Summary
Background
Complexin binds the SNARE complex at synapses and regulates exocytosis, but genetic studies indicate contradictory roles: in flies it predominantly inhibits synaptic vesicle fusion, whereas in mice it promotes evoked responses.
Results
Here we characterize the complexin mutant in the nematode Caenorhabditis elegans and reveal bipolar functions in neurotransmission: complexin inhibits spontaneous fusion of synaptic vesicles but is also essential for evoked responses. Complexin mutants exhibit a doubling of vesicle fusion in the absence of extracellular calcium. Even more profoundly, mutants exhibit an almost complete loss of evoked responses, current amplitudes are reduced by 94%. One possible interpretation is that spontaneous fusion leads to a severe depletion of primed vesicles and thereby eliminates the readily releasable pool. Consistent with this hypothesis, docked synaptic vesicles are reduced by 70% in complexin-1 mutants.
Conclusion
These data suggest that the main function of complexin is to maintain the docked state both by inhibiting fusion as well as by promoting priming.
Keywords: Complexin, C. elegans, synapse, tonic, evoked, spontaneous, vesicle
Introduction
During constitutive secretion, vesicles fuse to the cell membrane and release their cargo. Membrane fusion is mediated by the intertwining of SNARE proteins anchored in the plasma and vesicle membranes [1]. The SNARE complex also mediates exocytosis of synaptic vesicles; with SNARE protein interactions forming the primed state. However, the fusion process is interrupted and primed vesicles are held in a ready state until there is a stimulus. When the neuron is depolarized, calcium flows in through voltage-gated ion channels and relieves the block. Thus, regulated secretion requires a brake to block fusion and a calcium sensor to relieve the block. Synaptotagmin contains C2 domains capable of binding calcium and phospholipids, and it is widely accepted that synaptotagmin acts as the calcium sensor in synaptic vesicle fusion [2,3]. What protein interrupts fusion and holds vesicles in the docked state? Although synaptotagmin could also play an inhibitory role in fusion, recent studies suggest that another SNARE-interacting protein – complexin – may be responsible for blocking constitutive exocytosis [4–7]. Complexin forms an alpha helix that binds in the groove between syntaxin and synaptobrevin across the middle two-thirds of the SNARE complex and stabilizes the complex [8]. However, both biochemical and genetic experiments designed to test complexin function are contradictory and the role of complexin in calcium-driven fusion remains controversial.
In liposome and whole cell fusion assays complexin blocks fusion [9,10]. Complexin appears to block fusion by interfering with SNARE zippering [11,12]. The block is relieved by the addition of synaptotagmin and calcium [9,10,13,14]. The binding of complexin to the SNARE complex may simply recruit the blocking activity to the fusion complex, although it is possible that binding of complexin could promote zippering of the SNARE complex [8]. In fact, biochemical assays suggest that complexin might facilitate fusion [15,16]. In these assays, complexin was capable of increasing the rate of SNARE-mediated liposome fusion and in one report was even capable of conferring calcium sensitivity to this reaction [15]. Despite this study, the predominant activity observed for complexin in biochemical studies is to inhibit exocytosis.
By contrast, analysis of mutants indicates that the predominant activity of complexin is to promote exocytosis. In autaptic synapses from mutant mice lacking complexin-1 and -2, evoked release was reduced by 66% [17] and miniature postsynaptic currents were reduced by 30% [18]. Similarly, loss of complexin in Drosophila results in an approximately 40% reduction in evoked release [19,20].
Paradoxically genetic studies in Drosophila also suggest that complexin blocks exocytosis. In these mutants synaptic vesicles fuse to the plasma membrane constitutively. This increase in rate is due in part to a role in development: fly mutants exhibit a doubling of the number of active zones. However, the increase in the rate of spontaneous vesicle fusion is much larger, nearly 20-fold [19,20]. Knockdown of complexin-1 and -2 by RNAi in mouse cortical neurons caused a four-fold increase in spontaneous release [12], but the increase in spontaneous release is not observed in the mouse complexin knockouts [18]. It is not clear why the knockdown and knockout experiments in mice exhibit opposite effects on spontaneous vesicle fusion, but these results underscore the contradictory nature of complexin in synaptic vesicle fusion.
Here, we characterize complexin knockouts in the nematode Caenorhabditis elegans. We demonstrate that complexin-1 plays both facilitatory and inhibitory roles in synaptic vesicle exocytosis at the neuromuscular junction. Evoked release is largely absent in cpx-1 mutants; whereas tonic fusions are increased. These defects are accompanied by a severe decrease of docked vesicles at the synapse.
Results
The cpx-1 isoform functions at the nematode neuromuscular junction
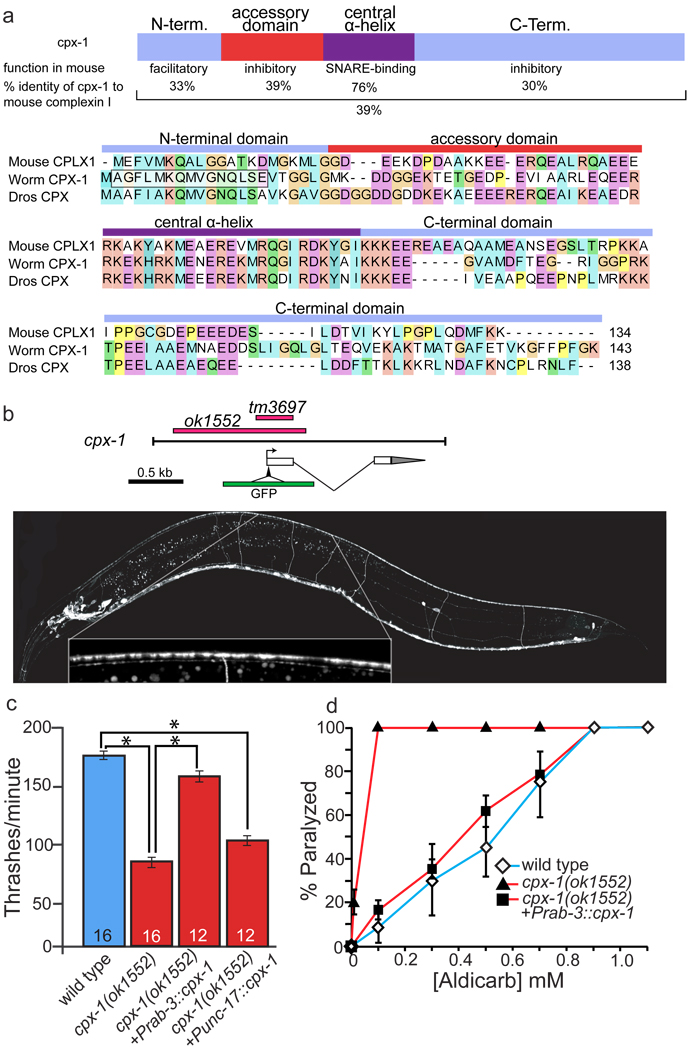
Two complexin genes, cpx-1 and cpx-2, are predicted in the C. elegans genome (Figure 1a, S1, S2). Worm cpx-1 exhibits greater identity to the fly and mouse complexins than cpx-2 (39% identity between CPX-1 and mouse Complexin-1 versus 32% identity between CPX-2 and mouse Complexin-1, (Figure 1, S2). For cpx-1, highest conservation is observed in the central helical domain (75%), which binds the SNARE complex, whereas the N-terminal facilitory domain, the accessory domain and the C-terminal domains each exhibit about 30% identity. The domains of complexin are defined based on sequence conservation across multiple animal phyla (See Figure S1 legend for full description of how the domains were defined). To assay for complexin function at neuromuscular junctions, we analyzed mutations in cpx-1 and cpx-2. cpx-1(ok1552) deletes the first of two exons of the gene which includes the N-terminus, the accessory domain, the central helix and part of the C-terminal domain. cpx-2(tm1871) deletes part of the accessory domain, the central helix and part of the C-terminal domain of cpx-2 (Figure 1b, S2). Complexins in mice and Drosophila are required for viability; complexin-1 complexin-2 double knockout mice die a few hours after birth and complexin null flies die before adult eclosion [17,19]. By contrast, C. elegans cpx-1(ok1552) and cpx-2(tm1871) mutants are viable and grow to adulthood; similarly cpx-1(ok1552) cpx-2(tm1871) double mutants develop into fertile adults.
Figure 1.
cpx-1 encodes the worm homolog of complexin and regulates locomotion and neurotransmitter release. (a). cpx-1 is the complexin gene with the highest identity to mouse and fly complexins. C. elegans CPX-1 was aligned with mouse CplxI (AAH14803) and fly complexin (AAF69518) using ClustalW2. Percent identities were calculated using Jalview [32]. (b). cpx-1 is expressed in the nervous system. Above, gene model and regions deleted in C. elegans complexin mutant alleles (red bars). The black line represents the region of the genome used to create the rescuing GFP construct. Gray triangle on transcript represents the 3’ UTR. GFP was inserted after the first three codons. Below, a reporter construct consisting of the cpx-1 gene with GFP inserted at the N-terminus of complexin is expressed in many neurons in the head and body of the animal. Neurons expressing CPX-1 include the motor neurons of the ventral nerve cord, neurons in the head ganglia and also neurons of the pharyngeal nervous system including the MC and M4 motorneurons. The inset shows the punctate localization of GFP::CPX-1 along the dorsal nerve cord. Confocal images were taken of young adult hermaphrodites oriented dorsal up and anterior to the left. Images are a stitch of multiple confocal Z-projections along the length of the animal. (c). cpx-1 mutants are defective for thrashing in liquid. cpx-1 thrashing behavior can be rescued by expressing cpx-1 under a pan-neuronal promoter (Prab-3::cpx-1 EG4633), but not by expressing it specifically in the acetylcholine neurons (Punc-17::cpx-1 EG4713). Tukey-Kramer multiple comparisons test: * P<0.001. The n values for each strain are listed at the bottom of each bar. (d). cpx-1 mutants are hypersensitive to the acetylcholinesterase inhibitor aldicarb, indicating that excessive acetylcholine is released at neuromuscular junctions. This sensitivity can be rescued by expressing cpx-1 in all neurons using the rab-3 promoter.
Nevertheless, cpx-1(ok1552) mutants are uncoordinated. To quantify body movement, we performed thrashing assays. Animals placed in a drop of water will swim by rapid reciprocal contractions of the body; locomotory activity can be quantified by counting flexures. Wild-type animals are capable of maintaining high rates of thrashing, whereas cpx-1(ok1552) worms exhibit reduced thrashing in liquid (Figure 1c). cpx-2(tm1871) mutants exhibit no swimming defect, suggesting that cpx-1, but not cpx-2, is important for the regulation of locomotion (Figure S2). To test whether loss of complexin results in defective neurotransmission, we tested the sensitivity of the complexin mutants to the acetylcholinesterase inhibitor aldicarb. Loss of cpx-1, but not cpx-2, resulted in hypersensitivity to aldicarb, suggesting there is excessive neurotransmitter release at the neuromuscular junction (Figure 1d; S2). The thrashing and aldicarb hypersensitivity phenotypes of cpx-1 mutants can be fully rescued by expressing the cpx-1 gene under its own promoter or under a pan-neuronal promoter, demonstrating that complexin is functioning in the nervous system (Figure 1d, S3). The aldicarb hypersensitivity defect can also be rescued by expressing cpx-1 in just the acetylcholine neurons demonstrating that excess neurotransmitter release arises from a cell-autonomous defect in the motor neurons (Figure S3a). However, thrashing was not rescued by expressing cpx-1 specifically in the acetylcholine motor neurons suggesting that cpx-1 is required more broadly in the nervous system for normal locomotion (Figure 1c). cpx-1 cpx-2 double mutants are not more severe than the cpx-1 single mutant for the thrashing or aldicarb assays demonstrating that cpx-1 plays the dominant role for the locomotory phenotypes (Figure S2). Together, these data suggest that cpx-1 functions in the motor neurons to prevent excess neurotransmitter release.
Complexins in other species are expressed mostly in neuronal tissue [4–6,19]. To determine the expression pattern of the C. elegans complexin genes, we tagged the N-termini of CPX-1 and CPX-2 with GFP. Each fusion construct includes the promoter, all introns and the native 3’ UTR (Figure 1b, S2). The cpx-1 transgenic reporter is widely expressed in the nervous system, including all the ventral cord motor neurons (Figure 1b). The CPX-1 protein is clustered into puncta along the dorsal and ventral nerve cords suggesting that the protein is likely localized to synapses (Figure 1b inset). Because the transgene rescues the cpx-1 aldicarb hypersensitivity phenotype, the tagged protein is functional (Figure S3). Since no obvious phenotype was detected for cpx-2 it was not possible to assay rescue. However the expression pattern suggests that cpx-2 is more narrowly expressed, including in a few neurons in the head and tail ganglia and in the coelomocyte scavenger cells, but not in the motor neurons of the ventral nerve cord (Figure S2). The expression patterns and behavioral phenotypes suggest that cpx-1 is the primary complexin functioning at neuromuscular junctions.
Loss of cpx-1 has contradictory effects on synaptic transmission
Loss of complexin results in a developmental defect in Drosophila neurons: the number of synaptic varicosities and active zones are nearly doubled [19]. It is has been suggested that the increase in tonic release leads to synaptic hypertrophy in Drosophila. To determine if there is a developmental defect at C. elegans synapses, we examined synaptic markers in cpx-1(ok1552) mutants. Normal numbers of neuromuscular junctions were observed in cpx-1 mutants as assayed by counting synaptic vesicle clusters (synaptobrevin-GFP) or dense projections (α-liprin/SYD-2::GFP) along the ventral nerve cord (Figure 2a, b). Normal postsynaptic receptor fields were observed in cpx-1 mutants by labeling the muscle GABA receptor (UNC-49::GFP; Figure 2c). Finally, axon outgrowth appeared normal in cpx-1(ok1552) mutants as determined by labeling the GABA motor neurons (Figure S3). It is possible that the synaptic phenotypes of cpx-1 mutants arose from long-term compensatory changes that occurred during development. To examine a developmental role for cpx-1 we expressed cpx-1(+) under the control of two different heat-shock promoters in cpx-1(ok1552) mutant animals (Figure S3). In the absence of heat shock, the transgenes failed to rescue the aldicarb hypersensitivity of cpx-1(ok1552); after heat shock in adult animals normal aldicarb sensitivity was restored. Taken together these results suggest that the development of the nervous system in a cpx-1 mutant is grossly normal and that the uncoordinated phenotype and hypersensitivity to aldicarb are due to defects in synaptic function rather than caused by an increase in synaptic number.
Figure 2.
cpx-1 mutants exhibit normal presynaptic and postsynaptic morphology. Left: Sample image of each synaptic reporter. Images for all strains were taken of the dorsal nerve cord near the posterior gonad reflex. Right: Quantification of the number of GFP positive puncta/10 µM. Ten images were analyzed for each strain. (a). cpx-1 mutation does not affect the number of synaptic vesicle clusters visualized by synaptobrevin::GFP (nIs52). (b). Normal numbers of synapses are observed in cpx-1 mutants as visualized by the dense projection marker α-liprin::GFP (hpIs3). (c). GFP-tagged GABA receptors (oxIs22) are clustered normally at postsynaptic sites. Images are confocal Z-stacks of a 70 µM section of the dorsal nerve cord. n = 10 sections of dorsal nerve cord for each strain.
Hypersensitivity to aldicarb suggests excessive neurotransmitter release at the neuromuscular junction. Three mechanisms of neurotransmitter release could be responsible for the increase: evoked, tonic or spontaneous release. Evoked release is generated by an action potential or a depolarizing stimulus from an electrode. The transient depolarization causes an influx of calcium at the synapse and stimulates the fusion of vesicles in the readily releasable pool. The coherent release of neurotransmitter produces a large postsynaptic current. Tonic release occurs even in the absence of a stimulus. If the membrane potential of the neuron at rest is sufficiently depolarized, a low level of calcium influx leads to sporadic vesicle fusion and miniature postsynaptic currents. Note that tonic release still requires calcium. In the absence of extracellular calcium, vesicle fusion is very rare and is called spontaneous fusion.
We measured these three types of neurotransmitter release using voltage-clamp recordings from neuromuscular junctions in cpx-1(ok1552) mutants [21]. At 5 mM calcium, tonic release in cpx-1 mutants was the same as in the wild type (Figure 3a, b). Tonic release is sensitive to extracellular calcium, so mini frequencies were measured over a range of calcium concentrations (Figure 3b). To measure spontaneous release, we recorded minis in the absence of extracellular calcium. Note that even in the absence of extracellular calcium that some minis are observed in C. elegans; these residual minis however are dependent on calcium from internal stores [22]. Interestingly, the cpx-1(ok1552) mutants still exhibited a >40% increase in mini frequency compared to the wild type at 0 mM calcium. These results suggest that CPX-1 is required to block spontaneous calcium-independent fusion of synaptic vesicles.
Figure 3.
cpx-1 mutants exhibit elevated spontaneous fusion and reduced evoked responses. (a). Representative traces of wild-type and cpx-1(ok1552) miniature postsynaptic currents (minis) from unstimulated motor neurons at 0 mM calcium. (b). Tonic neurotransmitter release at varying calcium concentrations. Each miniature current represents the fusion of a single synaptic vesicle. The increased frequency of miniature currents in cpx-1 mutants at 0mM extracellular calcium suggests that these vesicles may be fusing spontaneously in calcium-independent fusion. Mini frequencies in 5 mM calcium: wild type 64 ± 8, cpx-1(ok1552) 61 ± 11; in 1 mM calcium: wild type 55 ± 10, cpx-1(ok1552) 69 ± 9; in 0.4 mM calcium: wild type 44 ± 7, cpx-1(ok1552) 70 ± 10 (*P<0.05 vs. wild type); in 0 mM calcium: wild type 21 ± 2, cpx-1(ok1552) 45 ± 6 (** P<0.005 vs. wild type). Probabilities calculated using an unpaired independent T-test. (c). Representative traces of electrically evoked responses at 5 mM calcium of wild type, cpx-1(ok1552). (d). Summary of evoked responses from the wild type (1903 ± 182 nA) and cpx-1(ok1552) (113 ± 13 nA). Wild type vs. cpx-1(ok1552) P<0.00001 using an unpaired independent T-test.
To evaluate evoked release, we stimulated the ventral nerve cord with an electrode. Evoked release was reduced by 94% in cpx-1(ok1552) animals, demonstrating that the readily releasable pool was almost completely absent (Figure 3c, d). Once again, the defect in evoked release could be rescued by expressing cpx-1(+) in the nervous system of the mutant strain (Figure 5). These data suggest that in addition to an inhibitory role, complexin is playing a positive role in fusion.
Figure 5.
The N-terminus of cpx-1 is not required for evoked release at the C. elegans neuromuscular junction. (a). Models of the wild-type and N-terminal truncation mutant. The ΔN-cpx-1 mutant truncates the 15 amino acids immediately following the ATG (see box in Figure 1a). (b). Sample traces of tonic release in 0.4mM extracellular calcium. (c). The ΔN-cpx-1 does not rescue the spontaneous release phenotype of cpx-1(ok1552) mutants at 0 mM extracellular calcium. At 0.4 mM calcium, wild type 46 ± 6 minis/sec, cpx-1(ok1552) 70 ± 10 minis/sec (*P<0.05 vs. wild type), cpx-1(ok1552) + rescue (EG4633) 46 ± 9, ΔN-cpx-1 (EG6182) 54 ± 7 minis/sec. At 0 mM extracellular calcium, wild type 21 ± 2 minis/sec, cpx-1(ok1552) 45 ± 6 minis/sec (**P<0.005 vs. wild type), ΔN-cpx-1 (EG6182) 40 ± 5 minis/sec (***P<0.02 vs. wild type). Wild-type complexin is capable of fully rescuing the null mini frequency. (d). Sample traces of evoked release from the wild type, cpx-1(ok1552), cpx-1(ok1552)+rescue (EG4633), cpx-1(ok1552)+ΔN-cpx-1. (e). Evoked release is rescued in cpx-1(ok1552) mutants by expressing either wild-type complexin or the N-terminal truncation mutant ΔN-cpx-1 in neurons. Wild type 1903 ± 182 nA, cpx-1(ok1552) 113 ± 13 nA (*P<0.001), cpx-1(ok1552) + rescue (EG4633) 1768 ± 186 nA, ΔN-cpx-1 (EG6182) 2518 ± 215 nA (**P<0.05) at 5 mM extracellular calcium. The wild type and null mutant in c and e are recapitulated from Figure 3. n values are indicated in the bars.
Complexin plays dual roles in exocytosis
One possible interpretation of these data is that complexin plays one role at the synapse: it blocks spontaneous fusion of synaptic vesicles. In this model the absence of the evoked release is caused by a depletion of primed vesicles by spontaneous fusion. To assay for a depletion of docked vesicles, we examined the number and distribution of synaptic vesicles using electron microscopy. At a gross level the synapses seem normal in cpx-1 mutants: The morphology is normal (Figure 4a), synaptic vesicles have a normal diameter (Figure S4c) and vesicles are clustered around the dense projection (Figure S4a). However, the total number of synaptic vesicles is reduced to about 50% in cpx-1(ok1552) mutants compared to the wild type (Figure 4b). Moreover, the number of docked vesicles is severely reduced: there is a 70% reduction of docked vesicles at acetylcholine synapses and a 75% reduction of docked vesicles at GABA synapses (Figure 4c). Docking at the plasma membrane is reduced along the entire active zone (Figure S4b).
Figure 4.
cpx-1 mutants have reduced numbers of synaptic vesicles at the synapse. (a). Above are representative electron micrographs of a wild-type and a cpx-1 neuromuscular junction. ‘mt’, mitochondria. ‘DP’, dense projection. ‘SV’, synaptic vesicles. Below are high magnification images of a synapse from wild-type (left) and cpx-1(ok1552) (right) animals. Left, a vesicle docked to the plasma membrane adjacent to a dense projection at a wild-type neuromuscular junction. The arrow indicates the contact between the vesicle and plasma membrane. Right, a vesicle tethered to the plasma membrane next to the dense projection in a cpx-1(ok1552) animal. The arrow indicates the tether, connecting the plasma membrane to the vesicle. (b). Summary of the total number of synaptic vesicles per profile containing a dense projection. Wild-type acetylcholine neurons: 23.3 ± 0.8 vesicles; cpx-1(ok1552) acetylcholine neurons: 12.5 ± 0.8 vesicles (P<0.0001, unpaired T-test). Wild-type GABA neurons: 30.9 ± 1.0 vesicles; cpx-1(ok1552) GABA neurons:15.3 ± 1.5 vesicles (P<0.0001, unpaired T-test). (c). Total number of docked synaptic vesicles per profile containing a dense projection. Docked vesicles: wild-type acetylcholine neurons 2.3 ± 0.1 vesicles; cpx-1(ok1552) acetylcholine neurons 0.7 ± 0.1 vesicles (P<0.0001 unpaired T-test). Docked vesicles: wild-type GABA neurons 2.8 ± 0.2 vesicles; cpx-1(ok1552) GABA neurons 0.7 ± 0.1 vesicles (P<0.0001 unpaired T-test). A vesicle is considered docked if it is contacting the plasma membrane [31].
The ultrastructural data are superficially consistent with complexin having a single function: it blocks spontaneous fusion. If this model were true then one would expect that weak mutations would lead to an increase in spontaneous fusion and a concomitant decrease in evoked release; that is, the phenotypes should always be reciprocal. The first 15 amino acids of murine complexin are required for stimulating neurotransmission [12,23,24]. Specifically, the methionine and lysine residues at positions 5 and 6 in mouse complexin are believed to mediate the stimulatory effect [23] and these residues are conserved between mice and worms (Figure 1a). We deleted the 15 amino acids at the N-terminus of the complexin gene (‘ΔN-cpx-1’, Figure 5a). To insure that constructs were stable and not over-expressed, we inserted the transgenes as single copies into the cpx-1(ok1552) genome using Mos-mediated single copy insertion [25]. A cpx-1(+) gene expressed in neurons rescues the locomotory phenotypes, evoked responses and also inhibits the spontaneous fusion of vesicles (Figure 1c, d, Figure 5). The N-terminal deletion construct partially rescues the locomotory phenotype (Figure S5). Electrophysiological recordings demonstrate that spontaneous fusion is increased in the N-terminal deletion allele compared to the wild type (Figure 5b, c), suggesting that the N-terminus is required to inhibit spontaneous fusion. Surprisingly, the N-terminal deletion causes an increase rather than a decrease in evoked release (Figure 5d, e). These data tell us two important facts about complexin: (1) Unlike in the mouse, the N-terminal domain in the worm inhibits fusion, both evoked and spontaneous. (2) The negative and positive roles of complexin are separable. The N-terminal domain is required to inhibit fusion; the stimulatory functions must reside elsewhere. We conclude that complexin plays dual roles during vesicle fusion.
Discussion
Contradictory interpretations have been put forward for the role of complexin in synaptic vesicle fusion: some data suggest that complexin blocks spontaneous fusion of synaptic vesicles, other data suggest that complexin stimulates exocytosis. We find evidence for both of these roles in the nematode C. elegans.
Blocking fusion
Our data demonstrate that the complexin protein arrests SNARE-mediated fusion. First, in dissected preparations, spontaneous vesicle fusion is increased at cpx-1 neuromuscular junctions. Second, increased exocytosis occurs in undissected animals as well: complexin mutant animals exhibit an increased sensitivity to aldicarb, a drug that blocks the degradation of acetylcholine. The increased rate of release is not simply an increase in tonic release. Tonic release depends on extracellular calcium; whereas spontaneous release is independent of extracellular calcium. Thus, complexin may inhibit the spontaneous fusion of vesicles from both the evoked and tonic pools. An inhibitory role for complexin in tonic fusion is consistent with observations in both flies and mice. Complexin mutants in Drosophila exhibit elevated rates of spontaneous fusion as measured by field potentials at the neuromuscular junction of third instar larvae [19]. Similarly, knockdown of complexin in mouse cortical neurons by RNA interference led to a four-fold increase in the rate of spontaneous fusion [12].
An increased consumption of synaptic vesicles should lead to a depletion of vesicles at mutant synapses. In C. elegans spontaneous fusion occurs at high enough levels to deplete both docked and cytoplasmic pools of vesicles as observed by electron microscopy. There is a 70% decrease in the number of vesicles docked at active zones in cpx-1 mutants, presumably because vesicles spontaneously fuse after the priming of the SNARE complex. The loss of complexin from the C. elegans neuromuscular junction even depletes the reserve pool of vesicles by 50%, indicating that the high rates of fusion are outpacing the ability of the cell to replenish vesicles at the synapse. Although an increase in spontaneous fusion is not observed in mouse knockouts, there is a reduction in the readily releasable pool, that is docked and primed vesicles, as measured by applying moderately hyperosmotic media [23].
The inhibitory role of complexin in synaptic vesicle fusion is borne out by biochemical studies of membrane fusion. Complexin can inhibit SNARE-mediated fusion of whole cells [10] and also block SNARE-mediated fusion of liposomes [9]. The block of SNARE-mediated fusion appears to involve the accessory domain of complexin which may prevent synaptobrevin from fully zippering the SNARE complex [9–11].
Stimulating exocytosis
Complexin is also required for calcium-stimulated exocytosis in C. elegans: evoked currents are reduced by 94% in the complexin mutant. These data are also consistent with data from flies and mice. In Drosophila, complexin mutants exhibit an approximately 30–40% decrease in evoked potential [19]. In mouse cultured neurons, disruption of complexin by mutation or RNAi knockdown causes an approximately 70% decrease in evoked responses compared to the wild type [12,17,18]. Thus, loss of evoked synaptic vesicle exocytosis is a common feature of complexin mutants in all organisms studied.
How can complexin facilitate evoked release when it clearly inhibits spontaneous fusion of synaptic vesicles? There are two possible models for the role of complexin in evoked release: In the first model, complexin has only one function – to inhibit spontaneous vesicle fusion; the defect in evoked release is an indirect effect of depletion of the vesicle pool through spontaneous fusion. In the second model, complexin also has a domain that facilitates evoked release. In short, in this second model complexin has dual functions: to inhibit spontaneous release of the tonic pool and to facilitate fusion from the evoked pool.
Stimulating by inhibiting
In the inhibitory model, rapid consumption of vesicles via spontaneous fusion depletes vesicles from the evoked pool (Figure 6, middle). Thus, a role for complexin in stimulating exocytosis is an illusion of an empty readily releasable pool. An attractive aspect of this model is its simplicity: complexin would have a single, uncomplicated function in synaptic vesicle exocytosis. If this were true, mutations that partially disrupt the inhibitory function should disrupt evoked responses proportionally – the phenotypes should always be linked – losing vesicles to constitutive fusion should deplete the readily releasable pool. By contrast, mutations in the N-terminal domain increase spontaneous release but do not reduce evoked release (Figure 5). In fact, evoked release is even higher than in the wild type. Similarly, removal of the C-terminal prenylation motif of fly complexin increases spontaneous release and enhances evoked release [26]. Together, these data suggest that the synapses in worms and flies can maintain high rates of tonic release without depleting the evoked vesicle pool.
Figure 6.
Model for dual functions of complexin. Above, complexin functions to maintain primed vesicles in the readily-releasable pool by stabilizing the SNARE complex in a partially zippered state. Middle, in the absence of complexin the SNAREs can fully zipper and drive membrane fusion; thereby allowing spontaneous fusion. Below, in the absence of complexin the SNAREs might unzipper and reverse the docking of vesicles; thereby depleting evoked responses. Complexin might provide this stabilizing effect on its own or in conjunction with other synaptic proteins. The SNARE protein SNAP-25 is not shown for simplicity.
A stimulatory domain
In the dual function model, complexin inhibits spontaneous release from the tonic pool, but facilitates evoked release. We found in C. elegans that complexin is essential for evoked release, potentially supporting a dual role for complexin. In the mouse, the N-terminus of complexin is required for evoked responses possibly to promote a late step in the fusion process [20,12,23]. Surprisingly, when we deleted these residues in C. elegans complexin, evoked release was not disrupted. These data suggest that residues involved in promoting fusion are located in a different region of the molecule than the N-terminus in C. elegans.
Stimulating by docking
How then might complexin facilitate evoked release at a molecular level? An alternative model is that complexin facilitates evoked release via the central helix (Figure 6). The binding of the central helix domain of complexin to the partially zippered SNARE complex could prevent unzippering and the reversal of the primed state [7,8]. In an in vitro vesicle docking assay, binding by complexin stabilizes the trans configuration [15]. In the absence of complexin, trans-SNARE complexes are reversible and primed vesicles would become unprimed (Fig. 6, below). Such reversal of priming would contribute to the reduction of docked vesicles observed at the C. elegans neuromuscular junction and account for the unusually severe defect in evoked release. In this model, the function of complexin is to maintain the primed state, and the interactions of the alpha helical regions of complexin with the SNARE complex acts essentially as a fourth SNARE protein. Again, the elegance of this model is its simplicity – complexin stabilizes the docked state. However, we cannot exclude the possibility that complexin plays a more direct role in evoked release, for example via regulatory interactions with synaptotagmin.
Species diversity
How can we reconcile contradictory data for complexin mutants in different experimental systems? In the mouse, complexin primarily stimulates evoked release, whereas in flies it primarily blocks spontaneous release. In the worm, complexin plays a prominent role in both functions: complexin is essential for evoked release and it blocks spontaneous fusion. However, these differences among species are ones of degree: inhibitory and stimulatory functions for complexin are found in each organism. Given the strong sequence conservation, it is likely that complexin is playing similar roles at all synapses, its character shaped by the other players at the synapse. It seems that complexin encompasses schizophrenic roles in exocytosis – it both blocks and stimulates fusion.
Experimental Procedures
Constructs and strains
All constructs were made using standard molecular biology techniques and Gateway technology (Invitrogen). For a full description of how all constructs were made see the online supplemental materials and methods. Rescuing or reporter constructs were either microinjected into the worms gonads to produce progeny carrying extrachromosomal arrays or single copy insertions of rescuing or mutant complexins were produced using MosSCI as previously described [25]. For a full description of the methodologies used to create transgenic C. elegans see the online experimental procedures.
Behavioral assays
Thrashing assays were performed as previously described [27] and are fully described in supplemental online experimental procedures. For the aldicarb assays, 20–25 worms were added to the center of room-temperature aldicarb plates and scored for paralysis after four hours. The assays are fully described in the online experimental procedures. For heat-shock experiments, strains were heat shocked for one hour at 34°C and then allowed to recover at room temperature for six hours prior to assay. For heat shock aldicarb assays, 20–25 worms were added to plate containing 1 mM aldicarb and scored for paralysis every fifteen minutes.
Confocal Microscopy
Worms were immobilized in 2% phenoxypropanol in M9 solution on freshly made 2% agarose pads. Images were acquired on a Pascal LSM5 confocal microscope (Zeiss, Inc.) with a 63× 1.4NA oil objective. For imaging of the dorsal nerve cord worms were imaged with the dorsal surface facing towards the objective. For each of the synaptic markers a Z-stack was taken of a 70 µm section of nerve cord and the total number of GFP positive puncta were counted.
Electrophysiology
Young adult hermaphrodite animals were used for electrophysiological analysis. Miniature and evoked postsynaptic currents (mPSCs and ePSCs) at the neuromuscular junction were recorded as previously described [21,28]. For a full description of electrophysiological methods and analysis see the supplemental experimental procedures online. Data were imported into Origin, version 7.5 (OriginLab), for graphing and statistical analysis. Unpaired t-test was used for statistical comparisons. A P value of < 0.05 is considered statistically significant.
Electron microscopy
N2 and cpx-1(ok1552) worms were fixed in parallel for electron microscopy using high-pressure freezing followed by substitution of solvent-based fixatives as previously described [29–31]. For a full description of the electron microscopy methods see supplemental experimental procedures online.
All morphometry was conducted blind to genotype and included a matched wild-type worm that was fixed in parallel. The number of synaptic vesicles (~30 nm in diameter) in each synaptic profile was counted, and their distances from the dense projection and plasma membrane were measured. Docked vesicles were defined as touching the plasma membrane. Analysis included the acetylcholine neurons VA and VB and the GABA neuron VD.
Reagents
All chemicals were purchased from Sigma-Aldrich and all molecular reagents from Invitrogen and New England Biolabs, unless otherwise stated. Worm strains not generated in the lab were ordered from the Caenorhabditis genetics center (http://www.cbs.umn.edu/CGC/).
Supplementary Material
Acknowledgements
We would like to thank Jeremy Dittman for sharing unpublished results, M. Wayne Davis and Catherine Y. Dy for critical reading of the manuscript and David M. Hobson for advice on data analysis. Strains were obtained from the C. elegans Genetics Center (St Paul, MN, USA) and the National Bioresources Center (Tokyo, Japan). This work was supported by NIH NS034307 to E.M.J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
R.J.H. performed the initial characterization of cpx-1 and cpx-2 null mutants, all behavioral and pharmacological experiments and created all rescuing constructs and strains. Q.L. performed all electrophysiology experiments and analysis. S.W. Performed all electron microscopy experiments and analysis. E.M.J. and R.J.H. conceived the experiments and wrote the manuscript. E.M.J. supervised all authors and provided the necessary support. All authors discussed and helped in data interpretation for the manuscript.
Bibliography
- 1.Jahn R, Scheller RH. SNAREs-engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 2.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, et al. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Chacón R, Königstorfer A, Gerber SH, García J, Matos MF, Stevens CF, et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi S, Yamamoto H, Matsuda Z, Ogawa M, Yagyu K, Taniguchi T, et al. Identification of two highly homologous presynaptic proteins distinctly localized at the dendritic and somatic synapses. FEBS Lett. 1995;368:455–460. doi: 10.1016/0014-5793(95)00713-j. [DOI] [PubMed] [Google Scholar]
- 5.Ishizuka T, Saisu H, Odani S, Abe T. Synaphin: a protein associated with the docking/fusion complex in presynaptic terminals. Biochem. Biophys. Res. Commun. 1995;213:1107–1114. doi: 10.1006/bbrc.1995.2241. [DOI] [PubMed] [Google Scholar]
- 6.McMahon HT, Missler M, Li C, Südhof TC. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 7.Pabst S, Margittai M, Vainius D, Langen R, Jahn R, Fasshauer D. Rapid and selective binding to the synaptic SNARE complex suggests a modulatory role of complexins in neuroexocytosis. J. Biol. Chem. 2002;277:7838–7848. doi: 10.1074/jbc.M109507200. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Tomchick DR, Kovrigin E, Araç D, Machius M, Südhof TC, et al. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- 9.Schaub JR, Lu X, Doneske B, Shin Y, McNew JA. Hemifusion arrest by complexin is relieved by Ca2+-synaptotagmin I. Nat. Struct. Mol. Biol. 2006;13:748–750. doi: 10.1038/nsmb1124. [DOI] [PubMed] [Google Scholar]
- 10.Giraudo CG, Eng WS, Melia TJ, Rothman JE. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006;313:676–680. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- 11.Giraudo CG, Garcia-Diaz A, Eng WS, Chen Y, Hendrickson WA, Melia TJ, et al. Alternative zippering as an on-off switch for SNARE-mediated fusion. Science. 2009;323:512–516. doi: 10.1126/science.1166500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maximov A, Tang J, Yang X, Pang ZP, Südhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang J, Maximov A, Shin O, Dai H, Rizo J, Südhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Chicka MC, Chapman ER. Concurrent binding of complexin and synaptotagmin to liposome-embedded SNARE complexes. Biochemistry. 2009;48:657–659. doi: 10.1021/bi801962d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon T, Lu X, Diao J, Lee S, Ha T, Shin Y. Complexin and Ca2+ stimulate SNARE-mediated membrane fusion. Nat. Struct. Mol. Biol. 2008;15:707–713. doi: 10.1038/nsmb.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malsam J, Seiler F, Schollmeier Y, Rusu P, Krause JM, Söllner TH. The carboxy-terminal domain of complexin I stimulates liposome fusion. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2001–2006. doi: 10.1073/pnas.0812813106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reim K, Mansour M, Varoqueaux F, McMahon HT, Südhof TC, Brose N, et al. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 18.Xue M, Stradomska A, Chen H, Brose N, Zhang W, Rosenmund C, et al. Complexins facilitate neurotransmitter release at excitatory and inhibitory synapses in mammalian central nervous system. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7875–7880. doi: 10.1073/pnas.0803012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat. Neurosci. 2007;10:1235–1237. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- 20.Xue M, Lin YQ, Pan H, Reim K, Deng H, Bellen HJ, et al. Tilting the balance between facilitatory and inhibitory functions of mammalian and Drosophila Complexins orchestrates synaptic vesicle exocytosis. Neuron. 2009;64:367–380. doi: 10.1016/j.neuron.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richmond JE, Jorgensen EM. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat. Neurosci. 1999;2:791–797. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q, Chen B, Yankova M, Morest DK, Maryon E, Hand AR, et al. Presynaptic ryanodine receptors are required for normal quantal size at the Caenorhabditis elegans neuromuscular junction. J. Neurosci. 2005;25:6745–6754. doi: 10.1523/JNEUROSCI.1730-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue M, Craig TK, Xu J, Chao H, Rizo J, Rosenmund C. Binding of the complexin N terminus to the SNARE complex potentiates synaptic-vesicle fusogenicity. Nat Struct Mol Biol. 2010;17:568–575. doi: 10.1038/nsmb.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue M, Reim K, Chen X, Chao H, Deng H, Rizo J, et al. Distinct domains of complexin I differentially regulate neurotransmitter release. Nat. Struct. Mol. Biol. 2007;14:949–958. doi: 10.1038/nsmb1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frøkjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen S, et al. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet. 2008;40:1375–1383. doi: 10.1038/ng.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho RW, Song Y, Littleton JT. Comparative analysis of Drosophila and mammalian complexins as fusion clamps and facilitators of neurotransmitter release. Mol Cell Neurosci. 2010;45:389–397. doi: 10.1016/j.mcn.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller KG, Alfonso A, Nguyen M, Crowell JA, Johnson CD, Rand JB. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12593–12598. doi: 10.1073/pnas.93.22.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Hollopeter G, Jorgensen EM. Graded synaptic transmission at the Caenorhabditis elegans neuromuscular junction. Proc. Natl. Acad. Sci. U.S.A. 2009;106:10823–10828. doi: 10.1073/pnas.0903570106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rostaing P, Weimer RM, Jorgensen EM, Triller A, Bessereau J. Preservation of immunoreactivity and fine structure of adult C. elegans tissues using high-pressure freezing. J. Histochem. Cytochem. 2004;52:1–12. doi: 10.1177/002215540405200101. [DOI] [PubMed] [Google Scholar]
- 30.McDonald KL, Auer M. High-pressure freezing, cellular tomography, and structural cell biology. BioTechniques. 2006;41:137. doi: 10.2144/000112226. 139, 141 passim. [DOI] [PubMed] [Google Scholar]
- 31.Hammarlund M, Palfreyman MT, Watanabe S, Olsen S, Jorgensen EM. Open syntaxin docks synaptic vesicles. PLoS Biol. 2007;5:e198. doi: 10.1371/journal.pbio.0050198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.