Abstract
Orexin neurons contribute to cardiovascular, respiratory and analgesic components of the fight-or-flight response against stressors. Here, we examined whether the same is true for stress-induced hyperthermia. We used prepro-orexin knockout mice (ORX-KO) and orexin neuron-ablated mice (ORX-AB) in which the latter lack not only orexin, but also other putative neurotransmitter/modulators contained in the orexin neurons. In response to repetitive insertion of a temperature probe into their rectum (handling stress), ORX-KO mice showed a normal temperature change as compared to that of wild-type littermates (WT) while ORX-AB showed an attenuated response. Stress-induced expression of uncoupling protein-1, a key molecule in non-shivering thermogenesis in the brown adipose tissue (BAT), was also blunted in ORX-AB but not in ORX-KO. When the BAT was directly activated by a β3 adrenergic agonist, there was no difference in the resultant BAT temperature among the groups, indicating that BAT per se was normal in ORX-AB. In WT and ORX-KO, handling stress activated orexin neurons (as revealed by increased expression of c-Fos) and the resultant hyperthermia was largely blunted by pre-treatment with a β3 antagonist. This observation further supports the notion that attenuated stress-induced hyperthermia in ORX-AB mice was caused by a loss of orexin neurons and abnormal BAT regulation. This study pointed out, for the first time, the possible importance of co-existent neurotransmitter/modulators in the orexin neurons for stress-induced hyperthermia and the importance of integrity of the orexin neurons for full expression of multiple facets of the fight-or-flight response.
Introduction
Cold exposure and immune challenge inhibit GABAergic neurons in the preoptic area (Lazarus et al. 2007; Nakamura & Morrison, 2008) resulting in disinhibition of multiple thermogenic systems located in the dorsomedial hypothalamus (DMH) and medullary raphe pallidus nucleus (Cano et al. 2003; Nakamura et al. 2004; Hodges et al. 2008). These thermogenic nuclei activate sympathetic preganglionic neurons in the spinal cord, resulting in cutaneous vasoconstriction and activation of brown adipose tissue (BAT) that ultimately raises body temperature (Ootsuka & McAllen, 2006; Yoshida et al. 2009). Although we know less about the thermoregulatory pathways underlying stress-induced hyperthermia, involvement of the DMH–BAT system has been proposed (DiMicco & Zaretsky, 2007).
We previously demonstrated that cardiorespiratory excitation during the fight-or-flight response (defense response) was attenuated in orexin-deficient mice (Kayaba et al. 2003; Zhang et al. 2006a). However, it is currently unclear whether the same is true for stress-induced hyperthermia. We expected an affirmative result from two reasons. First, orexin neurons locate not only in the perifornical area (so-called defense area) and the lateral hypothalamic area but also in the DMH (Elias et al. 1998; Peyron et al. 1998; Nambu et al. 1999). Second, at least some orexin neurons project to the medullary raphe, one of the thermogenic nuclei described above (Peyron et al. 1998; Nambu et al. 1999).
A thermoregulatory role for orexin has previously been proposed. Intracerebroventricular administration of orexin increased body temperature (Monda et al. 2001; Zheng et al. 2005). Cold exposure increased orexin mRNA in the hypothalamus (Ida et al. 2000). Transneuronal retrograde transport of pseudorabies virus from the BAT identified the caudal raphe neurons with orexinergic innervation (Berthoud et al. 2005) and orexin-containing neurons in the hypothalamus (Oldfield et al. 2002). Orexin knockout (ORX-KO) mice showed elevated body temperature during sleep (Mochizuki et al. 2006) and orexin neuron-ablated (ORX-AB) mice had an attenuated body temperature fluctuation (Zhang et al. 2007).
Orexin neurons contain not only orexin but also other putative neurotransmitter/modulator candidates such as glutamate (Abrahamson et al. 2001; Rosin et al. 2003; Torrealba et al. 2003), dynorphin (Chou et al. 2001), galanin (Hakansson et al. 1999) and nitric oxide (Cheng et al. 2003). We can evaluate possible roles for these substances by comparing the phenotypes of ORX-KO and ORX-AB mice because the latter lack these substances in addition to orexin (Chou et al. 2001). However, the phenotypes tested thus far were very similar, at least in regard to feeding behaviour (Hara et al. 2005) and cardiorespiratory changes during the fight-or-flight response (Zhang et al. 2006b; Kuwaki et al. 2008). Regulation of rapid-eye-movement (REM) sleep appears to be an exception, since ORX-AB mice experienced more and longer REM sleep than ORX-KO mice (Kantor et al. 2009). Dynorphin and glutamate may act synergistically with orexin to promote wakefulness (Arrigoni et al. 2010). Nevertheless, the precise role(s) of the substances co-localized with orexin are largely unknown.
The aim of the present study was to examine whether orexin and co-localized substances in orexin neurons contribute to stress-induced hyperthermia. We hypothesized that both ORX-KO and ORX-AB mice would show a similar attenuation of stress-induced hyperthermia because this was the case in studies of the cardiorespiratory component of the fight-or-flight response (Zhang et al. 2006b; Kuwaki et al. 2008). In addition, we examined the relative importance of heat production in BAT and prevention of heat loss by vasoconstriction in a stress-induced hyperthermia protocol. We found, contrary to our expectations, that ORX-AB but not ORX-KO mice have a blunted stress-induced hyperthermia. The results indicate the possible importance of co-existent neurotransmitter/modulators in the orexin neurons for stress-induced hyperthermia and the importance of integrity of the orexin neurons for full expression of multiple facets of the fight-or-flight response.
Methods
Ethical approval
All experimental procedures were performed in accordance with the guiding principles for the care and use of animals in the field of physiological sciences published by the Physiological Society of Japan (2003) and were approved by the Institutional Animal Use Committees at Chiba University and Kagoshima University.
Animals
Animals used in this study were 5- to 8-month-old male ORX-KO mice, ORX-AB mice and corresponding wild-type littermates (WTKO and WTAB). The ORX-KO mice have a nuclear translocation signal plus the LacZ (also known as β-galactosidase) gene inserted into the prepro-orexin gene and do not produce orexin-A and -B (Chemelli et al. 1999). ORX-KO mice were maintained as heterozygotes and crossed to obtain null mutants and WTKO mice. In ORX-AB mice, almost all (>98%) of orexin neurons are ablated by 4 months of age through expression of the neurotoxin ataxin-3, under the control of the human orexin promoter (Hara et al. 2001). Thus, by comparison to the phenotype of ORX-KO mice, ORX-AB mice allow us to test the roles of other neurotransmitters (e.g. glutamate and dynorphin) produced in the orexin neurons (Chou et al. 2001; Rosin et al. 2003). ORX-AB were also maintained as heterozygotes and crossed with C57BL/6 (Clea, Japan) to obtain ORX-AB and WTAB mice. To maximize genetic homogeneity, we backcrossed the ORX-KO and ORX-AB mice with C57BL/6 for more than ten generations. The genotype of ORX-KO and ORX-AB mice was identified by PCR of DNA extracted from the tail, as has been reported (Terada et al. 2008; Zhang et al. 2009). All mice were housed in a room maintained at 22–24°C, with lights on at 07.00 h and off at 19.00 h. Mice had food and tap water available ad libitum. The animals were isolated 2–7 days preceding the experiment and singly housed in small cages. All the animal experiments were performed in a quiet air-conditioned (22–24°C) room from 13.00–19.00 h to avoid possible influence of circadian rhythm on body temperature.
Handling stress and measurement of rectal temperature
The temperature measurement itself was used as a stressor according to the method described elsewhere (van der Heyden et al. 1997; Bouwknecht et al. 2007). Animal cages were brought to the experimental room around 12.00 h and left there for 1–2 h as an acclimation period. Each mouse was picked up on the wire ceiling of its home cage and gently immobilized by hand. A thermistor probe (RET-3, Physitemp, Clifon, NJ, USA), dipped into mineral oil before inserting, was placed into the rectum. After a stable rectal temperature was measured for 10 s, the animal was released into its home cage. The whole procedure (picking up of the animal, insertion of probe, waiting for stabilization and release of the animal) was completed within 30 s. The same procedure was repeated at 10 min intervals for 2 h. We call these procedures ‘handling stress’ in this paper.
The relative importance of heat production in the BAT and the prevention of heat loss by vasoconstriction was also examined in the handling stress procedure. For this purpose, a β3 receptor selective antagonist, SR59230A (Tocris; dissolved in saline, 5 mg kg−1), or an α1 adrenergic antagonist, prazosin (Sigma; dissolved in 3.3% polyethylene glycol, 1 mg kg−1), was intraperitoneally administered 60 min prior to the handling stress. β3 and α1 receptors are the predominant subtypes at the sympathetic nerve terminals in the BAT and blood vessels, respectively. Only one drug or a vehicle was tested in a naïve animal. Doses of the drugs were chosen according to published reports (Lecci et al. 1990; Manara et al. 1996).
Telemetric measurement of body temperature
To assess the suitability of the repetitive probe insertion method to examine stress-induced hyperthermia, we compared the abdominal temperature measured by an indwelling telemetric device (TA10ETA-F10, Data Sciences International, MN, USA) with rectal temperature measured by probe insertion described above. For implantation of the telemetric transducer, mice were anaesthetized with 2–3% isoflurane and given an antibiotic (cephalosporin, 50 mg kg−1, s.c.). After a recovery period of 1 week, the same handling stress procedure was performed as described above together with the continuous telemetric measurement of abdominal temperature. Telemetric data were averaged for the 2 min just before the temperature probe was inserted into the rectum.
Quantitative real-time PCR
Expression of mitochondrial uncoupling protein (UCP)-1, a major contributor to non-shivering thermogenesis in the BAT (Cannon & Nedergaard, 2004), was determined using quantitative real-time PCR. For this purpose, interscapular BAT was quickly dissected on ice from decapitated mice just after the 4th measurement of rectal temperature when the temperature reached a maximal plateau in the WTAB mice (see Results). BAT tissues were weighed and stored in RNAlater solution (Qiagen, Tokyo, Japan) at –80°C before use. Total RNA was extracted using the RNeasy kit (Qiagen) according to the manufacturer's protocol and cDNA was reverse-transcribed using random primers. Possible contamination of genomic DNA was prevented by DNase-I treatment before cDNA synthesis and the quality of the obtained cDNA was checked by PCR using a primer set for β-actin DNA (5′-ATCCGTAAAGACCTCTATGC-3′ and 5′-ACGCAGCTCAGTAACAGTC-3′) spanning the intron between exon 5 and exon 6. We confirmed that the PCR product was only from cDNA (286 bp) but not from genomic DNA (411 bp).
Quantitative real-time PCR was performed (LightCycler, Roche Diagnostics, Mannheim, Germany) using TaqMan primers to amplify a fragment of mouse UCP-1 (Mm00494069_m1, Applied Biosystems, Carlsbad, CA, USA) and mouse β-actin (4352341E, Applied Biosystems). Each sample was determined in triplicate. Results of UCP-1 were normalized to β-actin.
BAT function test
To examine whether the BAT tissue per se was functional or not, the temperature response to a β3 receptor selective agonist, CL316243 (Stronberg & Pietri-Rouxel, 1996), was tested in chloralose- (75 mg kg−1, i.p.) and urethane- (750 mg kg−1, i.p.) anaesthetized mice warmed with a heating pad (set at 33°C). The adequacy of anaesthesia was confirmed by absence of the withdrawal reflex to a pinching stimulus. A thermo probe (IT-23, Physitemp) was implanted in the BAT and the skin sutured. After 30 min of control recording, saline (10 μl g−1, i.p.) and CL316243 (Tocris, dissolved in saline, 1 mg kg−1) were sequentially administered in this order with an interval of 2 h. BAT temperature was recorded for another 2 h until the temperature reached maximum.
Immunohistochemistry
Activation of orexin neurons by handling stress was examined by double immunohistochemical staining for orexin and c-Fos, a marker of neural activation (Hughes & Dragunow, 1995), using a similar method to those previously published (Watanabe et al. 2005; Sunanaga et al. 2009; Zhang et al. 2009). In brief, after the completion of 2 h of rectal temperature measurement, the mice were deeply anaesthetized by an i.p. injection of urethane (1.6 g kg−1) and transcardially perfused with 0.01 m phosphate-buffered saline (PBS) followed by a fixative solution containing 4% paraformaldehyde in PBS. Brains were excised and post-fixed in the same fixative solution for 48 h at 4°C. Control brains were obtained from naïve animals. After cryoprotection with 30% sucrose, serial transverse frozen sections (40 μm) were cut from the brain tissue that included the hypothalamus. Every fourth section was collected and sequentially incubated with PBS containing 0.3% Triton-X 100 and 1% normal goat serum for 30 min, rabbit anti-c-Fos antiserum (1/1000, Oncogene Research Products, San Diego, CA, USA) for 1 h, biotinylated goat anti-rabbit IgG antibody (1/200, Vector Laboratories, Burlingham, CA, USA) for 90 min and rabbit anti-orexin antiserum (1/1000, Peptide Institute, Minohshi, Osaka, Japan) for 1 h at the room temperature. The anti-orexin antiserum recognizes mouse/human orexin A and B but not glucagon-like peptide-1. Finally, tissue was incubated with Alexa Fluor 488 streptavidin conjugate (1/200, Molecular Probes, Eugene, OR, USA) and Alexa Fluor 568-labelled goat anti-rabbit IgG antibody (1/200, Molecular Probes) for 90 min in a dark box. The sections were then mounted on a glass slide and examined with a fluorescence microscope (Biorevo BZ-8000, Keyence, Osaka, Japan). Images were recorded at ×100 with a 48-bit digital camera (711 × 944 μm window). The photographic frame was set so that the fornix would be bottom and midline of the window (Fig. 5A). To confirm the specificity of the antibodies, incubations without primary or secondary antibody were conducted as a negative control in each experiment and no signal was observed.
Figure 5. Immunohistochemical evidence for activation of orexin neurons by handling stress.

A, schematic drawing of a coronal section of the mouse brain showing structure of the hypothalamus. A rectangle denotes examined area (711 × 944 μm). Both sides were examined in the actual experiment although only a right side window was depicted for simplicity. DMH, dorsomedial hypothalamus; f, fornix; LHA, lateral hypothalamic area; mt, mammillothalamic tract; PeF, perifornical area. B, representative photograph of double immunostaining for orexin and c-Fos in the hypothalamus of a wild-type (WT) mouse sampled after 2 h of repetitive handling stress. Orexin was stained in red and c-Fos was stained in green. Yellow designates cells stained for both orexin and c-Fos. Filled triangles indicate double-stained cells and open triangles indicate orexin-containing but not c-Fos-expressing cells. C, representative photograph of double immunostaining for LacZ (β-galactosidase) and c-Fos in the hypothalamus of an orexin knockout (ORX-KO) mouse sampled after 2 h of repetitive handling stress. LacZ gene was introduced into the ORX-KO mice instead of the normal orexin gene. LacZ was stained in red and c-Fos was stained in green. Yellow designates cells stained for both LacZ and c-Fos. Filled triangles indicate double-stained cells and open triangles indicate LacZ-containing but not c-Fos expressing cells. Bar, 100 μm in B and C. D, typical distribution of double-stained cells (filled circle), orexin-positive but not c-Fos positive cells (open circle), and c-Fos-positive but not orexin-positive cells (×) in the examination window of a WT mouse. E, typical distribution of double-stained cells (filled circle), LacZ-positive but not c-Fos-positive cells (open circle), and c-Fos positive but not LacZ-positive cells (×) in the examination window of a ORX-KO mouse. F, typical distribution of c-Fos-positive cells (×) in the examination window of a orexin neuron-ablated (ORX-AB) mouse. Note that there is no apparent difference in the distribution of c-Fos-positive cells among D, E and F. Bar, 200 μm in D–F. G, numbers of c-Fos-, orexin (ORX)- and LacZ-immunopositive cells and double-stained cells (c-Fos and ORX in WT or in ORX-AB, and c-Fos and LacZ in ORX-KO) in the hypothalamus. Every 4th section in an animal (6 sections per mouse) was examined. Data are presented as mean ±s.e.m. of 4 mice in a group except for handling-stressed ORX-KO mice (n = 3). *P < 0.05 vs. naïve. No orexin-immunopositive cell was detected (n.d.) in ORX-AB mice.
For ORX-KO mice that do not express orexin, we took advantage of the animal model which has a nuclear translocation signal plus the LacZ gene instead of the orexin gene (Chemelli et al. 1999). Brain sections were prepared by the same method described above and incubated with a mouse monoclonal anti-LacZ antibody (1/1000, Promega, Madison, WI, USA) instead of anti-orexin antiserum. In addition, Alexa Fluor 568-labelled goat anti-mouse IgG antibody (1/200, Molecular Probes) was used instead of Alexa Fluor 568-labelled goat anti-rabbit IgG antibody.
The number of single-labelled (c-Fos, orexin or LacZ) and double-labelled (orexin plus c-Fos or LacZ plus c-Fos) cells was determined in a blinded manner to the treatment (stressed or naïve).
Statistics
The effect of repeated handling stress on rectal temperature and that of β3 agonist on BAT temperature was assessed by ANOVA with genotype as the main factor and time as a repeated measure. When appropriate, within-subjects effect over time and between-subjects effects at each time point was determined by Dunnett's post hoc test. Effect of the β3- or α1-antagonist on handling stress-induced hyperthermia was assessed by one-way ANOVA with drugs (blocker or vehicle) as main factor and time as a repeated measure. When appropriate, a within-subjects effect over time was determined by Dunnett's test and between-subjects effects at each time point was determined by unpaired 2-tailed t test. UCP-1 measurements and immunohistochemistry results were analysed by one-way ANOVA. Data are presented as mean ±s.e.m. Differences were considered significant at P < 0.05.
Results
Handling stress-induced hyperthermia can be accurately determined by repetitive probe insertion
Temperature measurement itself was used as a stressor in the following study. Therefore, it may be questionable whether the first measurement can be thought as the baseline value without stimulation or whether it is already distorted by the handling process. To examine this question, six WTAB mice were used to compare abdominal temperature measured by an indwelling telemetric device with rectal temperature measured by probe insertion (Fig. 1). There was no difference between the rectal temperature obtained by the first probe insertion and the abdominal temperature obtained by the telemeter at the corresponding time point (36.3 ± 0.1°C in either method) and no deflection was observed (ΔT = 0.0 ± 0.1°C, range = −0.5 to +0.3). In addition, there was no significant difference in the measured values between the two methods at any time point.
Figure 1. Comparison between body temperature measurement by rectal temperature probe insertion and abdominal telemetry in wild-type mice.

To confirm that the temperature measurement by a rectal probe accurately reflects the body temperature just before probe insertion but subsequently triggers hyperthermia, rectal temperature was repetitively measured at 10 min intervals while abdominal temperature was continuously measured using radio-telemetry. Data are mean ±s.e.m. of 6 wild-type mice. Arrows indicate the timing of the insertion of temperature probe into the animal's rectum.
Blunted stress-induced hyperthermia in ORX-AB mice but not in ORX-KO mice
We have previously shown that the stress-induced cardiorespiratory responses were significantly attenuated in ORX-KO and ORX-AB mice (Kayaba et al. 2003; Zhang et al. 2006a). Here, we examined whether the same was true for stress-induced hyperthermia, a different facet of the fight-or-flight response.
There was no difference in initial rectal temperature among the genotypes. It was 36.3 ± 0.1°C in WTKO mice (n = 22), 36.2 ± 0.1°C in ORX-KO mice (n = 37), 36.1 ± 0.1°C in WTAB mice (n = 21) and 36.1 ± 0.1°C in ORX-AB mice (n = 32). In WTKO and WTAB mice, rectal temperature increased by >1.0°C at the third measurement (20 min after the initial measurement) (Fig. 2). Repetitive handling stress every 10 min did not result in a further increase and the rectal temperature remained higher than the baseline throughout the remainder of the experimental period, although a moderate decline was observed in the very late phase (100–120 min).
Figure 2. Effect of repeated handling stress on rectal temperature of mice of four genotypes.

The temperature measurement (insertion of thermistor probe into the animal's rectum) itself was used as a stressor and repeatedly applied at 10 min intervals for 2 h. Data are presented as mean ±s.e.m. of orexin knockout mice (ORX-KO, n = 37), orexin neuron-ablated mice (ORX-AB, n = 32) and their corresponding wild-type littermates (WTKO, n = 22 and WTAB, n = 21). *P < 0.05, **P < 0.01 compared with baseline value at time 0 (Dunnett's post hoc test).
In contrast to our expectation, a little slower but largely similar temperature change was observed in ORX-KO mice (Fig. 2). Meanwhile, responses in ORX-AB mice were very slow (peaking at 70 min) and small (0.4 ± 0.1°C at the peak). Overall, rectal temperature in ORX-AB mice was significantly lower than WTAB mice from 10 to 120 min after the initial application of the handling stress.
Blunted stress-induced expression of UCP-1 in ORX-AB mice but not in ORX-KO mice
To examine the possible contribution of UCP-1 expression to the stress-induced hyperthermia, we determined UCP-1 mRNA levels using the quantitative RT-PCR technique. After four repeated insertions of the temperature probe into the rectum, UCP-1 mRNA in the BAT increased by 100–160% as compared to the naïve control in WTKO, ORX-KO and WTAB mice (Fig. 3). In ORX-AB mice, however, the same handling stress did not increase UCP-1 in their BAT. Changes in rectal temperature between the 1st and 4th measurements was significantly smaller (P < 0.01, ANOVA followed by Tukey's post hoc test) in ORX-AB (0.5 ± 0.1°C, n = 8) mice than in WTAB (1.2 ± 0.1°C, n = 9), WTKO (1.1 ± 0.1°C, n = 9) and ORX-KO (1.0 ± 0.1°C, n = 7) mice.
Figure 3. Expression of uncoupling protein (UCP)-1 in the brown adipose tissue from stressed or naïve mice of four genotypes.

Brown adipose tissue (BAT) was dissected from orexin knockout mice (ORX-KO), orexin neuron-ablated mice (ORX-AB) and their corresponding wild-type littermates (WTKO and WTAB) after handling stress (4 rectal temperature measurements at 10 min intervals) and from naïve (unstressed) mice of the same genotypes. Total RNA was extracted from the BAT and cDNA was reverse transcribed. UCP-1 mRNA was determined by quantitative real-time PCR in triplicate and normalized with β-actin mRNA. Data are presented as mean ±s.e.m. of 7–9 animals. *P < 0.05 vs. naïve. Note that handling stress increased expression of UCP-1 in wild-type and ORX-KO mice but not in ORX-AB mice.
Among the four experimental groups, there was no difference in UCP-1 expression of naïve controls (Fig. 3) or in BAT tissue weight (WTKO: 4.7 ± 0.2; ORX-KO: 4.8 ± 0.4; WTAB: 4.5 ± 0.3; ORX-AB: 4.6 ± 0.2 mg g−1 body weight). These results indicated that the lack of stress-induced increase of UCP-1 in ORX-AB mice was not due to abnormality in the BAT in itself but to an abnormality in the central nervous system.
Preserved BAT function in ORX-AB mice
To further confirm the integrity of the BAT in ORX-AB mice, the change of BAT temperature in response to a β3 agonist, CL316243, was examined using a subset of anaesthetized mice. In WTAB mice, BAT temperature increased gradually after the administration of CL316243 and peaked within 120 min of the observation period. As expected, BAT temperature in ORX-AB mice increased in a similar time course and reached its maximum (Fig. 4) in a magnitude (ΔT = 2.0 ± 0.2°C, n = 5) similar to that of WTAB (ΔT = 2.0 ± 0.2°C, n = 5), WTKO (ΔT = 1.9 ± 0.3°C, n = 5) and ORX-KO (ΔT = 2.3 ± 0.2°C, n = 5) mice. On the other hand, administration of saline did not affect BAT temperature in any group of animals (Fig. 4). Initial BAT temperature was not different among the four groups, as was the case in rectal temperature during awake experiment (Fig. 2).
Figure 4. Brown adipose tissue (BAT) function test.
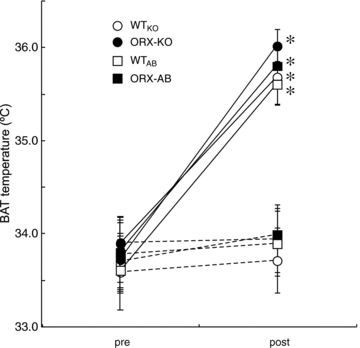
Change of BAT temperature in response to a β3-agonist, CL316243, was examined in chloralose- (75 mg kg−1, i.p.) and urethane- (750 mg kg−1, i.p.) anaesthetized mice. Orexin knockout mice (ORX-KO), orexin neuron-ablated mice (ORX-AB) and their corresponding wild-type litter mates (WTKO and WTAB) received i.p. injection of saline (dashed line) or CL316243 (1 mg kg−1, continuous line). Data are presented as mean ±s.e.m. of the peak values during the observation period of 120 min (n = 5 for each group). *P < 0.05 vs. pre-treatment value.
Activation of orexin neurons by handling stress
We next examined whether the orexin neurons were activated by the handling stress procedure. For this purpose, we performed immunohistochemical detection of orexin-like (in the WTKO mice and the ORX-AB mice) or LacZ-like (in the ORX-KO mice) immunoreactivity, together with c-Fos-like immunoreactivity in the hypothalamus (Fig. 5A) of the mice that received 2 h of handling stress (a subset of those used for Fig. 2) or naïve control mice.
Immunostaining for c-Fos was exclusively nuclear and that for orexin was confined to the cytoplasm and fibres (Fig. 5B). In WTKO mice, there was no difference in the total number of orexin-immunopositive cells between the treatments (541 ± 24 for stressed, n = 4 vs. 547 ± 18 for naïve, n = 4) showing highly reproducible results. Handling stress increased the numbers of c-Fos-immunopositive cells and double-labelled (orexin plus c-Fos) cells (Fig. 5G). Consequently, the percentage of double-labelled cells among the orexin-positive cells was significantly higher in the brains of stressed WTKO mice (47.3 ± 2.2%) than in naïve WTKO mice (28.1 ± 4.5%, P = 0.01). Double-labelled cells were distributed in the DMH, perifornical area and lateral hypothalamic area (Fig. 5D).
In the ORX-KO mice, immunostaining for both LacZ and c-Fos was exclusively nuclear (Fig. 5C). There was no difference in the total numbers of LacZ-immunopositive cells between the treatments (56 ± 6 for stressed, n = 3 vs. 54 ± 4 for naïve, n = 4) showing reproducible results. Handling stress significantly increased the numbers of c-Fos-immunopositive cells and double-labelled (LacZ plus c-Fos) cells (Fig. 5G). Consequently, the percentage of double-labelled cells in the LacZ-positive population was significantly higher in the brains of stressed ORX-KO mice (61.6 ± 4.4%) than in naïve ORX-KO mice (18.0 ± 8.2%, P = 0.01). As was the case in the WTKO mice, double-labelled cells were distributed in the DMH, perifornical area and lateral hypothalamic area (Fig. 5E).
No orexin-like immunoreactivity was observed in the ORX-AB mice, as expected (not shown). Of note, there was no difference in the distribution (Fig. 5D–F) or the numbers (Fig. 5G) of c-Fos-positive cells among stressed WTKO, stressed ORX-KO and stressed ORX-AB, indicating that handling stress activated the cells in the examined area in ORX-KO and ORX-AB similarly to those in WTKO.
Heat production in BAT as the main cause of handling stress-induced hyperthermia
Body temperature is regulated by the balance between heat production and heat loss. To examine the relative importance of these processes in handling stress-induced hyperthermia, a β3 receptor selective antagonist, SR59230A, or an α1 adrenergic antagonist, prazosin, was intraperitoneally administered 60 min prior to the start of the handling stress procedure.
SR59230A did not affect basal rectal temperature in either WTKO mice or ORX-KO mice (Fig. 6A). However, SR59230A significantly attenuated the hyperthermia that was induced by repetitive handling stress in both WTKO (ΔT from time 0 to the peak = 0.9 ± 0.1°C in vehicle-treated, n = 10 vs. 0.4 ± 0.1°C in SR59230A-treated, n = 10, P < 0.01) and ORX-KO mice (ΔT from time 0 to the peak = 0.9 ± 0.1°C in vehicle-treated, n = 10 vs. 0.3 ± 0.1°C in SR59230A-treated, n = 10, P < 0.01).
Figure 6. Effect of a β3-antagonist (SR59230A) and an α1-antagonist (prazosin) on handling stress-induced hyperthermia.

SR59230A (5 mg kg−1) and prazosin (1 mg kg−1) was intraperitoneally administered 1 h before the start (at time 0) of the handling stress procedure. Thereafter, temperature measurement was repeated 12 times with an interval of 10 min. Open circles indicate vehicle (saline for SR59230A and 3.3% polyethylene glycol in saline for prazosin)-treated wild-type (WT) mice, filled circles indicate antagonist (SR59230A in A and prazosin in B)-treated WT mice. Open triangles indicate vehicle-treated orexin knockout (KO) mice and filled triangles indicate antagonist-treated KO mice. Each point represents mean ±s.e.m. from 8–10 animals. *P < 0.05 compared with baseline value at time 0 (Dunnett's post hoc test). In A, initial rectal temperature was not different between pre-treatment with SR59230A and saline while, in B, initial rectal temperature was significantly lower in the prazosin-treated group than in the vehicle-treated group. Note that SR59230A prevented handling stress-induced hyperthermia in both WT and KO mice whereas prazosin did not or even exaggerated hyperthermia at the later observation periods (see also Results section).
Prazosin reduced rectal temperature in the resting condition in both WTKO mice and ORX-KO mice (Fig. 6B). Even though prazosin was effective when the handling stress was started, it did not inhibit or even exaggerate the subsequent handling stress-induced hyperthermia in both WTKO (ΔT from time 0 to the peak = 0.8 ± 0.1°C in vehicle-treated, n = 8 vs. 1.2 ± 0.1°C in prazosin-treated, n = 9, P < 0.01) and ORX-KO mice (ΔT from time 0 to the peak = 0.8 ± 0.1°C in vehicle-treated, n = 8 vs. 1.2 ± 0.1°C in prazosin-treated, n = 10, P < 0.01).
These data, together with the result of UCP-1 expression in Fig. 3, indicated that heat production in the BAT, rather than prevention of heat loss by cutaneous vasoconstriction, was the important determinant of the handling stress-induced hyperthermia, at least under these experimental conditions. On the other hand, regulation of skin blood flow seemed more important than BAT thermogenesis for basal body temperature regulation.
Discussion
In our previous studies using ORX-KO and ORX-AB mice, we demonstrated that endogenous orexin contributes to cardiovascular, respiratory and analgesic components of the fight-or-flight response (Kayaba et al. 2003; Watanabe et al. 2005; Zhang et al. 2006a,b, 2009). We hypothesized that the same would be true for stress-induced hyperthermia. Contrary to our expectations, ORX-KO mice showed normal temperature change in response to handling stress while ORX-AB mice showed the expected attenuation of stress-induced hyperthermia (Fig. 2). UCP-1, a key molecule in non-shivering thermogenesis, did not increase in the BAT of ORX-AB mice in response to the handling stress (Fig. 3). BAT of ORX-AB mice seemed to be functional since its temperature increased in response to a β3 agonist (Fig. 4). In addition, ORX-AB mice seemed to be able to sense stressful stimuli because c-Fos expression in their hypothalamus was comparable to those in WT and ORX-KO mice (Fig. 5). Therefore, it seems plausible that ORX-AB mice have normal BAT but its activation is attenuated during the handling stress, probably due to the absence of orexin neurons. In both WT and ORX-KO mice, handling stress activated orexin neurons (Fig. 5) and the subsequent hyperthermia largely depended on thermogenesis in the BAT but not on prevention of heat loss by vasoconstriction (Fig. 6). Together, these observations support the notion that attenuated stress-induced hyperthermia in ORX-AB was caused by a loss of orexin neurons and abnormal BAT regulation. This study pointed out, for the first time, the possible importance of the co-existent neurotransmitter/modulator candidates in the orexin neurons for stress-induced hyperthermia and the importance of integrity of the orexin neurons for full expression of multiple facets of the fight-or-flight response.
Methodological considerations
In this study, the temperature measurement itself was used as a stressor. Therefore, it may be questionable whether the result in the first measurement can be thought as the baseline value without stimulation or whether it is already distorted by the handling process. To examine this question, we compared core temperature measured by an indwelling telemetric device with rectal temperature measured by probe insertion. We confirmed that this was a needless concern (Fig. 1), at least in the present experimental procedure (temperature measurement by insertion of a probe into the rectum completed within 30 s), presumably because of the buffering effect of body mass to a rapid change in BAT temperature.
In ORX-KO mice that do not express orexin, we identified ‘orexin neurons’ by immunostaining for LacZ which is expressed in this neuronal population (Chemelli et al. 1999). Although some neurons did express LacZ, the number of LacZ-immunopositive neurons (∼50 per mouse) was far fewer than that of orexin-immunopositive neurons in the WT mice (∼500 per mouse). A similar low penetration of LacZ expression was observed in one ORX-KO heterozygote animal (56/547 = 10%). The reason for incomplete penetration of LacZ expression is unknown (Sakurai et al. 1999), although similar phenomena have been observed in other transgenic mice (McGowan et al. 1989; Mercer et al. 1991). LacZ immunostaining in ORX-KO mice is, therefore, not a complete substitute for orexin immunostaining in WT mice and the results were somewhat biased. Nevertheless, we think we can conclude from the present results that at least some of the ‘orexin neurons’ in ORX-KO mice were activated by handling stress because the total number of LacZ-positive cells was not different between naïve and stressed ORX-KO mice. In addition, activation of the hypothalamic area by handling stress seemed not different between WT and ORX-KO mice because the distribution and the total number of c-Fos-positive cells were comparable between the two.
State-dependent role of orexin neurons in thermoregulation
Basal body temperature was not different among three genotypes. Therefore, orexin and orexin neurons do not seem to participate in body temperature regulation when the animal is at rest. This notion is in line with the reports showing normal body temperature in ORX-KO (Mochizuki et al. 2006) and ORX-AB mice (Zhang et al. 2007) when the animal is awake without any specific stimulus.
On the other hand, this study showed that abnormalities in the thermoregulatory system became apparent when handling stress was applied to the ORX-AB mice. This observation was in line with our previous reports showing normal basal ventilation and an attenuated respiratory augmentation in response to the stimulation to the amygdala (Zhang et al. 2009) or the hypothalamus (Zhang et al. 2006a) that mimics the condition under stress. Abnormality in respiratory regulation was also apparent in ORX-KO and ORX-AB mice after repetitive intermittent hypoxia that mimics sleep apnoea (Kuwaki, 2008; Terada et al. 2008; Toyama et al. 2009). Taken together, orexin neurons plausibly regulate body temperature in a state-dependent and feedforward manner to fit with a bodily demand associated with behavioural and metabolic changes.
Location of the orexin neurons in the thermoregulatory circuit
There seems to be two possibilities for location of the orexin neurons in handling stress-induced thermogenic pathway. One possibility is that some of the orexin neurons per se function as DMH-raphe thermogenic neurons (Cano et al. 2003; DiMicco & Zaretsky, 2007). Anatomical connection data support this notion (Oldfield et al. 2002; Berthoud et al. 2005). Some orexin neurons in DMH were in fact activated by handling stress in this study (Fig. 5D and E). Another possibility is that some orexin neurons receive stress information (Zhang et al. 2009) and, in turn, activate the DMH-raphe thermogenic neurons through their projection to the DMH (Peyron et al. 1998). We cannot discriminate between the two possibilities from the current results. However, experimentation with different thermogenic stimuli such as cold exposure or inflammatory pyrogens may help to address this question.
Cold- and inflammatory pyrogen-exposure increases body temperature by activation of both heat production and vasoconstriction (Hodges et al. 2008; Rathner et al. 2008) whereas vasoconstriction had a minor role in handling stress-induced hyperthermia, at least in this study. Orexin neurons were activated by arousal-associated attention including fear conditioning but not by passive stress restraint or cold exposure (Furlong et al. 2009). It is plausible, therefore, that cold and/or pyrogen inhibits GABA neurons in the preoptic area that tonically inhibits thermogenic neurons and thus activates multiple thermogenic pathways (Ootsuka & McAllen, 2006; Nakamura & Morrison, 2008; Rathner et al. 2008; Yoshida et al. 2009) whereas handling or emotional stress activates only a part of the thermogenic pathways through a different neural connection, probably via the amygdala (Zhang et al. 2009). Actually, prostaglandin E2 evokes both BAT thermogenesis and cutaneous vasoconstriction but through different pathways in which only BAT thermogenic pathway relies on the DMH (Rathner et al. 2008). Therefore, the DMH–BAT thermogenic pathway seems to be a common pathway for all the stimuli (stress, cold, pyrogen) but the cutaneous vasoconstriction pathway seems to bypass DMH and orexin neurons.
Possible neurotransmitter/modulators in the orexin neurons for handling stress-induced hyperthermia
We have no data at present to determine which neurotransmitter/modulator in the orexin neurons is important for stress-induced hyperthermia. Dynorphin seems to be a hypothermic neuropeptide because intracerebroventricular (i.c.v.) administration decreased body temperature (Handler et al. 1994) and the content of dynorphin in the brain was high during hibernation when the body temperature is low (Cui et al. 1996). Galanin also seems to be hypothermic because i.c.v. administration inhibited endotoxin-induced fever (Lyudyno et al. 2001) and promoted sleep (Steiger, 2007). Glutamate seems to be a possible candidate because microinjection of glutamate receptor agonists into the raphe pallidus activated sympathetic nerve activity to the BAT and microinjection of glutamate receptor antagonists into the raphe pallidus inhibited an activation of BAT sympathetic nerve evoked by stimulation to the DMH (Cao & Morrison, 2006). However, it should be clarified whether the glutamate-synthesizing DMH thermoregulatory neurons are orexin neurons or not. Future experiments will be necessary to distinguish among these possibilities.
Acknowledgments
We thank Dr Thomas S. Kilduff for valuable discussion and help in editing the manuscript. We thank Ms Orie Iwaya for her technical assistance. We also thank Dr Mieko Kurosawa, Dr Takashi Miki and Dr Yoshitoshi Kasuya for their technical advice in identification of brown adipose tissue and PCR procedure. Part of the work was supported by the Grants-in Aid for Scientific Research from the Ministry of Education, Science, Culture and Sports, Japan.
Glossary
Abbreviations
- BAT
brown adipose tissue
- DMH
dorsomedial hypothalamus
- ORX-AB
orexin neuron-ablated mice
- ORX-KO
orexin knockout mice
- PBS
phosphate-buffered saline
- REM
rapid eye movement
- UCP
uncoupling protein
- WTAB
wild-type littermate of the orexin neuron-ablated mice
- WTKO
wild-type littermate of the orexin knockout mice
Author contributions
Conception and design: T.K. Data collection: W.Z., J.S., Y.T. and T.M. Analysis and interpretation: W.Z., J.S., T.S., Y.K. and T.K. Article drafting and revisions: W.Z., J.S. and T.K. All authors approved the final version of the manuscript. Experiments were done at Chiba University and Kagoshima University.
Author's present address
W. Zhang: Division of Cardiovascular Disease, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL 35294-0007, USA.
References
- Abrahamson EE, Leak RK, Moore RY. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12:435–440. doi: 10.1097/00001756-200102120-00048. [DOI] [PubMed] [Google Scholar]
- Arrigoni E, Mochizuki T, Scammell TE. Activation of the basal forebrain by the orexin/hypocretin neurons. Acta Physiol (Oxf) 2010;198:223–235. doi: 10.1111/j.1748-1716.2009.02036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H-R, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphé neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem Cell Biol. 2005;123:147–156. doi: 10.1007/s00418-005-0761-x. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. doi: 10.1016/j.neubiorev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Cao W-H, Morrison SF. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacol. 2006;51:426–437. doi: 10.1016/j.neuropharm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Cheng SB, Kuchiiwa S, Gao HZ, Kuchiiwa T, Nakagawa S. Morphological study of orexin neurons in the hypothalamus of the Long-Evans rat, with special reference to co-expression of orexin and NADPH-diaphorase or nitric oxide synthase activities. Neurosci Res. 2003;46:53–62. doi: 10.1016/s0168-0102(03)00026-9. [DOI] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, et al. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Lee TF, Wang LCH. In vivo microdialysis study on changes in septal dynorphin and β-endorphin activities in active and hibernating Columbian ground squirrels. Brain Res. 1996;710:271–274. doi: 10.1016/0006-8993(95)01528-0. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R47–R63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- Furlong TM, Vianna DML, Liu L, Carrive P. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci. 2009;30:1603–1614. doi: 10.1111/j.1460-9568.2009.06952.x. [DOI] [PubMed] [Google Scholar]
- Hakansson M, de Lecea L, Sutcliffe JG, Yanagisawa M, Meister B. Leptin receptor- and STAT3-immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus. J Neuroendocrinol. 1999;11:653–663. doi: 10.1046/j.1365-2826.1999.00378.x. [DOI] [PubMed] [Google Scholar]
- Handler CM, Piliero TC, Geller EB, Adler MW. Effect of ambient temperature on the ability of mu-, kappa- and delta-selective opioid agonists to modulate thermoregulatory mechanisms in the rat. J Pharmacol Exp Ther. 1994;268:847–855. [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Hara J, Yanagisawa M, Sakurai T. Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci Lett. 2005;380:239–242. doi: 10.1016/j.neulet.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, et al. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P, Dragunow M. Induction of immediate-early genes and the control of neurotransmitter-regulated gene expression within the nervous system. Pharmacol Rev. 1995;47:133–178. [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun. 2000;270:318–323. doi: 10.1006/bbrc.2000.2412. [DOI] [PubMed] [Google Scholar]
- Kantor S, Mochizuki T, Janisiewicz AM, Clark E, Nishino S, Scammell TE. Orexin neurons are necessary for the circadian control of REM sleep. Sleep. 2009;32:1127–1134. doi: 10.1093/sleep/32.9.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–R593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- Kuwaki T. Orexinergic modulation of breathing across vigilance states. Respir Physiol Neurobiol. 2008;164:204–212. doi: 10.1016/j.resp.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Kuwaki T, Zhang W, Nakamura A, Deng BS. Emotional and state-dependent modification of cardiorespiratory function: role of orexinergic neurons. Autonom Neurosci. 2008;142:11–16. doi: 10.1016/j.autneu.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Lecci A, Borsini F, Volterra G, Meli A. Pharmacological validation of a novel animal model of anticipatory anxiety in mice. Psychopharmacol. 1990;101:255–261. doi: 10.1007/BF02244136. [DOI] [PubMed] [Google Scholar]
- Lyudyno VI, Krasnova IN, Smirnova MP, Klimenko VM. Antipyretic effect of neuropeptide galanin in endotoxin-induced fever. Bull Exp Biol Med. 2001;131:60–63. doi: 10.1023/a:1017538814753. [DOI] [PubMed] [Google Scholar]
- McGowan R, Campbell R, Peterson A, Sapienza C. Cellular mosaicism in the methylation and expression of hemizygous loci in the mouse. Genes Develop. 1989;3:1669–1676. doi: 10.1101/gad.3.11.1669. [DOI] [PubMed] [Google Scholar]
- Manara L, Badone D, Baroni M, Boccardi G, Cecchi R, Croci T, et al. Functional identification of rat atypical ß-adrenoceptors by the first ß3-selective antagonists, aryloxypropanolaminotetralins. Brit J Pharmacol. 1996;117:435–442. doi: 10.1111/j.1476-5381.1996.tb15209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer EH, Hoyle GW, Kapur RP, Brinster RL, Palmiter RD. The dopamine ß-hydroxylase gene promoter directs expression of E. coli lacZ to sympathetic and other neurons in adult transgenic mice. Neuron. 1991;7:703–716. doi: 10.1016/0896-6273(91)90274-4. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Klerman EB, Sakurai T, Scammell TE. Elevated body temperature during sleep in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R533–R540. doi: 10.1152/ajpregu.00887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monda M, Viggiano A, Mondola P, Luca VD. Inhibition of prostaglandin synthesis reduces hyperthermic reactions induced by hypocretin-1/orexin A. Brain Res. 2001;909:68–74. doi: 10.1016/s0006-8993(01)02606-3. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hübschle T, Nakamura Y, Hioki H, Fujiyama F, et al. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermoregulatory pathway that controls body temperature. Nat Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, Mckinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110:515–526. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, McAllen RM. Comparison between two rat sympathetic pathways activated in cold defense. Am J Physiol Regul Integr Comp Physiol. 2006;291:R589–R595. doi: 10.1152/ajpregu.00850.2005. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, Van Den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathner J, Madden C, Morrison S. Central pathway for spontaneous and prostaglandin E2-evoked cutaneous vasoconstriction. Am J Physiol Regul Integr Comp Physiol. 2008;295:R343–R354. doi: 10.1152/ajpregu.00115.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465:593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Moriguchi T, Furuya K, Kajiwara N, Nakamura T, Yanagisawai M, Goto K. Structure and function of human prepro-orexin gene. J Biol Chem. 1999;274:17771–17776. doi: 10.1074/jbc.274.25.17771. [DOI] [PubMed] [Google Scholar]
- Steiger A. Neurochemical regulation of sleep. J Psych Res. 2007;41:537–552. doi: 10.1016/j.jpsychires.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Stronberg AD, Pietri-Rouxel F. Function and regulation of the ß3-adrenoceptor. Trends Pharmacol Sci. 1996;17:373. doi: 10.1016/s0165-6147(96)80011-3. [DOI] [PubMed] [Google Scholar]
- Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T. CO2 activates orexin-containing neurons in mice. Respir Physiol Neurobiol. 2009;166:184–186. doi: 10.1016/j.resp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, Kuwaki T. Ventilatory long-term facilitation in mice can be observed both during sleep and wake periods and depends on orexin. J Appl Physiol. 2008;104:499–507. doi: 10.1152/japplphysiol.00919.2007. [DOI] [PubMed] [Google Scholar]
- Torrealba F, Yanagisawa M, Saper CB. Colocalization of orexin A and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neurosci. 2003;119:1033–1044. doi: 10.1016/s0306-4522(03)00238-0. [DOI] [PubMed] [Google Scholar]
- Toyama S, Sakurai T, Tatsumi K, Kuwaki T. Attenuated phrenic long-term facilitation in orexin neuron-ablated mice. Respir Physiol Neurobiol. 2009;168:295–302. doi: 10.1016/j.resp.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Van Der Heyden JAM, Zethof TJJ, Olivier B. Stress-induced hyperthermia in singly housed mice. Physiol Behav. 1997;62:463–470. doi: 10.1016/s0031-9384(97)00157-1. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kuwaki T, Yanagisawa M, Fukuda Y, Shimoyama M. Persistent pain and stress activate pain-inhibitory orexin pathways. Neuroreport. 2005;16:5–8. doi: 10.1097/00001756-200501190-00002. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci. 2009;29:11954–11964. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zeitzer JM, Sakurai T, Nishino S, Mignot E. Sleep/wake fragmentation disrupts metabolism in a mouse model of narcolepsy. J Physiol. 2007;581:649–663. doi: 10.1113/jphysiol.2007.129510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Sakurai T, Fukuda Y, Kuwaki T. Orexin neuron-mediated skeletal muscle vasodilation and shift of baroreflex during defense response in mice. Am J Physiol Regul Integr Comp Physiol. 2006a;290:R1654–R1663. doi: 10.1152/ajpregu.00704.2005. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shimoyama M, Fukuda Y, Kuwaki T. Multiple components of the defense response depend on orexin: Evidence from orexin knockout mice and orexin neuron-ablated mice. Autonom Neurosci. 2006b;126–127:139–145. doi: 10.1016/j.autneu.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang N, Sakurai T, Kuwaki T. Orexin neurons in the hypothalamus mediate cardiorespiratory responses induced by disinhibition of the amygdala and bed nucleus of the stria terminalis. Brain Res. 2009;1262:25–37. doi: 10.1016/j.brainres.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud H-R. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol. 2005;485:127–142. doi: 10.1002/cne.20515. [DOI] [PubMed] [Google Scholar]


