Paraoxonase 1 (PON1) is a member of a tandem 3-gene family localized on human chromosome 7q21-22.1 High-density lipoprotein-associated PON12,3 and PON34,5 are synthesized primarily in the liver, whereas PON2 is ubiquitously expressed.1 PON1 was initially characterized and named for its ability to hydrolyze paraoxon, the toxic oxon metabolite of parathion.6 Although Aldridge6 proposed in 1953 that serum paraoxonase (POase) and arylesterase (AREase) activities were carried out by the same enzyme, controversy about 1 versus 2 enzymes persisted for many years, resulting in a reclassification of POase/AREase from EC 3.1.1.2 to EC 3.1.8.1 for PON1 as an example of an organophosphorus (OP) hydrolase.7 The controversy was finally settled when Sorenson et al8 demonstrated both activities in recombinant PON1. However, the revised nomenclature remains in place. Early studies of plasma PON1 found a large variability of POase activity among different species and in different tissues.6 Serum POase activity distribution studies in human populations revealed an activity polymorphism of high versus low POase activity. Studies on the polymorphic distribution of PON1 in human populations using a variety of different assays revealed bi or trimodal distributions of plasma POase activity (reviewed in Ref.9).
Our initial characterization of the human PON1 cDNA clones revealed 2 coding region polymorphisms Q192R and L55 M.10 Subsequently, it was shown that the Q192R polymorphism determined high versus low rates of paraoxon hydrolysis by the enzyme, with the PON1R192 alloform specifying high activity.11,12 After the demonstration that high-density lipoprotein-associated PON1 was implicated in reducing low-density lipoprotein13 and high-density lipoprotein14 oxidation, epidemiological studies were undertaken to explore the possible role of genetic variability of PON1 in cardiovascular disease15 (reviewed in Ref.16). Several meta-analyses of studies that examined only the association of PON1 genotypes with risk of vascular disease have been published in recent years. The first meta-analysis in 2001 by Mackness et al17 examined the 19 studies carried out up to that time as part of a study of PON1 status in 417 coronary heart disease subjects and 282 controls. A second meta-analysis examined 38 studies in addition to their own,18 whereas a third analyzed 43 previous studies.19 Unfortunately, the majority of the epidemiological studies examined only PON1 genotypes using DNA methodologies and ignored the large interindividual variability in plasma PON1 activity levels. Fundamental biochemical and physiological principles dictate that rates of detoxication or metabolism depend on the quantity of enzyme present. Thus, it is not surprising that many analyses examining disease or exposure risk using only single-nucleotide polymorphism (SNP) analysis and not enzyme activity levels have been inconclusive. Several of the most experienced investigators in PON1 research have pointed out the inadequacy of examining PON1 genotype alone as a risk factor for disease or exposure.15,20–27 We introduced the term PON1 status to include both plasma PON1 activity levels and PON1192 genotype.23 The few studies that have examined PON1 status have found that plasma PON1 activity level is indeed a risk factor for vascular disease,17,25,26,28–31 whereas there was no association observed with PON1 genotypes.17,25,26
The importance of plasma PON1 activity level in protecting against OP exposure has been clearly demonstrated in the mouse and genetically modified mouse model systems.23,32–36 Resistance to diazoxon exposure is modulated primarily by PON1 plasma activity level, whereas both PON1 activity level and PON1 genotype are important in modulating exposures to chlorpyrifos oxon, due to substrate-specific differences in catalytic efficiency between the PON1Q192 and the PON1R192 alloforms.34
The most convenient protocol for determining PON1 status—plasma activity levels as well as functional position 192 genotype—makes use of a 2-substrate assay, 2-dimensional enzyme activity plot that displays rates of diazoxonase activity versus POase activity under high salt conditions.24,27,37,38 The high salt conditions are used to separate the PON1192Q/R data points from the PON1192R/R data points. Unfortunately, this protocol involves the use of 2 highly toxic OP substrates. We report here a 2-substrate assay/analysis protocol that makes use of non-OP substrates and is convenient for general laboratory use. A third assay that measures rates of phenyl acetate (PA) hydrolysis (AREase activity) at low salt concentration reveals plasma PON1 activity levels for all 3 PON1192 genotypes. Factors are provided to allow the conversion of rates of hydrolysis of one substrate to another for each PON1192 phenotype.
Methods
Subjects
The plasma samples used for this study came from an institutional review board-approved project investigating the role of PON1 in vascular disease. Plasma samples were drawn into lithium-heparin tubes, and the cells were separated from the plasma by centrifugation for 15 minutes at 1800g.
4-(Chloromethyl)phenyl Acetate Assay
CMPA [4-(chloromethyl)phenyl acetate] was obtained from Sigma Chemical Co (St Louis, Mo). Rates of CMPA hydrolysis were determined in a SPECTRAmax PLUS Microplate Spectrophotometer (Molecular Devices, Sunnyvale, Calif) using ultraviolet transparent 96-well microplates from Costar (Cambridge, Mass.). Rates of hydrolysis were measured at 280 nm for 4 minutes at 25°C. Only initial linear rates were used for calculations, and results were normalized using the path-length correction software supplied by the system manufacturer. Replicate assays that varied by >10% were repeated. Plasma samples were diluted 1:40 in dilution buffer [20 mmol/L Tris-HCl (pH 8.0), 1.0 mmol/L CaCl2] and 20 μL was added per microplate well. The data points were run in triplicate. The substrate solution for CMPA determinations was 20 mmol/L Tris-HCl (pH 8.0), 1.0 mmol/L CaCl2 to which CMPA was added to a final concentration of 3 mmol/L. The substrate solution was shaken vigorously for 30 seconds in a screw-capped polypropylene tube before use. Substrate solution (200 μL) was added to initiate the assay. Activities were expressed in Units/mL, based on the molar extinction coefficient of 1.30 mmol/L–1cm–1 for the CMPA hydrolysis product, 4-(chloromethyl)phenol.
Arylesterase Assays (High Salt/No Salt)
Rates of PA hydrolysis were determined in the SPECTRAmax PLUS Microplate Spectrophotometer using ultraviolet transparent 96-well microplates from Costar. Rates of hydrolysis of PA were measured for 4 minutes at 270 nm, with only initial linear rates used for calculations, with results normalized using the path-length correction software provided by the manufacturer. Replicates that varied by >10% were repeated. AREase assays used plasma dilutions (in dilution buffer) of 1:40 for assays run at high salt concentration and 1:80 for assays run at low salt concentration. The assay used 20 μL of diluted plasma per well to which 200 μL of 3.26 mmol/L PA substrate was added in either high salt assay buffer, or no salt assay buffer. High salt PA buffer contained 2 mol/L NaCl, 20 mmol/L Tris-HCl (pH 8.0), 1.0 mmol/L CaCl2, and low salt assay buffer contained 20 mmol/L Tris-HCl (pH 8.0), 1.0 mmol/L CaCl2. Activities were expressed in Units/mL, based on the molar extinction coefficient of 1.31 mmol/L–1cm–1 for phenol.
Paraoxonase and Diazoxonase Assays
Plasma PON1 activities toward paraoxon (PO) and diazoxon (DZO) and were determined as described previously.24,38 Paraoxon and diazoxon were obtained from Chem Service (West Chester, Pa.). Rates of paraoxon and diazoxon hydrolysis were determined in the SPECTRA-max PLUS Microplate Spectrophotometer using either ultraviolet transparent 96-well microplates from Costar for UV diazoxonase readings (270 nm) or standard flat bottom 96-well microplates from Greiner One (Monroe, N.C.) for visible wavelength POase readings (405 nm). All assays were carried out in triplicate using a multi-channel pipette (Matrix, Hudson, N.H.). Outlier samples were reassayed. Rates of hydrolysis were measured for 4 minutes, with only initial linear rates used for calculations and results normalized using path-length correction. PO hydrolysis rates (POase) were expressed in Units/liter (U/L), based on the molar extinction coefficient of 18 mmol/L–1cm–1 for p-nitrophenol. DZO hydrolysis (DZOase) activities were expressed in Units/liter (U/L), based on the molar extinction coefficient of 3 mmol/L–1cm–1 for the diazoxon hydrolysis product, 2-isopropyl-4-methyl-6-hydroxypyrimidine.
Results
Identification and Characterization of Nontoxic Discriminatory Substrates
The aim of this study was to develop assays for the determination of PON1 status (plasma PON1 activity levels and functional position 192 genotype) that do not use the highly toxic organophosphate substrates paraoxon and diazoxon. More than 70 compounds with many assay conditions (variation of salt concentration and pH) were examined in attempts to find 2 substrates and assay conditions that would provide the same resolution of the PON1192 phenotypes as the toxic DZO/PO substrate pair.24 Of the many substrates and conditions tried, hydrolysis of PA at 2 mol/L salt and CMPA at low salt provided the best resolution of functional PON1192 phenotypes. The primary requirement for useful substrates is that the substrate and assay conditions reveal different rates of hydrolysis between the PON1Q192 and the PON1R192 alloforms.24
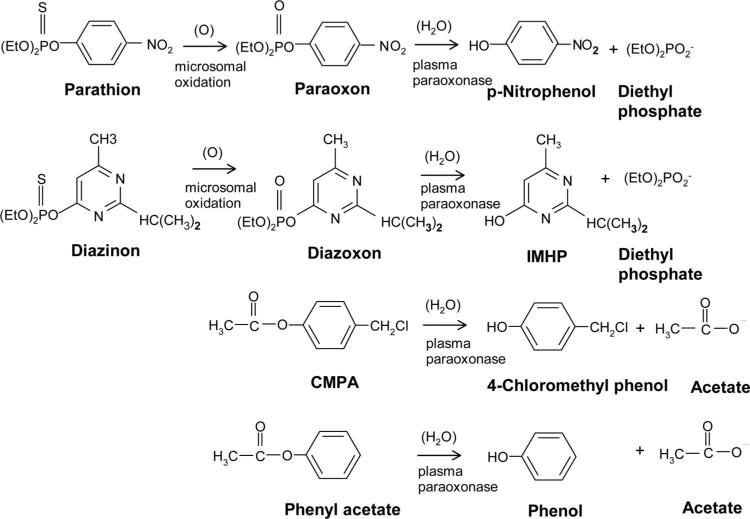
Figure 1 shows the structures of the 4 substrates used to determine PON1 status. The first step in the design of a spectrophotometric assay for substrate hydrolysis is to examine the spectra of the unhydrolyzed ester and the released alcohol. Aromatic alcohols in general provide useful spectral shifts on hydrolysis. Figure 2 shows the spectra of CMPA and 4-(chloromethyl)phenol. The wavelength of 280 nm was chosen for continuous monitoring of the hydrolysis of CMPA.
Figure 1.
Structures of the substrates used for determining PON1 status.
Figure 2.
Spectra of CMPA (●–●) and 4-(chloromethyl)phenol (○–○) and the difference spectrum (+–+).
In designing assays that best discriminate between the 2 PON1192 alloforms, it was necessary to find conditions where the kinetic properties of the 2 alloforms differed sufficiently to separate the 3 PON1192 phenotypes or “functional genotypes” (QQ, QR, and RR). Previous experience with PON1 assays has shown that either pH and/or salt conditions are most conveniently used to generate optimal assay conditions that will resolve the 3 PON1192 phenotypes. Our earlier work also showed that it is necessary to measure PON1 activity at or below pH 8.5 to avoid interference from the esterase activity of albumin, which catalyzes a higher rate of OP hydrolysis than PON1 at high pH values in plasma samples with low PON1 activity levels.9 A pH value of 8.0 proved to be optimal for measuring rates of hydrolysis of both PA and CMPA (data not shown).
The effects of varying NaCl concentration on rates of PA hydrolysis are shown in Figure 3A. As with diazoxon hydrolysis,24 PON1R192 was more sensitive to inhibition by NaCl than was PON1Q192. Because most of the currently used assays are run at 2 mol/L NaCl, and this level of salt provided a good differentiation of the activity of the 2 PON1192 alloforms, 2 mol/L NaCl was selected for optimizing the spread of the data points for the “y axis substrate” PA. Because we have previously shown that rates of PA hydrolysis in absence of NaCl may be used to compare levels of plasma PON1 across PON1192 genotypes, rates of PA hydrolysis were also determined in the absence of salt.
Figure 3.
Rates of hydrolysis of (A) PA by PON1Q192 (○–○) and PON1R192 (Δ–Δ); and (B) CMPA by PON1Q192 (○–○) and PON1R192 (Δ–Δ) as a function of sodium chloride concentration.
Figure 3B shows the dependence of rates of hydrolysis of CMPA by the 2 PON1192 alloforms as a function of salt. Interestingly, PON1R192 had higher rates of CMPA hydrolysis than PON1Q192 in the absence of salt, indicating that it would be a useful “x axis substrate.” Figure 4A shows the substrate dependence of PA hydrolysis by PON1Q192 (Km=443 μmol/L), and Figure 4B by PON1R192 (Km=279 μmol/L) at pH 8.0 in the presence of 2 mol/L NaCl. Figure 4C shows the substrate dependence of rates of hydrolysis of CMPA for PON1Q192 (Km=341 μmol/L), and Figure 4D by PON1R192 (Km=454 μmol/L) at pH 8.0 in the absence of NaCl. Km values were determined from plots of substrate concentration/velocity versus substrate concentration.39
Figure 4.
Substrate dependent rates of hydrolysis of (A) PA by PON1Q192, (B) PA by PON1R192, (C) CMPA by PON1Q192, and (D) CMPA by PON1R192. The assays for PA hydrolysis were run at high salt and those for CMPA at low salt as described in Methods section. Closed symbols indicate plots of velocity (v) versus substrate concentration [S]; open symbols, [S]/v versus [S].
Comparison of the 2 Protocols for Determining
PON1 Status
Figure 5 compares the population distribution of rates of hydrolysis of the substrate pair DZO/PO (Figure 5A) with those of the substrate pair PA/CMPA (Figure 5B) for 183 individuals (PON1 status). Both distributions clearly resolve the 3 functional PON1192 phenotypes (QQ, QR, and RR). These plots reveal not only the PON1192 alloform(s) present in an individual's plasma but also the relative levels of each individual's plasma PON1 activity within each PON1192 phenotype, data that is for most considerations much more relevant for estimating risk than the PON1 SNP data. All PON1192 genotypes were correctly inferred by both 2-substrate analyses as verified by polymerase chain reaction assays.12 We have reported previously, mutations discovered by discrepancies observed between the PON1 status determinations and polymerase chain reaction analyses.27
Figure 5.
Comparison of the 2 protocols for determining PON1 status. A, Assays using the highly toxic OP substrates DZO and PO. B, Assays using the non-OP substrates PA and CMPA. (○) indicates PON1Q/Q192;(■), PON1Q/R192;(Δ), PON1R/R192.
Generation of Assay Conversion Factors
To facilitate comparison of data obtained with this new protocol for establishing PON1 status with data generated with the DZO/PO substrate pair, plots of rates of hydrolysis of a given substrate versus rates of a second substrate for each PON1192 phenotype were prepared (data not shown) to obtain the conversion factors shown in the Table. It was necessary to determine the conversion factors for each PON1192 pheno-type separately because the catalytic efficiency of substrate hydrolysis differs for each phenotype. Because the rates of PA hydrolysis at low salt are not affected by PON1192 phenotype (Figure 6), they can be used to compare PON1 plasma activity levels across genotypes.40 Factors for converting rates of PA hydrolysis at high salt to low salt values were also determined so that it would not be necessary to run 3 different substrate assays for a study of PON1 status and plasma PON1 level determination (Table). If a laboratory environment has high ambient temperatures, it may be necessary to generate temperature correction factors.
Table.
Factors for Converting Rates of Hydrolysis of Substrates
| Phenotype | Conversion Factors | r2 |
|---|---|---|
| AREaseHS (U/mL)×185=DZOase (U/L) | 0.81 | |
| QR | AREaseHS (U/mL)×205=DZOase (U/L) | 0.90 |
| RR | AREaseHS (U/mL)×236=DZOase (U/L) | 0.88 |
| CMPAase (U/mL)×18.9=POase (U/L) | 0.92 | |
| QR | CMPAase (U/mL)×36.3=POase (U/L) | 0.90 |
| RR | CMPAase (U/mL)×54.3=POase (U/L) | 0.95 |
| AREaseHS (U/mL)×1.6 = AREaseLS (U/mL) | 0.95 | |
| QR | AREaseHS (U/mL)×2.0=AREaseLS (U/mL) | 0.66 |
| RR | AREaseHS (U/mL)×3.5=AREaseLS (U/mL) | 0.83 |
r2 indicates correlation coefficient squared; AREaseHS, arylesterase at high salt; AREaseLS, arylesterase at low salt.
Figure 6.

Rates of PA hydrolysis for each PON1192 phenotype/genotype at low salt concentration: PON1Q/Q192 (○–○), PON1Q/R192, (□–□) and PON1R/R192 (Δ–Δ). Horizontal bars represent the mean AREase activity values for each phenotype.
Discussion
The studies on the relationship of genetic variability of PON1 to risk of disease or exposure now number into the hundreds. Unfortunately, most of these studies have looked for association of PON1 SNPs with susceptibility and have ignored the more important factor, plasma PON1 activity levels.15–19,25,26,28–31 In our initial characterization of human PON1 cDNA sequences, we identified 2 coding region SNPs (L55 M and Q192R).10 It was subsequently shown that it was the Q192R polymorphism that determined the catalytic efficiency of PON1,11,12 with PON1R192 having approximately 9-times the catalytic efficiency for hydrolysis of paraoxon compared with PON1Q192.34 The effects of the Q192R polymorphism are substrate dependent, with PON1Q192 having higher activity against some of the nerve agents and PON1R192 having higher activity against PO and chlorpyrifos oxon.37 Both alloforms hydrolyze DZO34 and PA40 with approximately the same efficiency. Further research from our laboratory and 2 others examined the effects of 5 PON1 promoter region polymorphisms on plasma PON1 levels.41–44 The promoter region polymorphism that had the largest effect on PON1 activity levels was the C-108T polymorphism that occurs in an Sp1 transcription factor binding site.45 Homozygotes for PON1C-108 had on average twice the plasma level of PON1 activity compared with PON1T-108 homozygotes.42
There have been a number of reports linking low PON1 activity levels to the L55 M polymorphism with the PON1M55 allele being associated with low activity levels. However, most of this effect seems to be related to linkage disequilibrium of PON1M55 with the inefficient PON1T-108 allele.42 Leviev et al have reported that message levels46 and stability47 of the PON1M55 alloform may also contribute to the lower levels of PON1 activity associated with the PON1M55 genotype. AREase levels in a study of 1527 postmenopausal women reported by Roest et al48 were lower among PON1M55 homozygotes when compared with heterozygotes and PON1L55 homozygotes across all 3 PON1C-108T genotypes, lending support to an independent effect of the PON1M55 allele.
Of the 70 substrates tested, only PA (at high salt) and CMPA (in absence of salt) provided resolution of the 3 PON1 phenotypes comparable with that provided by the paraoxon/ diazoxon substrate pair. Another advantage of this substrate pair is that rates of hydrolysis can be determined at saturating substrate concentration. This was not feasible for diazoxon, where a nonsaturating concentration of 1 mmol/L was chosen for convenience and substrate solubility.24,38
Conclusions
The protocols described here will allow most laboratories to determine individuals’ PON1 status without the use of toxic substrates. The conversion factors presented here will also allow for the comparison of newly generated data with data reported from earlier studies. Again, it is important to note that epidemiological studies that examine only PON1 SNPs will be missing data on PON1 activity levels, which are more important than genotype in estimating an individual's risk of disease or exposure. For some exposures, genotype can also be important,34 but in no case are PON1 activity levels unimportant for estimating risk. Analysis of all ≈200 PON1 DNA polymorphisms will not provide the critical information generated by the 2-substrate PON1 status analysis protocol (functional PON1192 genotype and plasma activity levels).48
Sources of Funding
This work was supported by National Institutes of Environmental Health Sciences (ES09883) (Dr Furlong), (ES04696) (Dr Checkoway), (ES07033) (Dr Eaton), and the National Heart, Lung, and Blood Institute (RO1 HL67406 and HL074366) (Dr Jarvik).
Footnotes
Disclosures
None.
References
- 1.Primo-Parmo SL, Sorenson RC, Teiber J, La Du BN. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 1996;33:498–507. doi: 10.1006/geno.1996.0225. [DOI] [PubMed] [Google Scholar]
- 2.Mackness MI, Hallam SD, Peard T, Warner S, Walker CH. The separation of sheep and human serum “A”-esterase activity into the lipoprotein fraction by ultracentrifugation. Comp Biochem Physiol B. 1985;82:675–677. doi: 10.1016/0305-0491(85)90506-1. [DOI] [PubMed] [Google Scholar]
- 3.Blatter MC, James RW, Messmer S, Barja F, Pometta D. Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45. Identity of K-45 with paraoxonase. Eur J Biochem. 1993;211:871–879. doi: 10.1111/j.1432-1033.1993.tb17620.x. [DOI] [PubMed] [Google Scholar]
- 4.Draganov DI, Stetson PL, Watson CE, Billecke SS, La Du BN. Rabbit serum paraoxonase 3 (PON3) is a high density lipoprotein-associated lactonase and protects low density lipoprotein against oxidation. J Biol Chem. 2000;275:33435–33442. doi: 10.1074/jbc.M004543200. [DOI] [PubMed] [Google Scholar]
- 5.Reddy ST, Wadleigh DJ, Grijalva V, Ng C, Hama S, Gangopadhyay A, Shih DM, Lusis AJ, Navab M, Fogelman AM. Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler Thromb Vasc Biol. 2001;21:542–547. doi: 10.1161/01.atv.21.4.542. [DOI] [PubMed] [Google Scholar]
- 6.Aldridge WN. Serum esterases II. An enzyme hydrolysing diethyl p-nitrophenyl acetate (E600) and its identity with the A-esterase of mammalian sera. Biochem J. 1953;53:117–124. doi: 10.1042/bj0530117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Du BN. Historical considerations. In: Costa LG, Furlong CE, editors. Paraoxonase (PON1) in Health and Disease: Basic and Clinical Aspects. Kluwer Academic Press; Boston: 2002. pp. 1–25. [Google Scholar]
- 8.Sorenson RC, Primo-Parmo SL, Kuo CL, Adkins S, Lockridge O, La Du BN. Reconsideration of the catalytic center and mechanism of mammalian paraoxonase/arylesterase. Proc Nat Acad Sci USA. 1995;92:7187–7191. doi: 10.1073/pnas.92.16.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortigoza-Ferado J, Richter R, Hornung SK, Motulsky AG, Furlong CE. Paraoxon hydrolysis in human serum mediated by a genetically variable arylesterase and albumin. Am J Hum Genet. 1984;36:295–305. [PMC free article] [PubMed] [Google Scholar]
- 10.Hassett C, Richter RJ Humbert R, Chapline C, Crabb JW, Omiecinski CJ, Furlong CE. Characterization of cDNA clones encoding rabbit and human serum paraoxonase: the mature protein retains its signal sequence. Biochemistry. 1991;30:10141–10149. doi: 10.1021/bi00106a010. [DOI] [PubMed] [Google Scholar]
- 11.Adkins S, Gan KN, Mody M, La Du BN. Molecular basis for the polymorphic forms of human serum paraoxonase/arylesterase: glutamine or arginine at position 191 for the respective A or B allozymes. Am J Hum Genet. 1993;52:598–608. [PMC free article] [PubMed] [Google Scholar]
- 12.Humbert R, Adler DA, Disteche CM, Hassett C, Omiecinski CJ, Furlong CE. The molecular basis of the human serum paraoxonase activity polymorphism. Nat Genet. 1993;3:73–76. doi: 10.1038/ng0193-73. [DOI] [PubMed] [Google Scholar]
- 13.Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286:152–154. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- 14.Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest. 1998;101:1581–1590. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiz J, Blanché H, James RW, Garin MC, Vaisse C, Charpentier G, Cohen N, Morabia A, Passa P, Froguel P. Gln-Arg192 polymorphism of paraoxonase and coronary heart disease in type 2 diabetes. Lancet. 1995;346:869–872. doi: 10.1016/s0140-6736(95)92709-3. [DOI] [PubMed] [Google Scholar]
- 16.James RW. A long and winding road: defining the biological role and clinical importance of paraoxonases. Clin Chem Lab Med. 2006;44:1052–1059. doi: 10.1515/CCLM.2006.207. [DOI] [PubMed] [Google Scholar]
- 17.Mackness B, Davies GK, Turkie W, Lee E, Roberts DH, Hill E, Roberts C, Durrington PN, Mackness MI. Paraoxonase status in coronary heart disease: are activity and concentration more important than genotype? Arterioscler Thromb Vasc Biol. 2001;21:1451–1457. doi: 10.1161/hq0901.094247. [DOI] [PubMed] [Google Scholar]
- 18.Lawlor DA, Gaunt TR, Hinks LJ, Davey Smith G, Timpson N, Day IN, Ebrahim S. The association of the PON1 Q192R polymorphism with complications and outcomes of pregnancy: findings from the British Women's Heart and Health cohort study. Paediatr Perinat Epidemiol. 2006;20:244–250. doi: 10.1111/j.1365-3016.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 19.Wheeler JG, Keavney BD, Watkins H, Collins R, Danesh J. Four paraoxonase gene polymorphisms in 11212 cases of coronary heart disease and 12786 controls: meta-analysis of 43 studies. Lancet. 2004;363:689–695. doi: 10.1016/S0140-6736(04)15642-0. [DOI] [PubMed] [Google Scholar]
- 20.La Du BN, Billecke S, Hsu C, Haley RW, Broomfield CA. Serum paraoxonase (PON1) isozymes: the quantitative analysis of isozymes affecting individual sensitivity to environmental chemicals. Drug Metab Disp. 2001;29:566–569. [PubMed] [Google Scholar]
- 21.Draganov DI, La Du BN. Pharmacogenetics of paraoxonases, a brief review. Naunyn-Schmiedeberg's Arch Pharmacol. 2004;369:78–88. doi: 10.1007/s00210-003-0833-1. [DOI] [PubMed] [Google Scholar]
- 22.Deakin SP, James RW. Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin Sci (Lond) 2004;107:435–447. doi: 10.1042/CS20040187. [DOI] [PubMed] [Google Scholar]
- 23.Li W-F, Costa LG, Furlong CE. Serum paraoxonase status: a major factor in determining resistance to organophosphates. J Toxicol Environ Health. 1993;40:337–346. doi: 10.1080/15287399309531798. [DOI] [PubMed] [Google Scholar]
- 24.Richter RJ, Furlong CE. Determination of paraoxonase (PON1) status requires more than genotyping. Pharmacogenetics. 1999;9:745–753. [PubMed] [Google Scholar]
- 25.Jarvik GP, Rozek LS, Brophy VH, Hatsukami TS, Richter RJ, Schellenberg GD, Furlong CE. Paraoxonase phenotype is a better predictor of vascular disease than PON1192 or PON155 genotype. Arterioscler Thromb Vasc Biol. 2000;20:2442–2447. doi: 10.1161/01.atv.20.11.2441. [DOI] [PubMed] [Google Scholar]
- 26.Jarvik GP, Hatsukami TS, Carlson CS, Richter RJ, Jampsa R, Brophy VH, Margolin S, Rieder MJ, Nickerson DA, Schellenberg GD, Heagerty PJ, Furlong CE. Paraoxonase activity, but not haplotype utilizing the linkage disequilibrium structure predicts vascular disease. Arterioscler Thromb Vasc Biol. 2003a;23:1465–1471. doi: 10.1161/01.ATV.0000081635.96290.D3. [DOI] [PubMed] [Google Scholar]
- 27.Jarvik GP, Jampsa R, Richter RJ, Carlson C, Rieder M, Nickerson D, Furlong CE. Novel paraoxonase (PON1) nonsense and missense mutations predicted by functional genomic assay of PON1 status. Pharmacogenetics. 2003b;13:291–295. doi: 10.1097/00008571-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Mackness M, Mackness B. Paraoxonase 1 and atherosclerosis: is the gene or the protein more important? Free Radic Biol Med. 2004;37:1317–1323. doi: 10.1016/j.freeradbiomed.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 29.Warner S, Walker CH. Distribution of paraoxon hydrolyzing activity in the serum of patients after myocardial infarction. Clin Chem. 1986;32:671–673. [PubMed] [Google Scholar]
- 30.Navab M, Hama-Levy S, Van Lenten BJ, Fonarow GC, Cardinez CJ, Castellani LW, Brennan M-L, Lusis AJ, Fogelman AM, La Du BN. Mildly oxidized LDL induces an increased apolipoprotein J/paraoxonase ratio. J Clin Invest. 1997;99:2005–2019. doi: 10.1172/JCI119369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayub A, Mackness MI, Arrol S, Mackness B, Patel J, Durrington PN. Serum paraoxonase after myocardial infarction. Arterioscler Thromb Vasc Biol. 1999;19:330–335. doi: 10.1161/01.atv.19.2.330. [DOI] [PubMed] [Google Scholar]
- 32.Li W-F, Furlong CE, Costa LG. Paraoxonase protects against chlorpyrifos toxicity in mice. Toxicol Lett. 1995;76:219–226. doi: 10.1016/0378-4274(95)80006-y. [DOI] [PubMed] [Google Scholar]
- 33.Shih DM, Gu L, Xia Y-R, Navab M, Li W-F, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, Lusis AJ. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 34.Li W-F, Costa LG, Richter RJ, Hagen T, Shih DM, Tward A, Lusis AJ, Furlong CE. Catalytic efficiency determines the in vivo efficacy of PON1 for detoxifying organophosphates. Pharmacogenetics. 2000;10:767–780. doi: 10.1097/00008571-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Cole TB, Jampsa RL, Walter BJ, Arndt TL, Richter RJ, Shih DM, Tward A, Lusis AJ, Jack RM, Costa LG, Furlong CE. Expression of human paraoxonase (PON1) during development. Pharmacogenetics. 2003;13:357–364. doi: 10.1097/00008571-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Cole TB, Walter BJ, Shih DM, Tward AD, Lusis AJ, Timchalk C, Richter RJ, Costa LG, Furlong CE. Toxicity of chlorpyrifos and chlorpyrifos oxon in a transgenic mouse model of the human paraoxonase (PON1) Q192R polymorphism. Pharmacogenet Genomics. 2005;15:589–598. doi: 10.1097/01.fpc.0000167327.08034.d2. [DOI] [PubMed] [Google Scholar]
- 37.Davies H, Richter RJ, Keifer M, Broomfield C, Sowalla J, Furlong CE. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat Genet. 1996;14:334–336. doi: 10.1038/ng1196-334. [DOI] [PubMed] [Google Scholar]
- 38.Richter RJ, Jampsa RL, Jarvik GP, Costa LG, Furlong CE. Determination of paraoxonase 1 (PON1) status and genotypes at specific polymorphic sites. In: Mains MD, Costa LG, Reed DJ, Hodgson E, editors. Current Protocols in Toxicology. John Wiley and Sons; New York, NY: 2004. pp. 4.12.1–4.12.19. [DOI] [PubMed] [Google Scholar]
- 39.Dowd JE, Riggs DS. A comparison of estimates of Michaelis-Menten kinetic constants from various linear transformations. J Biol Chem. 1965;240:863–869. [PubMed] [Google Scholar]
- 40.Furlong C, Holland N, Richter R, Bradman A, Ho A, Eskenazi B. PON1 status of farmworker mothers and children as a predictor of organophosphate sensitivity. Pharmacogenet Genomics. 2006;16:183–190. doi: 10.1097/01.fpc.0000189796.21770.d3. [DOI] [PubMed] [Google Scholar]
- 41.Brophy VH, Hastings MD, Clendenning JB, Richter RJ, Jarvik GP, Furlong CE. Polymorphisms in the human paraoxonase (PON1) promoter. Pharmacogenetics. 2001;11:77–84. doi: 10.1097/00008571-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Brophy VH, Jampsa RL, Clendenning JB, McKinstry LA, Jarvik GP, Furlong CE. Effects of 5′ regulatory-region polymorphisms on paraoxonase gene (PON1) expression. Am J Hum Genet. 2001;68:1428–1436. doi: 10.1086/320600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suehiro T, Nakamura T, Inoue M, Shiinoki T, Ikeda Y, Kumon Y, Shindo M, Tanaka H, Hashimoto K. A polymorphism upstream from the human paraoxonase (PON1) gene and its association with PON1 expression. Atherosclerosis. 2000;150:295–298. doi: 10.1016/s0021-9150(99)00379-2. [DOI] [PubMed] [Google Scholar]
- 44.Leviev I, James RW. Promoter polymorphisms of human paraoxonase PON1 gene and serum paraoxonase activities and concentrations. Arterioscler Thromb Vasc Biol. 2000;20:516–521. doi: 10.1161/01.atv.20.2.516. [DOI] [PubMed] [Google Scholar]
- 45.Deakin S, Leviev I, Brulhart-Meynet MC, James RW. Paraoxonase-1 promoter haplotypes and serum paraoxonase: a predominant role for polymorphic position - 107, implicating the Sp1 transcription factor. Biochem J. 2003;372(Pt 2):643–649. doi: 10.1042/BJ20021670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leviev I, Negro F, James RW. Two alleles of the human paraoxonase gene produce different amounts of mRNA: an explanation for differences in serum concentrations of paraoxonase associated with the (Leu-Met54) polymorphism. Arterioscler Thromb Vasc Biol. 1997;17:2935–2939. doi: 10.1161/01.atv.17.11.2935. [DOI] [PubMed] [Google Scholar]
- 47.Leviev I, Deakin S, James RW. Decreased stability of the M54 isoform of paraoxonase as a contributory factor to variations in human serum paraoxonase concentrations. J Lipid Res. 2001;42:528–535. [PubMed] [Google Scholar]
- 48.Roest M, van Himbergen TM, Barendrecht AB, Peeters PH, van der Schouw YT, Voorbij HA. Genetic and environmental determinants of the PON-1 phenotype. Eur J Clin Invest. 2007;37:187–196. doi: 10.1111/j.1365-2362.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- 49.Furlong CE, Richter RJ, Li W-F, Brophy VH, Carlson C, Meider M, Nickerson D, Costa LG, Ranchalis J, Lusis AJ, Shih DM, Tward A, Jarvik GP. The functional consequences of polymorphisms in the human PON1 gene. In: Mackness B, Mackness M, Aviram M, Paragh G, editors. The Paraoxonases: Their Role in Disease, Development and Xenobiotic Metabolism. Springer; Dordrecht, The Netherlands: 2008. pp. 267–281. [Google Scholar]