Abstract
The corticotropin-releasing factor (CRF) peptide hormone family members coordinate endocrine, behavioral, autonomic, and metabolic responses to stress and play important roles within the cardiovascular, gastrointestinal, and central nervous systems, among others. The actions of the peptides are mediated by activation of two G-protein-coupled receptors of the B1 family, CRF receptors 1 and 2 (CRF-R1 and CRF-R2α,β). The recently reported three-dimensional structures of the first extracellular domain (ECD1) of both CRF-R1 and CRF-R2β (Pioszak, A. A., Parker, N. R., Suino-Powell, K., and Xu, H. E. (2008) J. Biol. Chem. 283, 32900–32912; Grace, C. R., Perrin, M. H., Gulyas, J., Digruccio, M. R., Cantle, J. P., Rivier, J. E., Vale, W. W., and Riek, R. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 4858–4863) complexed with peptide antagonists provided a starting point in understanding the binding between CRF ligands and receptors at a molecular level. We now report the three-dimensional NMR structure of the ECD1 of human CRF-R1 complexed with a high affinity agonist, α-helical cyclic CRF. In the structure of the complex, the C-terminal residues (23–41) of α-helical cyclic CRF bind to the ECD1 of CRF-R1 in a helical conformation mainly along the hydrophobic face of the peptide in a manner similar to that of the antagonists in their corresponding ECD1 complex structures. Unique to this study is the observation that complex formation between an agonist and the ECD1-CRF-R1 promotes the helical conformation of the N terminus of the former, important for receptor activation (Gulyas, J., Rivier, C., Perrin, M., Koerber, S. C., Sutton, S., Corrigan, A., Lahrichi, S. L., Craig, A. G., Vale, W., and Rivier, J. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 10575–10579).
Keywords: G Protein-coupled Receptors (GPCR), NMR, Peptide Biosynthesis, Peptide Chemical Synthesis, Peptide Conformation, Peptides, CRF, Corticotropin-releasing Factor, ECD1-CRF-R1, alphahcCRF.
Introduction
Corticotropin-releasing factor (5), a 41-residue peptide hormone, and other members of the CRF5 family (the urocortins) are important regulators of the stress response. They are involved in the control of appetite as well as in the functioning of the cardiovascular, reproductive, gastrointestinal, immune, and central nervous systems (6–8). Their actions are mediated by binding to their G-protein-coupled receptors that belong to the peptide hormone B1 family (family B1 GPCRs). The members of this family include receptors for growth hormone-releasing hormone, secretin, calcitonin, vasoactive intestinal peptide, glucagon, glucagon-like peptide-1 (GLP-1), gastric inhibitory peptide, pituitary adenylate cyclase-activating peptide, and parathyroid hormone (PTH). For the CRF family, two receptors, CRF-R1 and CRF-R2, have been cloned and characterized (9, 10). Structure-activity studies of mutant and chimeric CRF receptors identified residues that affect the binding of the peptide ligands and are located mainly in the extracellular domains of the receptors (11–15). On the one hand, structure-activity studies of the CRF family ligands showed that the first six residues of the N-terminal portion of the peptide are not necessary for receptor signaling as long as α-helicity is maintained (3, 4, 16–18). On the other hand, the C-terminal (∼15) residues contribute significantly to receptor binding affinities (16, 19). Similar data, both on signaling and binding properties, have been reported for the other members of the B1 GPCR family and their corresponding ligands (20–24).
Recently, three-dimensional structures of the ECD1s of several B1 family receptors in the presence and absence of their respective ligands have been solved. The structures reported include the NMR structure of the mouse CRF-R2β free and complexed with the CRF antagonist astressin (2), the NMR structure of the human splice variant PAC1-Rs complexed with the N-terminally truncated antagonist pituitary adenylate cyclase-activating peptide(6–38) (25), the crystal structure of human GIP-R complexed with its agonist incretin GIP(1–42) (26), the crystal structure of the human GLP-1R complexed with the antagonist exendin-4(9–39) (27), the crystal structure of human PTH-1R fused to maltose-binding protein (MBP) complexed with the peptide fragment PTH(15–34) (28), and the crystal structure of the human CRF-R1 fused to MBP complexed with CRF antagonists (1) (Fig. 1). These structures show that the short consensus repeat (SCR) is the common polypeptide fold for the ECD1 of the receptors (29), and the interaction of the C-terminal part of their cognate ligands is along the hydrophobic face of their helices.
FIGURE 1.
Ribbon diagram of the ECD1s of family B1 receptors. The following codes are used: GIP-R (PDB code 2QKH) (A), GLP-1R (PDB code 3C5T) (B), PTH-1R (PDB code 3C5T) (C), CRF-R2β (PDB code 2JND) (D), CRF-R1 (PDB code 3EHU) (E), and CRF-R1 (PDB code 2L27) (F) complexed with their respective ligands, namely incretin shown in blue (A), exendin-4 shown in magenta (B), parathyroid hormone shown in green (C), astressin shown in cyan (D), CRF(27–31) shown in dark green (E), and (αhcCRF shown in gold (F). The structures are superimposed along the β3 and β4 segments, and their orientations are retained. Highly conserved disulfides are shown in yellow. Note: the binding site and orientation of pituitary adenylate cyclase-activating peptide(6–38) complexed with its ECD1 receptor domain (25) is not included in this figure because they are completely different from that of the other ligands. Whether this difference is an artifact of the procedure used in structure determination or a true difference needs to be determined.
Here, we report the three-dimensional NMR structure of the human ECD1-CRF-R1 (14) complexed with the peptide agonist α-helical cyclic CRF (αhcCRF). The motivation for these studies is the identification of the binding surface involved in the recognition of a high affinity agonist, the subsequent comparison with that previously reported for the antagonists and the determination of reciprocal conformational changes in both the receptor and the agonist.
MATERIALS AND METHODS
Protein Expression and Purification
The purification and labeling of the ECD1-CRF-R1 expressed in Escherichia coli were carried out as described previously (15, 29).
Synthesis of αhcCRF, with C-13 and N-15 Isotopically Labeled Amino Acids
The synthesis was performed on a methyl-benzhydrylamine resin using the Fmoc (N-(9-fluorenyl)methoxycarbonyl) strategy. Purification and characterization used established procedures (see Refs. 30, 31). Full details are given in the Supplemental Information section.
Radioreceptor Assays
Radioreceptor assays for full-length receptors expressed in mammalian cells were carried out as described previously (14) and for soluble receptors as described previously (32).
NMR Experiments and Analysis
All NMR spectra were recorded at 35 °C using a Bruker 700-MHz spectrometer equipped with four radiofrequency channels and a triple resonance cryo-probe with shielded z-gradient coil. The NMR samples contained either 0.3 mm 13C,15N-labeled ECD1-CRF-R1 and an equimolar concentration of unlabeled αhcCRF or 0.3 mm 13C,15N-labeled αhcCRF (labeled uniformly by 15N and 13C except for residues Thr11, Glu30, and Lys33) and an equimolar concentration of unlabeled ECD1-CRF-R1 in 10 mm BisTris(HCl), 95% H2O, 5% D2O, pH 6.5. The sample for the two-dimensional 1H,1H NOESY was prepared with an equimolar concentration of 0.4 mm of unlabeled ECD1-CRF-R1 and αhcCRF, respectively, in 10 mm BisTris(HCl), pH 6.5, in 100% D2O.
Sequential assignment was performed with the standard protocol for 13C,15N-labeled samples (33). 1H,13C, and 15N backbone resonances were assigned using triple resonance HNCA (34), HNCACB (35), and three-dimensional 15N-resolved 1H,1H NOESY experiments (36). The side-chain 1H resonances were assigned using HCCH correlation spectroscopy (37) and 13C-resolved 1H,1H NOESY (38) experiments. Aromatic side-chain assignments were obtained with two-dimensional 13C,1H HSQC, two-dimensional 1H,1H NOESY (39) in D2O, and three-dimensional 13C aromatic-resolved 1H,1H NOESY (38) experiments. Distance constraints for the structure calculation were derived from three-dimensional 13C,15N-resolved 1H,1H NOESY and two-dimensional 1H,1H standard NOESY (39) spectra recorded with mixing times of either 100 or 120 ms. For all of the spectra quadrature detection in the indirect dimensions was achieved using States-TPPI (40). The water signal was suppressed using spin-lock pulses (41) or the WATERGATE sequence (42). All of the spectra were processed with the program PROSA (43) and were analyzed with the program CARA (44).
Determination of the Structure of ECD1-CRF-R1 Complexed with αhcCRF
Meaningful distance restraints (∼1795) and angle restraints (371) were collected for the calculation of the structure of the complex (Table 1). These structural restraints were used as an input for the structure calculation with the program CYANA (43) followed by restrained energy minimization using CNS (45). A total of 100 conformers was initially generated by CYANA, and the bundle of 20 conformers with the lowest target function was used to represent the three-dimensional NMR structure. The structures are validated with the program PROCHECK (46), and the structure has been deposited in the Protein Data Bank data base with code 2L27.
TABLE 1.
Structural statistics of the ECD1-CRF-R1 complexed with αhcCRF
| Parameters | Complex |
|---|---|
| Constraints | |
| No. of upper distance limits | 1795 |
| Intramolecular | |
| ECD1 | 1330 |
| αhcCRF | 324 |
| Intermolecular | 141 |
| No. of dihedral angle constraints | 382 |
| No. of hydrogen bonds | 20 |
| Residual target function | 1.7 ± 0.4 Å2 |
| Distance violations >0.2 Å | |
| Minimum violation | 3.6 ± 0.2 Å |
| Maximum violation | 9.4 ± 0.8 Å |
| Angle violation | |
| Minimum | 3.5 ± 0.2° |
| Maximum | 7.7 ± 0.3° |
| Energies | |
| Total | −2090 ± 109 kcal/mol |
| van der Waals | −310 ± 41 kcal/mol |
| Electrostatic | −1781 ± 99 kcal/mol |
| Atomic pairwise r.m.s.d.a | |
| Backbone atoms | 1.0 ± 0.2 Å |
| Heavy atoms | 1.5 ± 0.2 Å |
| Structural analysis | |
| Residues in allowed region | 93.9% |
| Residues in disallowed region | 6.1% |
a Backbone and heavy atom root mean square deviation (r.m.s.d.) is obtained by superposing residues 42–105 of ECD1-CRF-R1 and residues 27–38 of αhcCRF in the complex.
RESULTS
Selection of a Soluble High Affinity Agonist
A major challenge in the three-dimensional structure determination of ECD1-CRF-R1 complexed with an agonist was to obtain a high affinity agonist that was soluble above pH 5 at concentrations required for the NMR experiments. Comparison of several CRF analogs showed that the novel CRF agonist, αhcCRF, satisfied the requirements, i.e. it bound with low nanomolar affinity to both CRF-R1 (∼1 nm) and the ECD1-CRF-R1 (∼30 nm, Table 2) and was soluble at physiological pH. This peptide is in part only related to α-helical-CRF(1–41) and to astressin (3, 47). The sequence of αhcCRF is cyclo(30–33)Ac-PP5ISLDL10TFNLL15REVLE20IAKAE25QEAEE30AAK33NR35LLLEE40A-NH2. The lactam bridge connecting the side chains of residues Glu30 and Lys33 of αhcCRF stabilizes the α-helical domain first established for the antagonist astressin (3).
TABLE 2.
Inhibitory binding constants (Ki) (in nanomolar) of various CRF ligands bound to CRF receptors stably expressed in CHO cells and to their purified ECD1s
Values were derived from competitive displacement of bound [125I-d-Tyr0]astressin. Primary structures and residue numbering are as follows: r/hCRF, SEEPP5ISLDL10TFHLL15REVLE20MARAE25QLAQQ30AHSNR35KLMEI40I-NH2; rUcn1, DDPP5LSIDL10TFHLL15RTLLE20LARTQ25SQRER30AEQNR35IIFDS40V-NH2;astressin, cyclo(30–33)-f12HLL15REVLE20XARAE25QLAQE30AHK33NR35KLXEI40I-NH2; αhcCRF, cyclo(30–33)Ac-PP5ISLDL10TFNLL15REVLE20IAKAE25QEAEE30AAKNR35LLLEE40A-NH2; where X = Nle. Numbering of residues is adapted to give identical numbers to corresponding residues.
| Ligand/receptor | CHO-CRF-R1 | ECD1-CRF-R1 | CHO-CRF-R2β | ECD1-CRF-R2β |
|---|---|---|---|---|
| r/hCRF | 11 | 53.6 | 28 | 97 |
| (8.4–15) | (25.7–112) | (16–62) | (22–430) | |
| rUcn1 | 1.3 | 14.1 | 1.3 | 6.4 |
| (0.7–2.6) | (11.6–17.2) | (1.0–1.6) | (4.7–8.7) | |
| αhcCRF | 1.1 | 30 | 0.8 | 10 |
| (0.8–1.4) | (23–39) | (0.6–1.4) | (4.4–25) | |
| Astressin | 2 | 18 | 0.9 | 10.7 |
| (1.8–2.3) | (10–34) | (0.4–1.9) | (5.4–21.1) |
Slow Conformational Exchange Dynamics of Free ECD1-CRF-R1
The NMR structure of the free ECD1-CRF-R1 was not determined because resonances for many residues were not observed in the 15N,1H TROSY spectrum (Fig. 2A). These include the N-terminal residues 1–24 and the C-terminal residues 110–127 of the construct. In addition, several residues that are part of loop 2 involved in ligand binding (see below) (i.e. Arg66, Cys68, Phe71, Phe72, Gly74, Val75, Tyr77, Asn78, and Ala95) were absent in the 15N,1H TROSY spectrum (Fig. 2A). Changes in pH or temperature failed to improve the quality of the spectrum. The absence of these peaks in the spectrum may be due to slow conformational exchange dynamics involving the backbone, which results in line broadening so severe that peaks cannot be detected. A similar observation was documented for the free ECD1-CRF-R2β, but it was limited to only a few residues located in loop 2 (i.e. Tyr87 (corresponding to Phe71 in CRF-R1), Phe88 (Phe72); Asn89 (Tyr73), Gly90 (Gly74), Ile91 (Val75), Lys92 (Arg76), Arg97 (Asn81)) (2). For the ECD1-CRF-R2β, this slow conformational exchange was estimated to be on the time scale of 10−2 s, and a similar rate was also estimated for the ECD1-CRF-R1. However, because the number of cross-peaks absent in the 15N,1H TROSY spectrum is much larger for ECD1-CRF-R1 than for ECD1-CRF-R2β, the conformational exchange dynamics of ECD1-CRF-R1 must be of greater amplitude and/or must involve a larger segment of the ECD1-CRF-R1 compared with ECD1-CRF-R2β. For ECD1-CRF-R2β, this slow conformational exchange phenomenon was suppressed, at least in part, when complexed with the antagonist astressin, because all of the missing cross-peaks of the amide moieties appeared in the 15N,1H TROSY spectrum of the complex. Assuming that all of the peaks might appear in the spectrum of ECD1-CRF-R1 complexed with the agonist, we carried out the structural studies of the ECD1-CRF-R1 complexed with αhcCRF.
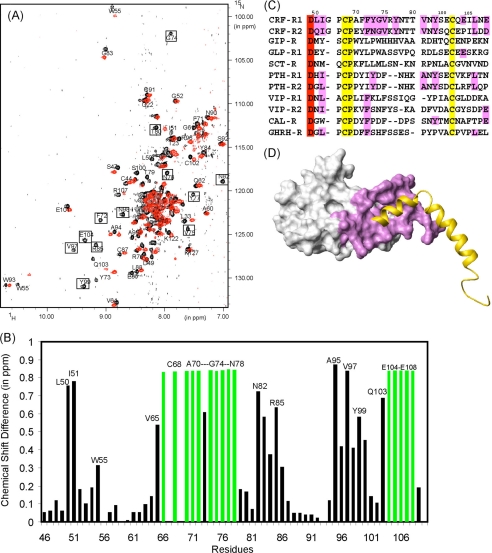
FIGURE 2.
Mapping onto the surface of the ECD1-CRF-R1-αhcCRF complex the residues that are chemically perturbed upon ligand binding. A, 15N,1H TROSY spectrum of the ECD1-CRF-R1 free (in red contours) and complexed with αhcCRF (in black contours). Residues enclosed in square boxes are observed only in the complex spectrum and are from the loop 2 region of ECD1-CRF-R1, suggesting that free ECD1 undergoes slow conformational dynamics. B, plot of the normalized chemical shift changes (Δ(δ(1H)2 + Δ(δ(15N))2/5)1/2 observed for the ECD1-CRF-R1 in the complex versus the amino acid sequence (34). For those residues that are not observed in the free ECD1-CRF-R1 spectrum (colored in green), chemical shift change of 1.5 ppm was assumed for 15N frequency and 0.5 ppm change in 1H frequency. C, sequence alignment of the ligand-binding site of the ECD1 of various members of the B1 family of GPCRs. Highly conserved residues are shown in yellow and residues in the ECD1 of CRF receptors, whose side chains interact with the ligand, are shown in violet. D, surface representation of ECD1-CRF-R1 showing the residues involved in binding. Residues with chemical shift perturbation (>0.5 ppm) are colored in violet and αhcCRF is shown as gold helical ribbon.
Mapping the Agonist-binding Site on ECD1-CRF-R1 by Agonist-induced Chemical Shift Changes
Insight into the agonist-binding site on ECD1-CRF-R1 can be obtained from an analysis of the resonance shifts in the NMR spectra upon addition of αhcCRF (34). Fig. 2A shows the 15N,1H TROSY spectrum of the ECD1-CRF-R1 in the absence and presence of equimolar αhcCRF. Following agonist binding, large chemical shift changes of resonances or appearance of cross-peaks was observed at four different regions of the ECD1 as follows: (i) Leu50 and Ile51, Val65; (ii) Arg66, Cys68, Phe71–Asn78; (iii) Asn82, Gly83, Arg85, Ala95–Ser100; and (iv) Gln103–Glu108 (Fig. 2B). Most of these residues are of hydrophobic nature and conserved between the two CRF receptors (Ile51, Arg66, Cys68, Phe72, Gly74, Val75, Tyr77, Asn78, Asn98, Tyr99) (Fig. 2C). This structural study, together with similar chemical shift perturbation data observed for the ECD1-CRF-R2β-astressin complex (2), suggests that both the agonist and antagonist interact with the same residues in the ECD1s.
Three-dimensional Structure of the ECD1-CRF-R1 Complexed with αhcCRF
Almost complete sequential assignment of the various resonances of the ECD1-CRF-R1-αhcCRF complex was obtained using standard procedures, and the three-dimensional structure was determined (see under “Materials and Methods”). The good quality of the three-dimensional structure is represented by the small root mean square deviation of 0.88 Å for residues 42–105 of the ECD1 and for residues 27–38 of αhcCRF (Fig. 3, A and B), as well as by the small value of residual constraint violations in the 20 refined conformers, and by the small deviations from ideal geometry (Table 1). In addition, the input data represent a self-consistent set, and the restraints are well satisfied in the calculated conformers, and similar energy values were obtained for all of the 20 conformers.
FIGURE 3.
Three-dimensional NMR structure of ECD1-CRF-R1 complexed with αhcCRF. A, superposition of 20 energy minimized conformers representing the three-dimensional NMR structure of the complex of ECD1-CRF-R1 at pH 6.5. The bundle is obtained by superimposing the backbone Cα atoms of residues 40–105 of ECD1-CRF-R1 and residues 27–41 of αhcCRF. The N-terminal residues 4–22 of αhcCRF do not interact with the ECD1-CRF-R1 and residues 10–41 of αhcCRF prefer a helical conformation with a kink at residues 27–29. Residues 25–108 of the ECD1-CRF-R1 are shown, and the disulfide bonds are colored in yellow. B, ribbon diagram of the lowest energy conformer representing the three-dimensional NMR structure. The N-terminal α-helix of ECD1-CRF-R1 is shown in gray; β-sheets are shown in cyan and the hydrophobic core residues W55 and W93 are shown in yellow. The salt bridge involving R85 (in blue) and D49 (in red) is shown as dashed line in gray. The backbone of αhcCRF is shown in gold. C, surface-charge representation of the ECD1-CRF-R1. Red represents negative; blue represents positive, and white represents neutral surface. The accumulation of negative charges on the obverse surface and of positive charges on the reverse surface suggest that the ECD1 may be oriented to the cell membrane along its positively charged surface.
As documented already in the structures of the ECD1 of CRF-R2β (2, 29) and of the ECD1s of the other members of the B1 receptor family (25–28), the overall fold of the ECD1 of CRF-R1 is the short consensus repeat (SCR) motif with the three disulfide bonds between cysteine residues, Cys30–Cys54, Cys44–Cys87, and Cys68–Cys102. The SCR includes a short N-terminal α-helix (Asp27–Glu31) and two anti-parallel β-sheet regions around residues Ser47–Val48 (β1 strand), Cys54–Trp55 (β2 strand), Leu63–Arg66 (β3 strand), and Gly83–Glu86 (β4 strand). The chemical shift values of 13Cα, 13Cβ, and 1Hα support the presence of these secondary structural elements. At the core of the SCR motif, the aliphatic side chain of the conserved Arg85 is sandwiched between the highly conserved Trp55 and Trp93 residues and is in close proximity to the side chain of the conserved Asp49 and thus may possibly be involved in a salt bridge interaction (Fig. 3B). Furthermore, the indole nitrogen of Trp55 forms a hydrogen bond with the carboxyl group of Asp49 stabilizing the β1-β2 hairpin (Fig. 3B). These structural features are very similar to those observed for the ECD1 of CRF-R2β and are supported by the up-field shifted side chain resonances of Arg85 (βCH2, 0.42 and −0.55; γCH2, 0.65 and 1.36; δCH2, 0.97 and 0.18 ppm) that require close proximity to an aromatic side chain because aromatic ring currents cause such high field shifts. Additional up-field shifted resonances are observed for the methyl protons of Ile51 (0.12 and −0.28 ppm), Thr53 (0.08 ppm), the α-protons of Gly83 (4.42 and 2.92 ppm), and methyl protons of Val97 (0.41 and −0.29 ppm), and they are all attributed to the close proximity of the residues to the aromatic ring of Tyr99.
Conformation of the Agonist αhcCRF Both Free and Complexed with the ECD1-CRF-R1
For the free 15N,13C-labeled αhcCRF (amino acid numberings per Fig. 4G) at pH 6.5 and 298 K, all of the expected 30 peaks were observed in the 15N,1H HMQC spectrum (Thr11, Glu30, and Lys33 were not labeled, see under “Materials and Methods”) (Fig. 4A, red contours). In addition to the major conformation, a minor conformation (∼⅓ of the major conformation) is also present for most of the N-terminal residues up to residue Leu10. This minor conformation is attributed to cis/trans isomerizations of the N-terminal proline residues. The chemical shift values for the Cα, Cβ, and Hα protons (Fig. 4, C and D, red bars) of the free peptide indicate that the peptide segment from residues Phe12 to Leu37 is partially in a helical conformation.
FIGURE 4.
αhcCRF binds to the ECD1 in an α-helical conformation. A, two-dimensional 15N,1H HMQC spectra of labeled αhcCRF free (red contours) and bound to unlabeled ECD1-CRF-R1 (black contours) at pH 6.5. B, schematic representation of the observed NOE interactions between αhcCRF and ECD1-CRF-R1. Hydrophobic residues are colored yellow; positively charged residues are colored blue, and negatively charged residues are colored red. C–G, plots of the chemical shift difference between αhcCRF and the corresponding “random coil” values for 13Cα, 13Cβ (C), and for 1Hα atoms shown as black and red bars for the complex and free αhcCRF (D), respectively. Chemical shift changes suggest that the ligand binding induces more helicity, not only in the C-terminal segment but also in the N-terminal segment. E, plot of the normalized chemical shift changes (Δ(δ(1H)2 + Δ(δ(15N))2/5)1/2 observed for αhcCRF in the complex versus the amino acid sequence (34). There is an overall 0.1 ppm chemical shift change due to the difference in the temperatures at which the free (298 K) and complex (308 K) spectra were recorded. F, plot of the observed chemical shifts of the 1HN moieties of αhcCRF free (in red) and bound (in black) to ECD1-CRF-R1. G, intramolecular NOEs observed in αhcCRF bound to ECD1-CRF-R1 along the amino acid sequence. Thin, medium, and thick lines represent weak (4–5 Å), medium (3–4 Å), and strong (<3 Å) NOEs observed between the residues connected by the line. The bar connecting Glu30 and Lys33 represents the lactam bridge connecting the side chains of these residues. Residues Thr11, Glu30, and Lys33 are not isotopically labeled, and hence their values are not shown.
Fig. 4A also shows the 15N,1H HMQC spectrum of 13C,15N-labeled αhcCRF complexed with unlabeled ECD1-CRF-R1 (black contours). When compared with the 15N,1H HMQC spectrum of free αhcCRF, two prominent features in the complex spectrum are observed as follows: (i) the larger dispersion of the cross-peaks attributed to a higher ordered structure of αhcCRF in the complex, and (ii) broad cross-peaks with large line widths due to the larger size of the complex and/or due to slow conformational exchange. Most prominent chemical shift changes as well as line broadening are observed for the C-terminal residues of αhcCRF with the maximum shift for Ala41 (Fig. 4E). The chemical shifts for the Cα, Cβ, and Hα protons (Fig. 4, C and D, black bars) suggest that αhcCRF prefers a helical conformation when bound to the ECD1-CRF-R1 and that the helicity is more pronounced upon complex formation not only at the C terminus but also toward the N terminus of the peptide (Fig. 4, A and B). This is further supported by the α-helical NOEs (i.e. αβ(i,i + 3), αN(i,i + 3), and αN(i,i + 4)) observed for residues Phe12 to Ala41 (Fig. 4G). In addition, amide proton chemical shifts between Ala31 and Ala41 (Fig. 4F) show a wave-like pattern attributed to the amphipathic nature of the helix in the complex, observed also for astressin bound to the ECD1 of CRF-R2β (2). In addition, a kink of the long helix is observed between residues Glu27 to Glu29 of αhcCRF (Fig. 3). The positioning of this kink is similar to the kinks observed for CRF family ligands in the solvent DMSO (48) but is absent in all the other ligands of family B1 when they are bound to their respective ECD1s in the crystal structures. Although the role of this kink may be understood only in the context of the full-length receptor, it enlarges the conformational space of the N-terminal peptide segment, which is possibly important for receptor-specific signaling (Fig. 3A) (49, 50).
Molecular Interactions between αhcCRF and the ECD1
The helical segment of αhcCRF bound to the ECD1 is along the hydrophobic face of the protein, covering an area of 2647 Å2 of the ECD1 (Fig. 5). The C-terminal αhcCRF residues Leu37, Leu38, and Ala41 are involved in hydrophobic contacts with Phe72, Tyr73, Tyr77, Ile51, Cys68, Pro69, Val97, Cys102, and Tyr99 of the ECD1. In particular, the side chain of Leu38 is located in a deep hydrophobic pocket surrounded by Ile51, Cys68, Pro69, Phe72, Tyr77, Tyr99, and Cys102 of the ECD1 (note that up-field shifted resonances for the β and methyl protons of Leu38 are indicative of its interactions with aromatic side chains of the ECD1). The amide group at the C terminus of αhcCRF is involved in an inter-molecular hydrogen bond with the backbone carbonyl of Val97. In return, the backbone amide proton of Val97 is involved in a hydrogen bond with the backbone carbonyl of Ala41 in αhcCRF (Fig. 5). These hydrogen bonds explain the necessity of C-terminal amidation for high affinity recognition of CRF ligands (48, 51). Although there are several NOEs observed between the side chain of Asn34 to Tyr73 and Val75, in the three-dimensional structure, these two side chains are not close enough to form an intermolecular hydrogen bond. The side chain of Ala31 of αhcCRF also interacts with Val75 and Tyr77 through hydrophobic interactions. The side chain of Arg35 is in close proximity to the side chain of Glu104, thereby facilitating a salt bridge interaction. Because the side chain resonances of Arg35 could not be observed in the 13C-resolved 1H,1H NOESY spectrum except for the δ protons, its interaction must be interpreted with care.
FIGURE 5.
Molecular anatomy of the residues in the interaction surface. Hydrophobic residues are colored yellow; positively charged residues are colored blue, and negatively charged residues are colored red. Front (obverse) (A) and back (reverse) (B) view of the side chain interactions between the ECD1-CRF-R1 and αhcCRF are shown in stereo. The backbone of αhcCRF is shown as gold and ECD1-CRF-R1 as cyan ribbons, respectively. Possible hydrogen bonds are shown in gray, and the potential salt bridge between Arg35 and Glu104 is shown also in gray. All the residues in the interaction surface are marked for clarity.
DISCUSSION
Comparison of the Structures of ECD1-CRF-R1 Complexed with the Agonist αhcCRF and ECD1-CRF-R2β Complexed with the Antagonist Astressin
Recently, our group reported the structure of the ECD1 of CRF-R2β complexed with the peptide antagonist astressin (2). Comparison of the structures of the complexes of ECD1-CRF-R1 and ECD1-CRF-R2β enables the identification of the common interaction sites. Both of the structures have the SCR motif characteristic of the ECD1s of family B1 members (Fig. 6A). There is a short N-terminal helix observed for ECD1-CRF1-R1, which was absent in the structure of ECD1-CRF-R2β because in the latter the N-terminal segment was truncated in the protein construct. Although conformation of loop 1 is not defined in either ECD1, loops 2 and 3 are structured and interact with the corresponding ligand in a slightly different manner (Fig. 6B). The backbone of loop 2 of ECD1-CRF-R2β folds closer to the ligand than the corresponding loop in ECD1-CRF-R1, although the opposite holds for loop 3.
FIGURE 6.
Comparison of the structures of the ECD1-CRF-R1 complexed with αhcCRF and ECD1-CRF-R2β complexed with astressin (PDB code 2JND). A, superposition of the ribbon diagrams of the ECD1 of CRF-R1 (in cyan) and that of CRF-R2β (in violet). The ligands αhcCRF and astressin are shown as gold and green ribbons, respectively. B, difference in the ligand binding loop 2 region and the side chains of the residues involved in binding. Various interactions between αhcCRF (C) and ECD1-CRF-R1 (D) astressin and ECD1-CRF-R2β are highlighted.
There are also structural differences of the ligand. Although both the agonist and the antagonist bind to almost the same region of the ECD1, the orientations of the ligands are slightly different with respect to loop 2 (Fig. 6, B–D). Furthermore, the C-terminal residues of astressin prefer a 310 helix, whereas in αhcCRF, they are in an α-helical conformation. In both ligands, the backbone carbonyl of the last residue (Ala/Ile41) is involved in a hydrogen bond with the amide proton of Val97/113. The side chains of the ligand residues, Glu39, Leu/Lys36, Ala/His32, and Gln29 are completely solvent-exposed in both of the structures. Also, the side chain of Glu40 of αhcCRF is completely solvent-exposed, whereas Ile40 of astressin interacts with Gln66 of CRF-R2β. The side chain of Leu/Nle38 is completely buried in a hydrophobic core in both structures (Fig. 6). Although the side chains of Phe72/88 of ECD1 are conserved in their positions and these residues interact with residues Leu37 and Asn34 of the ligand in both of the structures, the positions of Tyr73/Asn89 and Tyr77/93 are distinct. The orientation of Tyr77 of CRF-R1 is toward the ligand, whereas in CRF-R2β, Tyr93 points away from the ligand. Consistent with this is the observation that the mutation Y77A in CRF-R1 abrogated high affinity binding of both astressin and sauvagine and significantly increased the EC50 for sauvagine- and urocortin1-stimulated intracellular cAMP accumulation (data not shown). The side chain of Asn34 of astressin is in close proximity to the ECD1 to form a hydrogen bond with the backbone carbonyl of Phe88 in ECD1-CRF-R2β. Such a hydrogen bond is missing in the structure of the ECD1-CRF-R1 in complex with αhcCRF. The residues Val75/Ile91 are in almost the same position, and they interact with Ala31 of the ligand. The side chain of Arg35 does not directly interact with Glu104 of the ECD1-CRF-R1, although it is close enough to form a solvent-exposed intermolecular salt bridge. In contrast, in ECD1-CRF-R2β, Arg35 is involved in a buried salt bridge with Glu86, which, in ECD1-CRF-R1, is replaced by Ala70, and its side chain is solvent-exposed.
Comparison of the NMR Structure of ECD1-CRF-R1-αhcCRF Complex and X-ray Crystallographic Structures of ECD1-CRF-R1 Peptide Complex
The NMR structure of ECD1-CRF-R1 complexed with the high affinity agonist αhcCRF may be compared with the crystal structures of ECD1-CRF-R1 complexed with the low affinity fragments CRF(22–41) and CRF(27–41), which are presumed to be antagonists (1). Interestingly, the ECD1 of CRF-R1 was crystallized in a ligand-dependent manner in three different forms that differ mainly in the conformations of loop 2, to which the ligand binds (Fig. 7). Pioszak et al. (1) suggest that crystal form II is possibly more relevant, physiologically, than the others because only in crystal form II is loop 2 unhindered from crystal packing constraints. Indeed, our NMR structure of ECD-CRF-R1-αhcCRF (Fig. 7, E and F) is more similar to the crystal form II than to forms I and III (Fig. 7, A–D) (1). The differences between the NMR and x-ray structures are discussed in detail in the supplemental material. The comparison between all of the four structures suggests the presence of a structural plasticity of the SCR motif of the ECD1-CRF-R1 (see below).
FIGURE 7.
Comparison of the NMR and crystal structures of the ECD1 of CRF-R1. A and B, crystal structures of the MBP(A326E)-CRF-R1-ECD-H6 fusion protein complexed with the truncated CRF(22–41) (PDB code 3EHU, crystal form II). The backbone ribbon of the ECD1 along with the highly conserved residues, including the disulfide bonds, is shown in gray. Loop 2 residues that are observed are shown in light green; it must be noted that the side chains of Phe71, Tyr73, and Arg76 are missing. Ligand residues 27–41 that are observed are highlighted in dark green, and the ligand backbone is shown as a dark green ribbon. C and D, crystal structures of the MBP(F94E)-CRF-R1-ECD-H6 protein complexed with the truncated CRF(27–41) (PDB code 3EHT, crystal form III). The backbone ribbon of the ECD1 along with the highly conserved residues, including the disulfide bonds, is shown in orange. Loop 2 residues that are observed are shown in light green; it must be noted that the conformation of loop 2 is different from that of A, resulting in different orientations for the residues involved in ligand binding. Ligand residues 31–41 having electron density are highlighted in dark green, and the backbone is shown as a dark green ribbon. E and F, NMR structures of the ECD1-CRF-R1 complexed with αhcCRF. The backbone ribbon of the ECD1 along with the highly conserved residues including the disulfide bonds is shown in cyan. Side chains of loop 2 residues are shown in light green. The conformation of loop 2 is very close to that of A and is different from that of C. The side chains of Phe71, Tyr73, and Arg76 were assigned, and hence their interactions with the ligand could be observed.
Structural Plasticity of Both the ECD1 and the Ligand
The absence of cross-peaks for amide moieties of residues in loop 2 in the 15N,1H TROSY spectra of both of the ECD1s of CRF-R1 and CRF-R2β suggests the presence of slow conformational exchange dynamics in the ECD1s of CRF receptors. Although the cross-peaks appear in the 15N,1H TROSY spectra in the complex with the peptide for both receptor studies, the significant line broadening observed for these peaks suggests that the conformational exchange is only partially suppressed. The amide moieties of the ligand αhcCRF also show broad, weak cross-peaks in the 15N,1H HMQC spectra upon complex formation (Fig. 4). The presence of slow conformational exchange dynamics in the millisecond time range for segments of both the ECD1 and the ligand is in agreement with the presence of conformational heterogeneity observed in both the NMR and x-ray structures. Furthermore, the nanomolar binding affinity of the antagonist astressin for a CRF-R2β mutant whose corresponding ECD1 shows molten globule-like conformational states (52) supports the dynamic character of the ECD1. Although we can only speculate about the biological role of these conformational exchange dynamics, their presence could account for the multiple recognition, binding, and signaling observed for the various hormone ligands.
Refinement of the Two-step Binding Mechanism of CRF Peptides to Its Receptors
The two-step model for ligand binding and signaling of type B1 GPCRs (29, 53, 54) proposes that the C-terminal segment of the ligand binds to the ECD1, which then may position the N-terminal portion of the peptide hormone in close proximity to the serpentine regions of the receptor to initiate signaling. The ECD1 is therefore the major peptide-binding domain, and conversely, the C-terminal segment of the ligand is important for high binding affinity and selectivity to the receptors. All of the three-dimensional structures of the ECD1-receptor-ligand complexes are consistent with this model, because the C-terminal segment of the peptide ligand interacts with the ECD1 (1, 2, 26–28). Our NMR studies of the complex between ECD1-CRF-R1 and an agonist further indicate that the recognition of the ligand by the ECD1 not only binds the hormone and positions its N-terminal residues for signal activation but also induces helix formation toward the N terminus of the ligand to generate a conformationally active state. In a recent review (55), this additional function in receptor activation of the ECD1 in family B1 GPCRs was proposed to be based on the following: (i) a structural comparison between various hormone ligands, free and complexed with ECD1s and (ii) the importance of helix-capping residues in the N-terminal region of the numerous corresponding ligands (56–58). The induction of the helix in the ligand upon complex formation with the ECD1 is accompanied by a kink between residues Glu24 to Glu26 of αhcCRF (Figs. 3A and 8); this kink may also play a role in receptor activation by either enlarging the conformational space of the N-terminal peptide segment or by positioning the ECD1 relative to the serpentine region of the receptor important for signaling and/or co-receptor interactions (29). Hence, our three-dimensional structure of the ECD1 of CRF-R1 complexed with an agonist suggests a refined two-step model for receptor activation.
FIGURE 8.
Comparison of the three-dimensional structures of various CRF ligands bound to the ECD1 of either CRF-R1 or CRF-R2β or in DMSO. Comparison of the structures of αhcCRF (in gold) bound to ECD1-CRF-R1 and truncated CRF (22–41) (in dark green) complexed with ECD1-CRF-R1 (from the crystal structure with PDB code 3EHU) (A), astressin (in green) complexed with ECD1-CRF-R2β (from the NMR structure with PDB code 2JND) (B), and astressin (in royal blue) in DMSO (C). The structures are shown after superimposing the C-terminal residues 30–41 of astressin.
Agonist Versus Antagonist
Previous studies on chemically modified and truncated CRF ligands showed that the first seven residues at the N termini of CRF are not necessary for GPCR signaling (3, 4) and that residue 8 was critical, whereas the C-terminal (∼15) residues are important for binding (47, 53, 54). Hence, CRF analogs truncated by 8 residues or more at the N terminus are antagonists. This finding can easily be understood with the help of the two-step model for ligand binding and signaling of type B1 GPCR discussed above (16, 19, 29). If the N-terminal segment of the ligand is missing, the C-terminal fragment still binds to the receptor but is not able to produce activation. Such a ligand is then evidently an antagonist because it occupies the major binding site thereby blocking peptide agonist binding. The three-dimensional structure of the ECD1-CRF-R1 complexed with the agonist αhcCRF presented here is the first direct experimental proof that supports this hypothesis (Figs. 7 and 8) and shows clearly the similarity of the C-terminal binding of the peptide agonists and antagonists.
CONCLUSION
The information gained from our structural studies complements earlier knowledge of the molecular interactions between ligands and GPCRs of the B1 family. Specifically, the three-dimensional NMR structure presented here of ECD1-CRF-R1 complexed with a high affinity CRF agonist has identified the residues in the ECD1-CRF-R1 involved in ligand recognition and has highlighted the similarity between agonist and antagonist binding receptor domains. Furthermore, the structure of the complex revealed the extended helicity of the N-terminal domain of the agonist. These data provide further support for the model of ligand-induced receptor signaling in the B1 receptor family.
Supplementary Material
Acknowledgments
We thank Dr. W. Fischer for mass spectrometric analyses and S. Pisani, K. Sukhija, J. Erchegyi, and K. Lewis for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant DK026741 from the NIDDK. This work was also supported by Clayton Medical Research Foundation, Inc.

The on-line version of this article (available at http://www.jbc.org) contains supplemental text.
The atomic coordinates and structure factors (code 2L27) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- CRF
- corticotropin-releasing factor
- αhcCRF
- α-helical cyclic CRF
- CRF-R
- CRF receptor
- ECD
- extracellular domain
- GPCR
- G-protein-coupled receptor
- NOESY
- nuclear Overhauser enhancement spectroscopy
- PDB
- Protein Data Bank
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- MBP
- maltose-binding protein
- PTH
- parathyroid hormone
- SCR
- short consensus repeat.
REFERENCES
- 1.Pioszak A. A., Parker N. R., Suino-Powell K., Xu H. E. (2008) J. Biol. Chem. 283, 32900–32912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grace C. R., Perrin M. H., Gulyas J., Digruccio M. R., Cantle J. P., Rivier J. E., Vale W. W., Riek R. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 4858–4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gulyas J., Rivier C., Perrin M., Koerber S. C., Sutton S., Corrigan A., Lahrichi S. L., Craig A. G., Vale W., Rivier J. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 10575–10579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivier J., Lahrichi S. L., Gulyas J., Erchegyi J., Koerber S. C., Craig A. G., Corrigan A., Rivier C., Vale W. (1998) J. Med. Chem. 41, 2614–2620 [DOI] [PubMed] [Google Scholar]
- 5.Vale W., Spiess J., Rivier C., Rivier J. (1981) Science 213, 1394–1397 [DOI] [PubMed] [Google Scholar]
- 6.Bale T. L., Contarino A., Smith G. W., Chan R., Gold L. H., Sawchenko P. E., Koob G. F., Vale W. W., Lee K. F. (2000) Nat. Genet. 24, 410–414 [DOI] [PubMed] [Google Scholar]
- 7.Armario A. (2006) CNS Neurol. Disord. Drug Targets 5, 485–501 [DOI] [PubMed] [Google Scholar]
- 8.Smith G. W., Aubry J. M., Dellu F., Contarino L. M., Bilezikjian L. M., Gold L., Chen R., Marchuk Y., Hauser C., Koob G., Vale W., Lee K. F. (1998) Neuron 20, 1093–1102 [DOI] [PubMed] [Google Scholar]
- 9.Chen R., Lewis K. A., Perrin M. H., Vale W. W. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 8967–8971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrin M., Donaldson C., Chen R., Blount A., Berggren T., Bilezikjian L., Sawchenko P., Vale W. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 2969–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wille S., Sydow S., Palchaudhuri M. R., Spiess J., Dautzenberg F. M. (1999) J. Neurochem. 72, 388–395 [DOI] [PubMed] [Google Scholar]
- 12.Dautzenberg F. M., Kilpatrick G. J., Wille S., Hauger R. L. (1999) J. Neurochem. 73, 821–829 [DOI] [PubMed] [Google Scholar]
- 13.Dautzenberg F. M., Wille S., Lohmann R., Spiess J. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 4941–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrin M. H., Sutton S., Bain D. L., Berggren W. T., Vale W. W. (1998) Endocrinology 139, 566–570 [DOI] [PubMed] [Google Scholar]
- 15.Perrin M. H., Fischer W. H., Kunitake K. S., Craig A. G., Koerber S. C., Cervini L. A., Rivier J. E., Groppe J. C., Greenwald J., Møller, Nielsen S., Vale W. W. (2001) J. Biol. Chem. 276, 31528–31534 [DOI] [PubMed] [Google Scholar]
- 16.Rijkers D. T., Kruijtzer J. A., van Oostenbrugge M., Ronken E., den Hartog J. A., Liskamp R. M. (2004) ChemBioChem 5, 340–348 [DOI] [PubMed] [Google Scholar]
- 17.Coy D. H., Murphy W. A., Lance V. A., Heiman M. L. (1987) J. Med. Chem. 30, 219–222 [DOI] [PubMed] [Google Scholar]
- 18.Cervini L., Theobald P., Corrigan A., Craig A. G., Rivier C., Vale W., Rivier J. (1999) J. Med. Chem. 42, 761–768 [DOI] [PubMed] [Google Scholar]
- 19.Yamada Y., Mizutani K., Mizusawa Y., Hantani Y., Tanaka M., Tanaka Y., Tomimoto M., Sugawara M., Imai N., Yamada H., Okajima N., Haruta J. (2004) J. Med. Chem. 47, 1075–1078 [DOI] [PubMed] [Google Scholar]
- 20.Shimizu M., Carter P. H., Gardella T. J. (2000) J. Biol. Chem. 275, 19456–19460 [DOI] [PubMed] [Google Scholar]
- 21.Holtmann M. H., Hadac E. M., Miller L. J. (1995) J. Biol. Chem. 270, 14394–14398 [DOI] [PubMed] [Google Scholar]
- 22.Dong M., Li Z., Zang M., Pinon D. I., Lybrand T. P., Miller L. J. (2003) J. Biol. Chem. 278, 48300–48312 [DOI] [PubMed] [Google Scholar]
- 23.Hoare S. R., Sullivan S. K., Fan J., Khongsaly K., Grigoriadis D. E. (2005) Peptides 26, 457–470 [DOI] [PubMed] [Google Scholar]
- 24.López de Maturana R., Willshaw A., Kuntzsch A., Rudolph R., Donnelly D. (2003) J. Biol. Chem. 278, 10195–10200 [DOI] [PubMed] [Google Scholar]
- 25.Sun C., Song D., Davis-Tabor R. A., Barrett L. W., Scott V. E., Richardson P. L., Pereda-Lopez A., Uchic M. E., Solomon L. R., Lake M. R., Walter K. A., Hajduk P. J., Olejniczak E. T. (2007) Proc. Nat. Acad. Sci. U.S.A. 104, 7835–7880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parthier C., Kleinschmidt M., Neumann P., Rudolph R., Manhart S., Schlenzig D., Fanghänel J., Rahfeld J. U., Demuth H. U., Stubbs M. T. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 13942–13947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Runge S., Thøgersen H., Madsen K., Lau J., Rudolph R. (2008) J. Biol. Chem. 283, 11340–11347 [DOI] [PubMed] [Google Scholar]
- 28.Pioszak A. A., Xu H. E. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 5034–5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grace C. R., Perrin M. H., DiGruccio M. R., Miller C. L., Rivier J. E., Vale W. W., Riek R. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 12836–12841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivier J. E., Kirby D. A., Lahrichi S. L., Corrigan A., Vale W. W., Rivier C. L. (1999) J. Med. Chem. 42, 3175–3182 [DOI] [PubMed] [Google Scholar]
- 31.Rivier J., Gulyas J., Corrigan A., Martinez V., Craig A. G., Taché Y., Vale W., Rivier C. (1998) J. Med. Chem. 41, 5012–5019 [DOI] [PubMed] [Google Scholar]
- 32.Perrin M. H., DiGruccio M. R., Koerber S. C., Rivier J. E., Kunitake K. S., Bain D. L., Fischer W. H., Vale W. W. (2003) J. Biol. Chem. 278, 15595–15600 [DOI] [PubMed] [Google Scholar]
- 33.Cavanagh C., Palmer A. G., 3rd, Skelton N. J. (1996) Protein NMR Spectroscopy: Principles and Practice, Academic Press, San Diego [Google Scholar]
- 34.Grzesiek S., Bax A., Clore G. M., Gronenborn A. M., Hu J. S., Kaufman J., Palmer I., Stahl S. J., Wingfield P. T. (1996) Nat. Struct. Biol. 3, 340–345 [DOI] [PubMed] [Google Scholar]
- 35.Wittekind M., Mueller L. (1993) J. Magn. Reson. 101, 201–205 [Google Scholar]
- 36.Fesik S. W., Zuiderweg E. R. (1988) J. Mag. Reson. 78, 588–593 [Google Scholar]
- 37.Ikura M., Kay L. E., Bax A. (1991) J. Biomol. NMR 1, 299–304 [DOI] [PubMed] [Google Scholar]
- 38.Ikura K., Kay L. E., Tschudin R., Bax A. (1990) J. Magn. Reson. 86, 204–209 [DOI] [PubMed] [Google Scholar]
- 39.Kumar A., Ernst R. R., Wüthrich K. (1980) Biochem. Biophys. Res. Commun. 95, 1–6 [DOI] [PubMed] [Google Scholar]
- 40.Marion D., Ikura K., Tschudin R., Bax A. (1989) J. Magn. Reson. 85, 393–399 [Google Scholar]
- 41.Messerle B. A., Wider G., Otting G., Weber C., Wuthrich K. (1989) J. Magn. Reson. 85, 608–613 [Google Scholar]
- 42.Piotto M., Saudek V., Sklenár V. (1992) J. Biomol. NMR 2, 661–665 [DOI] [PubMed] [Google Scholar]
- 43.Güntert P., Dötsch V., Wider G., Wüthrich K. (1992) J. Biomol. NMR 2, 619–629 [Google Scholar]
- 44.Keller R. L. (2003) CANTINA, The Computer Aided Resonance Assignment Tutorial, Verlag, Zurich, Switzerland [Google Scholar]
- 45.Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 46.Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 47.Rivier J., Rivier C., Vale W. (1984) Science 224, 889–891 [DOI] [PubMed] [Google Scholar]
- 48.Grace C. R., Cervini L., Gulyas J., Rivier J., Riek R. (2007) Biopolymers 87, 196–205 [DOI] [PubMed] [Google Scholar]
- 49.Rivier J., Gulyas J., Kunitake K., DiGruccio M., Cantle J. P., Perrin M. H., Donaldson C., Vaughan J., Million M., Gourcerol G., Adelson D. W., Rivier C., Taché Y., Vale W. (2007) J. Med. Chem. 50, 1668–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivier J., Gulyas J., Kirby D., Low W., Perrin M. H., Kunitake K., DiGruccio M., Vaughan J., Reubi J. C., Waser B., Koerber S. C., Martinez V., Wang L., Taché Y., Vale W. (2002) J. Med. Chem. 45, 4737–4747 [DOI] [PubMed] [Google Scholar]
- 51.Kornreich W. D., Galyean R., Hernandez J. F., Craig A. G., Donaldson C. J., Yamamoto G., Rivier C., Vale W., Rivier J. (1992) J. Med. Chem. 35, 1870–1876 [DOI] [PubMed] [Google Scholar]
- 52.Perrin M. H., Grace C. R., Digruccio M. R., Fischer W. H., Maji S. K., Cantle J. P., Smith S., Manning G., Vale W. W., Riek R. (2007) J. Biol. Chem. 282, 37529–37536 [DOI] [PubMed] [Google Scholar]
- 53.Nielsen S. M., Nielsen L. Z., Hjorth S. A., Perrin M. H., Vale W. W. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 10277–10281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoare S. R. (2005) Drug. Discov. Today 10, 417–427 [DOI] [PubMed] [Google Scholar]
- 55.Parthier C., Reedtz-Runge S., Rudolph R., Stubbs M. T. (2009) Trends Biochem. Sci. 34, 303–310 [DOI] [PubMed] [Google Scholar]
- 56.Murage E. N., Schroeder J. C., Beinborn M., Ahn J. M. (2008) Bioorg. Med. Chem. 16, 10106–10112 [DOI] [PubMed] [Google Scholar]
- 57.Manhart S., Hinke S. A., McIntosh C. H., Pederson R. A., Demuth H. U. (2003) Biochemistry 42, 3081–3088 [DOI] [PubMed] [Google Scholar]
- 58.Neumann J. M., Couvineau A., Murail S., Lacapère J. J., Jamin N., Laburthe M. (2008) Trends Biochem. Sci. 33, 314–319 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.