Abstract
Nek2, a NIMA-related kinase, has been postulated to play a role in both the meiotic and mitotic cell cycles in vertebrates. Xenopus has two Nek2 splice variants, Nek2A and Nek2B, which are zygotic and maternal forms, respectively. Here we have examined the role of Nek2B in oocyte meiosis and early embryonic mitosis. Specific inhibition of Nek2B function does not interfere with the oscillation of Cdc2 activity in either the meiotic or mitotic cell cycles; however, it does cause abortive cleavage of early embryos, in which bipolar spindle formation is severely impaired due to fragmentation or dispersal of the centrosomes, to which endogenous Nek2B protein localizes. In contrast, inhibition of Nek2B function does not affect meiotic spindle formation in oocytes, in which functional centrosomes are absent. Thus, strikingly, Nek2B is specifically required for centrosome assembly and/or maintenance (and hence for normal bipolar spindle formation and cleavage) in early Xenopus embryos. Finally, (ectopic) Nek2A but not Nek2B is very labile in cleaving embryos, suggesting that Nek2A cannot replace the centrosomal function of Nek2B in early embryos.
Keywords: cell cycle/centrosome/Nek2/spindle formation/Xenopus embryo
Introduction
During cell divisions, the correct organization of microtubules in bipolar spindles is essential to distribute chromosomes to the daughter cells. In most animal cell types, centrosomes are instrumental for organization of the spindle poles and for determining both microtubule polarity and the spindle axis (Kellogg et al., 1994). The centrosome typically consists of a pair of centrioles and surrounding material called the pericentriolar material (PCM) (Andersen, 1999). γ-tubulin, a major component of the PCM (Stearns et al., 1991; Zheng et al., 1991), has been proposed to serve to nucleate microtubules with their minus-ends directed inward (or to the PCM) and their plus-ends outward (Joshi et al., 1992; Felix et al., 1994; Stearns and Kirschner, 1994). Centrosomes duplicate precisely once per cell cycle and, in mitosis, the daughter centrosomes separate to define the poles of the mitotic spindle. Recent studies show that centrosome duplication requires Cdk2–cyclin E in Xenopus eggs (Hinchcliffe et al., 1999; Lacey et al., 1999) and Cdk2–cyclin A in mammalian cells (Meraldi et al., 1999). In any systems, however, molecular mechanisms of centrosome assembly and maintenance are largely unknown (Kellogg et al., 1994; Andersen, 1999).
Although centrosomes play a dominant role in bipolar spindle formation during mitosis in most animal cells, they do not necessarily do so during female meiosis in some species. Thus, oocytes from mice, Caenorhabditis, Drosophila and Xenopus do not contain centrosomes, yet they form bipolar spindles during meiosis (Gard, 1992; Theurkauf and Hawley, 1992; Albertson and Thomson, 1993). In these systems, microtubules are nucleated from multiple sites near the chromatin and progressively self-organize into a bipolar spindle through the action of minus-end-directed motor proteins such as dynein (Heald et al., 1996; Merdes and Cleveland, 1997; Waters and Salmon, 1997). (Dynein also functions to focus microtubule minus-ends at the centrosome; Heald et al., 1997.) Thus, in a substantial number of cases, the mechanism of bipolar spindle formation changes during development from a centrosome-independent one (in meiosis) to a centrosome-dependent one (in mitosis).
The G2–M transitions in eukaryotic cells are brought about by the action of Cdc2–cyclin B complexes (Nurse, 1990). In the filamentous fungus Aspergillus nidulans, NIMA, a protein kinase, is also essential for the initiation of mitosis (S.A.Osmani et al., 1988; A.H.Osmani et al., 1991), playing a role in the nuclear localization of cyclin B (Wu et al., 1998). In mammals, many protein kinases structurally related to NIMA have been isolated (Fry and Nigg, 1997). Among these, Nek2 is most closely related to NIMA and has been best characterized. Thus, Nek2 shares ∼50% amino acid identity over the catalytic domain with NIMA and also has a putative C-terminal regulatory domain (Schultz et al., 1994). In humans, Nek2 is expressed in various cell lines with its highest expression in S and G2 phases of the cell cycle (Schultz et al., 1994; Fry et al., 1995). Moreover, in mice, Nek2 is highly expressed in both primary spermatocytes and oocytes as well as in early embryos and several adult tissues with high mitotic activity (Rhee and Wolgemuth, 1997; Tanaka et al., 1997). Thus, it has been postulated that Nek2 may play a role in both the mitotic and meiotic cell cycles. Specifically, chromosomal localization of mouse Nek2 in pachytene spermatocytes and oocytes suggests that Nek2 might be involved in chromosome condensation (Rhee and Wolgemuth, 1997) as NIMA is (O’Connell et al., 1994; Lu and Hunter, 1995). However, overexpression of Nek2 in human cells does not cause chromosome condensation or any other mitotic events (Fry and Nigg, 1997; Fry et al., 1998b), giving no clue to the role for Nek2 in G2–M regulation. In contrast, it has recently been shown that, in human cells, Nek2 localizes to the centrosome and its overexpression induces a splitting of centrosomes (Fry et al., 1998b), suggesting a role for Nek2 in centrosome separation. This idea has been strengthened by the isolation of C-Nap1, which is a centrosomal protein that physically interacts with Nek2 (Fry et al., 1998a). However, it is not known whether inhibition of Nek2 function can prevent centrosome separation in human cells.
In Xenopus, Nek2 exists as two splice variants, termed Nek2A and Nek2B (Uto et al., 1999). Nek2A, a C-terminally spliced form, corresponds to mammalian Nek2, while Nek2B is a novel, C-terminally unspliced form of Nek2. Interestingly, Nek2A and Nek2B are expressed differentially in adult tissues, the former strongly in the testis (and weakly in other tissues) and the latter predominantly in the ovary (Uto et al., 1999). Moreover, the two Nek2 splice variants are expressed differentially during development: Nek2A is zygotically expressed only in postneurula embryos, while Nek2B is maternally expressed in oocytes and early embryos up to the neurula stage (Uto et al., 1999). These results suggest that the two Nek2 variants may play a role in a spatially and temporally complementary manner during Xenopus development. However, it is not known at all why two Nek2 variants exist and what role(s) they play in development.
In this study, we have examined the role(s) of Nek2B, a maternal form of Nek2, in Xenopus oocyte meiosis and early embryonic mitosis by specifically inhibiting its function. We show that Nek2B is not essential for the oscillation of Cdc2 activity in either the meiotic or mitotic cell cycles, but is essential for bipolar spindle formation in embryonic mitosis but not in oocyte meiosis. Consistent with these results, Nek2B localizes to the mitotic centrosomes and is essential for their assembly or maintenance in early embryos. Moreover, we find that ectopically expressed Nek2A is much more unstable than Nek2B in cleaving embryos, suggesting that Nek2A cannot replace the centrosomal function of Nek2B in early embryos. These results allow us to propose that Nek2B specifically functions for the assembly and/or maintenance, rather than separation, of centrosomes in early Xenopus embryos.
Results
Non-essential role for Nek2B in the meiotic Cdc2 cycle
Nek2B is expressed maternally in oocytes and early embryos up to the neurula stage (Uto et al., 1999) (see Figure 1A). However, it is not known whether Nek2B plays any role in oocyte meiosis and embryonic mitosis. Therefore, we first determined whether Nek2B played a role in the initiation of meiotic maturation of oocytes. To do this, we took advantage of microinjection with either neutralizing anti-Nek2B antibody or mRNA encoding a kinase-deficient, dominant-negative Lys37Arg Nek2B mutant (Nek2B-KR; cf. Figure 1B) (for the properties of these reagents, see Materials and methods). Upon progesterone treatment, immature oocytes preinjected with 10 ng of Nek2B-KR mRNA (and hence expressing Nek2B-KR protein in 15-fold excess over endogenous Nek2B) underwent maturation or germinal vesicle breakdown (GVBD) essentially with the same kinetics as uninjected control oocytes and those injected with control mRNA encoding only the N-terminal half of the Nek2B kinase domain (Nek2B-ΔC; cf. Figure 1B) (Figure 2A). Likewise, the oocytes injected with anti-Nek2B antibody (300 ng/oocyte) matured with the same kinetics as control oocytes either uninjected or injected with preimmune antibody. (The doses of Nek2B-KR mRNA and anti-Nek2B antibody used in these experiments were those that were sufficient to inhibit endogenous Nek2B function in early embryos; see below.) Thus, apparently, Nek2B was not essential for the initiation of maturation of oocytes.
Fig. 1. Developmental expression and structural features of Nek2A and Nek2B. (A) Expression of Nek2A and Nek2B proteins during early Xenopus development. Data from Uto et al. (1999) are schematically represented here. FT, fertilization. (B) Structures of Nek2A, Nek2B and Nek2B mutants (used in the present study). Relevant amino acid numbers are indicated. For details, see Materials and methods and Uto et al. (1999).
Fig. 2. Effects of Nek2B inhibition on the meiotic Cdc2 cycle. (A) Effect on the initiation of oocyte maturation. Thirty immature oocytes were uninjected (None) or injected with Nek2B-ΔC mRNA (Nek2B-ΔC; 10 ng/oocyte), Nek2B-KR mRNA (Nek2B-KR;10 ng/oocyte), preimmune antibody (IgG; 300 ng/oocyte) or anti-Nek2B antibody (α-Nek2B; 300 ng/oocyte), and 2 h later were treated with progesterone and scored for the percentage GVBD. (B) Effect on the meiotic Cdc2 cycle. Oocytes treated as in (A) were collected at the times indicated and subjected to H1 kinase assays of Cdc2. H1 kinase activity is represented in arbitrary units. For both (A) and (B), similar results were obtained in two other independent experiments.
We then tested whether Nek2B played a role in the meiotic Cdc2 cycle of oocytes, by assaying histone H1 kinase activity of Cdc2. In both control oocytes and those injected with either Nek2B-KR mRNA or anti-Nek2B antibody, Cdc2 kinase activity increased 1.5 h after progesterone treatment, peaked at 3 h or at GVBD (representing entry into meiosis I), decreased transiently, and then increased again 1 h after GVBD (representing entry into meiosis II) (Figure 2B). Thus, specific inhibitions of Nek2B function did not appreciably affect the oscillation of Cdc2 activity during the meiotic cell cycle. These results indicate that Nek2B is not essential for the meiotic Cdc2 cycle of oocytes.
Non-essential role for Nek2B in the mitotic Cdc2 cycles
We also tested whether Nek2B had any role in the mitotic Cdc2 cycles. To do this, we injected unfertilized eggs with either Nek2B-KR mRNA (10 ng/egg) or anti-Nek2B antibody (300 ng/egg) and then subjected them to H1 kinase assays of Cdc2. (In these experiments, the action of injection itself caused egg activation, and the doses of Nek2B-KR mRNA and anti-Nek2B antibody used were those that affected cleavage of fertilized eggs, as shown below.) In both control eggs injected with Nek2B-ΔC mRNA and those injected with Nek2B-KR mRNA, Cdc2 activity decreased rapidly by 10 min after egg activation (representing release from metaphase II arrest), increased gradually, peaked at 70–80 min (representing the first mitotic M phase), decreased transiently, and then increased again at 100–110 min (representing entry into the second M phase) (Figure 3). The eggs injected with anti-Nek2B antibody as well as control eggs injected with preimmune antibody also showed essentially the same kinetics of Cdc2 activity (Figure 3). Thus, even in the absence of Nek2B function, Cdc2 kinase activity oscillated essentially normally in the mitotic cell cycles of activated eggs. These results suggest that Nek2B is not essential for the early embryonic Cdc2 cycles.
Fig. 3. Effect of Nek2B inhibition on the mitotic Cdc2 cycle. Thirty unfertilized eggs were injected with Nek2B-ΔC mRNA (Nek2B-ΔC; 10 ng/egg), Nek2B-KR mRNA (Nek2B-KR; 10 ng/egg), preimmune antibody (IgG; 300 ng/egg) or anti-Nek2B antibody (α-Nek2B;300 ng/egg); in these eggs, the action of injection itself caused egg activation. Activated eggs were collected at the times indicated and subjected to H1 kinase assays as in Figure 2B. Results similar to those presented here were obtained in two other independent experiments.
Requirement for Nek2B in normal cleavage of early embryos
Although Nek2B was not essential for the mitotic Cdc2 cycles, it might have been required for normal cell divisions or cleavage of early embryos. (In early embryos, the Cdc2 cycle can occur normally in the absence of cell divisions; Newport and Kirschner, 1984.) Thus, we tested whether inhibition of Nek2B had any effect on the cleavage of early embryos. We injected one-cell embryos (1 h after fertilization) with mRNA (10 ng/embryo) encoding either Nek2B-KR or control Nek2B-ΔC. Like uninjected control embryos (not shown), the embryos injected with control Nek2B-ΔC mRNA developed quite normally throughout the cleavage stage (Figure 4A). The embryos injected with Nek2B-KR mRNA also developed apparently normally until the four-cell stage (or until 1 h after injection), but then progressively showed abortive (or irregular) cleavage; at the 16- or 32-cell stages, some blastomeres were much larger but others were smaller than control blastomeres (Figure 4B). To test whether this effect was specific to inhibition of endogenous Nek2B, we also injected one-cell embryos with anti-Nek2B antibody (300 ng/embryo). While embryos injected with control preimmune antibody developed normally (Figure 4C), those embryos injected with anti-Nek2B antibody exhibited irregular cleavage essentially similar to that seen with Nek2B-KR mRNA injection (Figure 4D). These effects (of both Nek2B-KR mRNA and anti-Nek2B antibody) were very reproducible and occurred in a dose-dependent manner. Thus, strikingly, endogenous Nek2B function was specifically required for normal cleavage of early embryos.
Fig. 4. Effect of Nek2B inhibition on cleavage of early embryos. Ovulated eggs were injected 1 h after fertilization with (A) Nek2B-ΔC mRNA (Nek2B-ΔC; 10 ng/egg), (B) Nek2B-KR mRNA (Nek2B-KR; 10 ng/egg), (C) preimmune antibody (IgG; 300 ng/egg) or (D) anti-Nek2B antibody (α-Nek2B; 300 ng/egg); 2.5 h later they were photographed.
Requirement for Nek2B in bipolar spindle formation and centrosome assembly
In early embryos, bipolar spindle formation is essential for normal and regular cleavage of the cells. Because cleavage was severely impaired in Nek2B-inhibited embryos, we examined whether Nek2B inhibition influenced bipolar spindle formation in cleaving embryos. When immunostained with anti-α-tubulin antibody, control embryos injected with Nek2B-ΔC mRNA showed an apparently normal bipolar spindle (Figure 5A, a and b). However, the embryos injected with dominant-negative Nek2B-KR mRNA exhibited numerous dot-like structures instead of a bipolar spindle (Figure 5A, c); each of the dot-like structures had an associated microtubule aster (Figure 5A, d), indicating that it acted as a microtubule-organizing center (MTOC). Similar results were obtained with embryos that were injected with neutralizing anti-Nek2B antibody, but not with preimmune antibody (data not shown). Thus, apparently, endogenous Nek2B was required for normal bipolar spindle formation in cleaving embryos, its specific inhibitions disorganizing the bipolar spindle and, instead, producing numerous MTOCs. These results suggest that the defect in bipolar spindle formation was the primary and direct cause of the abortive cleavage of Nek2B-inhibited embryos.
Fig. 5. Effects of Nek2B inhibition on spindle formation and centrosome assembly. (A) α- and γ-tubulin stainings of Nek2B-inhibited embryos. Embryos 1 h after fertilization were injected with 10 ng of mRNA encoding either Nek2B-ΔC or Nek2B-KR; 2.5 h later they were immunostained with either anti-α- or anti-γ-tubulin antibody and examined by confocal microscopy. The boxed areas in panels a, c, e and g are enlarged in panels b, d, f and h, respectively. Bar, 100 µm. (B) γ-tubulin staining of Nek2B-inhibited eggs with or without injected sperm nuclei. Unfertilized eggs were first injected (and activated) with or without demembranated sperm nuclei and then (5 min later) with 10 ng of mRNA encoding either Nek2B-ΔC or Nek2B-KR; 2.5 h later they were immunostained with anti-γ-tubulin antibody and examined by confocal microscopy. Bar, 50 µm.
In most animal cells including Xenopus embryos, the MTOC is equivalent to the centrosome or its PCM, and duplication and separation of intact centrosomes are fundamental for bipolar spindle formation (Krioutchkova and Onishchenko, 1999). Therefore, we next examined centrosome morphology in Nek2B-inhibited embryos by immunostaining γ-tubulin, a major component of the PCM (Stearns et al., 1991; Zheng et al., 1991). Most of the cells in control embryos injected with Nek2B-ΔC mRNA showed two closely located dots typical of separated centrosomes (Figure 5A, e and f). In contrast, a significant fraction of cells in Nek2B-KR mRNA-injected embryos exhibited numerous tiny dots that were dispersed throughout the cytoplasm (Figure 5A, g and h). These tiny dots resembled, in their number and random distribution, those dot-like structures that were immunostained with anti-α-tubulin antibody (cf. Figure 5A, c and d), suggesting that they did act as MTOCs. (Often the tiny dots or MTOCs looked to be linked together by microtubules; cf. Figure 5A, d and h.) Similar tiny dots, instead of two centrosomes, were observed by γ-tubulin staining of the embryos injected with anti-Nek2B antibody but not with preimmune antibody (data not shown). Thus, without Nek2B function, centrosomes or PCMs were apparently disorganized and, instead, numerous γ-tubulin-containing MTOCs were produced, probably accounting for the defects in bipolar spindle formation and cleavage in Nek2B-inhibited embryos.
The appearance of numerous MTOCs in Nek2B- inhibited embryos could have been either due to a failure to assemble or maintain the centrosome (or PCM) structure, or due to a spontaneous development of MTOCs. (In the eggs of many species including Xenopus, MTOCs can develop spontaneously from cytoplasmic precursors under certain conditions; Longo, 1997). To distinguish between these two possibilities, we injected (and hence also activated) unfertilized eggs with Nek2B-KR mRNA (10 ng/egg) with or without demembranated sperm nuclei and, 2.5 h later, stained them with anti-γ-tubulin antibody. In the eggs containing injected sperm nuclei, pairs of two closely located dots (i.e. separated centrosomes) were clearly detected in the presence (and absence; not shown) of control Nek2B-ΔC mRNA (Figure 5B, b), but such centrosomes looked to be fragmented or dispersed in the presence of Nek2B-KR mRNA (Figure 5B, d). Thus, these results were essentially similar to those obtained with normally fertilized eggs (cf. Figure 5A, g and h). In the eggs containing no sperm nuclei, on the other hand, multiple dots were observed on a single, globular structure (probably a nucleus) in the presence (and absence; not shown) of Nek2B-ΔC mRNA (Figure 5B, a). In the presence of Nek2B-KR mRNA, however, such dots looked to be fragmented around the nucleus-like structure (which itself was somewhat disorganized) and, importantly, no extra (free) dots were observed in the other cytoplasmic regions (Figure 5B, c). Thus, even without sperm nuclei, inhibition of Nek2B could not induce spontaneous development of γ-tubulin-containing MTOCs in the egg cytoplasm. (The nature of multiple dots on the nucleus-like structure is presently unknown, but they seem to be structurally related to the PCM of the centrosome as they were also fragmented by Nek2B inhibition.) These results strongly suggest that the numerous dots or MTOCs observed in Nek2B-inhibited embryos (Figure 5A, g and h) were produced by fragmentation or dispersal of centrosomes, rather than by spontaneous development of MTOCs. Thus, it appears that Nek2B is required for the assembly and/or maintenance of centrosomes (or, more strictly, of the PCM) in cleaving embryos.
Nek2B localizes to the centrosome
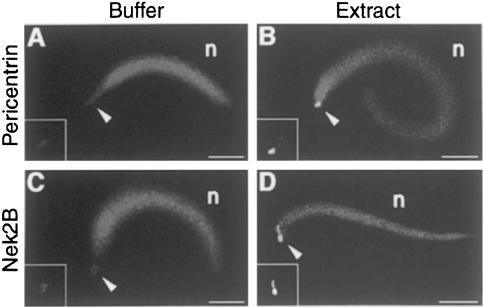
The fragmentation or dispersal of the PCM by Nek2B inhibition suggests that Nek2B might be an integral component of the PCM. To test this possibility, we initially undertook to immunostain Nek2B protein in intact cleaving embryos. However, this staining was not successful, presumably because of technical problems such as fixation of the embryo. Therefore, we took advantage of unfertilized egg extracts, to which demembranated sperm nuclei were added; in such a system, the centrosome can assemble at the basal body of the sperm flagellum (Sawin and Mitchison, 1991). Before incubation with egg extracts (or upon incubation with buffer alone), the sperm basal body was very faintly stained both with antibody specific to pericentrin, a component of the PCM (Doxsey et al., 1994), and with anti-Nek2B antibody (Figure 6A and C). (The validity of this faint staining with anti-Nek2B antibody is currently unknown, but that with anti-pericentrin antibody seems to be a bona fide one; cf. Stearns and Kirschner, 1994). Upon incubation with egg extracts (for 7 min), however, the sperm basal body was strongly stained with both anti-pericentrin antibody and anti-Nek2B antibody (Figure 6B and D), indicating that Nek2B as well as pericentrin was recruited from the egg extracts (or cytoplasm) to the basal body. At this time point, γ-tubulin was also detected at the basal body (data not shown), confirming that the centrosome was being assembled on it (cf. Felix et al., 1994; Stearns and Kirschner, 1994). Thus, these results strongly suggest that Nek2B is recruited to the sperm centrosome upon fertilization and is thus a component of the centrosome (or PCM) in cleaving embryos.
Fig. 6. Recruitment of Nek2B to the sperm centrosome. Demembranated sperm nuclei were incubated with either buffer alone (Buffer) or unfertilized egg (or CSF) extracts (Extract) and, 7 min later, stained with DAPI (to position the sperm nucleus, n) and either anti-pericentrin or anti-Nek2B antibody. The arrowhead denotes the position of the basal body. Staining with anti-pericentrin or anti-Nek2B antibody alone is shown in the corner of each panel. Bar, 20 µm.
Nek2B is dispensable for meiotic bipolar spindle formation
The results obtained thus far strongly suggest that Nek2B localizes to the centrosome and functions for its assembly and/or maintenance (and hence for normal bipolar spindle formation and cleavage) in early embryos. If this is truly the case, then Nek2B might not be essential for bipolar spindle formation in oocytes, in which functional centrosomes are absent and spindle formation occurs independently of centrosomes (Gard, 1992; Heald et al., 1996). To test this possibility, we examined spindle morphology in maturing and mature oocytes in which Nek2B function had been inhibited as described earlier (cf. Figure 2). Immunostaining of α-tubulin revealed that the oocytes preinjected with dominant-negative Nek2B-KR mRNA had a typical bipolar spindle just beneath the animal pole both at metaphase I (1 h after GVBD) and metaphase II (4 h after GVBD) (Figure 7B and E), as did uninjected control oocytes (Figure 7A and D) and those injected with control Nek2B-ΔC mRNA (not shown). Similarly, the oocytes injected with neutralizing anti-Nek2B antibody showed a normal bipolar spindle at both metaphase I and metaphase II (Figure 7C and F). Thus, specific inhibitions of Nek2B did not appreciably affect bipolar spindle formation in oocytes, which lack centrosomes (Gard, 1992). These results contrast sharply with those with Nek2B-inhibited cleaving embryos (in which bipolar spindle formation was severely impaired), and further support the idea that Nek2B functions specifically for the assembly and/or maintenance of centrosomes in early embryos.
Fig. 7. Effect of Nek2B inhibition on meiotic spindle formation. Immature stage VI oocytes were left uninjected (Control; A and D) or injected with either 10 ng of Nek2B-KR mRNA (Nek2B-KR; B and E) or 300 ng of anti-Nek2B antibody (α-Nek2B; C and F) and, 2 h later, treated with progesterone. At metaphase I (1 h after GVBD) or metaphase II (4 h after GVBD), the oocytes were immunostained with anti-α-tubulin antibody and examined by confocal microscopy. Bar, 50 µm.
Nek2B, but not Nek2A, is stable in early embryos
Compared with Nek2B, which is maternally expressed in oocytes and preneurula embryos, the zygotic form of Nek2 or Nek2A has a longer C-terminal half (due to alternative splicing) and is expressed only in postneurula embryos (Uto et al., 1999) (see Figure 1). An important question is then why only Nek2B exists and functions in early (e.g. cleaving) embryos. In cleaving Xenopus embryos, centrosome assembly, duplication and separation can all occur in the absence of new protein synthesis (Gard et al., 1990). Therefore, all of the components necessary for centrosome cycles, including Nek2B, would be stable in cleaving embryos. To test this possibility, we examined the metabolic stability of Nek2B as well as (ectopic) Nek2A in cleaving embryos. For this, we injected fertilized eggs with mRNA encoding either Myc-tagged Nek2A or Myc-tagged Nek2B and, 2 h later (or at the 16-cell stage), treated them with the protein synthesis inhibitor cycloheximide (which arrests the cell cycle at G2; Miake-Lye et al., 1983). Immunoblot analysis with anti-Nek2B antibody (which reacts with both Nek2A and Nek2B; Uto et al., 1999) revealed that both Myc-Nek2B and endogenous Nek2B persisted long (for at least 2.5 h) after the cycloheximide treatment, but the level of Myc-Nek2A decreased drastically as early as 30 min after the treatment and remained very low thereafter (Figure 8A). (A similar drastic decrease was observed with Nek2A having no Myc tag.) Thus, Nek2B was very stable, but a large fraction of Nek2A was unstable probably at the G2 phase of the embryonic cell cycle. To confirm these results further, we also examined the stability of Nek2A and Nek2B in cycling egg extracts (Murray, 1991), to which Myc-Nek2A or Myc-NekB protein that was synthesized in reticulocyte lysates was added. Myc-Nek2B as well as endogenous Nek2B was very stable throughout the first mitotic cell cycle, in which M phase (determined by the morphology of added sperm nuclei) occurred 50–60 min after incubation of the extracts (Figure 8B). In contrast, Myc-Nek2A showed a substantial decrease in levels 30–40 min after incubation of the extracts or 10–20 min after its addition to the extracts. Thus, again, Nek2B was stable, but a large fraction of Nek2A was very labile probably at the G2 phase of the mitotic cell cycle. These results establish that Nek2B is much more stable than Nek2A in cleaving embryos, probably explaining why only Nek2B exists and functions in early embryos, in which centrosome cycles occur autonomously.
Fig. 8. Stability of Nek2A and Nek2B in embryos and cell-free egg extracts. (A) Embryos 1 h after fertilization were injected with 10 ng of mRNA encoding either Myc-Nek2A or Myc-Nek2B and, 2 h later, treated with cycloheximide. Embryos were sampled at the times indicated (after mRNA injection) and subjected to Western blot analysis with anti-Nek2B antibody, which can recognize not only Nek2B but also Nek2A (cf. Uto et al., 1999). The arrowhead denotes endogenous Nek2B. (B) Twenty minutes after incubation, cycling extracts were mixed with reticulocyte lysates containing Myc-Nek2A or Myc-Nek2B (see Materials and methods) and incubated further. A portion of the extracts was sampled at the times indicated (after the initial incubation) and analyzed as in (A). The time of M phase was determined by the morphology of added sperm nuclei. The arrowhead denotes endogenous Nek2B.
Discussion
In the present study, we have examined the role for Nek2B, a maternal form of Nek2, in Xenopus oocyte meiosis and embryonic mitosis. Our results show that Nek2B is not essential for either the meiotic or mitotic Cdc2 cycles, but is essential for the assembly and/or maintenance of centrosomes (to which Nek2B localizes) in cleaving embryos. We also suggest that Nek2A, a zygotic form of Nek2, cannot replace the centrosomal function of Nek2B in cleaving embryos, probably due to its extreme instability.
Non-essential role for Nek2B in the meiotic and mitotic Cdc2 cycles
Based on its structural similarity to the NIMA kinase of A.nidulans and its cell cycle-regulated expression, mammalian Nek2 has been thought to play a role in G2–M transitions of the cell cycle (Fry and Nigg, 1997). Specifically, chromosomal association of Nek2 in mouse spermatocytes and oocytes has been postulated to play a role in chromosome condensation (Rhee and Wolgemuth, 1997). Unlike overexpression of NIMA (O’Connell et al., 1994; Lu and Hunter, 1995), however, overexpression of Nek2 or Nek2B cannot induce premature mitotic events such as chromosome condensation in human cells or Xenopus oocytes (Fry et al., 1998b; Uto et al., 1999), giving no clue to the role for Nek2 in G2–M regulation. In the present study, we have directly tested the possible role for Nek2B in the meiotic and mitotic cell cycles of Xenopus. Overexpression of a dominant-negative Nek2B mutant or injection of neutralizing anti-Nek2B antibody did not interfere with the oscillation of Cdc2 activity in either oocytes or activated eggs (Figures 2 and 3). Essentially similar results were obtained using cell-free egg extracts that were immunodepleted of Nek2B (our unpublished data). Thus, although Nek2B might have a subtle role in cell cycle (or G2–M) regulation, it does not seem to be essential for either the meiotic or mitotic Cdc2 cycles. In addition, it seems clear that Nek2A, a genuine homolog of mammalian Nek2, is dispensable for the meiotic and mitotic cell cycles, because it is absent in both oocytes and early embryos (Uto et al., 1999). Thus, at least in early Xenopus development, Nek2 does not seem to play a role such as that played by NIMA in the cell cycle (cf. Fry and Nigg, 1995; Wu et al., 1998).
Essential role for Nek2B in centrosome assembly or maintenance in early embryos
Despite no appreciable effect on the mitotic Cdc2 cycles, specific inhibitions of Nek2B in cleaving embryos had a dramatic effect on the pattern of cell divisions or cleavage: fertilized eggs either overexpressing a dominant-negative Nek2B mutant or injected with neutralizing anti-Nek2B antibody showed abortive, irregular cleavage and could not develop normally (Figure 4). Immunostaining of these embryos with anti-α-tubulin antibody revealed that bipolar spindle formation was severely impaired in a significant fraction of the cells (Figure 5). Thus, the abortive cleavage of Nek2B-inhibited embryos was probably due to a failure to form bipolar spindles, which are essential for nor mal cleavage of early embryos (Krioutchkova and Onishchenko, 1999). (In early embryos, however, spindle defects cannot inhibit the Cdc2 cycle due to the absence of the spindle checkpoint; cf. Newport and Kirschner, 1984). In sharp contrast with this, specific inhibitions of Nek2B in oocytes had no apparent effect on bipolar spindle formation at either metaphase I or metaphase II (Figure 7). Thus, strikingly, Nek2B was specifically required for mitotic, but not meiotic, bipolar spindle formation in Xenopus eggs.
To explore the mechanical basis of the spindle defects in Nek2B-inhibited embryos (but not oocytes), we examined centrosome morphology in Nek2B-inhibited embryos by immunostaining γ-tubulin, because centrosomes play a dominant role in bipolar spindle formation in Xenopus embryos (as in most other animal cells), but not in oocytes, which lack centrosomes (Gard, 1992; Merdes and Cleveland, 1997; Waters and Salmon, 1997). We found that functional inhibition of endogenous Nek2B either by dominant-negative Nek2B or by neutralizing anti-Nek2B antibody does cause fragmentation or dispersal of the centrosomes in cleaving embryos (Figure 5), and that Nek2B is rapidly recruited to the sperm basal body (or the centrosome precursor) in egg extracts (Figure 6). Thus, these results do suggest that Nek2B localizes to the centrosome and is essential for its assembly and/or maintenance in early embryos. This can explain well why specific inhibition of Nek2B impaired bipolar spindle formation (and cell divisions) in cleaving embryos but not in oocytes.
In cultured human cells, Nek2, a homolog of Xenopus Nek2A, localizes to the centrosome and its overexpression induces a premature splitting of centrosomes (Fry et al., 1998b). This result suggests a role for Nek2 in centrosome separation (although it is not known whether inhibition of Nek2 can prevent centrosome separation). At first sight, this result with human cells seems to conflict with our result with Xenopus embryos, in which Nek2B was required for the assembly and/or maintenance, rather than separation, of centrosomes. (In egg extracts, immunodepletion of Nek2B also seems to affect centrosome assembly at the sperm basal body; A.M.Fry, personal communication.) This apparent discrepancy between the human and Xenopus systems may be due to the difference in cell types and/or the difference in intrinsic activities of the two Nek2 isoforms. Intriguingly, however, human Nek2 is also likely to function for centrosome assembly or maintenance, because overexpression of its kinase-deficient (and hence probably dominant-negative) mutant has been shown to induce dispersal of centrosomes in cultured cells (cf. Fry et al., 1998b). Thus, human Nek2 might have multiple centrosomal functions, although it is also possible that the centrosome splitting induced by overexpression of wild-type Nek2 was, in fact, due to a premature assembly or maturation of centrosomes (by the centrosome-assembling activity of the overexpressed Nek2). Although Nek2B might also play a role in centrosome separation (in addition to its assembly or maintenance) in Xenopus embryos, our results on Nek2B inhibition (Figure 5) and wild-type Nek2B overexpression (our unpublished data) do not give any clue to such a role for Nek2B in early embryos.
A possible function of Nek2B in centrosome assembly or maintenance
How might Nek2B be involved in the assembly or maintenance of centrosomes in cleaving embryos? So far, the precise mechanisms by which centrosomes are assembled and nucleate microtubules are not known. In many species, however, γ-tubulin, a well known centrosomal component, has been shown to be required for centrosome-dependent microtubule nucleation (Joshi et al., 1992; Felix et al., 1994; Stearns and Kirschner, 1994). Specifically, γ-tubulin forms, together with several other proteins, the so-called γ-tubulin ring complexes (γ-TuRCs), which are recruited to the PCM of the centrosome and probably serve as the templates for the nucleation of microtubules (Moritz et al., 1995b; Zheng et al., 1995; Raff, 1996; Andersen, 1999). A recent study also shows that Xgrip109, a component of the Xenopus γ-TuRC, is necessary for the assembly and centrosomal recruitment of γ-TuRCs in egg extracts (Martin et al., 1998). In the present study, inhibition of Nek2B function in cleaving embryos specifically induced fragmentation or dispersal of centrosomes; however, the fragmented or dispersed centrosomes possessed microtubule asters (cf. Figure 5A, d and h), implying that they retained intact γ-TuRCs. Thus, apparently, Nek2B was required for the assembly and/or maintenance of the PCM architecture, but not for the formation or activity of γ-TuRCs. Then what role might Nek2B play in the assembly or maintenance of the PCM? For the formation of functional centrosomes, γ-TuRCs are first recruited to the centrosomes and are probably bound and retained by the PCM (Moritz et al., 1995a; Zheng et al., 1995; Vogel et al., 1997). The PCM has an intricate lattice structure containing pericentrin, and γ-TuRCs are usually embedded in the lattice (Dictenberg et al., 1998). Moreover, the PCM (in Spisula oocytes) is structurally organized around another filament-based structure called the ‘centromatrix’ that serves as a scaffold to which γ-TuRCs bind (Schnackenberg et al., 1998). (Similar structures have been shown in Drosophila; Moritz et al., 1998.) Thus, if Nek2B were involved in the formation of the lattice structure or the centromatrix, then it could function for the assembly or maintenance of the basic PCM architecture to which γ-TuRCs should be recruited. In Xenopus egg extracts, sperm aster assembly requires ATP (Felix et al., 1994; Stearns and Kirschner, 1994), which could occur at the assembly step of the lattice or the centromatrix (Schnackenberg et al., 1998). It is tempting to speculate, therefore, that Nek2B, as a protein kinase, might phosphorylate and regulate some ‘glue’ protein(s) that is required for the assembly of the lattice or the centromatrix of the PCM.
Another interesting possibility is that Nek2B might display the centrosomal function via the action of centrioles. Indeed, in several cell types, the presence of intact centrioles has been suggested to be required for the normal assembly of the PCM (Gueth-Hallonet et al., 1993; Debec et al., 1995; Bobinnec et al., 1998). For instance, when introduced into HeLa cells, monoclonal antibodies specific to centriolar tubulin (or polyglutamylated tubulin) can cause not only a disappearance of centrioles but also a scattering of the PCM within the cytoplasm (Bobinnec et al., 1998). Hence, if Nek2B were involved in the correct organization of centrioles, then it could function indirectly for the assembly or maintenance of the PCM. In this context, it is worth noting that human Nek2 physically interacts with C-Nap1, a novel coiled-coil protein that localizes to the proximal ends of centrioles (Fry et al., 1998a). To test these as well as other possibilities, reconstitution of centrosome assembly in vitro and determination of precise Nek2B localization in the centrosome will be very useful.
Why does only Nek2B exist and function in early embryos?
Besides Nek2B, Xenopus has another Nek2 splice variant, or Nek2A, which corresponds to mammalian Nek2 and, unlike Nek2B, is expressed only in postneurula embryos (Uto et al., 1999). Why then might only Nek2B be expressed and play a centrosomal function in early embryos such as cleaving embryos? In cleaving Xenopus embryos, centrosome assembly, duplication and separation can all occur autonomously or in the absence of any new protein synthesis (Gard et al., 1990). Therefore, all of the components that are necessary for centrosome cycles should already be present and metabolically stable in cleaving embryos. Indeed, both Cdk2 and cyclin E, which are required for centrosome duplication in Xenopus egg extracts (Hinchcliffe et al., 1999; Lacey et al., 1999), have been shown to be very stable in cleaving embryos (Hartley et al., 1996; Howe and Newport, 1996). We have shown here that Nek2B is also very stable in cycling egg extracts and cleaving embryos (Figure 8), consistent with it being a component required for centrosome assembly and/or maintenance. In contrast, a large fraction of (ectopic) Nek2A was very labile in both egg extracts and cleaving embryos; it was rapidly degraded apparently at G2 phase during the mitotic cell cycles, as mammalian Nek2 is at late G2 phase in the somatic cell cycles (Schultz et al., 1994). Thus, due to its extreme instability, Nek2A, even if it had a centrosomal function similar to Nek2B, would not be able to replace the Nek2B function in early embryos. This may be one of the reasons why only Nek2B, but not Nek2A, is expressed and functions in early embryos. Structurally, Nek2A has a significantly longer C-terminal half than Nek2B (Uto et al., 1999) (cf. Figure 1B), and a C-terminally truncated form of Nek2A is metabolically as stable as Nek2B in cleaving embryos (our unpublished data). Thus, it appears that early Xenopus embryos employed Nek2B rather than Nek2A, at least in part, for their autonomous centrosome cycles. If so, it remains to be elucidated whether Nek2A has any other different properties or functions from Nek2B in postneurula embryos.
In conclusion, we have shown here that Nek2B, a maternal form of Nek2, plays an important role in the assembly and/or maintenance of centrosomes in early Xenopus embryos. Like those of centrosome duplication and separation, molecular mechanisms of centrosome assembly, maintenance and maturation are largely unknown (cf. Kellogg et al., 1994; Andersen, 1999). Further analyses of Nek2B or Nek2A function will greatly contribute to our understanding of centrosome cycle regulation in animal cells.
Materials and methods
cDNA constructions and in vitro transcription and translation
A cDNA encoding N-terminal 151 amino acids of Nek2B (Nek2B-ΔC) was prepared by PCR of the Nek2B cDNA (Uto et al., 1999) using a 5′ primer (5′-GCGGATCCCCATGCCGTCACGGGTCGAG-3′) and a 3′ primer (5′-GCGGATCCTTATGGCATCTAGAAATATGTTGGC-3′). The cDNA was cut with BamHI and inserted into the BamHI site of the pT7G (UK–) plasmid vector (Uto et al., 1999). A cDNA encoding a kinase-deficient Lys37Arg Nek2B mutant (Nek2B-KR) was made by mutagenesis of the pT7G (UK–)-Nek2B plasmid (Uto et al., 1999) using the Quick Change Mutagenesis kit (Ambion) and a primer (5′-GAA GCTGTTGGTATGGAGAGAACTGGACTATGG-3′) for the sense strand. To make a cDNA encoding N-terminally Myc-tagged Nek2A or Nek2B, a cDNA encoding Nek2A or Nek2B (Uto et al., 1999) was first subjected to PCR using a 5′ primer (5′-CTAGCTAGCTAGCCG TCACGGGTCGAG-3′ for both Nek2A and Nek2B) and a 3′ primer (5′-CGGGATCCAGCGTTACATAAGCAAGTAAGA-3′ for Nek2A or 5′-CGGGATCCTTAGAATTTGCTCCATTCATTCC-3′ for Nek2B). The amplified cDNAs were then digested with NheI and BamHI and inserted into the NheI and BamHI sites of the Myc-tagged pT7G (UKII+) transcription vector, which had a human c-Myc tag downstream of the T7 promoter. All of the constructs were cut singly with NotI and then transcribed in vitro. In vitro transcription and translation (in reticulocyte lysates) were performed as described previously (Uto et al., 1999).
Antibodies
For the detection and functional inhibition of Nek2B, affinity-purified polyclonal anti-Nek2B (N1) antibody was used throughout the present study (cf. Uto et al., 1999). For microinjection, the N1 antibody was dialyzed against a buffer (88 mM NaCl, 1 mM KCl, 15 mM Tris–HCl pH 7.5; Vize et al., 1991) and concentrated to 10 mg/ml. (Upon in vitro kinase assays of Nek2B, added N1 antibody was found to act as a neutralizing antibody in a dose-dependent manner.) For Western blotting and immunostaining, the N1 antibody was dialyzed against Tris-buffered saline (TBS) and concentrated to 1 mg/ml. Anti-α-tubulin antibody (DM1A) and anti-γ-tubulin antibody (GTU88) were from Sigma, and anti-pericentrin antibody (PRB-432C) was from Covance. Rhodamine-conjugated anti-mouse IgG antibody (R0270) and fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG antibody (F0205) were from Dako.
Western blot analysis and histone H1 kinase assays
Routinely, five oocytes or embryos were homogenized in 50 µl of an extraction buffer (80 mM β-glycerophosphate, 15 mM MgCl2, 20 mM EGTA pH 7.3; Gerhart et al., 1984) and centrifuged briefly. A portion (usually 2 µl) of the supernatant was then subjected to H1 kinase assays and another portion (usually 10 µl) to Western blot analysis, essentially as described previously (Furuno et al., 1994).
Whole-mount immunostaining and confocal microscopy
Fixation and immunostaining of albino oocytes or embryos were performed essentially as described previously (Gard et al., 1990). In brief, oocytes or embryos were fixed in TGF buffer for 6 h, postfixed with methanol, rehydrated with TBS, bisected, and treated with 100 mM NaBH4 for 12 h. Samples were then washed with TBS, incubated with anti-α-tubulin antibody (1:250) or anti-γ-tubulin antibody (1:250) for 48 h, stained with rhodamine-conjugated anti-mouse IgG antibody (1:50) in TBS plus 0.1% Tween-20 (TBST) for 48 h, and washed with TBST for 48 h. Stained samples were dehydrated with methanol and cleared with a clearing solution consisting of 1 vol. of benzylalcohol and 2 vols of benzylbenzoate. Immunostained samples were analyzed by confocal microscopy using an LSM 410 (Zeiss) and images were processed by Adobe photoshop.
Preparation of egg extracts and sperm nuclei
Cytostatic factor (CSF) extracts (from unfertilized eggs), cycling extracts (from activated eggs) and demembranated sperm nuclei were prepared as described previously (Murray, 1991). CSF extracts and sperm nuclei were frozen in liquid nitrogen and stored at –80°C until use. Cycling extracts were used freshly within 3 h of preparation.
Sperm centrosome assembly and immunostaining
Sperm centrosomes were assembled in vitro as described previously (Stearns and Kirschner, 1994). Briefly, demembranated sperm nuclei were laid on a poly-l-lysine-coated cover slip, which was then applied with CSF extracts and incubated for 7 min. These cover slips were washed with TBS and fixed with methanol at –30°C for 5 min. Fixed cover slips were rehydrated in TBS, blocked with 3% bovine serum albumin (BSA) in TBST, incubated with anti-Nek2B antibody (20 µg/ml) or anti-pericentrin antibody (1:500) for 30 min, and then stained with FITC-conjugated anti-rabbit IgG antibody (1:50) for 30 min. Immunostained cover slips were mounted on glass slides with TBS containing 50% glycerol and 0.1 µg/ml 4′,6-diamidino-2-phenylindole (DAPI) and were subjected to microscopic observation.
Cell-free Nek2 degradation assays
Twenty minutes after incubation at 23°C, 30 µl of cycling extracts were mixed with 3 µl of reticulocyte lysates (containing translated Myc-Nek2A or Myc-Nek2B proteins) and were further incubated. At appropriate time points, 1.5 µl of the extracts were sampled and subjected to Western blot analysis with anti-Nek2B antibody.
Preparation, culture and microinjection of oocytes and embryos
Fully grown stage VI oocytes, unfertilized eggs and fertilized eggs were obtained as described previously (Sagata et al., 1989; Uto et al., 1999). Stage VI oocytes were cultured in 50% Leivovitz-15 medium and maturation was induced by the addition of progesterone (5 µg/ml). Unfertilized and fertilized eggs were cultured in 100% modified Barth’s solution (MBS) and 30% MBS, respectively. Microinjection of oocytes, eggs and embryos was performed in each culture medium containing 3% Ficoll by using a Nanoject Microinjector (Drummond Scientific Co.). After injection, unfertilized and fertilized eggs were cultured in 100% MBS containing 3% Ficoll and 30% MBS containing 1% Ficoll, respectively. To inhibit protein synthesis in embryos, cycloheximide (500 µg/ml) was added to the culture medium.
Acknowledgments
Acknowledgements
We thank Drs N.Furuno and N.Nakajo for valuable discussions and K.Gotoh for typing the manuscript. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan. K.U. is a research fellow of the Japan Society for the Promotion of Science.
References
- Albertson D.G. and Thomson,J.N. (1993) Segregation of holocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans. Chromosome Res., 1, 15–26. [DOI] [PubMed] [Google Scholar]
- Andersen S.S. (1999) Molecular characteristics of the centrosome. Int. Rev. Cytol., 187, 51–109. [DOI] [PubMed] [Google Scholar]
- Bobinnec Y., Khodjakov,A., Mir,L.M., Rieder,C.L., Edde,B. and Bornens,M. (1998) Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol., 143, 1575–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debec A., Detraves,C., Montmory,C., Geraud,G. and Wright,M. (1995) Polar organization of γ-tubulin in acentriolar mitotic spindles of Drosophila melanogaster cells. J. Cell Sci., 108, 2645–2653. [DOI] [PubMed] [Google Scholar]
- Dictenberg J.B., Zimmerman,W., Sparks,C.A., Young,A., Vidair,C., Zheng,Y., Carrington,W., Fay,F.S. and Doxsey,S.J. (1998) Pericentrin and γ-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol., 141, 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S.J., Stein,P., Evans,L., Calarco,P.D. and Kirschner,M.W. (1994) Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell, 76, 639–650. [DOI] [PubMed] [Google Scholar]
- Felix M.A., Antony,C., Wright,M. and Maro,B. (1994) Centrosome assembly in vitro: role of γ-tubulin recruitment in Xenopus sperm aster formation. J. Cell Biol., 124, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry A.M. and Nigg,E.A. (1995) The NIMA kinase joins forces with Cdc2. Curr. Biol., 5, 1122–1125. [DOI] [PubMed] [Google Scholar]
- Fry A.M. and Nigg,E.A. (1997) Characterization of mammalian NIMA-related kinases. Methods Enzymol., 283, 270–282. [DOI] [PubMed] [Google Scholar]
- Fry A.M., Schultz,S.J., Bartek,J. and Nigg,E.A. (1995) Substrate specificity and cell cycle regulation of the Nek2 protein kinase, a potential human homolog of the mitotic regulator NIMA of Aspergillus nidulans. J. Biol. Chem., 270, 12899–12905. [DOI] [PubMed] [Google Scholar]
- Fry A.M., Mayor,T., Meraldi,P., Stierhof,Y.D., Tanaka,K. and Nigg,E.A. (1998a) C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J. Cell Biol., 141, 1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry A.M., Meraldi,P. and Nigg,E.A. (1998b) A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J., 17, 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno N., Nishizawa,M., Okazaki,K., Tanaka,H., Iwashita,J., Nakajo,N., Ogawa,Y. and Sagata,N. (1994) Suppression of DNA replication via Mos function during meiotic divisions in Xenopus oocytes. EMBO J., 13, 2399–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D.L. (1992) Microtubule organization during maturation of Xenopus oocytes: assembly and rotation of the meiotic spindles. Dev. Biol., 151, 516–530. [DOI] [PubMed] [Google Scholar]
- Gard D.L., Hafezi,S., Zhang,T. and Doxsey,S.J. (1990) Centrosome duplication continues in cycloheximide-treated Xenopus blastulae in the absence of a detectable cell cycle. J. Cell Biol., 110, 2033–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J., Wu,M. and Kirschner,M. (1984) Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J. Cell Biol., 98, 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueth-Hallonet C., Antony,C., Aghion,J., Santa-Maria,A., Lajoie-Mazenc,I., Wright,M. and Maro,B. (1993) γ-tubulin is present in acentriolar MTOCs during early mouse development. J. Cell Sci., 105, 157–166. [DOI] [PubMed] [Google Scholar]
- Hartley R.S., Rempel,R.E. and Maller,J.L. (1996) In vivo regulation of the early embryonic cell cycle in Xenopus. Dev. Biol., 173, 408–419. [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize,R., Blank,T., Sandaltzopoulos,R., Becker,P., Hyman,A. and Karsenti,E. (1996) Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature, 382, 420–425. [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize,R., Habermann,A., Karsenti,E. and Hyman,A. (1997) Spindle assembly in Xenopus egg extracts: respective roles of centrosomes and microtubule self-organization. J. Cell Biol., 138, 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe E.H., Li,C., Thompson,E.A., Maller,J.L. and Sluder,G. (1999) Requirement of Cdk2–cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science, 283, 851–854. [DOI] [PubMed] [Google Scholar]
- Howe J.A. and Newport,J.W. (1996) A developmental timer regulates degradation of cyclin E1 at the midblastula transition during Xenopus embryogenesis. Proc. Natl Acad. Sci. USA, 93, 2060–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi H.C., Palacios,M.J., McNamara,L. and Cleveland,D.W. (1992) γ-tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature, 356, 80–83. [DOI] [PubMed] [Google Scholar]
- Kellogg D.R., Moritz,M. and Alberts,B.M. (1994) The centrosome and cellular organization. Annu. Rev. Biochem., 63, 639–674. [DOI] [PubMed] [Google Scholar]
- Krioutchkova M.M. and Onishchenko,G.E. (1999) Structural and functional characteristics of the centrosome in gametogenesis and early embryogenesis of animals. Int. Rev. Cytol., 185, 107–156. [DOI] [PubMed] [Google Scholar]
- Lacey K.R., Jackson,P.K. and Stearns,T. (1999) Cyclin-dependent kinase control of centrosome duplication. Proc. Natl Acad. Sci. USA, 96, 2817–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo F.J. (1997) Fertilization, 2nd edn. Chapman & Hall, London, UK. [Google Scholar]
- Lu K.P. and Hunter,T. (1995) Evidence for a NIMA-like mitotic pathway in vertebrate cells. Cell, 81, 413–424. [DOI] [PubMed] [Google Scholar]
- Martin O.C., Gunawardane,R.N., Iwamatsu,A. and Zheng,Y. (1998) Xgrip109: a γ tubulin-associated protein with an essential role in γ tubulin ring complex (γTuRC) assembly and centrosome function. J. Cell Biol., 141, 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P., Lukas,J., Fry,A.M., Bartek,J. and Nigg,A.E. (1999) Centrosome duplication in mammalian somatic cells requires E2F and Cdk2–cyclinA. Nature Cell Biol., 1, 88–93. [DOI] [PubMed] [Google Scholar]
- Merdes A. and Cleveland,D.W. (1997) Pathways of spindle pole formation: different mechanisms, conserved components. J. Cell Biol., 138, 953–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miake-Lye R., Newport,J. and Kirschner,M. (1983) Maturation-promoting factor induces nuclear envelope breakdown in cycloheximide-arrested embryos of Xenopus laevis. J. Cell Biol., 97, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M., Braunfeld,M.B., Fung,J.C., Sedat,J.W., Alberts,B.M. and Agard,D.A. (1995a) Three-dimensional structural characterization of centrosomes from early Drosophila embryos. J. Cell Biol., 130, 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M., Braunfeld,M.B., Sedat,J.W., Alberts,B. and Agard,D.A. (1995b) Microtubule nucleation by γ-tubulin-containing rings in the centrosome. Nature, 378, 638–640. [DOI] [PubMed] [Google Scholar]
- Moritz M., Zheng,Y., Alberts,B.M. and Oegema,K. (1998) Recruitment of the γ-tubulin ring complex to Drosophila salt-stripped centrosome scaffolds. J. Cell Biol., 142, 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A.M. (1991) Cell cycle extracts. Methods Cell Biol., 36, 581–605. [PubMed] [Google Scholar]
- Newport J.W. and Kirschner,M.W. (1984) Regulation of the cell cycle during early Xenopus development. Cell, 37, 731–742. [DOI] [PubMed] [Google Scholar]
- Nurse P. (1990) Universal control mechanism regulating onset of M-phase. Nature, 344, 503–508. [DOI] [PubMed] [Google Scholar]
- O’Connell M.J., Norbury,C. and Nurse,P. (1994) Premature chromatin condensation upon accumulation of NIMA. EMBO J., 13, 4926–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani A.H., McGuire,S.L. and Osmani,S.A. (1991) Parallel activation of the NIMA and p34cdc2 cell cycle-regulated protein kinases is required to initiate mitosis in A.nidulans. Cell, 67, 283–291. [DOI] [PubMed] [Google Scholar]
- Osmani S.A., Pu,R.T. and Morris,N.R. (1988) Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell, 53, 237–244. [DOI] [PubMed] [Google Scholar]
- Raff J.W. (1996) Centrosomes and microtubules: wedding with a ring. Trends Cell Biol., 6, 248–251. [DOI] [PubMed] [Google Scholar]
- Rhee K. and Wolgemuth,D.J. (1997) The NIMA-related kinase 2, Nek2, is expressed in specific stages of the meiotic cell cycle and associates with meiotic chromosomes. Development, 124, 2167–2177. [DOI] [PubMed] [Google Scholar]
- Sagata N., Watanabe,N., Vande Woude,G.F. and Ikawa,Y. (1989) The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature, 342, 512–518. [DOI] [PubMed] [Google Scholar]
- Sawin K.E., and Mitchison,T.J. (1991) Mitotic spindle assembly by two different pathways in vitro. J. Cell Biol., 112, 925–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnackenberg B.J., Khodjakov,A., Rieder,C.L. and Palazzo,R.E. (1998) The disassembly and reassembly of functional centrosomes in vitro. Proc. Natl Acad. Sci. USA, 95, 9295–9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S.J., Fry,A.M., Sutterlin,C., Ried,T. and Nigg,E.A. (1994) Cell cycle-dependent expression of Nek2, a novel human protein kinase related to the NIMA mitotic regulator of Aspergillus nidulans. Cell Growth Differ., 5, 625–635. [PubMed] [Google Scholar]
- Stearns T. and Kirschner,M. (1994) In vitro reconstitution of centrosome assembly and function: the central role of γ-tubulin. Cell, 76, 623–637. [DOI] [PubMed] [Google Scholar]
- Stearns T., Evans,L. and Kirschner,M. (1991) γ-tubulin is a highly conserved component of the centrosome. Cell, 65, 825–836. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Parvinen,M. and Nigg,E.A. (1997) The in vivo expression pattern of mouse Nek2, a NIMA-related kinase, indicates a role in both mitosis and meiosis. Exp. Cell Res., 237, 264–274. [DOI] [PubMed] [Google Scholar]
- Theurkauf W.E. and Hawley,R.S. (1992) Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol., 116, 1167–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uto K., Nakajo,N. and Sagata,N. (1999) Two structural variants of Nek2 kinase, termed Nek2A and Nek2B, are differentially expressed in Xenopus tissues and development. Dev. Biol., 208, 456–464. [DOI] [PubMed] [Google Scholar]
- Vize P.D., Hemmati-Brivanlou,A., Harland,R.M. and Melton,D.A. (1991) Assays for gene function in developing Xenopus embryos. Methods Cell Biol., 36, 367–387. [DOI] [PubMed] [Google Scholar]
- Vogel J.M., Stearns,T., Rieder,C.L. and Palazzo,R.E. (1997) Centrosomes isolated from Spisula solidissima oocytes contain rings and an unusual stoichiometric ratio of α/β tubulin. J. Cell Biol., 137, 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J.C. and Salmon,E.D. (1997) Pathways of spindle assembly. Curr. Opin. Cell Biol., 9, 37–43. [DOI] [PubMed] [Google Scholar]
- Wu L., Osmani,S.A. and Mirabito,P.M. (1998) A role for NIMA in the nuclear localization of cyclin B in Aspergillus nidulans. J. Cell Biol., 141, 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Jung,M.K. and Oakley,B.R. (1991) γ-tubulin is present in Drosophila melanogaster and Homo sapiens and is associated with the centrosome. Cell, 65, 817–823. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Wong,M.L., Alberts,B. and Mitchison,T. (1995) Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature, 378, 578–583. [DOI] [PubMed] [Google Scholar]