Abstract
In the past few years there has been an explosion in the characterization of skin-resident dendritic cells (DC). This is due in large part to the development of several lines of mice with genetic alterations that allow for selective targeting of many of these subsets. There is now considerable data derived from in vivo experiments using these mice. This review will focus on the relative contribution of murine skin-resident DC in the generation of immune responses to epicutaneous application of ovalbumin and during contact hypersensitivity. We describe a model in which the two best characterized skin-resident DC, langerhans cells (LC) and Langerin+ dermal DC (dDC) have distinct functions: Langerin+ dDC initiate T cell responses and LC suppress T cell responses.
Dendritic cells of the skin
The skin can be broadly divided into two distinct regions, the epidermis and dermis. The epidermis is derived from ectoderm and is an epithelial layer composed primarily of keratinocytes. Keratinocytes provide for the integrity of the skin and also produce the stratum corneum which is the water impermeable outer-most layer of skin. Langerhans cells (LC) are the only dendritic cells found in the epidermis during the steady-state. LC ontogeny depends on a number of soluble factors including TGFβ and MCSF1–3. LC precursors first populate the epidermis on embryonic day 18 and proliferate in-situ to form a radio-resistant, self-renewing population that is a unique among dendritic cells (DC)4,5. Like all peripheral DC, LC efficiently acquire antigen in the periphery and migrate to regional LN where they can present antigen to naïve and memory T cells6. LC extend dendritic processes in the horizontal plane of the epidermis which allows them to sample a large area of epidermis and contact many keratinocytes. They have access to antigen from pathogens that invade the epidermis, self antigen from keratinocytes and other epidermal-resident cell types as well as tumor antigen from epidermal neoplasia. LC also extend dendritic processes in the vertical axis towards the stratum corneum they acquire antigen from at or near the external surface of the skin akin to lamina propria DC in the gut that sample the luminal contents7,8. Thus, LC are also ideally situated to sample external antigen such as commensal microorganisms or benign environmental antigen.
LC can be readily identified as the only cell expressing MHC-II in the epidermis. LC also express the C-type lectin, Langerin, which is responsible for the generation of Birbeck’s granules, the ultra-structural hallmark of LC6. LC that are transiting through the dermis or that have migrated to skin-draining LN can be identified based on expression of Langerin, EpCam and CD11b and the absence of CD8 and CD103 expression (Table 1)9,10.
Table 1.
Comparison of DC subsets markers.
| Epidermal LCs |
Dermal Langerin+ DCs |
Dermal Langerin− DCs |
CD8+ DCs |
|
|---|---|---|---|---|
| Tissue of Residence | Skin, Epidermis |
Skin, Dermis |
Skin, Dermis |
LN, Spleen, Thymus |
| Langerin | +++ | +++ | − | Strain dependent |
| CD8 | − | − | − | + |
| CD103 | − | +/het | − | |
| CD11b | + | − | het | |
| Ep-Cam | + | − | − | |
| Origin in Chimeras | Host | Donor | Donor | Donor |
Inflammation from a variety of sources (UV light, application of hapten, infection) induces LC activation and migration from the epidermis to the cortical and paracortical T cell areas of the LN5,11,12. LC arrive in the LN relatively late (~72 hours) after an inflammatory stimulus which may reflect the time needed to cross the basement membrane that separates the epidermis and dermis13. In the steady-state, LC appear to be largely sessile, though migration to skin-draining LN does occur at an unknown and presumable low rate in the absence of inflammation1. DC migration in the absence of inflammation-induced activation has been hypothesized as a mechanism by which peripheral tolerance is maintained14,15.
The dermis is a relatively cell-sparse tissue derived from mesoderm that forms a stromal layer immediately below the epidermis. It is populated by fibroblasts that secrete components of the complex extracellular matrix. In the steady-state, a variety of immune cells can be found in the dermis including memory T cells16, mast cells17, and DC. Unlike LC, which have been recognized for many years, dermal DC (dDC) have only recently been studied in detail.
Although dDC can be divided into subsets based on many markers, perhaps the simplest distinction is between those dDC that express Langerin and those that do not. Langerin+ dDC are a distinct DC subset that arise from bone marrow precursors in a Flt3-dependent manner9,18–21. Like LC, they migrate to skin-draining LN both in the steady-state state and in response to inflammation. They are also able to present antigen acquired in the periphery to T cells. Unlike LC, they arrive at LN more quickly and can be identified within 18 hours after stimulation. Langerin+ dDC, although only a small percentage of the total DC population in the dermis (~3%), are in a high state of flux and are continually replaced by new recruits from the blood10,18.
Langerin+ dDC are most often identified in the dermis and LN based on expression of Langerin, CD103 and the absence of CD11b, CD8 and EpCam expression (Table 1). Recently, a population of Langerin+ CD103− dDC has been identified in the dermis that are distinct from LC, though little is known about this population10. Langerin− dDC make-up the bulk of dDC (~80%) and are heterogenous with at least two subsets that can be distinguished based on expression of CD11b+. Importantly, careful examination of subset ontogeny has clearly identified that LC and Langerin+ dDC are distinct DC subsets1,4,21,22. A key question in the field is whether the other skin-derived DC (i.e. Langerin− dDC and Langerin+ CD103− dDC) represent phenotypic heterogenity or if they are also distinct subsets.
Thus, skin-resident DC are clearly quite heterogeneous but individual subsets can be identified by careful use of markers. Successful functional analysis of these DC subsets, however, requires systems that allow for the examination of subsets in isolation.
Methods to ablate skin DC in vivo
Many techniques to ablate LC from the epidermis have been developed. Topical application of glucocorticoids and irradiation with UV light deplete LC from the epidermis but also have many effects on other cell types including dermal DC23,24. This complicates interpretation of experiments that use these strategies. The discovery that LC are radio-resistant has allowed for the generation of bone-marrow chimeras where LC remain of host origin while other hematopoietic cells including dermal DC are donor derived5,25. By reconstituting knock-out mice with WT bone-marrow, LC can be made selectively deficient in a setting of an otherwise normal hematopoietic system. While, this is a powerful technique, it can be complicated by incomplete chimerism10,25.
Genetic approaches to ablate skin DC are quite selective but, as we will discuss, also come with caveats. huLangerin-DTA are transgenic mice in which the active subunit of diphtheria toxin (DTA) is expressed in LC under control of the human CD207 (Langerin) promoter (Table 2)26. Expression of DTA in LC leads to their ablation early in ontogeny. Using a similar transgenic strategy, huLangerin-Cre mice were engineered to express the Cre recombinase under control of the human Langerin promoter27. When huLangerin-Cre mice are bred to strains in which loxP sites have been inserted into genes of interest, the result is a conditional, LC-specific ablation. Both mouse lines have a constitutive and durable LC deficit. As discussed above, murine Langerin is expressed by DC other than LC. Importantly, dDC including those that express Langerin are not affected in huLangerin transgenic mice. The specific targeting of LC in huLangerin-DTA/Cre mice likely results from differences between the murine and human Langerin promoters.
Table 2.
Comparison of cells targeted.
| Mouse Lines | Epidermal LCs |
Dermal Langerin+ DCs |
Dermal Langerin− DCs |
CD8+ DCs |
|---|---|---|---|---|
| Constitutive systems | ||||
| huLangerin-DTA | absent | nl | nl | nl |
| huLangerin-Cre | defective | nl | nl | nl |
| Batf3−/− | nl | absent | nl | absent |
| Inducible systems | ||||
| muLangerin-DTR Day+1 | absent | absent | nl | 25% |
| muLangerin-DTR Day+7/13 | absent | ~20–50% | nl | nl |
| huLangerin-DTR | absent | nl | nl | nl |
Recently, Batf3−/− mice have been described28. The ablation of this transcription factor selectively prevents development of Langerin+ CD103+ dDC as well as CD103+ DC in other peripheral tissues22. CD8+ DC that reside in the LN and spleen are also ablated28. LC and other DC subsets, however, are unaffected. As with huLangerin-DTA and huLangerin-Cre, Batf3−/− mice have a constitutive absence of DC subsets.
Mice do not express the high affinity diphtherhia toxin receptor (DTR) and are relatively resistant to injection of diphtherhia toxin (DT)29. MuLangerin-DTR mice (generated by 2 groups using a similar strategy) exploit this absence to develop an inducible ablation system by introducing the primate DTR into the endogenous murine Cd207 (Langerin) locus11,30. All Langerin+ DC in these mice express DTR and become sensitive to DT. This results in an efficient ablation of LC and Langerin+ dDC after administration of DT. Interestingly, the rate of repopulation of Langerin+ dDC and LC differs after DT ablation9. LC do not repopulate the epidermis in appreciable numbers until approximately 2–3 weeks after depletion. Langerin+ dDC, however, quickly repopulate the dermis. Within two weeks, they have returned to ~50% of their steady-state numbers. Thus, there is a window during which LC are selectively absent but Langerin+ dDC have returned to near their steady-state levels. This can be exploited to examine the effect of ablation of LC vs. Langerin+ dDC by comparing muLangerin-DTR mice +1 day and +7/13 days after DT treatment.
We have recently generated huLangerin-DTR mice, that express DTR under control of the human Langerin promoter31. Like the other lines of huLangerin mice, Langerin+ dDC are unaffected. Thus, administration of DT leads to selective and inducible ablation of LC. HuLangerin-DTR mice allow for the direct examination of functional consequences following acute LC ablation.
Although this review focuses on skin DC, it is important to note that CD103+ Langerin+ DC can be found in many other organs including lung, gut, spleen and thymus32–34. Langerin+ cells in these tissues are ablated in muLangerin-DTR mice. In addition, a variable percentage of LN/spleen-resident CD8+ DC also express low levels of Langerin and are ablated in muLangerin-DTR mice11. The percentage of CD8+ DC that express Langerin varies by genetic background32,35. In the case of muLangerin-DTR approximately 70% of CD8+ DC are ablated11. This is in contrast to Batf3−/− mice which lack all CD8+ DC, at least on the genetic background on which it has been described28.
Thus, mouse models allow for the inducible or constitutive targeting of either LC and/or Langerin+ dDC. These mice have greatly facilitated the exploration of the function of these DC subsets in vivo. We will next examine data from esetwo commonly employed functional assays of the skin immune response – cross presentation of ovalbumin (ova) and contact hypersensitivity (CHS).
Langerin+ dDC, not LC cross-present antigen
A key function of some DC is to cross-present antigen to CD8 T cells and initiate CTL responses. The most commonly used system to examine cross-presentation relies on the protein ovalbumin (ova) and ova-specific OT-I (CD8) transgenic T cells. In one of the early papers using this technique in the skin, Langerin+ DC were isolated by allowing them to crawl out of mouse skin explants in vitro36. When these cells were pulsed with whole ovalbumin protein, Langerin+ cells were able to efficiently drive proliferation of OT-I. Langerin+ cells isolated from the skin or LN of transgenic K14-OVA mice, which have been engineered so that keratinocytes express the immunodominant peptide from ovalbumin, also induced OT-I proliferation in vitro37. Although, this data was initially used as evidence that LC are required for cross-presentation of soluble and keratinocyte-derived antigen to CD8 T cells, these studies were performed prior to the discovery of Langerin+ CD103+ dDC and did not distinguish between these cells and LC. Even so, these data are important and support the conclusion that skin-derived DC can cross-present antigen.
More recently, adoptive transfer of OT-I cells into K14-OVA mice has been used to explore the role of cross-presentation by skin-resident DC to CD8 T cells in vivo38. In this model, ova is a “self” protein and cross-presentation results in activation of autoreactive OT-I. In a series of bone-marrow chimera experiments in which only radio-resistant cells (i.e. LC) or radio-sensitive cells (i.e. dermal DC) could present OVA antigen obtained from keratinocytes, it was observed that OT-I were activated regardless of which cell type was capable of presenting antigen Similar results were obtained using muLangerin-DTR mice, where Langerin+ DC were ablated, leading to the conclusion that radio-resistant cells other than LC were sufficient to cross-present antigen in this system. Indeed, a radio-resistant LN-resident cell has been shown to mediate deletion of self-reactive CD8 cells in other systems39. In addition, in certain circumstances, keratinocytes can present antigen to OT-I directly40. Thus, the ability of multiple cell types to cross-present OVA in K14-OVA mice renders this approach unsuitable for examining the relative function of skin-resident DC.
In a related, but more direct approach, ova protein was applied onto the shaved flank skin of muLangerin-DTR mice that had received adoptive transfer of OT-I cells41. Application of ova 1 day after DT administration when LC and Langerin+ dDC were absent resulted in greatly diminished OT-I proliferation. Proliferation was normal, however, when ova was applied 7 days after DT, a time point at which LC remained absent but Langerin+ dDC had partially returned. Thus Langerin+ dDC but not LC were required to present antigen to OT-I. Since ova applied to flank skin concentrated primarily in the epidermis, this suggested a model in which Langerin+ dDC have a non-redundant role in the cross-presentation of epidermal antigen but LC are dispensable.
Indeed, strong support for this model comes from recent reports that examined the ability of various skin-derived DC isolated from mice in which keratinocytes expressed ova (K5-OVA) to activate OT-I in vitro10,42. Langerin+ CD103+ dDC were unique in their ability to cross-present antigen to OT-I. Other DC including LC, Langerin+ CD103− dDC, Langerin− dDC and LN-resident CD8+ DC were unable to induce OT-I proliferation. A redundant role for LC is further supported by the observation that OT-I proliferation is unaffected in huLangeirn-DTR and huLangerin-DTA mice after application of OVA to flank or ear skin31. Finally, recent work elegantly demonstrated that cross-presentation during recurrent herpes skin infection was mediated by CD103+ dDC and not by LC42.
LC and Langerin+ dDC determine CHS responses
Contact hypersensitivity is a mouse model for allergic contact dermatitis (e.g. eczema that can develop after skin contact with metals containing nickel) and has become a widely used technique to assay adaptive skin immune responses. Mice are sensitized by application of a small organic hapten such as DNFB (2-dinitrofluorobenzene) onto shaved abdominal skin. Five to six days later, the same hapten is applied to the ear. The degree of swelling that develops at 24 hours correlates with the magnitude of the effector response43.
In the original descriptions of muLangerin-DTR mice, CHS was used to determine the effect of LC ablation. In one study decreased CHS was observed, while another study did not observe any effect after administration of DT11,30. Although this was initially quite confusing, it appears that differences in the timing of DT administration can affect the outcome. CHS in both muLangerin-DTR lines is diminished if DT is administered only prior to sensitization41. Thus, it appears quite likely that there are no intrinsic differences between the two mouse lines. Indeed, a recent review has noted that this has been confirmed6. Importantly, the elimination of Langerin+ DC affects sensitization and not challenge30.
As discussed above, both LC and Langerin+ dDC are ablated in muLangerin-DTR mice. The absence of both cell types results in reduced CHS. Interestingly, muLangerin-DTR mice sensitized 7 or 13 days after DT administration, a time when only the epidermal LC remain absent, develop normal CHS responses9. From these studies, it appears that dermal Langerin+ DC are critical for the development of efficient CHS responses and that LC are not involved which is analogous to the findings with cross-presentation.
This conclusion should be tempered somewhat by the observation that Batf3−/− mice generate normal CHS responses despite the lack of Langerin+ dDC22. Unlike muLangerin-DTR, Batf3−/− mice have a constitutive ablation of Langerin+ dDC. Other DC population(s) could compensate in the setting of chronic absence Langerin+ dDC. Alternatively, CD8+ DC are completely absent in Batf3−/− mice but only partially depleted in muLangerin-DTR mice. Differences in the composition of DC available to participate in CHS as well as still undiscovered defects in the Batf3−/− mice may also explain the normal CHS response. In addition, the selective ablation of Langerin+ dDC but not LC using Langerin-DTR bone-marrow chimeras does not reduce CHS responses44. Thus, LC may be able to compensate for the absence Langerin+ dDC in this chimeric system.
HuLangerin-DTA mice have a constitutive absence of LC and normal numbers of Langerin+ dDC. In contrast to muLangerin-DTR mice, CHS responses are increased in huLangerin-DTA mice26,45. This increase is due to the absence of LC at the time of hapten sensitization and occurs in response to multiple haptens at multiple doses. Thus, these data suggest that LC have a suppressive function.
Given the suppressive role for LC in huLangerin-DTA mice, it is surprising, that enhanced CHS was not observed in muLangerin-DTR mice at time points after DT administration when LC remain absent but Langerin+ dDC have largely returned. LC migrate to skin-draining LN in the steady-state and have been proposed to participate in the maintenance of peripheral tolerance14,15. Thus, alterations in peripheral tolerance or compensation by other DC subsets in the constitutive absence of LC could potentially account for exaggerated CHS responses. This does not appear to be the case, however, as acute ablation of LC in huLangerin-DTR mice produces exaggerated CHS responses31. Since Langerin+ dDC are unaffected in these mice, it argues that the still-decreased numbers of Langerin+ dDC in muLangerin-DTR mice +7/13 days after DT, or a functional impairment that occurred during ongoing repopulation of Langerin+ dDC reduced their pro-inflammatory capacity. As a result, the suppressive properties of LC would be masked and CHS response would appear unchanged. Regardless of the precise mechanism, muLangerin-DTR 7/13 days after DT injection behave differently than mice with an acute selective ablation of LC. Interpretations of experiments using the delayed immunization technique with muLangerin-DTR should be mindful of this fact.
CHS against DNFB produces a mix of cytotoxic, Th1 and Th17 type responses46,47. To explore potential mechanisms of LC-mediated suppression, anti-hapten responses have been examined in huLangerin-DTA mice. Analysis of antigen-specific T cells after hapten treatment revealed exaggerated numbers of both CD4 and CD8 T cells in huLangerin-DTA mice but cytokine expression by CD4 and CD8 T cells was unaltered. Number and homeostasis of Treg was also unaffected. Thus, LC appear to inhibit anti-hapten responses by reducing the numbers of antigen-specific T cells without affecting the nature of the response45. CHS has also been examined in huLangerin-Cre mice bred to I-Aβ-flox mice thereby generating a LC-specific “knock-out” of MHC-II. As in huLangerin-DTA mice, CHS responses and numbers of hapten-specific CD4 and CD8 T cells were increased in these mice. Interestingly, expression of FasL by LC, a marker of CD40L-dependent LC activation, was absent in huLangerin-Cre MHC-II-flox mice45,48. Thus, direct cognate interaction between LC and CD4 cells appears to be required for LC activation and suppression of CHS. CHS was also enhanced in huLangerin-Cre IL-10-flox mice demonstrating a non-redundant role for LC-derived IL-10 in the suppression of CHS.
Although the data is far from complete, it is possible to construct a model of antigen-presentation by skin DC subsets during the development of anti-hapten responses (Figure 1). Epicutanous application of standard doses of hapten penetrates through the epidermis and is acquired by both LC and dermal DC41. Langerin+ dDC and Langerin− dDC acquire antigen and arrive in skin-draining LN soon after sensitization. Langerin+ dDC are required for cross-presentation of antigen to CD8 T cells and for the development of CTL responses. Both dDC subsets probably also initiate Th responses as well, though this has not been explored. LC arrive in the skin-draining LN 3–4 days after hapten application. Upon cognate interaction with CD4 cells LC become fully activated presumably via CD40-CD40L interaction and secrete IL-10 which, along with other unknown factors, inhibits expansion of hapten-specific CD4 and CD8 effectors.
Figure 1. Participation of skin DC during contact hypersensitivity.
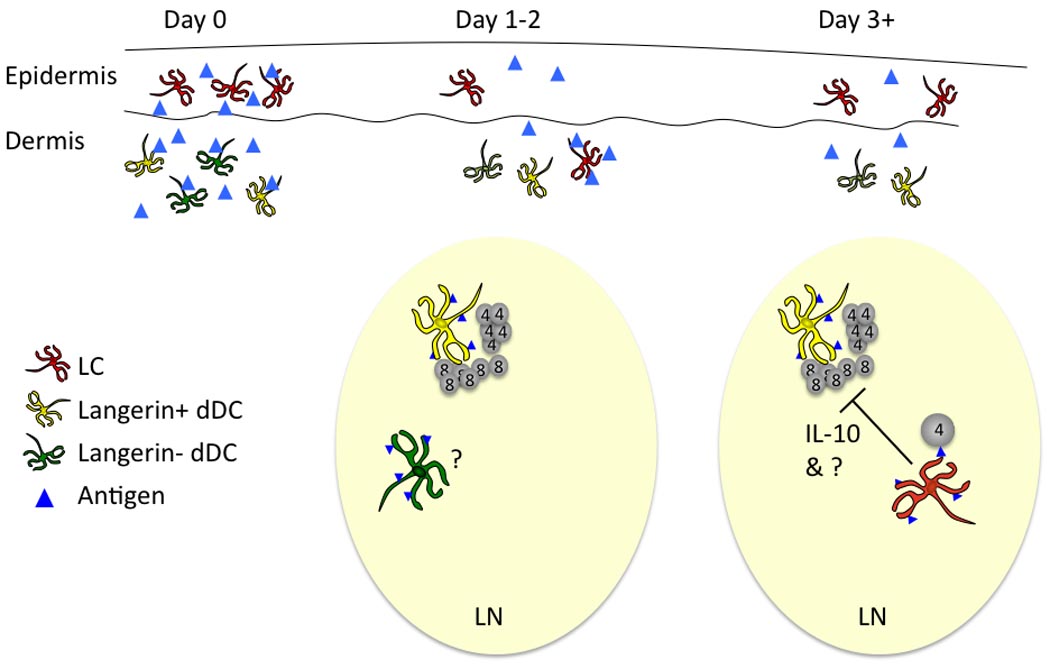
Haptens (blue triangles) penetrate though the stratum corneum and into the epidermis and dermis soon after application (Day 0). They are acquired by LC (red cells in the epidermis), Langerin+ dDC (yellow cells in the dermis) and Langerin− dDC (green cells in the dermis). Approximately 1–2 days after application of hapten, dDC arrive in the skin draining lymph node. Langerin+ dermal DC cross-present antigen to CD8 T cells and initiate an adaptive response. Langerin+ and Langerin− dermal DC also likely present antigen to CD4 Th cells, though this has been less well explored. By day +3 LC arrive in the lymph node. After cognate interaction with CD4 T cells, LC suppress the development of effector CD4 and CD8 T cells though elaboration of IL-10 along with other unidentified factors.
Emerging concepts
In the preceding section, only CHS responses to hapten used at standard doses were discussed. However, CHS experiments that used low dose of the hapten Oxazalone to sensitize muLangerin-DTR mice have been performed. They find that CHS is reduced when mice are sensitized at both early and late time points after DT administration30,49. They hypothesize that low doses of oxazalone concentrate preferentially in the epidermis and conclude that LC are required for efficient presentation of antigen found primarily in the epidermis. These data are at odds with the normal CHS observed in muLangerin-DTR at late time-points after DT and the exaggerated CHS observed in huLangerin-DTA/DTR mice even at low doses of DNFB. To better understand the mechanism of low-dose CHS, it would be important to determine the exact depth of penetration of the Oxazalone and whether this effect occurs with multiple haptens. Never-the-less, these data suggest two exciting possibilities.
The first is that LC are specialized to acquire antigen from the outer-most regions of the epidermis (i.e. stratum granulosum or stratum corneum). Ova cross-presentation studies clearly demonstrate that Langerin+ dDC can access epidermal antigen. However, if low dose oxazalone concentrates in the superficial epidermis, LC may be the only DC with access. This would be consistent with the recent observation that LC dendrites penetrate into the upper epidermis7. It would also raise the possibility that LC may specialize in sampling commensal microorganisms from the surface of the skin.
The second is that the consequence of antigen presentation by LC is determined by the nature of the immunogen/adjuvant. During CHS against standard doses of DNFB, Oxazalone, and FITC, LC have a suppressive phenotype that, at least for DNFB, inhibits development of Th1 and Th17-type responses. Low dose Oxazalone may promote a different type of immune response in which LC are not suppressive. Indeed, selective targeting of antigen to LC using a gene gun led to the production of Th2-related antibody isotypes 50. In addition, LC stimulated in vitro promoted the development of Th2 cells51,52 and topical application of vitamin D analog appears to selectively activate LC to promote Th2-type responses53.
The interaction of LC with commensal microorganims and the potential for LC to promote different Th responses are exciting future directions. These questions can probably not be addressed using the ovalbumin and CHS systems described in this review. Rather, new models will be required in which antigen-specific CD4 and CD8 responses can be examined in vivo both during the steady-state and after physiologically relevant activation.
Functional conclusions about LC and dDC
-
-
Differences in the DC populations ablated in various mouse models and in experimental techniques account for much of the conflicting data from LC-targeted mice.
-
-
Langerin+ dDC cross-present antigen. LC do not.
-
-
Langerin+ dDC likely promote CHS responses.
-
-
LC actively suppress CHS responses.
-
-
LC suppression requires cognate interaction with CD4 Th cells and LC-derived IL-10.
Acknowledgements
We would like to thank Drs. Botond Igyarto and Kristen Hogquist for their careful reading of the manuscript. D.H.K. is supported by the Al Zelickson professorship in dermatology and by NIH grant R01AR056632.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Merad M, et al. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8(12):935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 2.Borkowski TA, et al. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184(6):2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginhoux F, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7(3):265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chorro L, et al. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med. 2009;206(13):3089–3100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merad M, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3(12):1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romani N, et al. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234(1):120–141. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubo A, et al. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med. 2009;206(13):2937–2946. doi: 10.1084/jem.20091527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rescigno M. Identification of a new mechanism for bacterial uptake at mucosal surfaces, which is mediated by dendritic cells. Pathol Biol (Paris) 2003;51(2):69–70. doi: 10.1016/s0369-8114(03)00100-7. [DOI] [PubMed] [Google Scholar]
- 9.Bursch LS, et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204(13):3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henri S, et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 2010;207(1):189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kissenpfennig A, et al. Dynamics and Function of Langerhans Cells In Vivo Dermal Dendritic Cells Colonize Lymph Node AreasDistinct from Slower Migrating Langerhans Cells. Immunity. 2005;22(5):643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Eidsmo L, et al. Differential migration of epidermal and dermal dendritic cells during skin infection. J Immunol. 2009;182(5):3165–3172. doi: 10.4049/jimmunol.0802950. [DOI] [PubMed] [Google Scholar]
- 13.Henri S, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167(2):741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 14.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99(1):351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang A, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27(4):610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 17.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9(11):1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginhoux F, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204(13):3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poulin LF, et al. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204(13):3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shklovskaya E, et al. Epidermal and dermal dendritic cells display differential activation and migratory behavior while sharing the ability to stimulate CD4+ T cell proliferation in vivo. J Immunol. 2008;181(1):418–430. doi: 10.4049/jimmunol.181.1.418. [DOI] [PubMed] [Google Scholar]
- 21.Ginhoux F, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206(13):3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edelson BT, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207(4):823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furio L, et al. UVA radiation impairs phenotypic and functional maturation of human dermal dendritic cells. J Invest Dermatol. 2005;125(5):1032–1038. doi: 10.1111/j.0022-202X.2005.23904.x. [DOI] [PubMed] [Google Scholar]
- 24.Aubin F. Mechanisms involved in ultraviolet light-induced immunosuppression. Eur J Dermatol. 2003;13(6):515–523. [PubMed] [Google Scholar]
- 25.Merad M, et al. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10(5):510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan DH, et al. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23(6):611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan DH, et al. Autocrine/paracrine TGF{beta}1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204(11):2545–2552. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322(5904):1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito M, et al. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19(8):746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 30.Bennett CL, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169(4):569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bobr A, et al. Acute ablation of Langerhans Cells enhances skin immune responses. J Immunol. 2010 doi: 10.4049/jimmunol.1001802. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douillard P, et al. Mouse lymphoid tissue contains distinct subsets of langerin/CD207 dendritic cells, only one of which represents epidermal-derived Langerhans cells. J Invest Dermatol. 2005;125(5):983–994. doi: 10.1111/j.0022-202X.2005.23951.x. [DOI] [PubMed] [Google Scholar]
- 33.Sung SS, et al. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176(4):2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 34.del Rio ML, et al. Development and functional specialization of CD103+ dendritic cells. Immunol Rev. 2010;234(1):268–281. doi: 10.1111/j.0105-2896.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- 35.Flacher V, et al. Expression of langerin/CD207 reveals dendritic cell heterogeneity between inbred mouse strains. Immunology. 2008;123(3):339–347. doi: 10.1111/j.1365-2567.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoitzner P, et al. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A. 2006;103(20):7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waithman J, et al. Skin-derived dendritic cells can mediate deletional tolerance of class I-restricted self-reactive T cells. J Immunol. 2007;179(7):4535–4541. doi: 10.4049/jimmunol.179.7.4535. [DOI] [PubMed] [Google Scholar]
- 38.Bursch LS, et al. Langerhans cells are not required for the CD8 T cell response to epidermal self-antigens. J Immunol. 2009;182(8):4657–4664. doi: 10.4049/jimmunol.0803656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols LA, et al. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179(2):993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- 40.Kim BS, et al. Keratinocytes function as accessory cells for presentation of endogenous antigen expressed in the epidermis. J Invest Dermatol. 2009;129(12):2805–2817. doi: 10.1038/jid.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, et al. Langerin Expressing Cells Promote Skin Immune Responses under Defined Conditions. J Immunol. 2008;180(7):4722–4727. doi: 10.4049/jimmunol.180.7.4722. [DOI] [PubMed] [Google Scholar]
- 42.Bedoui S, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10(5):488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 43.Gaspari AA, Katz SI. Contact hypersensitivity. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im0402s08. Chapter 4, Unit 4 2. [DOI] [PubMed] [Google Scholar]
- 44.Honda T, et al. Compensatory role of Langerhans cells and langerin-positive dermal dendritic cells in the sensitization phase of murine contact hypersensitivity. J Allergy Clin Immunol. 2010;125(5):1154–1156. doi: 10.1016/j.jaci.2009.12.005. e1152. [DOI] [PubMed] [Google Scholar]
- 45.Igyarto BZ, et al. Langerhans cells suppress contact hypersensitivity responses via cognate CD4 interaction and langerhans cell-derived IL-10. J Immunol. 2009;183(8):5085–5093. doi: 10.4049/jimmunol.0901884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang B, et al. Cytokine knockouts in contact hypersensitivity research. Cytokine Growth Factor Rev. 2003;14(5):381–389. doi: 10.1016/s1359-6101(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 47.Nakae S, et al. IL-1-induced tumor necrosis factor-alpha elicits inflammatory cell infiltration in the skin by inducing IFN-gamma-inducible protein 10 in the elicitation phase of the contact hypersensitivity response. Int Immunol. 2003;15(2):251–260. doi: 10.1093/intimm/dxg028. [DOI] [PubMed] [Google Scholar]
- 48.Shibaki A, Katz SI. Activation through CD40 ligation induces functional Fas ligand expression by Langerhans cells. Eur J Immunol. 2001;31(10):3006–3015. doi: 10.1002/1521-4141(2001010)31:10<3006::aid-immu3006>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 49.Bennett CL, et al. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179(10):6830–6835. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]
- 50.Nagao K, et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci U S A. 2009;106(9):3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klechevsky E, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29(3):497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding W, et al. Calcitonin gene-related peptide biases Langerhans cells toward Th2-type immunity. J Immunol. 2008;181(9):6020–6026. doi: 10.4049/jimmunol.181.9.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elentner A, et al. Langerhans cells are critical in the development of atopic dermatitis-like inflammation and symptoms in mice. J Cell Mol Med. 2009;13(8B):2658–2672. doi: 10.1111/j.1582-4934.2009.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


