Abstract
Objectives
This clinical trial evaluated whether supplementation with flax oil, containing the omega-3 fatty acid α-linolenic acid (α-LNA), safely reduced symptom severity in youth with bipolar disorder.
Methods
Children and adolescents aged 6-17 years with symptomatic bipolar I or bipolar II disorder (n = 51), manic, hypomanic, mixed, or depressed, were randomized to either flax oil capsules containing 550 mg α-LNA per 1 gram or an olive oil placebo adjunctively or as monotherapy. Doses were titrated to 12 capsules per day as tolerated over 16 weeks. Primary outcomes included changes in the Young Mania Rating Scale, Child Depression Rating Scale-Revised, and Clinical Global Impressions-Bipolar ratings using Kaplan-Meier survival analyses.
Results
There were no significant differences in primary outcome measures when compared by treatment assignment. However, clinician-rated Global Symptom Severity was negatively correlated with final serum omega-3 fatty acid compositions: % α-LNA (r = −0.45, p < 0.007), % eicosapentaenoic acid (EPA) (r = −0.47, p < 0.005), and positively correlated with final arachidonic acid (AA) (r = 0.36, p < 0.05) and docosapentaenoic acid (DPA) n-6 (r = 0.48, p < 0.004). The mean duration of treatment for α-LNA was 11.8 weeks versus 8 weeks for placebo; however, the longer treatment duration for α-LNA was not significant after controlling for baseline variables. Subjects discontinued the study for continued depressive symptoms.
Conclusions
Studies of essential fatty acid supplementation are feasible and well tolerated in the pediatric population. Although flax oil may decrease severity of illness in children and adolescents with bipolar disorder who have meaningful increases in serum EPA percent levels and/or decreased AA and DPA n-6 levels, individual variations in conversion of α-LNA to EPA and docosahexaenoic acid as well as dosing burden favor the use of fish oil both for clinical trials and clinical practice. Additionally, future research should focus on adherence and analysis of outcome based on changes in essential fatty acid tissue compositions, as opposed to group randomization alone.
Keywords: adolescents, bipolar disorder, children, flax oil, omega-3 fatty acid
Introduction
Pediatric bipolar disorder is a difficult-to-treat, recurrent mental illness characterized by a predominant mood state of irritability and often mixed, rapid-cycling, and psychotic symptoms (1). Two systematic reviews support further study of omega-3 fatty acids in mood disorders, with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) as the studied agents (2, 3). Results of randomized, controlled trials of lithium, valproic acid, and antipsychotics for early-onset bipolar disorder offer hope of improvement for many (4, 5), yet also demonstrate the need for additional treatment options for those children who do not respond adequately to, or cannot tolerate, a first-line mood stabilizer alone or in combination with an atypical antipsychotic.
Rationale for omega-3 fatty acids
Omega-3 fatty acids are naturally occurring, diet-essential plant- or marine-based lipids that are an important component for cell membrane fluidity (6). In addition, omega-3 fatty acids may help stabilize mood by multiple mechanisms, including suppression of neuronal signaling, second messenger generation, calcium channel and protein kinase C activity, proinflammatory cytokines, and kindling (7). Seafood and eggs are rich in the longer chain (20-22 carbon) fatty acids DHA and EPA, while plant seed oils are rich in the shorter chain (18 carbon) precursor plant-derived α-linolenic acid (α-LNA). Western diets are deficient in omega-3 fats, largely due to relatively low seafood consumption and food industry preference for corn and soy oils high in the short-chain omega-6 fatty acid linoleic acid (8).
As popular over-the-counter dietary supplements, omega-3 fatty acids represent an appealing option for treatment in the younger bipolar population as they are likely to be better tolerated and cost less compared with conventional mood-stabilizing agents. In addition, they have appeal to parents and adolescents due to their perception as a ‘natural’ substance and relative lack of systemic side effects. Restoration of adequate long-chain omega-3 tissue status may be considered a reversal of a nutritional deficiency (9).
Previous work
Evidence that omega-3 fatty acids are potentially helpful in the treatment and prevention of bipolar disorder comes from a small body of epidemiologic, basic, and interventional lines of investigation. A cross-national comparison of seafood consumption and rates of bipolar disorder determined that lower rates of bipolar I, bipolar II, and spectrum disorders were correlated with increased seafood consumption based on national estimates, with a threshold dose of 50 pounds/year per capita intake for bipolar II disorder and bipolar disorder not otherwise specified (10).
Peripheral and central free fatty acid tissue compositional studies in patients and controls with unipolar and bipolar disorders have examined erythrocyte (11-13), serum (13-16), adipose (17-19), and brain (20, 21) phospholipids, and have fairly consistently reported omega-3 fatty acid deficits and/or elevated arachidonic acid (AA):EPA or AA:DHA ratios. Community samples in the U.S. (22) and of elderly (23, 24), adolescents (18), and healthy males (19) in Europe have also found inverse associations between specific or total plasma ω–3 long chain fatty acids and depressive symptoms. Specific to our population, red blood cell DHA was negatively correlated with clinician ratings of depression in a small sample of children and adolescents with juvenile bipolar disorder compared to healthy age and sex-matched controls (25). Depressive symptoms were also positively associated with the ω–6 fatty acid dihomo-γ-linolenic acid in adipose tissue of adolescents (18), and negatively associated with DHA levels in adipose tissue in healthy males (19). EPA levels in erythrocytes have also been shown to be lower in suicide attempters than in control subjects (26). The most specific evidence for central tissue compositional deficits is the finding of lower DHA in the postmortem orbitofrontal cortex of patients with major depressive disorder or bipolar disorder (20, 27). The pathophysiology of depressive disorders is thought to involve deficits in orbitofrontal cortex function. Lipid alterations in gray and white matter as well as red blood cell membranes in drug-naïve bipolar patients compared with control data have also been shown using high-throughput ultra performance liquid chromatography-mass spectrometry (21). Despite these correlations between phospholipid compositions and affective illness symptom severity, dietary or metabolic causal mechanisms have yet not been established.
Treatment studies show mixed results. Rudin (28) first reported using flax oil in an open trial of patients with mixed diagnoses and proposed that major psychotic disorders could be due to deficiencies in omega-3 fatty acids. Stoll et al. (29) found that high-dose fish oil (9.6 grams/day) improved the short-term course of bipolar disorder and was well tolerated in adults with recent mania or hypomania in a placebo-controlled, randomized, double-blind trial. The study was terminated at four months due to marked differences between the omega-3 fatty acid and olive oil groups on a planned interim analysis. The most common side effects were gastrointestinal, primarily loose stools, with no other adverse effects noted. Two small open-label add-on studies of EPA in adults with bipolar disorder reported improvement in symptoms of depression and irritability (30, 31). A 12-week double-blind study of adults with bipolar depression assigned to placebo versus 1 or 2 grams of ethyl-EPA found improvement in depressive symptoms (32). Yet Keck et al. (33) described a negative double-blind trial in adults with bipolar depression or rapid-cycling bipolar disorder treated with up to 6 grams of EPA. A randomized clinical trial (RCT) of EPA and DHA in mainstreamed schoolchildren ages 5-12 years found no improvement in the primary outcome of motor skills, but did find significant improvement in reading, spelling, attention, oppositional behavior, and impulsivity over three months of treatment (34). Following up on a successful adjunctive study of omega-3 fatty acids in adults with depression, a small RCT of EPA and DHA in children with depression ages 6-12 years showed significant improvement in depressive symptoms and clinical global improvement (35). A prospective open-label trial of EPA and DHA monotherapy found a modest improvement in manic symptoms in 7 of 20 children and adolescents with clinical bipolar disorder given up to 4300 mg of combined DHA and EPA per day over eight weeks (36). In a six-week open trial of 360 mg/day EPA and 1560 mg/day DHA among 18 subjects with juvenile bipolar disorder, red blood cell EPA and DHA were significantly higher and clinician ratings of mania and depression were significantly lower after supplementation. Parent ratings of internalizing and externalizing behaviors were also significantly lower following supplementation (37). A meta-analysis of the antidepressant efficacy of long-chain omega-3 fatty acids found significant treatment effects but cautioned that publication bias and heterogeneity of study methodology required more large-scale and well-controlled trials to determine appropriate target subjects, and best dose and composition (38). It is unknown what the best combination and dose requirements of EPA and DHA may be to obtain improvement in bipolar symptoms, and whether depression versus manic symptoms may respond preferentially to different doses.
To our knowledge, there are no prospective, randomized, controlled trials of flax oil for the treatment of bipolar disorder or selectively evaluating omega-3 fatty acids in the child and adolescent bipolar population. At the time study funding was awarded, highly palatable, concentrated forms of fish oil were not available. Additionally, the limited extent of conversion from α-LNA to EPA in the brain and the genetic variations in activity of conversion enzymes were not yet readily known. Flax oil was chosen for study as confusion exists among the public and healthcare providers about what omega-3 fatty acids are possibly beneficial. Also, in the primary author’s mood disorder specialty clinic, young patients frequently refused to consider ‘fish oil’ due to fear of fishy taste or odor, or reflux; in addition, some patients were vegetarian. Thus, flax oil presented with greater potential for acceptance and compliance for many young patients. Our primary objective was to determine if flax oil is efficacious in the pediatric bipolar population for reducing symptoms of mania and depression. A secondary objective was to examine fatty acid levels as predictors of treatment response and symptom severity.
Methods and materials
Design and human subjects
A 16-week double-blind, parallel-group, placebo-controlled design was used to study the efficacy and safety of flax oil in patients meeting eligibility criteria. Study procedures took place from November 2001 to March 2005 at the University Hospitals of Cleveland (first five patients) and at the University of Rochester Strong Memorial Hospital. Study approval was granted from the U.S. Food and Drug Administration (FDA) as Investigational New Drug #62,599; the FDA specifically requested the dose be titrated to 12 grams per day to assess tolerability fully. The Research Subject Review Board of the University of Rochester and the University Hospitals of Cleveland Institutional Review Board for Human Investigation approved the study.
Parents/guardians provided written informed consent and all subjects provided written informed consent or assent if under 16. All study subjects discussed participation with their prescribing mental health practitioner prior to enrollment; practitioners were informed that previously prescribed medication dosing should not change throughout the course of the study unless advised by the principal investigator.
Inclusion and exclusion criteria
Eligible subjects were outpatient males and females ages 6-17, with a DSM-IV diagnosis of bipolar I or bipolar II disorder, with a Clinical Global Impression-Bipolar version (CGI-BP) (39) score ≥ 3, Young Mania Rating Scale (YMRS) (37) score ≥ 4, or Children’s Depression Rating Scale (CDRS-R) (40) score ≥ 22. The subjects had failed stabilization with or were intolerant to lithium and/or valproate and/or atypical antipsychotic therapy, with therapeutic levels documented as appropriate or if subjects desired participation in the study without conventional treatment concomitantly. Subjects with comorbid attention-deficit hyperactivity disorder (ADHD) were maintained on their stimulant medication under two conditions: (i) subjects had been treated with a stimulant with good response; and (ii) there was no evidence that the stimulant medication had contributed to worsening of mood temporally or with previous prescribing changes. Subjects ineligible for enrollment included those with IQ < 70, comorbid autism, pervasive developmental disorder, history of substance abuse or positive toxicology screen, acute posttraumatic stress disorder, presence of a serious chronic medical illness, inability to swallow capsules, or female and pregnant or sexually active without reliable contraception.
Study evaluations
The study generally followed the methodology of Stoll et al. (29). Subjects were scheduled for eight visits, at baseline and end of weeks 2, 4, 6, 8, 10, 12, and 16.
At baseline, parents and children were interviewed separately by the principal investigator (PI) or research coordinator using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL) to verify bipolar diagnosis, current state and subtype, and comorbid diagnoses (41, 42). Confirmation of the diagnosis by the PI then took place. The child’s past and current medication treatment and medical histories were collected, as were height, weight, and vital signs. Parents and children were interviewed separately with the following rating scales: YMRS (37), CDRS-R (40), Children’s Psychiatric Rating Scale (CPRS) (43) modified for psychosis, Side Effects Form for Children and Adolescents (SEFCA) (44), and the K-SADS Suicidality subscale. The PI completed the Global Assessment of Functioning (GAF) (45) and CGI-BP (39). Subjects and parents were asked to complete the Child Life Chart Method prospectively. The SEFCA, a 54-item structured rating scale that rates frequency and severity of adverse events, was also completed by the research coordinator interviewing both child and parent separately.
Laboratory testing included: complete blood count, comprehensive metabolic profile, hepatic function panel, thyroid stimulating hormone (TSH) level, urine toxicology screen, pregnancy test, urinalysis, and serum lithium, valproate, or carbamazepine levels as appropriate. All subjects had a minimum eight-hour fasting fatty acid profile drawn and sent to Kennedy Kreiger Institute, Baltimore, MD, USA, for analysis.
At all follow-up visits, these measurements and ratings were recored: clinical assessment by the PI, height/weight, YMRS, CDRS-R, CPRS, CGI-BP, SEFCA, K-SADS Suicidality subscale, and concomitant medication form. Life charts were reviewed to confirm adequacy of parent report during the rating form interviews. At the end of weeks 4, 8, 12, and as needed clinically, 10- to 12-hour trough serum lithium, carbamazepine, and/or valproate levels were drawn for subjects taking any of those medications. At the end of week 16 or at the end of study, subjects also had a repeat fasting fatty acid profile, comprehensive metabolic profile, TSH level, and serum lithium, carbamazepine, and/or valproate levels as appropriate.
Diagnostic interview and rating form procedures
The K-SADS interview was administered by the PI or one of four research coordinators, who were trained to an overall κ > 0.85 over 10 interviews with an experienced rater prior to interviewing subjects and their families independently.
Study medication and dosing procedures
Research-grade flax oil and olive oil placebo were obtained through the Omega-3 Research Institute, Bethesda, MD, USA, and were analyzed for quality and purity; sufficient bioactivity was confirmed for the flax oil independently at the University of Massachusetts midway through the study. Each capsule of omega-3 fatty acid concentrate contained 550 mg of α-LNA from flax seed oil (Table 1). No flavoring was added. Eligible subjects were randomized to study medication by the research pharmacy, blocked by concomitant lithium treatment to control for any alterations in absorption of lithium as a result of flax oil use. Subjects were asked to take lithium at least two hours apart from their study medication dose. Subjects and their parents, raters, and study psychiatrist were blinded to placebo versus active compound status. The unblinded statistician (EAY) was the only member of the team with access to both assignment and outcome information in the same file prior to closure of the trial. Rater and clinician guesses about assignment status were recorded at each visit, and patient guesses about assignment status were also recorded at later visits. Subjects were provided identical light-proof bottles with identical gelatin capsules containing flax oil or research-grade olive oil labeled with the appropriate dose for that visit interval. Anticipated side effects from flax seed oil included: unpleasant taste, nausea, loose stools, and potentially mania or hypomania. Parents were advised to contact the study team about any significant worsening in the child’s condition between scheduled visits; interim visits were made as necessary. Compliance was monitored via direct pill count in returned bottles at each visit as well as through a medication log given to parents also returned at each visit.
Table 1.
Fatty acid composition of flax oil (1000 mg) and virgin olive oil (1000 mg)
| Standard specifications (active ingredients) | Formulation |
|---|---|
| Flax oil (GMO free) | 1000 mg |
| C18:3n3 (alpha linolenic acid) | 530.0 mg/capsulea |
| C18:2n6 (linoleic acid) | 121.0–177.0 mg/capsule |
| C18:1n9 (oleic acid) | 143.0–239.0 mg/capsule |
| C16:0 (palmitic acid) | 45.0–64.0 mg/capsule |
| Virgin olive oil | 1000 mg |
| C16:0 (palmitic acid) | 90 mg/capsule |
| C16:1 (palmitoleic acid) | 6 mg/capsule |
| C18:0 (stearic acid) | 27 mg/capsule |
| C18:1 (oleic acid) | 803 mg/capsule |
| C18:2 (linoleic acid) | 63 mg/capsule |
| C18:3 (linolenic acid) | 7 mg/capsule |
| C20:0 (arachidic acid) | 4 mg/capsule |
GMO = genetically modified organism.
not less than.
A stepped but flexible dose-titration schedule was carried out with doses increased by 1-2 grams at each visit as tolerated, up to an attempted total dose of 6 capsules twice per day, as suggested by the FDA (up to 6.6 grams of daily α-LNA). Psychotropic medication doses were required to be unchanged except for adjustments of mood stabilizers, to maintain serum levels within 10% of the baseline level (standardized dose adjustment protocol available on request). Subjects were considered a treatment failure and withdrawn from the study if their condition worsened over the course of two visits (either > 30% increase from baseline YMRS score, a K-SADS suicidality subscale score of 4, or clinical judgment).
Serum fatty acid levels
Plasma C8:0 to C30:0 total lipid fatty acid quantitation was performed by capillary gas chromatography-electron-capture negative-ion mass spectrometry using a modification of the method of Lagerstedt et al. (46) by the Peroxisomal Diseases Laboratory at Kennedy Kreiger Institute, Baltimore, MD, USA. Details of the sample preparation, standards, and reference values are available on request. Fatty acids were reported as γg/ml plasma as well as % total fatty acids. Flax oil versus placebo group assignment was redesignated in a secondary analysis based on whether subjects had EPA levels > 0.8% of total plasma fatty acids, redefining the placebo group as subjects who did not achieve an EPA level > 0.8% of total plasma fatty acids, based on the highest baseline individual subject EPA level of 0.8%.
Statistics
Power analysis
Given mixed findings in previous trials of omega-3 fatty acids, it was important to establish the power of the study to detect clinically meaningful effects. Because the size of the sample was set by the level of funding available in a grant mechanism, we conducted ‘sensitivity’ power analyses to determine the critical effect size (given set N, alpha, and power) (47). For the survival analyses, with 25 participants per arm, power would be 0.82 to detect a hazard ratio of ~3 or larger, as would be obtained if 24% of participants on placebo completed the maintenance period versus 62% of the flax oil participants (48). This would correspond to a number needed to treat (NNT) of 2.6, meaning that if flax oil demonstrated this degree of efficacy, then for every 2.6 youths receiving flax oil treatment, an additional youth would show good maintenance for at least four months (49). With 25 participants per arm, the detectable difference between groups (assuming alpha = 0.05 and power = 0.80 and no correlation between baseline and outcome rank) would be 0.7 points on the CGI-BP, 5.6 points on the YMRS, 7.7 points on the CDRS-R, or 1.8 points on the CPRS (50), roughly corresponding to a Cohen’s d value of 0.8. These were conservative estimates, because there is likely to be correlation between scores at baseline and study end, which would increase power to detect differences. Thus, power was adequate to detect effects that would be considered large and clinically meaningful on symptom measures as well as primary outcome.
Statistical analysis
T-tests and chi-squared analyses tested whether there were significant differences between treatment arms on demographic or clinical characteristics despite random assignment. Chi-squared analyses evaluated whether the rater, clinician, or family was able to guess accurately whether patients were assigned to the placebo or active arm. The primary outcome, whether subjects assigned to flax oil remained stable and compliant with study procedures longer than the group receiving placebo, was tested via Kaplan-Meier survival analyses, comparing the length of time in the study across the two arms. The Breslow statistic (also referred to as the ‘generalized Wilcoxon’ test) was used to compare arms, weighting observations by the number of cases still at risk at each time point (51). The Breslow test was selected a priori because both recurrent symptoms and side effects were likely to occur rapidly, whereas dropout due to unrelated factors such as moving away would be expected to occur at an even rate throughout the study. Follow-up Cox regressions evaluated, in order of entry, whether baseline severity of mania or depression, patient age or gender, or the use of concomitant lithium or antipsychotic medication had any effect on length of maintenance. All of these covariates were tested in a forward stepwise manner after entering treatment arm into the model to maximize statistical power to detect potential significant covariates for further exploration. For the survival models, endpoints were defined in three progressively broader ways: (i) leaving the study early due to clinically significant worsening mood symptoms; (ii) exiting for any clinical reason, including side effects as well as mood disturbance; (iii) exiting for any reason at all, such as moving out of the region. The Cochrane Collaborative recommends the last and most conservative analysis as the primary definition of efficacy, because it can be difficult to determine when clinical issues contribute to otherwise unrelated exit reasons (52). Supplementing the broad definition of endpoints with more narrow endpoints focusing on mood response may be valuable, however (53).
The second way of evaluating efficacy evaluated change in symptoms using mixed effects models, where repeated measures were nested within individuals (54). These analyses were run separately for YMRS, CDRS-R, CPRS, and GAF ratings to avoid obscuring efficacy by combining different aspects of treatment response (Mixed procedure, SPSS version 15, SPSS, Inc., Chicago, IL, USA). A first-order autoregressive covariance structure for the repeated measurements was used, with restricted maximum likelihood estimation, and treatment assignment treated as a fixed factor. Holm’s stepdown Bonferroni procedure was used to control Type I error rates due to the multiple outcome measures (55). Last observation carried forward (LOCF) analyses were also conducted for each outcome measure to facilitate comparison with prior published studies, although the mixed model approach is a stronger methodology. All p-values reported are two-tailed, except for chi-squared and Fisher’s exact tests, which only have one-tailed distributions.
Results
Participants
Table 2 provides a summary of participant demographic and clinical characteristics by treatment arm. Treatment arms were successfully balanced with regard to participant age, lithium, stimulant and antipsychotic use, sex, race, bipolar I disorder, current episode type, psychosis, rapid cycling, and comorbid ADHD status. The placebo arm entered the study with somewhat higher severity of mania and slightly worse global functioning based on the GAF and the CGI-BP global scores. These differences could make it slightly harder to detect a true treatment effect for omega-3 fatty acids, as statistical artifacts (regression to the mean) might create the illusion of improvement in the placebo arm (boosting the placebo effect) (56). Key outcome analyses were rerun using CGI-BP or baseline GAF as covariates to ensure that these factors did not change results substantively.
Table 2.
Demographic and clinical characteristics
| Characteristic | Placebo | Flax oil |
|---|---|---|
| Demographics | ||
| Baseline age, years, mean (SD) | 13.0 (3.6) | 13.4 (2.8) |
| Male, % | 52 | 54 |
| White, % | 86 | 88 |
| Black, Hispanic, or mixed, % | 14 | 12 |
| Clinical features | ||
| Bipolar I disorder, % | 72 | 85 |
| Psychosis, % | 32 | 15 |
| Rapid cycling, % | 40 | 23 |
| Comorbid ADHD, % | 52 | 54 |
| Baseline YMRS total, mean (SD) | 18.9 (7.3) | 16.3 (6.5) |
| Baseline CDRS-R total, mean (SD) | 36.4 (10.7) | 33.3 (8.5) |
| Baseline CPRS total, mean (SD) | 1.6 (2.9) | 0.5 (1.2) |
| Baseline CGI bipolar, mean (SD) | 3.7 (0.9)a | 3.0 (0.8) |
| Baseline CGI mania, mean (SD) | 3.4 (1.1)a | 2.8 (0.9) |
| Baseline CGI depression, mean (SD) | 2.8 (1.1) | 2.6 (0.9) |
| Euthymic at entry, n (%) | 1 (4) | 4 (15) |
| Manic at entry, n (%) | 4 (16) | 1 (4) |
| Mixed at entry, n (%) | 15 (60) | 13 (50) |
| Hypomanic only at entry, n (%) | 1 (4) | 3 (12) |
| Depressed at entry, n (%) | 4 (16) | 5 (19) |
| Past week GAF (baseline), mean (SD) | 56.0 (6.8)b | 60.5 (5.3) |
| Antipsychotic, % | 40 | 40 |
| Lithium, % | 28 | 31 |
| Last visit in study, weeks, mean (SD) | 8.4 (6.3) | 11.9 (5.2)a |
Comparisons based on t-tests for continuous variables, and chi-square for categorical variables. ADHD = attention-deficit hyperactivity disorder; YMRS = Young Mania Rating Scale; CDRS-R = Children’s Depression Rating Scale; CPRS = Children’s Psychiatric Rating Scale; CGI = Clinical Global Impression; GAF = Global Assessment of Functioning.
p < 0.05.
p < 0.01, two-tailed.
Integrity checks for randomization
Chi-squared analyses indicated that none of the guesses of subject, parent, rater, or psychiatrist matched true assignment status at any time point in the study at better than chance levels. The largest χ2 value was 1.32 (1 df), p > 0.05, even without correction for the multiple comparisons. Blinding procedures appeared effective.
Compliance
Subject compliance, as reported by medication log and direct pill count of study medication returned at each visit, is displayed in Figure 1. Based on direct pill counts, more than 75% of participants were within two pills of the dosing regimen at each visit.
Fig. 1.
Compliance: direct pill counts.
Subject exit reasons and tolerability of treatment
Table 3 presents the reasons for study exit. There were no significant differences between flax oil and placebo in the exit rate or reasons for exit [χ2 (1 df) = 1.68, n.s.], including mood problems or other adverse events There were no differences in the rates at which subjects experienced adverse events. The summary of the total adverse events were nonsignificant for count and severity comparing the two arms (p > 0.05, two-tailed), even prior to adjustments for post-hoc analysis. Participants physiologically tolerated both flax oil and placebo well, with only one patient in each arm discontinuing due to intolerable side effects. There were no differences in the rates of urinary, cardiovascular, central nervous system, ocular, dermal, muscular, or other adverse events, based on the total frequency or severity, or weekly comparisons of severe events. There were no differences in the total number of gastrointestinal or mouth/nose adverse events or the total severity; however, at week 8 there were four adverse gastrointestinal and mouth/nose events in the placebo arm and none in the flax oil arm (Fisher’s exact p = 0.051). Specific summary information by system is listed in Table 3. No patients experienced persistent or worsening manic or hypomanic symptoms that led to study discontinuation. Instead, the intractable mood issues pertained primarily to depression. There was one serious adverse event, with a patient in the placebo arm attempting suicide. Seven (14%) discontinued for mood-related issues, 15 (30%) due to other clinical issues, and 27 (53%) for any other reason.
Table 3.
Discontinuation reasons by treatment arm and safety data
| Exit reason | Placebo n (%) |
Flax oil n (%) |
Total n (%) |
|---|---|---|---|
| Mood reasons | 5 (20) | 3 (12) | 8 (16) |
| LOE: mania | 0 (0) | 0 (0) | 0 (0) |
| LOE: hypomania | 0 (0) | 0 (0) | 0 (0) |
| LOE: continued cycling | 2 (8) | 1 (4) | 3 (6) |
| LOE: depression | 1 (4) | 2 (8) | 3 (6) |
| LOE: psychosis | 1 (4) | 0 (0) | 1 (2) |
| SAE: suicide attempt | 1 (4) | 0 (0) | 1 (2) |
| Other clinical reasons | 6 (24) | 5 (19) | 11 (22) |
| Medication noncompliance | 2 (8) | 4 (15) | 6 (12) |
| Substance abuse | 2 (8) | 0 (0) | 2 (4) |
| Intolerable side effects | 1 (4) | 1 (4) | 2 (4) |
| Protocol violation | 1 (4) | 0 (0) | 1 (2) |
| Other reasons | 5 (20) | 3 (12) | 8 (16) |
| Withdrew consent | 4 (16) | 3 (12) | 7 (14) |
| Lost to follow-up | 0 (0) | 0 (0) | 0 (0) |
| Refusal to swallow capsules | 1 (4) | 0 (0) | 1 (2) |
| All cause discontinuation | 16 (64) | 11 (42) | 27 (53) |
| Completed all visits | 9 (36) | 15 (58) | 24 (47) |
| Total | 25 (100) | 26 (100) | 51 (100) |
| Safety data | Mean (SD) | Mean (SD) | F |
|---|---|---|---|
| Gastrointestinala | 2.6 (3.2) | 0.6 (0.8) | 7.57c |
| Mouth/nosea | 7.0 (7.8) | 4.7 (4.9) | 0.88 |
| Central nervous system AEb | 11.4 (7.4) | 9.6 (7.9) | 0.64 |
| Cardiovascular AEb | 0.6 (1.9) | 1.0 (2.2) | 0.23 |
Comparisons based on chi-square for categorical variables. LOE = lack of efficacy; SAE = serious adverse event; AE = adverse event.
Total events by week 16
Total events.
p < 0.05.
Survival analyses
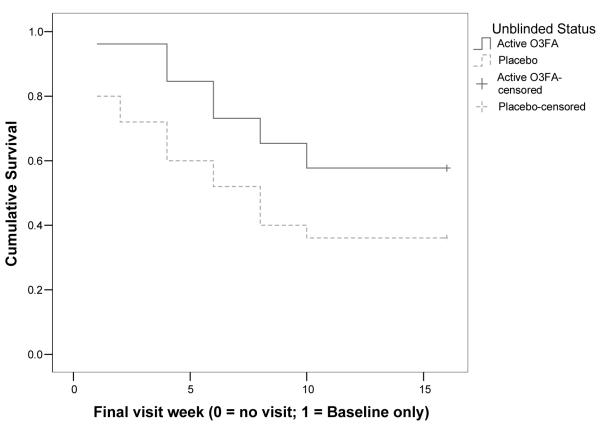
Examination of the survival curves in Figure 1 demonstrates a difference between arms driven by a larger amount of discontinuation within the first week in the placebo arm (5 cases versus 1 case in the flax oil arm); after that week the survival curves remain parallel. The flax oil group had significantly lower rates of discontinuation due to any reason [Breslow χ2 (1 df) = 4.17, p = 0.041]. Results did not change when stratified on antipsychotic use. The number of cases on lithium within each arm (n = 7 and 8) was too small to stratify based on lithium assignment. In the Cox regression, baseline CGI-BP levels predicted discontinuation. After controlling for treatment status and baseline CGI-BP, the treatment arms no longer separated. Baseline YMRS, CDRS-R, CPRS, and concomitant lithium or antipsychotic use did not enter the model (all p’s > 0.10). The mean length maintained on flax oil was 11.8 weeks, versus 8.4 weeks on placebo; median survival could not be estimated for the flax oil arm because more than 50% of participants continued to the end of the study.
There was no difference between treatments on time until mood issues forced discontinuation [mean = 14.8 weeks for flax oil versus 13.1 weeks placebo; Breslow χ2 (1 df) = 1.95, n.s.]. Similarly, time until discontinuation due to other clinical concerns did not differ for the treatments [mean = 13.0 weeks flax oil versus 10.3 weeks placebo; Breslow χ2 (1 df) = 3.17, n.s.]. No covariates predicted mood events. The baseline level of psychotic symptoms significantly increased the risk of adverse clinical events (p = 0.045), but the difference between treatment arms did not achieve significance even when controlling for baseline levels of psychotic symptoms post hoc. Median survival times could not be estimated because more than 50% of the sample did not have the index events.
Differences in symptom reduction or global functioning
No differences between flax oil and placebo were detected or found on either the slope or the intercept of any of the measures of mood symptoms or global functioning in the mixed models (all p’s > 0.05). The first order autoregressive covariance structure fit appropriately. Adding concomitant antipsychotics or lithium as covariates, along with sex, did not change the significance of the difference between treatment arms (all p’s > 0.05, even before correction for multiple outcomes). Both placebo and flax oil groups showed essentially parallel rates of improvement. The LOCF analyses produced similar conclusions to the mixed models. Both groups experienced small amounts of improvement over the course of the study: overall, there was a 1.5 point increase in GAF (p < 0.05), a 2.3 point reduction in YMRS (p < 0.05), and a 2.9 point reduction in CDRS-R scores (p = 0.055). There were no significant differences in amount of change between treatment arms. Effect sizes for treatment effects were consistently small (i.e., largest partial eta squared value of 0.01). Given the modest amounts of change, negative findings are unlikely to be due to high placebo response.
Reanalysis of data based on serum fatty acid levels
Comparison analyses were rerun examining LOCF data redefining the placebo group as subjects who did not achieve an EPA level > 0.8% of total plasma fatty acids, based on the highest baseline individual subject EPA level of 0.8%. Complete fatty acid data were available for 34 subjects. At baseline, only one subject had an EPA level > 0.8%. At final analysis, only 8 subjects had EPA levels > 0.8%, i.e., clearly had evidence of conversion of flax oil supplementation to EPA. Groups were therefore reassigned with 8 subjects now in the flax oil group and 26 in the placebo group. Results are shown in Tables 4 and 5 and Figures 2, 3, and 4. For CGI mania and overall severity of illness, there is a significant protective effect from elevated levels of 18:3n-3 (α-LNA) and 20:5n-3 (EPA), but not for 22:6n-3 (DHA). Significant worsening in CGI mania and overall severity of illness scores were correlated with elevated levels of most of n-6 highly unsaturated fatty acids, particularly notable for AA (20:4n-6) and docosapentaenoic acid [(DPA) (n-3; 22:5n-6)]. No effect was noted for depression or YRMS, CDRS-R, or CPRS at baseline versus LOCF. The final plasma EPA level (classified as > 0.8% or < 0.8%) was associated with Global Severity of Bipolar Illness at the last visit (Fig. 4). Serum lithium levels did not change significantly as a result of flax oil treatment; nor did lithium treatment significantly change the level of absorption of fatty acids (all p’s > 0.05).
Table 4.
Percent final fatty acid composition and clinician-rated Global Severity of Illness
| C18:3(n-3) α-LNA | C20:5(n-3) EPA |
C22:6(n-3) DHA |
C20:3(n-6) Dihomo-γ- linolenic |
C20:4(n-6) AA |
C22:2(n-6) Docosadienoic |
C22:4(n-6) Adrenic |
C22:5(n-6) DPA |
AA/EPA | AA/DHA | n-3 HUFA | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CGI-maniaa | |||||||||||
| r = | −0.41 | −0.38 | −0.01 | 0.32 | 0.38 | 0.36 | 0.42 | 0.35 | 0.39 | 0.29 | −0.46 |
| p < | 0.017 | 0.026 | 0.970 | 0.065 | 0.025 | 0.036 | 0.015 | 0.041 | 0.022 | 0.094 | 0.007 |
| CGI-depressiona | |||||||||||
| r = | −0.25 | −0.17 | 0.28 | 0.22 | 0.30 | 0.14 | 0.15 | 0.39 | 0.26 | −0.12 | −0.18 |
| p < | 0.159 | 0.328 | 0.103 | 0.222 | 0.090 | 0.440 | 0.411 | 0.022 | 0.138 | 0.508 | 0.306 |
| CGI-overall bipolar illnessa |
|||||||||||
| r = | −0.45 | −0.47 | 0.04 | 0.35 | 0.36 | 0.44 | 0.31 | 0.48 | 0.50 | 0.19 | −0.51 |
| p < | 0.007 | 0.005 | 0.824 | 0.045 | 0.036 | 0.009 | 0.071 | 0.004 | 0.002 | 0.289 | 0.002 |
Bolded values indicate significant results. α-LNA = alpha linolenic acid; EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid; AA = arachidonic acid; DPA = docosapentaenoic acid; HUFA = highly unsaturated fatty acids; CGI = Clinical Global Impressions.
n = 34.
Table 5.
Subject fasting plasma n-3 and n-6 fatty acid (FA) compositions at baseline and endpoint
| Percent total fatty acids | |||||
|---|---|---|---|---|---|
| Placebo group | Flax group | ||||
| Pre (n = 19) | Post (n = 15) | Pre (n = 26) | Post (n = 20) | p < | |
| 18:3n-3 (Alpha linolenic) | 0.52 (0.23) | 0.45 (0.13) | 0.80 (0.81) | 1.41 (1.17) | 0.00002 |
| 20:5n-3 [Eicosapentaenoic (EPA)] | 0.37 (0.18) | 0.35 (0.16) | 0.45 (0.35) | 0.56 (0.36) | 0.00002 |
| 22:5n-3 [Docosapentaenoic (n-3)] | 0.38 (0.08) | 0.38 (0.10) | 0.38 (0.09) | 0.61 (0.22) | 0.0002 |
| 22:6n-3 [Docosahexanoic (DHA)] | 1.03 (0.37) | 1.09 (0.39) | 1.09 (0.32) | 1.09 (0.26) | ns |
| 18:2n-6 (Linoleic) | 26.76 (3.90) | 24.60 (3.11) | 26.85 (2.57) | 25.62 (3.27) | ns |
| 20:3n-6 (Dihomo-γ-linolenic) | 1.84 (0.38) | 1.71 (0.34) | 1.66 (0.41) | 1.64 (0.33) | ns |
| 20:4n-6 [Arachidonic (AA)] | 7.74 (1.58) | 6.48 (1.38) | 7.14 (1.50) | 6.28 (1.33) | 0.02 |
| 22:4n-6 (Adrenic) | 0.01 (0.00) | 0.01 (0.00) | 0.28 (0.06) | 0.26 (0.05) | ns |
| 22:5n-6 (Docosapentaenoic) | 0.34 (0.14) | 0.37 (0.18) | 0.28 (0.11) | 0.26 (0.10) | 0.0002 |
| AA/EPA ratio | 23.59 (8.49) | 22.85 (13.14) | 22.72 (10.80) | 16.88 (11.14) | 0.000009 |
| AA/DHA ratio | 8.02 (1.88) | 6.30 (1.31) | 7.10 (2.72) | 5.98 (1.57) | ns |
| Total monounsaturated FA | 20.26 (1.93) | 21.11 (1.48) | 20.17 (1.93) | 19.70 (1.48) | 0.007 |
| Total saturated FA | 32.67 (1.68) | 34.86 (0.83) | 32.31 (1.48) | 35.21 (1.51) | ns |
| EPA < 0.8% group | EPA > 0.8% group | ||||
|---|---|---|---|---|---|
| Pre (n = 26) | Post (n = 26) | Pre (n = 8) | Post (n = 8) | p < | |
| 18:3n-3 (Alpha linolenic) | 0.43 (0.18) | 0.58 (0.42) | 0.50 (0.16) | 2.11 (1.06) | 0.00003 |
| 20:5n-3 [Eicosapentaenoic (EPA)] | 0.34 (0.17) | 0.31 (0.12) | 0.33 (0.13) | 0.90 (0.31) | 0.000002 |
| 22:5n-3 [Docosapentaenoic (n-3)] | 0.37 (0.10) | 0.37 (0.10) | 0.40 (0.08) | 0.67 (0.19) | 0.00004 |
| 22:6n-3 [Docosahexanoic (DHA)] | 0.96 (0.34) | 1.02 (0.33) | 1.04 (0.22) | 1.25 (0.30) | 0.02 |
| 18:2n-6 (Linoleic) | 26.91 (3.90) | 25.44 (3.52) | 26.17 (2.11) | 24.97 (2.97) | ns |
| 20:3n-6 (Dihomo-γ-linolenic) | 1.84 (0.34) | 1.67 (0.32) | 1.79 (0.29) | 1.47 (0.33) | ns |
| 20:4n-6 [Arachidonic (AA)] | 7.63 (1.51) | 6.40 (1.22) | 7.71 (1.35) | 5.70 (1.03) | ns |
| 22:4n-6 (Adrenic) | 0.34 (0.07) | 0.31 (0.06) | 0.32 (0.06) | 0.24 (0.06) | 0.05 |
| 22:5n-6 (Docosapentaenoic ) | 0.33 (0.14) | 0.35 (0.17) | 0.33 (0.10) | 0.20 (0.06) | 0.06 |
| AA/EPA ratio | 25.82 (9.01) | 23.62 (11.70) | 25.73 (7.90) | 7.20 (3.30) | 0.00005 |
| AA/DHA ratio | 8.41 (1.83) | 6.59 (1.29) | 7.59 (1.32) | 4.69 (0.91) | 0.008 |
| Total monounsaturated FA | 23.47 (2.38) | 23.74 (2.35) | 23.88 (1.92) | 23.88 (1.92) | ns |
| Total saturated FA | 30.06 (2.00) | 33.27 (0.97) | 30.14 (1.17) | 30.14 (1.17) | ns |
Values presented as mean (SD) of each fatty acid. P-value is indicated for repeated measures MANOVA comparisons of pre- and post-testing by group assignment (flax or placebo) and by change in plasma EPA percent (0.08 cut-off) total fatty acids. ns = not significant.
Fig. 2.
Comparing time maintained successfully in study for flax oil versus placebo. O3FA = omega-3 fatty acids.
Fig. 3.
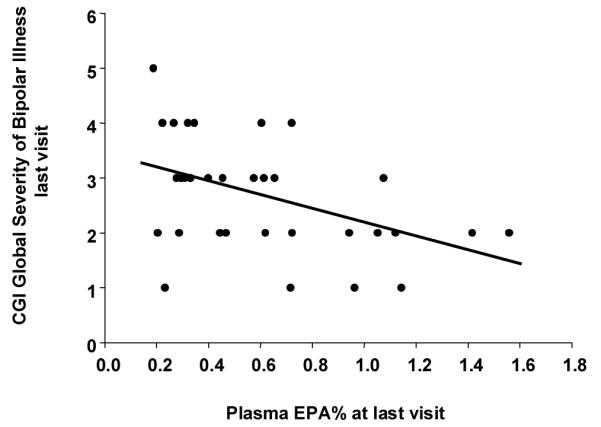
Correlation between plasma eicosapentaenoic acid (EPA) (% total fatty acid) and Global Severity of Bipolar Illness at last visit illustrated by a scattergram and linear regression line (r = 0.47, p < 0.005, n = 34). Subjects with final plasma EPA% of < 0.8% had greater symptom severity compared to those with final plasma EPA% of > 0.8% (ANOVA, p < 0.1). CGI = Clinical Global Impression.
Fig. 4.
Comparison between plasma eicosapentaenoic acid (EPA) and Global Severity of Illness. Boxes represent mean, and whiskers equal 95% confidence interval of Global Severity of Bipolar Illness at last visit. Subjects with final plasma EPA% of < 0.8% were compared to those with final plasma EPA% of > 0.8% (ANOVA, p < 0.01). At baseline, plasma EPA (% total fatty acid) did not exceed 0.8% for all but one subject.
Discussion
The goal of the present study was to evaluate whether flax oil augmentation led to better mood stabilization than placebo using a double-blinded RCT. The results appear valid as raters, clinicians, and participants did not penetrate the blind. Although the group receiving flax oil stayed in the study significantly longer than the group receiving placebo (58% versus 36% at 16 weeks), time maintained on flax oil versus placebo was not significantly different after controlling for baseline severity of bipolar illness, patient sex, or concomitant lithium or antipsychotic use.
In addition, analyzed by randomization to group assignment alone, flax oil did not appear to have any major effect on symptom reduction, as measured by clinician ratings on the CDRS-R, YMRS, CPRS, GAF, or CGI-BP. On all of these measures, mixed-effects regression models found that patients in both arms showed small but significant improvement in symptoms and functioning over time, but the trajectories of improvement were essentially identical comparing the flax oil and the placebo groups.
When data were analyzed by reassigning groups based on meaningful change in EPA levels, only 8 of 34 (24%) were considered to have adequate exposure to flax oil. In this group, there was clinician-rated evidence of response, with improvement in overall illness and mania (Fig. 4). Producing this change in EPA levels required a dose of 10-12 capsules per day, and the majority of subjects were either unable to comply or could have had a variant in fatty acid desaturase enzyme production limiting conversion of α-LNA to EPA. Thus, persistence with similar regimens in future study is not practical.
In terms of adverse events, flax oil was well tolerated up to a dose of 12 grams per day in children as young as 6 years of age. This study provides tolerance data that may be useful to investigators in other areas of medicine examining flax oil in the pediatric population, such as rheumatology or preventive cardiology. In addition, we found no evidence that lithium inhibits absorption of flax oil.
Limitations
Although the 16-week follow-up is much longer than typical acute trials, and may have been associated with ‘study fatigue’ given the possibility of low compliance rates with capsule dosage, this may not have been an adequate length of follow-up to evaluate long-term risks and clinical benefits of flax oil usage. In all three survival models, the length of follow-up was too short to allow estimation of the median survival time for the flax oil group.
A second limitation is that many of the secondary analyses are based on small numbers of cases. Given the growing evidence that genetic polymorphisms moderate treatment response to a variety of other compounds, it is reasonable to expect that flax oil will have different efficacy within certain subgroups. The present study, although one of the larger RCTs of flax oil to date, is too small to provide more than exploratory analyses with regard to subgroups. For all analyses, statistical power was adequate only for large-sized effects, but not for medium or smaller effects. However, given the tolerability of treatment, it is possible that small effects would still provide a good risk/benefit ratio; thus, this study may have been underpowered to detect effects that still might have some utility. The enrollment criteria would accept cases that were potentially euthymic at baseline. This could have reduced the severity and range of mood symptoms, possibly reducing the estimates of treatment effects. These concerns were mitigated by the high median levels of mood symptoms in both arms, with fewer than 10% of the cases being euthymic at entry and more than 50% experiencing at least moderate levels of manic or depressive symptomatology. Thus, restriction in range of mood symptoms does not appear to be a large contributor to the pattern of findings.
A third limitation is that participants in the trial were predominantly white and middle class. This is important to consider when generalizing these findings to other settings, not only because of differences or similarities in culture, but also because genetic polymorphisms could potentially result in varying efficacy for flax oil augmentation.
Another limitation is in the secondary analysis of the reanalysis of data based on EPA levels > 0.8%. As the sample was extremely small, the results are hypothesis-generating and provide possible explanations for the overall negative result.
Finally, other factors may be operating that are unknown but confounding. Potential factors may be that if flax oil has an effect on mood symptoms for those who are able to respond, it may be subtle and slow, and not detectable within 16 weeks. It may also be that if individuals with bipolar disorder are to benefit from omega-3 fatty acids, intervention may be most helpful prior to the time point at which symptoms are persisting despite active psychotropic treatment. Other dietary factors may also result in poor absorption or conversion of α-LNA to EPA and DHA, including a diet high in linoleic acid, in the n-6 family of fatty acids (57). In addition, there are now known variants in the fatty acid desaturases which are the rate-limiting enzymes in the desaturation of linoleic acid to AA, and α-LNA to EPA and DHA. Minor alleles of human delta-5 and delta-6 desaturase genes FADS1 and FADS2 have resulted in increased α-LNA, decreased AA, and decreased EPA (58). Thus, for flax oil to be useful to brain, its active omega-3 fatty acid present in flax oil, α-LNA, requires transformation in patients’ bodies. As we do not know the exact proportion of these patients in general and specific populations, flax oil is not likely to be the most appropriate omega-3 fatty acid supplementation strategy for further research or clinical use. It is possible that these single nucleotide polymorphisms contributed to our findings for an unknown proportion of subjects.
In summary, our study finds highly preliminary, possible evidence of improvement in clinical global impression of illness and mania in children and adolescents with bipolar I or bipolar II disorder taking flax oil at the full dose of 10-12 capsules a day for subjects who demonstrated an increase in EPA. Our study also confirms the findings of Sublette et al. (15) that manic symptom severity correlated negatively with levels of free AA and free EPA. Although flax oil is an inexpensive, well-tolerated compound with few deleterious effects and with other potential benefits for general physical health, including cholesterol metabolism (59) and arterial compliance in the obese (60), the number of capsules needed to produce an EPA level > 0.8% is prohibitive for most children in real-world settings. Combined EPA and DHA are therefore preferable as conversion rate limitations of α-LNA to EPA are not present and a lower overall dose with fewer capsules is recommended. Other publications utilizing EPA and DHA in the pediatric population support this recommendation.
When modest gain is set against low cost and negligible risk, adjunctive omega-3 fatty acid treatment is a relatively inexpensive way of increasing positive outcomes at the population level for many children followed by developmental pediatric and mental health professionals. Due to the limitations of α-LNA supplementation, including the large dosing required and the variable ability to convert α-LNA to EPA and DHA, future research in the child and adolescent mood disorder population should be directed toward fish oil. Larger, more definitive intervention studies for children with clinical and subclinical symptoms, as well as prevention studies for children at risk, that carefully focus on adherence and changes in free fatty acid levels as mediating variables may help further define the role of omega-3 fatty acids in pediatric bipolar disorders.
Acknowledgements
This research was funded by the Stanley Medical Research Foundation, and National Research Service Award Institutional Training Grant T32-MH-018911. This publication was made possible by Grant #1-KL2-RR024136-1 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or the NIH. Information on the NCRR is available at http://www.ncrr.nih.gov/. Information on re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overviewtranslational.asp.
We thank the families who participated in the study and also thank Jeffrey Swan, Resa Whipkey, Lauren Rios, Stephen Bean, Michael Banchy, and Carolyn Myers for their assistance.
Footnotes
BLG has served as a consultant for Janssen/Johnson & Johnson. MCC, SC, TLF, EAY, and JRH have no conflicts of interest to report in relation to this manuscript.
These data were presented in part at the NIMH Pediatric Bipolar conference, April 1, 2006, Chicago, IL, USA and at the American Academy of Child and Adolescent Psychiatry 54th Annual Meeting, Boston, MA, USA, October 23, 2007 poster session.
References
- 1.Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry. 2005;162:58–64. doi: 10.1176/appi.ajp.162.1.58. [DOI] [PubMed] [Google Scholar]
- 2.Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 3.Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. Am J Psychiatry. 2006;163:969–978. doi: 10.1176/ajp.2006.163.6.969. [DOI] [PubMed] [Google Scholar]
- 4.McClellan J, Kowatch R, Findling RL. Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:107–125. doi: 10.1097/01.chi.0000242240.69678.c4. [DOI] [PubMed] [Google Scholar]
- 5.Nandagopal JJ, DelBello MP, Kowatch R. Pharmacologic treatment of pediatric bipolar disorder. Child Adolesc Psychiatr Clin N Am. 2009;18:455–469. doi: 10.1016/j.chc.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 7.Stahl LA, Begg DP, Weisinger RS, Sinclair AJ. The role of omega-3 fatty acids in mood disorders. Curr Opin Investig Drugs. 2008;9:57–64. [PubMed] [Google Scholar]
- 8.Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutri. 2006;83:1483S–1493S. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- 9.Hibbeln JR. Depression, suicide and deficiencies of omega-3 essential fatty acids in modern diets. World Rev Nutr Diet. 2009;99:17–30. doi: 10.1159/000192992. [DOI] [PubMed] [Google Scholar]
- 10.Noaghial S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–2227. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- 11.Edwards R, Peet M, Shay J, et al. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 12.Peet M, Murphe B, Shay J, et al. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 13.Maes M, Smith RS, Christophe A, et al. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20:4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord. 1996;38:35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 14.Adams PB, Lawson S, Sanigorski A, et al. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31:S157–S161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- 15.Sublette ME, Bosetti F, DeMar JC, et al. Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord. 2007;9:759–765. doi: 10.1111/j.1399-5618.2007.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered omega–3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 1999;85:275–291. doi: 10.1016/s0165-1781(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 17.Mamalkis G, Kiriakakis M, Tsibinos G, Kafatos A. Depression and adipose polyunsaturated fatty acids in the survivors of the Seven Countries Study population of Crete. Prostagladins Leukot Essent Fatty Acids. 2004;70:495–501. doi: 10.1016/j.plefa.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Mamalakis G, Jansen E, Cremers H, Kiriakakis M, Tsibinos G, Kafatos A. Depression and adipose and serum cholesteryl ester polyunsaturated fatty acids in the survivors of the seven countries study population of Crete. Eur J Clin Nutr. 2006;60:1016–1023. doi: 10.1038/sj.ejcn.1602413. [DOI] [PubMed] [Google Scholar]
- 19.Mamalakis G, Tornaritis M, Kafatos A. Depression and adipose essential polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2002;67:311–318. doi: 10.1054/plef.2002.0435. [DOI] [PubMed] [Google Scholar]
- 20.McNamara RK, Jandacek R, Rider T, et al. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160:285–299. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarz E, Prabakaran S, Whitfield P, et al. High throughput lipidomic profiling of schizophrenia and bipolar disorder brain tissue reveals alterations of free fatty acids, phosphatidylcholines, and ceramides. J Proteome Res. 2008;7:4266–4277. doi: 10.1021/pr800188y. [DOI] [PubMed] [Google Scholar]
- 22.Conklin SM, Manuck SB, Yao JK, Flory JD, Hibbeln JR, Muldoon MF. High omega–6 and low omega–3 fatty acids are associated with depressive symptoms and neuroticism. Psychosom Med. 2007;69:932–934. doi: 10.1097/PSY.0b013e31815aaa42. [DOI] [PubMed] [Google Scholar]
- 23.Feart C, Peuchant E, Letenneur L, et al. Plasma eicosapentaenoic acid is inversely associated with severity of depressive symptomatology in the elderly: data from the Bordeaux sample of the Three-City Study. Am J Clin Nutr. 2008;87:1156–1162. doi: 10.1093/ajcn/87.5.1156. [DOI] [PubMed] [Google Scholar]
- 24.Tiemeier H, van Tuijl HR, Hofman A, Kiliaan AJ, Breteler MM. Plasma fatty acid composition and depression are associated in the elderly: the Rotterdam Study. Am J Clin Nutr. 2003;78:40–46. doi: 10.1093/ajcn/78.1.40. [DOI] [PubMed] [Google Scholar]
- 25.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids. 2008;43:1031–1038. doi: 10.1007/s11745-008-3224-z. [DOI] [PubMed] [Google Scholar]
- 26.Huan M, Hamazaki K, Sun Y, et al. Suicide attempt and n–3 fatty acid levels in red blood cells: a case-control study in China. Biol Psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 27.McNamara RK, Hahn CG, Jandacek R, et al. Selective deficits in the omega–3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 28.Rudin DO. The major psychoses and neuroses as omega-3 essential fatty acid deficiency syndrome: substrate pellagra. Biol Psychiatry. 1981;16:837–50. [PubMed] [Google Scholar]
- 29.Stoll AL, Severus WE, Freeman MP, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- 30.Osher Y, Bersudsky Y, Belmaker RH. Omega-3 eicosapentanoic acid in bipolar depression: report of a small open-label study. J Clin Psychiatry. 2005;66:726–739. doi: 10.4088/jcp.v66n0608. [DOI] [PubMed] [Google Scholar]
- 31.Sagduyu K, Dokucu ME, Eddy BA, Craigen G, Baldaswsano CF, Yildiz A. Omega-3 fatty acids decreased irritability of patients with bipolar disorder in an add-on, open label study. Nutr J. 2005;4:6. doi: 10.1186/1475-2891-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosoapentaenoic acid in bipolar depression: randomized double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50. doi: 10.1192/bjp.188.1.46. [DOI] [PubMed] [Google Scholar]
- 33.Keck PE, Jr, Mintz J, McElroy SL, et al. Double-blind, randomized, placebo-controlled trials of ethyl-sicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry. 2006;60:1020–1022. doi: 10.1016/j.biopsych.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 34.Richardson AJ, Montgomery P. The Oxford-Durham study: a randomized controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics. 2005;115:1360–1366. doi: 10.1542/peds.2004-2164. [DOI] [PubMed] [Google Scholar]
- 35.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled double-blind pilot study. Am J Psychiatry. 2006;163:1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- 36.Wozniak J, Biderman J, Mick E, et al. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17:440–447. doi: 10.1016/j.euroneuro.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 38.Lin P, Su K. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 39.Spearing MK, Robert MP, Leverich GS, Brandt D, Nolen W. Modification of the clinical global impressions (CGI) scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73:159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 40.Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64:442–450. [PubMed] [Google Scholar]
- 41.Lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 43.Spencer EK, Alpert M, Pouget ER. Scales for the assessment of neuroleptic response in schizophrenic children: specific measures derived from the CPRS. Psychopharmacol Bull. 1994;4:199–202. [PubMed] [Google Scholar]
- 44.Klein RG, Abikoff H, Barkley RA, et al. Clinical trials in children and adolescents. In: Prien RF, Robinson DS, editors. Clinical Evaluation of Psychotropic Drugs: Principles and Guidelines. Raven Press; New York: 1994. pp. 508–509. [Google Scholar]
- 45.Moos RH, Nichol A, Moos B. Global assessment of functioning ratings and the allocation and outcome of mental health care. Psychiatr Serv. 2002;53:730–737. doi: 10.1176/appi.ps.53.6.730. [DOI] [PubMed] [Google Scholar]
- 46.Lagerstedt SA, Hinrichs DR, Batt SM, Magera MJ, Rinaldo P, McConnell JP. Quantitative determination of plasma c8-c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol Genet Metab. 2001;73:38–45. doi: 10.1006/mgme.2001.3170. [DOI] [PubMed] [Google Scholar]
- 47.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 48.Borenstein M, Rothstein H, Cohen J, Schoenfeld D, Berlin J. Power and Precision. Version 2.1 Lawrence Erlbaum Assoc, Inc; New York: 2001. [Google Scholar]
- 49.Sackett DL, Straus SE, Richardson WS, Rosenberg W, Haynes RB. Evidence-Based Medicine: How to Practice and Teach EBM. 2nd ed Churchill Livingstone; New York: 2000. [Google Scholar]
- 50.van Belle G. Statistical Rules of Thumb. Wiley; New York: 2002. [Google Scholar]
- 51.Breslow N. Covariance analysis of censored survival data. Biometrics. 1974;30:89–99. [PubMed] [Google Scholar]
- 52.Guyatt GH, Rennie D. Users’ Guides to the Medical Literature. AMA Press; Chicago: 2002. [PubMed] [Google Scholar]
- 53.Findling RL, McNamara NK, Youngstrom EA, Stansbrey R, Gracious BL, Reed MD. Double-blind 18-month trial of lithium versus divalproex maintenance treatment in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:409–417. doi: 10.1097/01.chi.0000155981.83865.ea. [DOI] [PubMed] [Google Scholar]
- 54.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed Sage Publications, Inc.; Newbury Park, CA: 2002. [Google Scholar]
- 55.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 56.Campbell DT, Kenny DA. A Primer on Regression Artifacts. The Guilford Press; New York: 1999. [Google Scholar]
- 57.Gerster H. Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Intern J Vit Nutr Res. 1998;68:159–173. [PubMed] [Google Scholar]
- 58.Schaeffer L, Gohlke H, Muller M, et al. Common genetic variants of the FDAS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet. 2006;15:1745–1756. doi: 10.1093/hmg/ddl117. [DOI] [PubMed] [Google Scholar]
- 59.Bierenbaum ML, Reichstein R, Watkins TR. Reducing atherogenic risk in hyperlipemic humans with flax seed supplementation: a preliminary report. J Am Coll Nutr. 1993;12:501–504. doi: 10.1080/07315724.1993.10718342. [DOI] [PubMed] [Google Scholar]
- 60.Nestel PJ, Pomeroy SE, Sasahara T, et al. Arterial compliance in obese subjects is improved with dietary plan n-3 fatty acid from flaxseed oil despite increased LDL oxidizability. Arterioscler Thromb Vasc Biol. 1996;17:1163–1170. doi: 10.1161/01.atv.17.6.1163. [DOI] [PubMed] [Google Scholar]