Abstract
Cells of the mammary gland are in intimate contact with other cells and with the extracellular matrix (ECM), both of which provide not only a biochemical context, but a mechanical context as well. Cell-mediated contraction allows cells to sense the stiffness of their microenvironment, and respond with appropriate mechanosignaling events that regulate gene expression and differentiation. ECM composition and organization are tightly regulated throughout development of the mammary gland, resulting in corresponding regulation of the mechanical environment and proper tissue architecture. Mechanical regulation is also at play during breast carcinoma progression, as changes in ECM deposition, composition, and organization accompany breast carcinoma. These changes result in stiffer matrices that activate mechanosignaling pathways and thereby induce cell proliferation, facilitate local tumor cell invasion, and promote progression. Thus, understanding the role of forces in the mammary gland is crucial to understanding both normal developmental and pathological processes.
An increase in the stiffness of the extracellular matrix causes cells to activate mechanosignaling pathways that induce cell proliferation and promote local tumor cell invasion.
While it has long been appreciated that the biochemical environment provided by the extracellular matrix (ECM) is a key determinant of normal and pathological progression in the mammary gland, recently the realization that there is also a mechanical aspect to cell responses to the ECM has emerged. The signal-transduction response of cells to the physical aspects of their environment is termed “mechanosignaling,” and is the general subject of this article. It is anticipated that the mammary gland will prove to be a powerful developmental model to investigate mechanosignaling due to its postnatal development, which extends the time-course and provides a large tissue source for biochemical studies. However, ultimately, the power of this model lies in the fact that the normal mammary gland exists in many different developmental states, each with unique tissue tension requirements. For this review, we include studies from non-mammary cells to help inform what may be occurring in the context of the mammary gland, and try to indicate where information is specifically obtained in a mammary system.
The recognition that a cell is in a physical continuum with its ECM was discerned early on from images obtained by quick freeze, deep-etch electron microscopy, a rapid, chemical-fixation-free method that preserves native macromolecular structure with high fidelity. Using this approach to study cells within their tissue context, fine ECM fibers were found to radiate orthogonally from the plasma membrane surface and to exist as a continuum with the cytoplasmic cytoskeleton (Mecham and Heuser 1990; Singer 1979; Singer et al. 1984). The clear registry of cellular microtubules and actin cytoskeleton with extracellular matrix fibers in fibroblasts (Tomasek et al. 1982) and corneal epithelium (Sugrue and Hay 1981) led Dr. Elizabeth D. Hay to propose that the physical continuum between ECM and cytoskeletal organization reflects a functional continuum (Bissell 1981; Emerman et al. 1981; Hay 1981).
Independently, involvement of the cytoskeletal components in growth regulation (Teng et al. 1977), the connection between extracellular matrix (ECM) and gene expression in mammary epithelial cells (Emerman et al. 1981; Bissell 1981), and the importance of 3D context in functional differentiation (Hall et al. 1982; Hall and Bissell 1986) led Mina Bissell to state that the microenvironment regulates gene expression (Bissell 1981; Bissell et al. 1982). Subsequent studies evaluating human embryonic lung epithelial cells showed concomitant organization of fibronectin fibers secreted by and deposited beneath the cell and the microfilament bundles within the cell (Hynes and Yamada 1982) as well as collagen fibers with intermediate filaments (Hall and Bissell 1986). This non-random orientation of ECM fibers with respect to the cell surface is called anisotropy and leads to spatially oriented matrix networks that serve as adhesion sites, migration routes, as well as concentration gradients of fibrils that generate differential tension and distinct gene expression patterns.
The major structural protein in the mammary gland, and indeed in the entire body, is fibrillar collagen. In addition to providing a biochemical ligand for several receptors, collagen provides structural support for the gland, which is appreciated when one sees the relationship of collagen fibers to the epithelial cells (Fig. 1A). Fibrillar collagen is closely associated with the basal lamina, a highly organized and specialized ECM region that separates the epithelium from the less structured underlying collagen1-rich stromal compartment (Monaghan et al. 1983). The proteins comprising the basal lamina were classically identified as collagen IV, the laminins, entactin, and proteoglycans. While the mechanical properties of the basal lamina per se are currently not clear, no doubt several basal lamina proteins contribute to the mechanical properties of the ECM, and their roles are expected to emerge in coming years. In this article, we focus predominantly on the stromal ECM and the fibrillar collagens as the major structural proteins that affect the mechanical environment of mammary epithelial cells, as this is the aspect that is best understood.
Figure 1.
Mammary epithelial cells respond to the stiffness of a collagen matrix. (A) Scanning electron microscopy image of mammary acini showing individual mammary epithelial cells (M) surrounded by oriented collagen fibers (arrows). (SEM image courtesy of Paolo Provenzano.) Bar, 30 um. (B) Elastic modulus of collagen gels increases with increased collagen concentration. Modulus was measured by tension as described (Provenzano et al. 2009). Triangles represent data from Provenzano et al. (2009), squares from Roeder et al. (2002). Data was fitted and the resulting equation shown. (C) MCF10A cells cultured in a low-density (1.0 mg/ml) collagen gel form polarized acinar structures (a), while the same cells cultured in a high-density (2.0 mg/ml) collagen gel proliferate in a disorganized manner (c). Addition of HGF to low-density cultures results in tubule formation (b), while HGF added to cells in high-density culture results in an invasive phenotype (d). (Panels B and C reprinted from Provenzano et al. 2009 with permission from Nature Publishing Group © 2009.)
Cells adhere to the ECM through several different receptors, the most prominent of which belong to the integrin family. Several reviews of integrin structure and function exist, and the reader is referred there for more information (Liu et al. 2000; Hynes 2002; Katsumi et al. 2004). Beta-1 family integrins in particular are implicated in maintaining the normal phenotype of breast epithelial cells (Streuli et al. 1991), and contribute to the architecture and branching morphogenesis of the gland. Knockout of the β1 integrin subunit results in disrupted differentiation and alveologenesis, presumably through loss of critical adhesion sites (Li et al. 2005; Naylor et al. 2005). Moreover, knockout of the α2 subunit, a key receptor for collagens in the mammary gland, leads to a loss of branching complexity (Chen et al. 2002). While a classic biochemical receptor/ligand relationship between integrins and ECM molecules has been the model, emerging is the concept that receptors for ECM work at the interface of linking mechanical signals into biochemical signals in the cell.
The mechanical properties of the ECM are regulated by numerous growth factors acting on cells such as fibroblasts, which deposit stroma and arrange collagen. TGF-β1 is a primary activator of quiescent fibroblasts to highly contractile myofibroblasts involved in tissue fibrosis. Using culture conditions that permit tension modulation of the ECM, primary rat lung fibroblasts activated TGF-β1 stored in the ECM only under high tension conditions (Wipff et al. 2007). The authors propose that a requirement of high tissue tension for TGF-β activation restricts generation of myofibroblasts to a stiffened ECM, thus imposing a physical limitation to fibrosis. Relevance to the mammary gland is likely, as latent TGF-β stored within the mammary matrix, and known to be activated in response to irradiation-induced fibrosis (Barcellos-Hoff and Ravani 2000), is ovarian-hormone regulated.
Changes in the composition of the ECM during both mammary development and with tumor progression will affect the mechanical environment of the cells. Tumor progression in particular is characterized by increased ECM deposition, termed “desmoplasia.” Moreover, collagen V is increased in desmoplastic stroma (Barsky et al. 1982), which is mechanically significant as collagen V changes the structure of collagen I fibrils (Wenstrup et al. 2004; Berendsen et al. 2006; Breuls et al. 2009). In addition, several other proteins such as fibronectin and proteoglycans bind to collagens and affect the organization of collagen fibers, and thereby have effects on the mechanical milieu. The properties of the ECM are further changed by remodeling and the function of several matrix proteases, which will not be extensively covered in this article. The reader is referred to a couple of recent reviews on this topic (Page-McCaw et al. 2007; Rowe and Weiss 2008; Wolf and Friedl 2009). Finally, cross-linking of collagen fibers and collagen arrangement will also affect the mechanical properties of the ECM (Raub et al. 2007, 2008).
In addition to the ECM, several other aspects of mammary gland structure contribute to the mechanical environment. Normal mammary epithelial cells are polarized, and adhere in part via α6β4 integrin to the basement membrane at hemidesmosomes, which will have their own mechanical properties. In addition, tight junctions and adherens junctions between cells provide sites of attachment and mediate forces exerted between cells. This aspect of mechanosignaling is not well understood in the mammary gland, and will not be extensively covered here. The reader is referred to recent reviews on this subject regarding other cell types and developmental systems (Pokutta and Weis 2007; Wozniak and Chen 2009). The actin cytoskeleton, linked to force and contractility by interaction with myosin, links the adherens junctions with the integrin-mediated adhesions and hemidesmosomes, creating a potentially integrated tensile structure. Finally, myoepithelial cells, which tightly surround ductal epithelium and loosely encase the luminal epithelial cells, are specifically designed for contractility, and likely create mechanical events within the gland that differ between ductal and alveolar cells and with hormone status. Thus, every aspect of the mammary gland is a source of possible mechanical stimuli that can impact the behavior of mammary epithelial cells.
DYNAMIC NATURE OF TENSILE FORCES IN THE MAMMARY GLAND
Forces exerted on cells can occur in multiple dimensions: Tissues can be compressed or stretched in axial or multiaxial directions. The mammary gland is subject to several forces of both types in the course of daily activities such as walking, exercising, or physical work. Compression in the mammary gland is probably most relevant during lactation and tumor growth, where the cells may be pushing out on their local environment, as well as during mammography and other examinations where external pressure is applied. In order to sense a tensile mechanical environment, a cell needs to adhere, experience force exerted upon it, and meet that force with some level of resistance. It is proposed that forces within the cell reach a tensional balance with the stiffness outside the cell, a concept termed “tensional homeostasis” (Paszek et al. 2005), and thus understanding and measuring cellular forces and matrix stiffness has become an area of intense interest.
DEFINING MATRIX STIFFNESS
Each ECM will have material properties based on the structure and arrangement of its component parts. Collagen itself is a viscoelastic material, meaning that it exhibits behaviors of both viscous and elastic materials, and its deformation depends on the rate at which it is strained. The viscoelastic property is quantified by applying force as tension, shear, or compression, and measuring the relationship between stress and displacement in order to determine a property termed the elastic modulus. A full description is beyond the scope of this article, and the reader is referred to a few excellent texts on the subject (Fung 1993; Humphrey and Delang 2004; Janmey et al. 2007).
The tensional moduli of gels composed of predominantly collagen I have been reported (Roeder et al. 2002; Provenzano et al. 2009) and demonstrate that an increase in the concentration of collagen from 1.0 to 4.0 mg/ml results in a non-linear increase in the elastic modulus of the gel (Fig. 1B). Compression studies of collagen gels across the same concentration range give a similar finding: Modulus increases as collagen concentration increases (Paszek et al. 2005; Gehler et al. 2009). Note that the modulus measured by tensional forces results in a value in the kPa range, while modulus measured by compression is in the Pa range. Importantly, when actual mammary tissue, more complex because it contains heterogeneous stroma and cells, is measured by compression, there is an increase in the modulus for mammary tumors compared to normal tissue (Paszek et al. 2005). This makes sense, as mammary tumors can be found by their increased stiffness upon palpation. It is important to note here that the compression modulus of normal mammary tissue is similar to the compression modulus of lower concentration collagen gels, while the compression modulus of tumor tissue is similar to the compression modulus of higher concentration collagen gels (Paszek et al. 2005). Engineered environments differing in stiffness can also be created by coating flexible polyacrylamide gels with collagen, fibronectin, or other proteins. Further, by altering the proportion of bis-acrylamide, the modulus can be altered in precise ways (Wang et al. 2000).
Functionally, differences in matrix stiffness result in profound cellular responses. Mammary epithelial cells cultured in lower concentration 3D collagen gels organize into polarized acinar and ductal structures, while those in higher concentration collagen gels, which are stiffer, lose this polarization and instead become locally invasive (Fig. 1C). Cells cultured in stiffer matrices have an increase in cellular proliferation and changes in gene expression compared to cells in less stiff or compliant matrices. This finding is true in mammary epithelial cells (Wozniak et al. 2003; Paszek et al. 2005; Provenzano et al. 2009) as well as several other cell types (Chen et al. 1997; Wang et al. 2000; Discher et al. 2005). Moreover, stem cells differentiate in a manner that parallels the stiffness of their environment, such that stem cells cultured in a matrix with a stiffness similar to muscle become myogenic, while those in even stiffer matrices that mimic bone become osteogenic (Engler et al. 2006). This differentiation may be related to the Wnt pathway, as canonical Wnt signaling is enhanced when bone is mechanically loaded (Robinson et al. 2006). Moreover, cells migrate preferentially onto stiffer surfaces (Wang et al. 2001), a process fundamental to embryonic morphogenesis and termed “durotaxis.”
MEASUREMENTS OF CELL FORCE
Several means have emerged to measure cell-generated forces. Force applied to cells with “laser tweezers” to optically trap beads coated with fibronectin results in a strengthening of the integrin-cytoplasmic linkages (Choquet et al. 1997). By altering this force, the point at which the bond to the bead is broken occurs at 2 pN (picoNewtons), suggesting this is the force of the integrin-mediated link between the ECM and the cytoskeleton (Jiang et al. 2003). Because contraction of a collagen gel is a balance between the stiffness of the collagen gel and the force exerted by the cells therein, a change in collagen gel contraction suggests a change in the force exerted by the cells. Thus, another force measure can be implied by cellular displacement of collagen fibers (Miron-Mendoza et al. 2008). Using this approach, it was found that enhanced filamin binding to integrins results in an increased ability to contract a stiff collagen gel, and relates to increased contractility signaling (Gehler et al. 2009). When the elastic modulus of the environment is known, force can also be computed from the displacement of beads within that environment. Such measurements demonstrate that cellular forces are greatest and more dynamic at the leading edge of a migrating cell compared to the trailing edge (Pelham and Wang 1997, 1999). Cell-mediated forces also have been measured by placing cells on micro-patterned flexible posts that vary in geometry and stiffness, and measuring the displacement of those posts, which demonstrates that cells exert greater forces as they become more spread (Tan et al. 2003). Similarly, as cells crawl over a micromachined device, cell forces are demonstrated to move toward the center of the cell (Galbraith and Sheetz 1997).
The question of whether cell force exerted on matrix influences large-scale anisotropic patterning of ECM, such as that observed in the mammary gland, is of obvious interest. In vivo, individual mammary acini and ducts are frequently circumscribed with 10–100 µm or larger bands of organized collagen-rich fibrillar stroma. In vitro, force exertion experiments, such as those described above, may provide insight into this macro-scale patterning. Pellets of fibroblast cells on an isotropic collagen-1 gel and separated by distances of 1000 µm reorganize the collagen into bundles running parallel between the cell foci (Sawhney and Howard 2002, 2004). The initial rate of collagen traction was 0.28 µm/min with an estimated collagen translocation of ≤10 µm at the cell surface. Surprisingly, this localized cell surface force was sufficient to rearrange the collagen over the 1000 µm gap between cell pellets. Further, while the fibroblasts interacted with the collagen at their cell surface, the induction of the bands of collagen was transmitted simultaneously throughout the gel, rather than progressively from the cell surface as predicted if the gel was a collection of distinct fibers. Instead, these data indicate that the collagen gel has properties of an interconnected mesh, where tension exerted in one location is rapidly transmitted to distant sites due to its interconnected nature (Sawhney and Howard 2002, 2004).
Global self-organization of ECM by mammary epithelial cells has been observed in a 3D culture model designed to evaluate rapid effects of endogenous matrices on acinar organization in the absence of cell proliferation. Using Matrigel spiked with either FN or mammary matrix isolated from nulliparous rats, highly organized bands of matrix were found to extend 20–30 µm from the basal side of the acini, with further matrix organization persisting even deeper into the matrix pad (Fig. 2A) (Schedin et al. 2004, 2000b). Thus, organizing antistrophic ECM at the cell periphery and at a distance appears to be an intrinsic property of mammary epithelial cells, much like the properties of cell and acinar size regulation and polarity. Further, these observations indicate that tissue-extracted matrix, whether from EHS sarcoma or from rat mammary gland, retains viscoelastic and mechanosignaling properties and acts more like an interconnected mesh than as a collection of individual matrix components.
Figure 2.
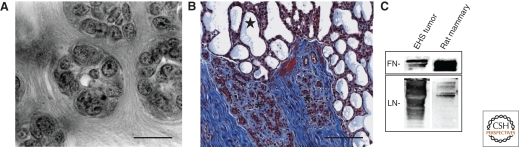
Anisotropic organization of matrix is an intrinsic property of MECs and is developmentally regulated. (A) Macro-patterning of anisotropic ECM by mammary epithelial cells in 3D culture. Bar, 15 um. (B) Collagen fibers in blue (Trichrome stain) show increased deposition in regressing mammary lobules (white asterisks) in comparison to lactating lobules (black star). Bar, 100 µm. (C) Comparison of fibronectin and laminin ratios between rat mammary ECM and EHS reconstituted basement membrane (Matrigel). Lane 1: 10.8 µg EHS tumor matrix. Lane 2: 10.8 µg Day 4 involution rat mammary matrix. Based on scanning densitometry, rat mammary matrix has ∼five-fold more FN per μg protein than EHS matrix, whereas EHS matrix has ∼10-fold more LN per μg protein than rat mammary matrix, with a FN/LN ratio of 50 or higher in rat mammary matrix compared to EHS matrix.
CONTRACTILE PATHWAYS GENERATE FORCE
In large part, cells sense the stiffness of their environment by pulling against the ECM using intracellular contractile mechanisms derived from actin-myosin interactions. Thus, in addition to the concept that external forces may be exerted upon cells, cells exert forces upon themselves when they contract against the ECM. In a compliant matrix, the cells will pull the components of that matrix in toward themselves, and when in a stiffer matrix, the cells will generate forces that result in focal adhesions and stress fiber formation (Grinnell 2000; Wozniak et al. 2003; Miron-Mendoza et al. 2008; Gehler et al. 2009).
Contractility is positively affected by phosphorylation of the myosin regulatory light chain (MLC). Both MLC-kinase and the Rho effector, Rho-Kinase (ROCK), can directly phosphorylate MLC (Amano et al. 1996). Also, ROCK can inhibit the phosphatase that acts on MLC, further enhancing phosphorylation of MLC (Kimura et al. 1996). Of these two pathways, the Rho-ROCK pathway is the one predominantly linked to mammary epithelial cell response to matrix stiffness (Wozniak et al. 2003; Paszek et al. 2005; Wyckoff et al. 2006). Rho activity is itself linked to matrix stiffness, as a compliant matrix leads to down-regulation of Rho-GTP (Wozniak et al. 2003). It appears that part of the mechanism by which Rho plays a role involves controlling the degree of cell spreading, as endothelial cells confined in their shape on a patterned 2D surface have increased proliferation only if allowed to spread (Chen et al. 1997). This effect involves a bi-modal regulation of focal adhesion kinase (FAK) function, such that phosphorylated FAK promotes proliferation, while unphosphorylated FAK in rounded cells prevents proliferation (Pirone et al. 2006). Moreover, cell shape regulates the differentiation of mesenchymal stem cells in a Rho-ROCK dependent manner (McBeath et al. 2004). The relationship of cell spreading to Rho is also bi-directional, as spread cells are able to activate the Rho-ROCK pathway (Bhadriraju et al. 2007).
FOCAL COMPLEXES AS MECHANICAL SENSORS
Mechanosignaling entails the conversion of a mechanical event, such as cellular deformation, into a biochemical event typically thought of in signal transduction, such as protein phosphorylation or generation of second messengers (Fig. 3). There is much speculation regarding the identity of putative mechano-responsive sensors within cells, but much more information is needed before understanding this process fully. It makes intuitive sense that mechanosensing proteins or cellular structures should have the property of being conformationally regulated when forces are exerted upon them and that these conformational changes should alter the signaling properties of the sensor, for example by regulating catalytic activity or by exposing binding sites for other molecules.
Figure 3.
Mechanical signaling pathways. (A) Mammary cells in a compliant matrix organize into polarized acinar and ductal structures. Local deposition and remodeling of extracellular matrices accompanies tumor progression (green = collagen fibers). (B) Diagram of signaling pathways related to focal contacts and cellular contractility. (T) talin, (V) vinculin. (C) Diagrams representing conformational changes in putative mechanosensors: filamin, talin, and integrins.
Integrin-mediated focal complexes as a unit could represent a mechanical sensor, as these structures are assembled in response to mechanical strain and substratum stiffness (Choquet et al. 1997; Pelham and Wang 1997; Katz et al. 2000) (for review, see Galbraith et al. 2002). While some have suggested that focal adhesions do not exist in three-dimensional matrices (Fraley et al. 2010), it should be noted that this observation was made in the context of a compliant 3D matrix. In stiff matrices, specialized focal adhesions termed “3D matrix adhesions” (Cuikerman et al. 2001) have been noted (Wozniak et al. 2003; Pasek et al. 2005; Provenzano et al. 2009). Integrins are proposed to themselves represent mechano-sensors (Katsumi et al. 2004, 2005), as they have profound conformational changes upon ligand binding. Moreover, integrin-cytoskeletal linkages are enhanced when force is exerted on integrins, suggesting that binding sites within the complex are exposed under strain (Choquet et al. 1997). Many of the molecules resident in focal complexes are implicated in mechanosensing, including talin (Giannone et al. 2003; Jiang et al. 2003), vinculin, FAK, Src, and p130Cas (Felsenfeld et al. 1999; Wang et al. 2001; Li et al. 2002; Frame 2004; Kostic and Sheetz 2006; Sawada et al. 2006), and indeed the proteins resident in focal adhesions may function cooperatively as a mechanosensing complex.
Talin in particular is a candidate mechano-sensor in focal complexes. Talin is uniquely involved in direct activation of integrin function, as talin binding to integrin β cytoplasmic tails induces conformational changes that propagate across the membrane and enhance ligand binding (Critchley 2000; Calderwood and Ginsberg 2003; Garacia-Alvarez et al. 2003; Calderwood et al. 2004; Wegener et al. 2007; Himmel et al. 2009). Talin binding to integrins involves both the N-terminal FERM domain (Calderwood et al. 1999; Wegener et al. 2007) as well as a C-terminal binding site in the rod domain (Gingras et al. 2009). The N- and C-terminal sites work in a potentially cooperative manner, as talin is auto-inhibitory when not bound to integrins (Goksoy et al. 2008). Calpain cleavage can release some of this auto-inhibition (Yan et al. 2001). As well, there is likely a mechanical component to talin function. Force applied to the talin rod domain stretches a series of helical bundles in the rod domain, opening up not only the cryptic C-terminal integrin binding site, but also binding sites for vinculin and actin (Hytonen and Vogel 2008; del Rio et al. 2009). Thus, integrin activation by talin is likely mechanically regulated and indeed is necessary for vinculin recruitment and cytoskeletal strengthening when force is applied to integrins (Giannone et al. 2003). Vinculin, too, is conformationally regulated such that it is active at focal adhesions (Chen et al. 2005).
The scaffolding molecule, p130Cas, has recently emerged as a putative mechanosensor. p130Cas contains a highly phosphorylated substrate domain in the middle of the molecule that is phosphorylated predominantly by Src, and may be the means by which Src participates in mechano-signaling (Honda et al. 1999; Sawada et al. 2006). Stretching of p130Cas exposes this domain, and results in increased phosphorylation through the Src-family kinase, fyn (Kostic and Sheetz 2006; Sawada et al. 2006). Once phosphorylated, p130Cas scaffolds several molecules, and results in signaling through myosin light chain to promote cellular contractility (Cheresh et al. 1999), as well as activation of Dock180 to promote Rac activation (Kiyokawa et al. 1998). Importantly, exposure of the p130Cas substrate domain occurs at protrusive edges of spreading cells (Sawada et al. 2006), suggesting spatial regulation that may allow p130Cas to translate mechanical cues into migration, and may explain part of the mechanism underlying durotaxis.
Filamin is a large scaffolding molecule that has several folded domains, and is hypothesized to unfold upon mechanical forces placed on the molecule, exposing several binding sites for signaling molecules such as Rho, ROCK, PAK, and PKC (Stossel et al. 2001; Vadlamudi et al. 2002; reviewed in Feng and Walsh 2004). Filamins are poised to mediate mechanical cues from the ECM, as they bind directly on one end to integrins and on the other to the actin cytoskeleton. Importantly, filamin-integrin linkages regulate mechanical responses such as cell migration and collagen gel contraction, and may serve to “tune” the response of mammary epithelial cells to matrix stiffness (Calderwood et al. 2001; Gehler et al. 2009). Moreover, filamin alters the mechanical properties of actin networks (DiDonna and Levine 2006; Gardel et al. 2006).
FAK is also implicated in mechanosensing. In particular, phosphorylation of FAK at Y397 is up-regulated when cells are on stiff matrices compared to compliant matrices (Yano et al. 1996; Cukierman et al. 2001; Wozniak et al. 2003). Cells devoid of FAK lose the ability to migrate in response to mechanical cues (Wang et al. 2001; Li et al. 2002). FAK is also recruited to focal adhesions in cells undergoing mechanical strain within stiff 3D matrices (Wozniak et al. 2000; Provenzano et al. 2009), where it is necessary for subsequent mechanically responsive signaling (Schober et al. 2007). Src is intimately linked to FAK function, and has also emerged as a molecule that is both responsive to and necessary for cellular responses to mechanical cues (Felsenfeld et al. 1999; Frame 2004). Src and focal adhesion regulation are implicated in cellular responses to osmotic forces (Volonte et al. 2001). Src activation has been used as an in situ mechano-sensitive reporter, with data suggesting that mechanical signaling to Src propogates through the cell via both actin filaments and microtubules (Wang et al. 2005).
Integrating these various signaling pathways (Fig. 3), the model that emerges is one in which cells pull against the ECM using Rho-ROCK-mediated contraction of the actin cytoskeleton. If the ECM around the cell is stiff and the cell meets with resistance, the forces generated result in tension across the adhesion receptors and focal complexes. This in turn results in adhesion strengthening, recruitment of signaling molecules into the focal complexes, and focal adhesion signaling. Moreover, a stiff matrix provides enough traction that the cell is able to spread and proliferate, or to migrate. If the matrix is compliant, the cells will instead deform the matrix and round up, resulting in different signaling and phenotypic outcomes.
MECHANICAL SIGNALING REGULATES PROLIFERATION AND DIFFERENTIATION
Signaling events related to mechanical stimuli clearly have effects on gene expression downstream from these signals. A predominant outcome found in cells of all types when they are in rigid matrices or allowed to spread is cellular proliferation (Chen et al. 1997; Fringer and Grinnell 2001; Thery et al. 2005). Specifically, mammary cells in a stiff matrix are more proliferative (Wozniak et al. 2003; Paszek et al. 2005) and up-regulate proliferative signals including cyclin D1 (Klein et al. 2009) as well as an entire set of genes identified as a proliferation signature in human breast carcinoma (Provenzano et al. 2009). The pathway leading to proliferation is linked to function of MEK/ERK, as inhibition of this pathway reverses expression of the proliferation signature induced by rigid substratum (Assoian and Klein 2008; Provenzano et al. 2009). Involvement of the MEK/ERK pathway also mediates the proliferation of fibroblasts cultured in a stiff matrix (Fringer and Grinnell 2001, 2003).
In contrast to proliferation, mammary cells within compliant matrices demonstrate growth control, organization of glandular architecture, and express proteins that are consistent with a more “differentiated” phenotype (Emerman and Pitelka 1977; Streuli et al. 1995; Wozniak et al. 2003; Paszek et al. 2005; Provenzano et al. 2009). For example, the expression of β-casein, a marker of breast epithelial differentiation, is coordinately regulated by both adhesion to laminin and a compliant matrix (Alcaraz et al. 2008). The polarization of the epithelial cell is thought to drive these events, with compliant matrices supporting and stiff matrices abolishing polarity. During lactation, the mammary epithelial cells is at its most polarized state, as tight junction complexes close with lactation, absolutely restricting transport between the basolateral and luminal compartments of the gland (Neville 2009). Proper lactogenesis and signaling involves contact with the BM, as well as the down-regulation of Rho activity (Lee et al. 2009). The role of integrins in this regulation is demonstrated by the finding that inhibition of β1 integrin adhesion to the ECM that mimics a more compliant environment reverts the disorganized phenotype of mammary carcinoma cells, resulting in restoration of acinar structure (Weaver et al. 1997). Conversely, matrix cross-linking, which is associated with and enhances tumor progression, acts to stimulate integrin signaling (Levental et al. 2009). Compliance works in concert with growth factor pathways to regulate cellular proliferation and phenotype. For example, when the ErbB2 receptor is driven to dimerize in cells within compliant rBM, otherwise normal MECs lose growth control and proliferate into the lumen of ascini (Muthuswamy et al. 2001). This result demonstrates that compliance can be “overruled” by strong mitogenic signals, but intriguingly raise the possibility that compliance may also normally down-regulate these pathways.
Given that apical/basal polarity defines epithelium, the role of cell adhesion in regulating polarity has been investigated in retinal pigment epithelial cells using defined micro-patterned ECM substrates that force single cells to have patterned areas of adhesion and non-adhesion. In this study, geometry of the ECM determined positioning of the nucleus-centrosome complex, with orientations reproducibly directed toward cell adhesive edges. These data add the property of ECM geometry, in concert with composition and mechanical properties, in regulating gene expression patterns critical for cell fate decisions (Thery et al. 2006).
CHANGES IN MAMMARY ECM DURING NORMAL DEVELOPMENT—FOCUS ON COLLAGEN AND FIBRONECTIN
While the mammary anlagen is formed embryologically, the majority of ductal development occurs with onset of sexual maturation and alveolar development with pregnancy. The mammary gland is also unique in that terminal differentiation does not occur unless lactation ensues, and further, with each estrous cycle, the “immature” gland undergoes a cyclic expansion followed by a modest regression phase (Schedin et al. 2000a; Fata et al. 2001). Consequently, the normal adult mammary gland exists in many different developmental states that vary with time.
Based on pioneering work from Mina Bissell's laboratory showing mammary epithelial cells differentiate to a milk-secreting phenotype when cultured on floating collagen pads (Lee et al. 1984) or embedded in thick pads of reconstituted basement membrane proteins (Matrigel) (Li et al. 1987), the functional unit of the mammary gland was defined as the epithelial cell plus its ECM (Bissell and Barcellos-Hoff 1987). A corollary to this hypothesis is the ECM tensional requirements of the gland would change to accommodate the distinct demands required of the nulliparous, pregnant, lactating, or involuting mammary epithelium. Indeed, the microenvironment of the mammary gland is highly dynamic as each developmental state has a unique ECM protein signature, and further, the responsiveness of the epithelium to systemic hormones is facilitated through concomitant modification of the ECM (Warburton et al. 1982; Silberstein and Daniel 1984; Schedin et al. 2004).
A role for fibrillar collagen and thus tissue tension in the development of the rudimentary mammary anlagen to the fully elaborated ductal tree was observed early on. Terminal end buds (TEBs) are transient, mitotically active, and motile structures that drive ductal elongation and penetration through the mammary fat pad. TEBS are characterized by a basement membrane devoid of collagen-1 bundles. Collagen deposition occurs along the flank of the nascent ducts, where epithelial cell proliferation is inhibited (Silberstein and Daniel 1982). By implanting a slow release pellet containing exogenous TGF-β at the tip of the growing ductal tree, ductal growth was rapidly inhibited concurrent with thick fibrillar collagen deposition around the TEBs (Silberstein and Daniel 1987). However, not only the presence, but also the orientation of collagen is important for proper mammary gland development. Aligned collagen fibers are found radiating from the terminal end bud (TEB) of the developing mammary gland just prior to the invasion of the cells into the mammary fat pad (Ingman et al. 2006). That these fibers might assist morphogenesis of the mammary gland is suggested by the finding that branching morphogenesis follows patterns of ECM topography in engineered matrices (Nelson et al. 2006).
To date, a relatively limited number of mammary ECM proteins have been quantitatively evaluated across gland development. Nonetheless, evidence that developmentally regulated changes in mammary ECM composition result in distinct viscoelastic and mechanical properties is compelling. Gene expression of numerous collagens are differentially regulated by reproductive state, including fibrillar collagens type I, III, and V, bead-filament collagen VI, FACIT family member collagen IX, and basal lamina collagen IV (Schedin et al. 2007). Collagen-associated proteins known to influence cross linking are similarly regulated including elastin, fibrillin 1, decorin, lumican, and biglycan (Schedin et al. 2007). Recently, fibrillar collagen deposition has been quantitated in the rat mammary gland across the pregnancy, lactation, and involution cycle, and the ratio of collagen to epithelial cells differs by an order of magnitude depending on developmental state (O'Brien et al. 2010). The lactating gland has very few fibrils of collagen between individual acini, an observation consistent with previous studies, demonstrating that a compliant ECM is required for MEC differentiation in vitro. Following weaning, high levels of fibrillar collagen are deposited between involuting ascini. This increase has been observed in mouse, rat and human, suggesting the associated tensional changes are required for the massive remodeling that occurs with involution (Fig. 2B). In addition, numerous ECM proteins including LN5 (Giannelli et al. 1997), entactin (Alexander et al. 1996), FN (Schedin et al. 2000b), and collagen 1 (O'Brien et al. 2010) are targeted for partial proteolysis during involution, which alters the viscoelastic properties of the matrix significantly, as well as releasing cryptic ECM fragments capable of engaging additional integrin and non-integrin signaling pathways (Werb et al. 1989; Schenk and Quaranta 2003; Mott and Werb 2004). In conclusion, when considering the single ECM protein collagen 1 and the myriad of regulatory controls such as expression, cross linking, proteolysis, and cellular engagement, the potential for fine tuning mechanosignaling in the mammary gland is evident and likely integral to tissue function.
FN IS UNIQUELY POISED TO REGULATE MAMMARY MECHANICAL PROPERTIES
FN is found in abundant quantities within the normal rat mammary gland intra- and inter-lobular stroma (Schedin et al. 2004). By Western blot analysis, the relative abundance of FN in mammary ECM has been compared to reconstituted basement membrane preparations derived from EHS tumors (Matrigel), and mammary stroma has 50–100 fold higher levels of FN than Matrigel (Fig. 2C) (Schedin et al. 2004). In what was somewhat of a surprise, given that FN is not classically considered a basal lamina protein, FN is also found in the highly organized and specialized mammary basal lamina (Monaghan et al. 1983), implying that FN can interact directly with α5β1-integrin expressing mammary epithelial cells. Like mammary epithelial cells themselves, FN and α5β1-integrin expression are under endocrine control, with expression up-regulated during the pubertal and pregnancy windows of gland expansion (Haslam and Woodward 2001; Woodward et al. 2001), and precipitously down-regulated at late pregnancy after completion of the proliferative phase, and with full differentiation of the lactating gland (Schedin et al. 2004). This is in contrast to mammary LN mRNA, which appears to be constitutively expressed across the pregnancy, lactation, and involution cycle (Woodward et al. 2001; Schedin et al. 2004). The in vivo FN studies are supported by in vitro experiments where intact FN induced proliferation, increased acinar size in 3D culture, and stimulated proliferation of growth-arrested mammary epithelial cells (Barkan et al. 2008; Williams et al. 2008).
Results from embryonic lethal FN knockout mice demonstrating a requirement for anisotropic FN fibrils in embryonic cell migration has peaked interest in the potential role of FN in mechanosignaling (Yang et al. 1999). Recently, force generation has been identified as the mechanism by which FN is required for branching morphogenesis in the salivary gland (Larsen et al. 2006). In an elegant series of experiments, FN was observed to translocate directionally by addition of new FN to the ends of older FN fibrils. Old FN fibril accumulated at the sites of cleft formation in a pattern consistent with FN, driving a physical wedge between cells at the cleft, resulting in bifurcation.
Numerous physical attributes enable fibrillar FN to contribute to tensional status of the microenvironment. FN is a large approximately 440,000 kD glycoprotein consisting of two similar 220,000 kD subunits bridged by a disulfide bond. FN has a modular structure and interacts directly with cells through two heparin-binding domains and two distinct RGD containing sites. Assembly of secreted FN into fibrils appears to be nucleated through α5β1 integrin binding, as α5β1 blocking antibodies inhibit FN matrix formation in vitro (Fogerty et al. 1990; Mao and Schwarzbauer 2005). It is thought that the requirement for α5β1 permits precise tempo-spatial integration between location of FN matrix assembly, local tissue tension, and specific requirements of the cell or tissue. FN fibrils also incorporate directly into the ECM due to specific heparin, fibrin I, fibrin II, and multiple collagen binding domains. In fact, while fibrillar collagen formation in vitro is driven by self assembly thermodynamics, in vivo fibrillar collagen formation has been demonstrated to be FN-dependent due to a required conformational change in FN (Kadler et al. 2008). This conformational change is likely induced by contractile forces exerted on FN by cells, as FN assembly and thus subsequent collagen assembly is dependent on Rho-mediated contractility (Zhang et al. 1997). Evidence from biochemical studies shows that FN directly modifies mechanical and structural properties of collagen fibers. The addition of FN to type I collagen prior to collagen fibrillogenesis increases the percentage of linear fibers and reduces the number of collagen cross-links, suggesting that FN shifts the balance towards linear collagen growth (Guarnieri et al. 2007). This shift correlates with a reduction in elasticity at high FN concentrations (Guarnieri et al. 2007). Recent data from Viola Vogel's laboratory has shown that FN fibers display extraordinary extensibility, which is reversible (Klotzsch et al. 2009). Further, they demonstrate that FN extension increases matrix rigidity and cryptic epitope exposure (Klotzsch et al. 2009), directly implicating FN in mechanotransduction processes (Smith et al. 2007). Finally, bioactivity of FN fragments was initially demonstrated in rabbit synovial fibroblasts, where exposure to FN fragments, but not intact FN, induced collagenase and stromelysin gene expression (Werb et al. 1989). FN fragments were subsequently demonstrated to up-regulate MMP2 activity in human breast cancer cell lines as well as induce cell motility and invasion (Schedin et al. 2000b). More recently, the ability of FN fragments and a splice variant named migration-stimulating factor (MSF) to stimulate fibroblast migration has been mapped to specific sites (Schor et al. 2003). Further, FN domains that mask the motogenic site when FN is intact have been identified (Ellis et al. 2010). Questions such as how matrix tension influences protease access to cryptic cleavage sites, and how specific FN fragments, in turn, alter matrix tension remain intriguing and to date, unanswered.
In summary, in the mammary gland, FN fibers present at the epithelial cell membrane and within the intra and inter-lobular stromal compartment can exert influence on micropatterning at the cellular scale, such as actin cytoskeleton organization, cell polarity, signal transduction, and altered gene expression, as well as macropatterning at the tissue level, including matrix rigidity, collagen fiber density, orientation, and gradient formation. Further studies to understand the relationships between FN fibril thickness, length, orientation, partial proteolysis, and mechanical strength are clearly required to more fully understand its importance in mammary epithelial cell function.
CANCER IMPLICATIONS FOR HORMONAL CONTROL OF MATRIX ASSEMBLY
The endocrine control of FN expression and subsequent hormone-dependent matrix assembly observed in the normal mammary gland and described above has been documented in breast cancer cells in vitro (Quinn et al. 2009). Additional mechanisms for ECM regulation of estrogen signaling in breast cancer have been reported as well. MCF-7 cells cultured on stiff collagen 1 matrix responded to estrogen stimulation with up-regulation of the Rac1/JNK/c-Jun pathway, cyclin D1 expression, and proliferation, whereas this response was significantly dampened in cells cultured on compliant LN (Xie and Haslam 2008). ECM regulation of ER expression has also been reported, with ERβ expressed when MDA-MB-231 cells are cultured on rigid plastic, and expression lost when cultured on basal lamina proteins collagen IV and Laminin-111 (Neubauer et al. 2009). While these studies clearly demonstrate ECM control over endocrine responsiveness in both normal and transformed mammary epithelial cells, the specific role mechanosignaling plays remains to be confirmed. Nonetheless, a recurrent theme is that substrata with higher tension correlate with increased ER signaling, even in the absence of ligand. The implications for collagen deposition as one primary determinant of increased breast cancer risk associated with high mammographic density are clear, especially since mammographic density is hormonally responsive (Boyd et al. 2009).
ECM DENSITY AND ALIGNMENT IN TUMOR PROGRESSION
Mammographic density is associated with a 4–6 fold increased relative risk of developing breast carcinoma and is largely associated with an increase in stromal collagen (Boyd et al. 2001; Guo et al. 2001). While the underlying mechanism for the risk factor is not entirely known, it was possible that density could be merely a co-associated risk factor, but itself had no contribution to carcinoma. However, recent studies suggest that density per se contributes to carcinoma progression, as tumor formation and metastasis is enhanced in the background of a mouse mammary gland enriched in collagen because the collagen is protease resistant (Provenzano et al. 2008b). A collagen-rich ECM is mechanically stiffer (Paszek et al. 2005; Provenzano et al. 2009), and this will promote the pro-tumorigenic changes in signaling and gene expression discussed above. While it is not currently known whether ECM stiffness is an initiating event in mammary carcinoma, this possibility is consistent with the fact that normal MECs cultured on stiff substratum activate proliferation genes and oncogenic signaling pathways such as ERK (Paszek et al. 2005; Provenzano et al. 2009).
During breast carcinoma progression, additional ECM deposition, or desmoplasia, occurs and is associated with a poorer predicted outcome (Walker 2001). In a study of triple-negative breast carcinomas, it was found that fibrosis was associated with distant metastasis (Kreike et al. 2007). Not only does the total amount of ECM components increase, but the anisotropy of the ECM also increases. Collagen fibers thicken and straighten during tumor progression, and ultimately align perpendicular to the tumor boundary concordant with tumor invasion (Fig. 4) (Provenzano et al. 2006). These changes, termed “Tumor Associated Collagen Signatures” (TACS) manifest in specific ways during mammary tumor progression in mice (Provenzano et al. 2006). Changes in collagen fiber organization may be due in part to FN as described above or to an increase in the intermolecular cross-links between collagen fibers. Lysyl oxidase, which catalyzes collagen cross-links, is increased during tumor progression (Peyrol et al. 1997; Decitre et al. 1998; Kirschmann et al. 1999). Cross-linking is expected to make the ECM stiffer, and therefore would have consequences for mechanosignaling.
Figure 4.
Collagen alignment facilitates invasion. (A) Parallel alignment of collagen fibers (arrows) around a non-invasive tumor (T), imaged in a live mammary tumor ex vivo by multiphoton laser scanning microscopy and second harmonic generation (SHG) to image collagen. (B) Diagram of TACS-2 (example in A), in which collagen fibers are denoted by tan-colored lines. (C) Perpendicular alignment of collagen fibers (arrows) at a tumor (T) boundary, which is depicted by the red line. (Panel reprinted from Provenzano et al. 2006 with permission from BioMed Central Ltd. © 2006.) Live tumor was imaged as in A. (D) Diagram of TACS-3 (example in C), with cells invading out from the tumor along aligned collagen fibers. (E) Human T47D breast carcinoma cells (yellow) aligns relative to collagen fibers (green) within an engineered collagen matrix. The orientation of collagen fibers is depicted by the arrow. Live cell in matrix was imaged as in A.
Studies in vitro help to suggest mechanisms by which collagen might become aligned in vivo. Collagen fibers are aligned when subjected to externally applied mechanical load (Vader et al. 2009). The load placed on collagen fibers by cellular contractility can also align the collagen in a Rho-ROCK-myosin-dependent manner (Provenzano et al. 2009). This result suggests that tumor cells themselves may help set up the matrix alignment associated with cellular invasion. Alignment of the ECM may occur during the process of cell invasion, as mammary carcinoma cells invading through 3D collagen matrices can simultaneously move and align collagen fibers (Wolf et al. 2007).
Carcinoma-associated fibroblasts (CAFs) also contribute to the ECM changes that accompany tumor progression. CAFs differ from normal mammary fibroblasts in their ability to promote cellular proliferation and tumor progression (Olumi et al. 1999; Orimo et al. 2005; Orimo and Weinberg 2006). In addition to the mechanisms discussed above for α5β1 integrin-mediated FN deposition, there is also a role for the cell-surface proteoglycan, syndecan-1 (Sdc-1), which is expressed on CAFs but not on normal mammary fibroblasts (Su et al. 2007). Sdc-1 expressing fibroblasts promote tumor progression in vivo, and lead to a desmoplastic stroma (Maeda et al. 2006). The signal that induces CAFs to express Sdc-1 in mammary stroma is not yet known, but may be a response of fibroblasts to matrix density or early desmoplasia, as mechanical strain induces Sdc-1 expression in smooth muscle cells (Julien et al. 2007). Since FN can scaffold and direct collagen fiber assembly (Kadler et al. 2008), it is likely that the aligned matrix observed near tumors is due, at least in part, to the role of CAFs. Physiologically regulated changes in FN fibrillogenesis, as discussed above, may inadvertently provide signals for Sdc-I expression and anisotropic FN assembly by CAFs. Relevance to cancer progression is suggested, as FN is a classic marker of epithelial to mesenchymal transition (EMT) as well as mammary cancer initiating cells (Mani et al. 2008).
CELL INVASION AND MECHANOSIGNALING
Aligned matrices found near tumors are not only a sign of a reactive stroma, but likely act to facilitate tumor cell invasion. Carcinoma cells preferentially invade along aligned fibers, versus randomly organized fibers (Provenzano et al. 2008c). Elegant intra-vital imaging demonstrates that mammary carcinoma cells traverse along radial collagen fibers in vivo (Wyckoff et al. 2004). It is intriguing that tumor cells may have co-opted the normal process of TEB invasion that occurs along aligned fibers in the developing mammary gland (see above) (Ingman et al. 2006). In addition to carcinoma cells, fibroblasts and myeloid cells also demonstrate increased migration proximal to the tumor (Egeblad et al. 2008), suggesting that the local increase in ECM deposition and alignment may serve to recruit additional stromal cells. This is significant for metastasis, as macrophages promote the local invasion of tumor cells and their extravasation into blood vessels (Goswami et al. 2005). Moreover, the production of an aligned matrix may be facilitated by macrophages (Ingman et al. 2006). Among the many areas that are currently understudied, the question of how collagen fibers are oriented with respect to the involuting mammary epithelium is of intense interest, given the relationships between collagen fiber orientation (O'Brien et al. 2010), cancer metastasis, and the poor prognosis of breast cancers diagnosed in the post-partum window (Lyons et al. 2009; O'Brien et al. 2010). Further research separating roles of collagen deposition from alignment is highlighted by recent evidence showing that collagen levels increase in mammary glands of rats treated with doses of tamoxifen that prevent tumor progression, in part due to decreased MMP activity (Hattar et al. 2009).
The precise mechanisms by which cells recognize and migrate along aligned ECMs are not fully known. In vitro, cells preferentially migrate along stiffer substrata in a manner dependent on mechanical signaling through FAK, as FAK-depleted cells lose their preference for stiff substrata and will migrate as well on compliant surfaces (Wang et al. 2001). A role for FAK in tumor cell invasion in vivo was recently demonstrated by four independent groups (Lahlou et al. 2007; Provenzano et al. 2008a; Pylayeva et al. 2009). Tumors can arise in the FAK-/- mouse mammary epithelium, but are largely non-invasive hyperplasias (Lahlou et al. 2007; Provenzano et al. 2008a). Interestingly, this points to a specific role for FAK in the epithelium, as these animals have normal FAK expression in the stromal compartment (Provenzano et al. 2008a).
Recent findings suggest that specific cytoskeletal regulators, including Arp2/3, cofilin, and vinculin, are associated with an invasive phenotype (Wang et al. 2004). An intriguing possibility is that expression of these molecules in invasive tumors reflects a role in sensing ECM stiffness or alignment. A recent exciting finding is the identification of splice variants of the actin-organizing protein, Mena, that are specifically up-regulated in breast carcinoma cells and that increase invasion in 3D matrices (Philippar et al. 2008; Goswami et al. 2009). Mena serves to alter the branching of the actin cytoskeleton, and regulates migration of several cell types (Bear et al. 2001). Mena is also implicated in regulation of adherens junctions (Vasioukhin and Fuchs 2001), suggesting an important role in epithelial polarity as well. Moreover, the expression of several cytoskeletal-regulating proteins is altered in non-invasive FAK-/- mammary carcinoma cells (Provenzano et al. 2008a). Because of the role of actin-myosin contractility in sensing matrix stiffness, molecules that regulate the cytoskeleton may contribute to invasion along aligned matrices.
SUMMARY
While it is understood that the extracellular microenvironment around normal tissues affects cellular behavior, more recently it has become clear that the ECM serves not only scaffolding and biochemical functions, but also affects the mechanical environment. The demonstration that cells within the mammary gland respond to changes in the stiffness of their environment points to an important role for cellular force and mechanosignaling events in the normal development and differentiation of the gland at puberty, pregnancy, lactation, and involution. The complexity and potential for fine-tuning of tissue tension is highlighted by the distinct yet interconnected roles of ECM deposition, alignment, cross-linking, and partial proteolysis. Moreover, force also plays an important role in tumor progression, as stiff matrices are associated with increased risk of breast carcinoma, and promote cellular proliferation as well as progression. In addition to a general increase in the deposition of ECM components with cancer progression, collagen and fibronectin are deposited as aligned matrices with various levels of cross-linking and proteolysis that provide preferred surfaces on which carcinoma cells invade, thus promoting breast cancer metastasis. As our understanding of mechanical signaling events evolves, we may find novel means by which to predict outcome and hopefully target breast carcinoma progression.
ACKNOWLEDGMENTS
The authors would like to thank members of their laboratories, Ori Maller, Traci Lyons, Paolo Provenzano, Steve Trier, and Kristin Riching for providing data presented in Figures 1, 2, and 4, and for critical review of the manuscript. Work pertinent to this article has been funded by grants to P.J.K. (American Cancer Society RPG-00-339; Susan G Komen Foundation, and DOD Postdoctoral Fellowship W81XWH-04-1-042 to P. Provenzano) and to P.S. (DOD Synergistic Idea Award #BC060531 and Susan G Komen, Avon and Mary Kay Ash Foundations).
Footnotes
Editors: Mina J. Bissell, Kornelia Polyak, and Jeffrey Rosen
Additional Perspectives on The Mammary Gland as an Experimental Model available at www.cshperspectives.org
REFERENCES
- Alcaraz J, Xu R, Mori H, Nelson CM, Mroue R, Spencer VA, Brownfield D, Radisky DC, Bustamante C, Bissell MJ 2008. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. Embo J 27: 2829–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander CM, Howard EW, Bissell MJ, Werb Z 1996. Rescue of mammary epithelial cell apoptosis and entactin degradation by a tissue inhibitor of metalloproteinases-1 transgene. J Cell Biol 135: 1669–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246–20249 [DOI] [PubMed] [Google Scholar]
- Assoian RK, Klein EA 2008. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol 18: 347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Ravani SA 2000. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res 60: 1254–1260 [PubMed] [Google Scholar]
- Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, Liu ZY, Costes SV, Cho EH, Lockett S, et al. 2008. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res 68: 6241–6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsky SH, Rao CN, Grotendorst GR, Liotta LA 1982. Increased content of Type V Collagen in desmoplasia of human breast carcinoma. Am J Pathol 108: 276–283 [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Krause M, Gertler FB 2001. Regulating cellular actin assembly. Curr Opin Cell Biol 13: 158–166 [DOI] [PubMed] [Google Scholar]
- Berendsen AD, Bronckers AL, Smit TH, Walboomers XF, Everts V 2006. Collagen type V enhances matrix contraction by human periodontal ligament fibroblasts seeded in three-dimensional collagen gels. Matrix Biol 25: 515–522 [DOI] [PubMed] [Google Scholar]
- Bhadriraju K, Yang M, Alom Ruiz S, Pirone D, Tan J, Chen CS 2007. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp Cell Res 313: 3616–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ 1981. The differentiated state of normal and malignant cells or how to define a ‘normal’ cell in culture. Int Rev Cytol 70: 27–100 [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Barcellos-Hoff MH 1987. The influence of extracellular matrix on gene expression: Is structure the message? J Cell Sci Suppl 8: 327–343 [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG, Parry G 1982. How does the extracellular matrix direct gene expression? J Theor Biol 99: 31–68 [DOI] [PubMed] [Google Scholar]
- Boyd NF, Martin LJ, Rommens JM, Paterson AD, Minkin S, Yaffe MJ, Stone J, Hopper JL 2009. Mammographic density: A heritable risk factor for breast cancer. Methods Mol Biol 472: 343–360 [DOI] [PubMed] [Google Scholar]
- Boyd NF, Martin LJ, Stone J, Greenberg C, Minkin S, Yaffe MJ 2001. Mammographic densities as a marker of human breast cancer risk and their use in chemoprevention. Curr Oncol Rep 3: 314–321 [DOI] [PubMed] [Google Scholar]
- Breuls RG, Klumpers DD, Everts V, Smit TH 2009. Collagen type V modulates fibroblast behavior dependent on substrate stiffness. Biochem Biophys Res Commun 380: 425–429 [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Ginsberg MH 2003. Talin forges the links between integrins and actin. Nat Cell Biol 5: 694–697 [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Huttenlocher A, Kiosses WB, Rose DM, Woodside DG, Schwartz MA, Ginsberg MH 2001. Increased filamin binding to β-integrin cytoplasmic domains inhibits cell migration. Nat Cell Biol 3: 1060–1068 [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Tai V, Di Paolo G, De Camilli P, Ginsberg MH 2004. Competition for talin results in trans-dominant inhibition of integrin activation. J Biol Chem 279: 28889–28895 [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Zent R, Grant R, Rees DJG, Hynes RO, Ginsberg MH 1999. The talin head domain binds to integrin β subunit cytoplasmic tails and regulates integrin activation. J Biol Chem 274: 28071–28074 [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE 1997. Geometric control of cell life and death. Science 276: 1425–1428 [DOI] [PubMed] [Google Scholar]
- Chen H, Cohen DM, Choudhury DM, Kioka N, Craig SW 2005. Spatial distribution and functional significance of activated vinculin in living cells. J Cell Biol 169: 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM 2002. The α2 integrin subunit-deficient mouse: A multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol 161: 337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh DA, Leng J, Klemke RL 1999. Regulation of cell contraction and membrane ruffling by distinct signals in migratory cells. J Cell Biol 146: 1107–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP 1997. Extracellular matrix rigidity causes strengthening of integrin–cytoskeleton linkages. Cell 88: 39–48 [DOI] [PubMed] [Google Scholar]
- Critchley DR 2000. Focal adhesions—the cytoskeletal connection. Curr Opin Cell Biol 12: 133–139 [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM 2001. Taking cell-matrix adhesions to the third dimension. Science 294: 1708–1712 [DOI] [PubMed] [Google Scholar]
- Decitre M, Gleyzal C, Raccurt M, Peyrol S, Aubert-Foucher E, Csiszar K, Sommer P 1998. Lysyl oxidase-like protein localizes to sites of de novo fibrinogenesis in fibrosis and in the early stromal reaction of ductal breast carcinomas. Lab Invest 78: 143–151 [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP 2009. Stretching single talin rod molecules activates vinculin binding. Science 323: 638–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonna BA, Levine AJ 2006. Filamin cross-linked semiflexible networks: Fragility under strain. Phys Rev Lett 97: 068104. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL 2005. Tissue cells feel and respond to the stiffness of their substrate. Science 310: 1139–1143 [DOI] [PubMed] [Google Scholar]
- Egeblad M, Ewald AJ, Askautrud HA, Truitt ML, Welm BE, Bainbridge E, Peeters G, Krummel MF, Werb Z 2008. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech 1: 155–167; discussion 165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis IR, Jones SJ, Staunton D, Vakonakis I, Norman DG, Potts JR, Milner CM, Meenan NAG, Raibaud S, Schor AM, et al. 2010. Multi-factorial modulation of IGD motogenic potential in MSF (migration stimulating factor). Exp Cell Res doi: 101016/jyexcr201004003 [DOI] [PubMed] [Google Scholar]
- Emerman JT, Bartley JC, Bissell MJ 1981. Glucose metabolite patterns as markers of functional differentiation in freshly isolated and cultured mouse mammary epithelial cells. Exp Cell Res 134: 241–250 [DOI] [PubMed] [Google Scholar]
- Emerman JT, Pitelka DR 1977. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro 13: 316–328 [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE 2006. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689 [DOI] [PubMed] [Google Scholar]
- Fata JE, Chaudhary V, Khokha R 2001. Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17β-estradiol during the estrous cycle. Biol Reprod 65: 680–688 [DOI] [PubMed] [Google Scholar]
- Felsenfeld DP, Schwartzberg PL, Venegas A, Tse R, Sheetz MP 1999. Selective regulation of integrin-cytoskeleton interactions by the tyrosine kinase Src. Nature Cell Biology 1: 200–206 [DOI] [PubMed] [Google Scholar]
- Feng Y, Walsh CA 2004. The many faces of filamin: A versatile molecular scaffold for cell motility and signalling. Nat Cell Biol 6: 1034–1038 [DOI] [PubMed] [Google Scholar]
- Fogerty FJ, Akiyama SK, Yamada KM, Mosher DF 1990. Inhibition of binding of fibronectin to matrix assembly sites by anti-integrin (α5 β1) antibodies. J Cell Biol 111: 699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley SI, Feng Y, Krishnamurthy R, Kim DH, Celedon A, Longmore GD, Wirtz D 2010. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol 12: 598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame MC 2004. Newest findings on the oldest oncogene; how activated src does it. J Cell Sci 117: 989–998 [DOI] [PubMed] [Google Scholar]
- Fringer J, Grinnell F 2001. Fibroblast quiescence in floating or released collagen matrices: Contribution of the ERK signaling pathway and actin cytoskeletal organization. J Biol Chem 276: 31047–31052 [DOI] [PubMed] [Google Scholar]
- Fringer J, Grinnell F 2003. Fibroblast quiescence in floating collagen matrices—decrease in serum activation of MEK and RAF but not Ras. J Biol Chem 278: 20612–20617 [DOI] [PubMed] [Google Scholar]
- Fung Y 1993. Biomechanics: Mechanical properties of living tissue Springer-Verlag, New York [Google Scholar]
- Galbraith CG, Sheetz MP 1997. A micromachined device provides a new bend on fibroblast traction forces. Proc Natl Acad Sci U S A 94: 9114–9118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith CG, Yamada KM, Sheetz MP 2002. The relationship between force and focal complex development. J Cell Biol 159: 695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alvarez B, de Pereda JM, Calderwood DA, Ulmer TS, Critchley D, Campbell ID, Ginsberg MH, Liddington RC 2003. Structural determinants of integrin recognition by talin. Mol Cell 11: 49–58 [DOI] [PubMed] [Google Scholar]
- Gardel ML, Nakamura F, Hartwig JH, Crocker JC, Stossel TP, Weitz DA 2006. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc Natl Acad Sci U S A 103: 1762–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehler S, Baldassarre M, Lad Y, Leight JL, Wozniak MA, Riching KM, Eliceiri KW, Weaver VM, Calderwood DA, Keely PJ 2009. Filamin A-β1 integrin complex tunes epithelial cell response to matrix tension. Mol Biol Cell 20: 3224–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V 1997. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277: 225–228 [DOI] [PubMed] [Google Scholar]
- Giannone G, Jiang G, Sutton DH, Critchley DR, Sheetz MP 2003. Talin1 is critical for force-dependent reinforcement of initial integrin-cytoskeleton bonds but not tyrosine kinase activation. J Cell Biol 163: 409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AR, Ziegler WH, Bobkov AA, Joyce MG, Fasci D, Himmel M, Rothemund S, Ritter A, Grossmann JG, Patel B, et al. 2009. Structural determinants of integrin binding to the talin rod. J Biol Chem 284: 8866–8876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goksoy E, Ma YQ, Wang X, Kong X, Perera D, Plow EF, Qin J 2008. Structural basis for the autoinhibition of talin in regulating integrin activation. Mol Cell 31: 124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S, Philippar U, Sun D, Patsialou A, Avraham J, Wang W, Di Modugno F, Nistico P, Gertler FB, Condeelis JS 2009. Identification of invasion specific splice variants of the cytoskeletal protein Mena present in mammary tumor cells during invasion in vivo. Clin Exp Metastasis 26: 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS 2005. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res 65: 5278–5283 [DOI] [PubMed] [Google Scholar]
- Grinnell F 2000. Fibroblast-collagen-matrix contraction: Growth-factor signalling and mechanical loading. Trends Cell Biol 10: 362–365 [DOI] [PubMed] [Google Scholar]
- Guarnieri D, Battista S, Borzacchiello A, Mayol L, De Rosa E, Keene DR, Muscariello L, Barbarisi A, Netti PA 2007. Effects of fibronectin and laminin on structural, mechanical and transport properties of 3D collageneous network. J Mater Sci Mater Med 18: 245–253 [DOI] [PubMed] [Google Scholar]
- Guo YP, Martin LJ, Hanna W, Banerjee D, Miller N, Fishell E, Khokha R, Boyd NF 2001. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev 10: 243–248 [PubMed] [Google Scholar]
- Hall HG, Bissell MJ 1986. Characterization of the intermediate filament proteins of murine mammary gland epithelial cells. Response to collagen substratum. Exp Cell Res 162: 379–89 [DOI] [PubMed] [Google Scholar]
- Hall HG, Farson DA, Bissell MJ 1982. Lumen formation by epithelial cell lines in response to collagen overlay: A morphogenetic model in culture. Proc Natl Acad Sci U S A 79: 4672–4676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam SZ, Woodward TL 2001. Reciprocal regulation of extracellular matrix proteins and ovarian steroid activity in the mammary gland. Breast Cancer Res 3: 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar R, Maller O, McDaniel S, Hansen KC, Hedman KJ, Lyons TR, Lucia S, Wilson RS Jr, Schedin P 2009. Tamoxifen induces pleiotrophic changes in mammary stroma resulting in extracellular matrix that suppresses transformed phenotypes. Breast Cancer Res 11: R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED 1981. Cell biology of the extracellular matrix Plenum Press, New York [Google Scholar]
- Himmel M, Ritter A, Rothemund S, Pauling BV, Rottner K, Gingras AR, Ziegler WH 2009. Control of high affinity interactions in the talin C terminus: How talin domains coordinate protein dynamics in cell adhesions. J Biol Chem 284: 13832–13842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H, Nakamoto T, Sakai R, Hirai H 1999. p130(Cas), an assembling molecule of actin filaments, promotes cell movement, cell migration, and cell spreading in fibroblasts. Biochem Biophys Res Commun 262: 25–30 [DOI] [PubMed] [Google Scholar]
- Humphrey J, Delang S 2004. An introduction to biomechanics: Solids and fluids, analysis and design Springer-Verlag, New York [Google Scholar]
- Hynes RO 2002. Integrins: Bidirectional, allosteric signaling machines. Cell 110: 673–687 [DOI] [PubMed] [Google Scholar]
- Hynes RO, Yamada KM 1982. Fibronectins: Multifunctional modular glycoproteins. J Cell Biol 95: 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hytonen VP, Vogel V 2008. How force might activate talin's vinculin binding sites: SMD reveals a structural mechanism. PLoS Comput Biol 4: e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW 2006. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn 235: 3222–3229 [DOI] [PubMed] [Google Scholar]
- Janmey PA, Georges PC, Hvidt S 2007. Basic rheology for biologists. Methods Cell Biol 83: 3–27 [DOI] [PubMed] [Google Scholar]
- Jiang GY, Giannone G, Critchley DR, Fukumoto R, Sheetz MP 2003. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 424: 334–337 [DOI] [PubMed] [Google Scholar]
- Julien MA, Haller CA, Wang P, Wen J, Chaikof EL 2007. Mechanical strain induces a persistent upregulation of syndecan-1 expression in smooth muscle cells. J Cell Physiol 211: 167–173 [DOI] [PubMed] [Google Scholar]
- Kadler KE, Hill A, Canty-Laird EG 2008. Collagen fibrillogenesis: Fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol 20: 495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA 2005. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem 280: 16546–16549 [DOI] [PubMed] [Google Scholar]
- Katsumi A, Orr AW, Tzima E, Schwartz MA 2004. Integrins in mechanotransduction. J Biol Chem 279: 12001–12004 [DOI] [PubMed] [Google Scholar]
- Katz BZ, Zamir E, Bershadsky A, Kam Z, Yamada KM, Geiger B 2000. Physical state of the extracellular matrix regulates the structure and molecular composition of cell-matrix adhesions. Mol Biol Cell 11: 1047–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. 1996. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248 [DOI] [PubMed] [Google Scholar]
- Kirschmann DA, Seftor EA, Nieva DR, Mariano EA, Hendrix MJ 1999. Differentially expressed genes associated with the metastatic phenotype in breast cancer. Breast Cancer Res Treat 55: 127–136 [DOI] [PubMed] [Google Scholar]
- Kiyokawa E, Hashimoto Y, Kobayashi S, Sugimura H, Kurata T, Matsuda M 1998. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev 12: 3331–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian RK 2009. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol 19: 1511–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotzsch E, Smith ML, Kubow KE, Muntwyler S, Little WC, Beyeler F, Gourdon D, Nelson BJ, Vogel V 2009. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc Natl Acad Sci U S A 106: 18267–18272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic A, Sheetz MP 2006. Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge. Mol Biol Cell 17: 2684–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ 2007. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res 9: R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC, Muller WJ 2007. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci U S A 104: 20302–20307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M, Wei C, Yamada KM 2006. Cell and fibronectin dynamics during branching morphogenesis. J Cell Sci 119: 3376–3384 [DOI] [PubMed] [Google Scholar]
- Lee EY, Parry G, Bissell MJ 1984. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol 98: 146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Hsu TC, Du JY, Valentijn AJ, Wu TY, Cheng CF, Yang Z, Streuli CH 2009. Extracellular matrix controls insulin signaling in mammary epithelial cells through the RhoA/Rok pathway. J Cell Physiol 220: 476–484 [DOI] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. 2009. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139: 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ 1987. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A 84: 136–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, Schuetz G, Mueller U, Streuli CH, Hynes NE 2005. β1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. Embo J 24: 1942–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Butler P, Wang Y, Hu Y, Han DC, Usami S, Guan JL, Chien S 2002. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci U S A 99: 3546–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SC, Calderwood DA, Ginsberg MH 2000. Integrin cytoplasmic domain-binding proteins. J Cell Sci 113: 3563–3571 [DOI] [PubMed] [Google Scholar]
- Lyons TR, Schedin PJ, Borges VF 2009. Pregnancy and breast cancer: When they collide. J Mammary Gland Biol Neoplasia 14: 87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Desouky J, Friedl A 2006. Syndecan-1 expression by stromal fibroblasts promotes breast carcinoma growth in vivo and stimulates tumor angiogenesis. Oncogene 25: 1408–1412 [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. 2008. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133: 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE 2005. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol 24: 389–399 [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS 2004. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6: 483–495 [DOI] [PubMed] [Google Scholar]
- Mecham RP, Heuser J 1990. Three-dimensional organization of extracellular matrix in elastic cartilage as viewed by quick freeze, deep etch electron microscopy. Connect Tissue Res 24: 83–93 [DOI] [PubMed] [Google Scholar]
- Miron-Mendoza M, Seemann J, Grinnell F 2008. Collagen fibril flow and tissue translocation coupled to fibroblast migration in 3D collagen matrices. Mol Biol Cell 19: 2051–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan P, Warburton MJ, Perusinghe N, Rudland PS 1983. Topographical arrangement of basement membrane proteins in lactating rat mammary gland: Comparison of the distribution of type IV collagen, laminin, fibronectin, and Thy-1 at the ultrastructural level. Proc Natl Acad Sci U S A 80: 3344–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott JD, Werb Z 2004. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 16: 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS 2001. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol 3: 785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, et al. 2005. Ablation of β1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol 171: 717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ 2006. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 314: 298–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer H, Ruoff A, Paessler N, Solomayer E, Wallwiener E, Fehm T 2009. A laminin-rich basement membrane matrix influences estrogen receptor β expression and morphology of MDA-MB-231 breast cancer cells. Oncol Rep 21: 475–481 [PubMed] [Google Scholar]
- Neville MC 2009. Introduction: Tight junctions and secretory activation in the mammary gland. J Mammary Gland Biol Neoplasia 14: 269–270 [DOI] [PubMed] [Google Scholar]
- O'Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, Man YG, Borges V, Schedin P 2010. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol 176: 1241–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR 1999. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59: 5002–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA 2005. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121: 335–348 [DOI] [PubMed] [Google Scholar]
- Orimo A, Weinberg RA 2006. Stromal fibroblasts in cancer: A novel tumor-promoting cell type. Cell Cycle 5: 1597–1601 [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z 2007. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8: 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. 2005. Tensional homeostasis and the malignant phenotype. Cancer Cell 8: 241–254 [DOI] [PubMed] [Google Scholar]
- Pelham RJ Jr, Wang Y 1997. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A 94: 13661–13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham RJ Jr, Wang Y 1999. High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Mol Biol Cell 10: 935–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrol S, Raccurt M, Gerard F, Gleyzal C, Grimaud JA, Sommer P 1997. Lysyl oxidase gene expression in the stromal reaction to in situ and invasive ductal breast carcinoma. Am J Pathol 150: 497–507 [PMC free article] [PubMed] [Google Scholar]
- Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, et al. 2008. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell 15: 813–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirone DM, Liu WF, Ruiz SA, Gao L, Raghavan S, Lemmon CA, Romer LH, Chen CS 2006. An inhibitory role for FAK in regulating proliferation: A link between limited adhesion and RhoA-ROCK signaling. J Cell Biol 174: 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S, Weis WI 2007. Structure and mechanism of cadherins and catenins in cell–cell contacts. Annu Rev Cell Dev Biol 23: 237–261 [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ 2006. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med 4: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Beggs HE, Keely PJ 2008a. Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. Am J Pathol 173: 1551–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ 2008b. Collagen density promotes mammary tumor initiation and progression. BMC Med 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ 2008c. Contact guidance mediated three-dimensional cell migration is regulated by rho/ROCK-dependent matrix reorganization. Biophys J 95: 5374–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Keely PJ 2009. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene 28: 4326–4343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva Y, Gillen KM, Gerald W, Beggs HE, Reichardt LF, Giancotti FG 2009. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J Clin Invest 119: 252–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JA, Graeber CT, Frackelton AR Jr, Kim M, Schwarzbauer JE, Filardo EJ 2009. Coordinate regulation of estrogen-mediated fibronectin matrix assembly and epidermal growth factor receptor transactivation by the G protein-coupled receptor, GPR30. Mol Endocrinol 23: 1052–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raub CB, Suresh V, Krasieva T, Lyubovitsky J, Mih JD, Putnam AJ, Tromberg BJ, George SC 2007. Noninvasive assessment of collagen gel microstructure and mechanics using multiphoton microscopy. Biophys J 92: 2212–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raub CB, Unruh J, Suresh V, Krasieva T, Lindmo T, Gratton E, Tromberg BJ, George SC 2008. Image correlation spectroscopy of multiphoton images correlates with collagen mechanical properties. Biophys J 94: 2361–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, et al. 2006. Wnt/β-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 281: 31720–31728 [DOI] [PubMed] [Google Scholar]
- Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL 2002. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. J Biomech Eng 124: 214–222 [DOI] [PubMed] [Google Scholar]
- Rowe RG, Weiss SJ 2008. Breaching the basement membrane: Who, when and how? Trends Cell Biol 18: 560–574 [DOI] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP 2006. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127: 1015–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney RK, Howard J 2002. Slow local movements of collagen fibers by fibroblasts drive the rapid global self-organization of collagen gels. J Cell Biol 157: 1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney RK, Howard J 2004. Molecular dissection of the fibroblast-traction machinery. Cell Motil Cytoskeleton 58: 175–185 [DOI] [PubMed] [Google Scholar]
- Schedin P, Mitrenga T, Kaeck M 2000a. Estrous cycle regulation of mammary epithelial cell proliferation, differentiation, and death in the Sprague–Dawley rat: A model for investigating the role of estrous cycling in mammary carcinogenesis. J Mammary Gland Biol Neoplasia 5: 211–225 [DOI] [PubMed] [Google Scholar]
- Schedin P, Mitrenga T, McDaniel S, Kaeck M 2004. Mammary ECM composition and function are altered by reproductive state. Mol Carcinog 41: 207–220 [DOI] [PubMed] [Google Scholar]
- Schedin P, O'Brien J, Rudolph M, Stein T, Borges V 2007. Microenvironment of the involuting mammary gland mediates mammary cancer progression. J Mammary Gland Biol Neoplasia 12: 71–82 [DOI] [PubMed] [Google Scholar]
- Schedin P, Strange R, Mitrenga T, Wolfe P, Kaeck M 2000b. Fibronectin fragments induce MMP activity in mouse mammary epithelial cells: Evidence for a role in mammary tissue remodeling. J Cell Sci 113 (Pt 5): 795–806 [DOI] [PubMed] [Google Scholar]
- Schenk S, Quaranta V 2003. Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol 13: 366–375 [DOI] [PubMed] [Google Scholar]
- Schober M, Raghavan S, Nikolova M, Polak L, Pasolli HA, Beggs HE, Reichardt LF, Fuchs E 2007. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J Cell Biol 176: 667–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor SL, Ellis IR, Jones SJ, Baillie R, Seneviratne K, Clausen J, Motegi K, Vojtesek B, Kankova K, Furrie E, et al. 2003. Migration-stimulating factor: A genetically truncated onco-fetal fibronectin isoform expressed by carcinoma and tumor-associated stromal cells. Cancer Res 63: 8827–8836 [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW 1982. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev Biol 90: 215–222 [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW 1984. Glycosaminoglycans in the basal lamina and extracellular matrix of serially aged mouse mammary ducts. Mech Ageing Dev 24: 151–162 [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW 1987. Reversible inhibition of mammary gland growth by transforming growth factor-β. Science 237: 291–293 [DOI] [PubMed] [Google Scholar]
- Singer II 1979. The fibronexus: A transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell 16: 675–685 [DOI] [PubMed] [Google Scholar]
- Singer II, Kawka DW, Kazazis DM, Clark RA 1984. In vivo co-distribution of fibronectin and actin fibers in granulation tissue: Immunofluorescence and electron microscope studies of the fibronexus at the myofibroblast surface. J Cell Biol 98: 2091–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Gourdon D, Little WC, Kubow KE, Eguiluz RA, Luna-Morris S, Vogel V 2007. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol 5: e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS 2001. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol 2: 138–145 [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ 1991. Control of mammary epithelial differentiation: Basement membrane induces tissue-specific gene expression in the absence of cell–cell interaction and morphological polarity. J Cell Biol 115: 1383–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, Bissell MJ 1995. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol 129: 591–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G, Blaine SA, Qiao D, Friedl A 2007. Shedding of Syndecan-1 by stromal fibroblasts stimulates human breast cancer cell proliferation via FGF2 activation. J Biol Chem 282: 14906–14915 [DOI] [PubMed] [Google Scholar]
- Su G, Blaine SA, Qiao D, Friedl A 2008. Membrane type 1 matrix metalloproteinase-mediated stromal syndecan-1 shedding stimulates breast carcinoma cell proliferation. Cancer Res 68: 9558–9565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue SP, Hay ED 1981. Response of basal epithelial cell surface and cytoskeleton to solubilized extracellular matrix molecules. J Cell Biol 91: 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS 2003. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci U S A 100: 1484–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng MH, Bartholomew JC, Bissell MJ 1977. Synergism between anti-microtubule agents and growth stimulants in enhancement of cell cycle traverse. Nature 268: 739–741 [DOI] [PubMed] [Google Scholar]
- Thery M, Racine V, Pepin A, Piel M, ChenY, Sibarita MB, Bornens M 2005. The extracellular matrix guides the orientation of the cell division axis. Nat Cell Biol 7: 947–953 [DOI] [PubMed] [Google Scholar]
- Thery M, Racine V, Piel M, Pepin A, Dimitrov A, Chen Y, Sibarita JB, Bornens M 2006. Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proc Natl Acad Sci U S A 103: 19771–19776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Hay ED, Fujiwara K 1982. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: Distribution of actin, α-actinin, and myosin. Dev Biol 92: 107–122 [DOI] [PubMed] [Google Scholar]
- Vader D, Kabla A, Weitz D, Mahadevan L 2009. Strain-induced alignment in collagen gels. PLoS One 4: e5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, Kumar R 2002. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol 4: 681–690 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Fuchs E 2001. Actin dynamics and cell–cell adhesion in epithelia. Curr Opin Cell Biol 13: 76–84 [DOI] [PubMed] [Google Scholar]
- Volonte D, Galbiati F, Pestell RG, Lisanti MP 2001. Cellular stress induces the tyrosine phosphorylation of caveolin-1 (Tyr14) via activation of p38 mitogen-activated protein kinase and c-Src kinase. Evidence for caveolae, the actin cytoskeleton, and focal adhesions as mechanical sensors of osmotic stress. J Biol Chem 276: 8094–8103 [DOI] [PubMed] [Google Scholar]
- Walker R 2001. The complexities of breast cancer desmoplasia. Breast Cancer Res 3: 143–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Hanks SK, Wang Y 2001. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci U S A 98: 11295–11300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Wang YL 2000. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol 279: C1345–C1350 [DOI] [PubMed] [Google Scholar]
- Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS 2004. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res 64: 8585–8594 [DOI] [PubMed] [Google Scholar]
- Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S 2005. Visualizing the mechanical activation of Src. Nature 434: 1040–1045 [DOI] [PubMed] [Google Scholar]
- Warburton MJ, Mitchell D, Ormerod EJ, Rudland P 1982. Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating, and involuting rat mammary gland. J Histochem Cytochem 30: 667–676 [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ 1997. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 137: 231–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID 2007. Structural basis of integrin activation by talin. Cell 128: 171–182 [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE 2004. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem 279: 53331–53337 [DOI] [PubMed] [Google Scholar]
- Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH 1989. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol 109: 877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Engler AJ, Slone RD, Galante LL, Schwarzbauer JE 2008. Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Res 68: 3185–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B 2007. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol 179: 1311–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Friedl P 2009. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin Exp Metastasis 26: 289–298 [DOI] [PubMed] [Google Scholar]
- Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P 2007. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol 9: 893–904 [DOI] [PubMed] [Google Scholar]
- Woodward TL, Mienaltowski AS, Modi RR, Bennett JM, Haslam SZ 2001. Fibronectin and the alpha(5)beta(1) integrin are under developmental and ovarian steroid regulation in the normal mouse mammary gland. Endocrinology 142: 3214–3222 [DOI] [PubMed] [Google Scholar]
- Wozniak M, Fausto A, Carron CP, Meyer DM, Hruska KA 2000. Mechanically strained cells of the osteoblast lineage organize their extracellular matrix through unique sites of αvβ3-integrin expression. J Bone Miner Res 15: 1731–1745 [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Chen CS 2009. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol 10: 34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ 2003. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol 163: 583–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J 2004. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 64: 7022–7029 [DOI] [PubMed] [Google Scholar]
- Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E 2006. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol 16: 1515–1523 [DOI] [PubMed] [Google Scholar]
- Xie JW, Haslam SZ 2008. Extracellular matrix, Rac1 signaling, and estrogen-induced proliferation in MCF-7 breast cancer cells. Breast Cancer Res Treat 110: 257–268 [DOI] [PubMed] [Google Scholar]
- Yan B, Calderwood DA, Yaspan B, Ginsberg MH 2001. Calpain cleavage promotes talin binding to the β 3 integrin cytoplasmic domain. J Biol Chem 276: 28164–28170 [DOI] [PubMed] [Google Scholar]
- Yang JT, Bader BL, Kreidberg JA, Ullman-Cullere M, Trevithick JE, Hynes RO 1999. Overlapping and independent functions of fibronectin receptor integrins in early mesodermal development. Dev Biol 215: 264–277 [DOI] [PubMed] [Google Scholar]
- Yano Y, Geibel J, Sumpio B 1996. Tyrosine phosphorylation of pp125FAK and paxillin in aortic endothelial cells induced by mechanical strain. Am J Physiol 271: C635–C649 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Magnusson MK, Mosher DF 1997. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell 8: 1415–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]