.tp.0000267918.07906.08. [DOI] [PubMed] [Google Scholar]
- 227.Hainz U, Jurgens B, Heitger A. The role of indoleamine 2,3-dioxygenase in transplantation. Transpl Int. 2007;20(2):118–127. doi: 10.1111/j.1432-2277.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 228.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 229.Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Alwayn IP, Weimar W, Hoogduijn MJ. Donor-derived mesenchymal stem cells suppress alloreactivity of kidney transplant patients. Transplantation. 2009;87(6):896–906. doi: 10.1097/TP.0b013e31819b3d72. [DOI] [PubMed] [Google Scholar]
- 230.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 231.Ge W, Jiang J, Baroja ML, Arp J, Zassoko R, Liu W, Bartholomew A, Garcia B, Wang H. Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am J Transplant. 2009;9(8):1760–1772. doi: 10.1111/j.1600-6143.2009.02721.x. [DOI] [PubMed] [Google Scholar]
- 232.Wan CD, Cheng R, Wang HB, Liu T. Immunomodulatory effects of mesenchymal stem cells derived from adipose tissues in a rat orthotopic liver transplantation model. Hepatobiliary Pancreat Dis Int. 2008;7(1):29–33. [PubMed] [Google Scholar]
- 233.Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, Shpall EJ, McCarthy P, Atkinson K, Cooper BW, Gerson SL, Laughlin MJ, Loberiza FR, Jr, Moseley AB, Bacigalupo A. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11(5):389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 234.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 235.Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, Locatelli F, Fibbe WE. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110(7):2764–2767. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- 236.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 237.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56(4):1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 238.Park HK, Cho KS, Park HY, Shin DH, Kim YK, Jung JS, Park SK, Roh HJ. Adipose-derived stromal cells inhibit allergic airway inflammation in mice. Stem Cells Dev. 2010 March 17; doi: 10.1089/scd.2009.0513. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 239.Weiss DJ, Kolls JK, Ortiz LA, Panoskaltsis-Mortari A, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2008;5(5):637–667. doi: 10.1513/pats.200804-037DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, Hodges MG, Jelinek I, Madala S, Karpati S, Mezey E. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010;107(12):5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cho KS, Park HK, Park HY, Jung JS, Jeon SG, Kim YK, Roh HJ. IFATS collection: Immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells. 2009;27(1):259–265. doi: 10.1634/stemcells.2008-0283. [DOI] [PubMed] [Google Scholar]
- 242.Iyer SS, Co C, Rojas M. Mesenchymal stem cells and inflammatory lung diseases. Panminerva Med. 2009;51(1):5–16. [PubMed] [Google Scholar]
- 243.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62(13):3603–3608. [PubMed] [Google Scholar]
- 244.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96(21):1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 245.Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15(10):739–752. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- 246.Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y, Hamada H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11(14):1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 247.Grisendi G, Bussolari R, Cafarelli L, Petak I, Rasini V, Veronesi E, De Santis G, Spano C, Tagliazzucchi M, Barti-Juhasz H, Scarabelli L, Bambi F, Frassoldati A, Rossi G, Casali C, Morandi U, Horwitz EM, Paolucci P, Conte P, Dominici M. Adipose-Derived Mesenchymal Stem Cells as Stable Source of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Delivery for Cancer Therapy. Cancer Res. 2010 Apr 13; doi: 10.1158/0008-5472.CAN-09-1865. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 248.Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69(10):4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Menon LG, Kelly K, Yang HW, Kim SK, Black PM, Carroll RS. Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells. 2009;27(9):2320–2330. doi: 10.1002/stem.136. [DOI] [PubMed] [Google Scholar]
- 250.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, Figueiredo JL, Martuza RL, Weissleder R, Shah K. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci U S A. 2009;106(12):4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hamada H, Kobune M, Nakamura K, Kawano Y, Kato K, Honmou O, Houkin K, Matsunaga T, Niitsu Y. Mesenchymal stem cells (MSC) as therapeutic cytoreagents for gene therapy. Cancer Sci. 2005;96(3):149–156. doi: 10.1111/j.1349-7006.2005.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]