Abstract
Parkinson's disease (PD) presents clinically with varying degrees of resting tremor, rigidity, and bradykinesia. For decades, striatal-thalamo-cortical (STC) dysfunction has been implied in bradykinesia and rigidity, but does not explain resting tremor in PD. To understand the roles of cerebello-thalamo-cortical (CTC) and STC circuits in the pathophysiology of the heterogeneous clinical presentation of PD, we collected functional magnetic resonance imaging (fMRI) data from 17 right-handed PD patients [nine tremor predominant (PDT) and eight akinetic-rigidity predominant (PDAR)] and 14 right-handed Controls while they performed internally-guided (IG) sequential finger tapping tasks. The percentage of voxels activated in regions constituting the STC and CTC [divided as cerebellar hemisphere-thalamo-cortical (CHTC) and vermis-thalamo-cortical (CVTC)] circuits was calculated. Multivariate analysis of variance compared the activation patterns of these circuits between study groups. Compared to Controls, both PDAR and PDT subjects displayed an overall increase in the percentage of voxels activated in both STC and CTC circuits. These increases reached statistical significance in contralateral STC and CTC circuits for PDT subjects, and in contralateral CTC pathways for PDAR subjects. Comparison of PDAR and PDT subjects revealed significant differences in ipsilateral STC (p=0.005) and CTC (p=0.043 for CHTC and p=0.003 for CVTC) circuits. These data support the differential involvement of STC and CTC circuits in PD subtypes, and help explain the heterogeneous presentation of PD symptoms. These findings underscore the importance of integrating CTC circuits in understanding PD and other disorders of the basal ganglia.
Keywords: Parkinson's disease, motor pathways, cerebellum, striatum, fMRI
Parkinson's disease (PD)1 is characterized by resting tremor, rigidity, bradykinesia, and gait disorder, each expressed in varying proportions and progressing in a highly individual manner. Hoehn and Yahr (1967) first described the marked clinical diversity exhibited in PD, and later studies (Jankovic et al., 1990; Spiegel et al., 2007) provided empirical support for PD subgroup differences. Patients may have either primary rigidity and bradykinesia with minimal tremor (akinetic/rigidity predominant, PDAR), or tremor with minimal rigidity, bradykinesia and other symptoms (tremor-predominant, PDT).
Several lines of evidence suggest PD tremor may have different underlying pathophysiology processes from those of bradykinesia and rigidity [see Zaidel et al. (2009) for a recent review]. For example: factor analysis of PD signs shows rest tremor is relatively independent of other cardinal signs of PD (Zetusky et al., 1985), is less reliably responsive to dopaminergic modulation (Marjama-Lyons and Koller, 2000), and does not worsen at the same rate as bradykinesia and rigidity (Zetusky et al., 1985; Louis et al., 1999; Jankovic and Kapadia, 2001). Recent PD imaging studies suggest dopamine transporter levels and myocardial sympathetic degeneration correlate with hypokinesia/rigidity but not tremor (Spiegel et al., 2007). Finally, there is no correlation between rest tremor and striatal 18F-fluorodopa uptake in PD patients (Vingerhoets et al., 1997).
The pathological hallmark of PD is dopamine neuron loss in the substantia nigra pars compacta (SNc) of the basal ganglia (BG). For decades, a classic model emphasized the role of the BG in modulating cortical function through striatal-thalamo-cortical (STC) circuits (DeLong et al., 1984; Alexander et al., 1986; Albin et al., 1989), the dysfunction of which may lead to bradykinesia and rigidity. This model does not, however, explain PD resting tremor. Emerging evidence suggests the necessity of incorpating cerebello-thalamo-cortical (CTC) circuitry into discussions of motor function in both normal (Kelly et al., 2009) and dysfunctional (Deiber et al., 1993; Neychev et al., 2008; Argyelan et al., 2009; Benninger et al., 2009; Zaidel et al., 2009) states. CTC dysfunction is implied in tremorgenesis in PDT, as PET studies indicate increased regional cerebral blood flow (rCBF) in the vermis, whereas stimulation of the thalamic Vim nucleus (site of cerebellar output) can capture and ameliorate PD tremor (Deiber et al., 1993). Several recent imaging studies suggest altered/compensatory activity in cerebellar pathways in PD (Cerasa et al., 2006; Yu et al., 2007; Sen et al., 2009) and its progression (Sen et al., 2009).
To understand the clinical heterogeneity of PD presentation, and the role of STC and CTC circuits in PD pathophysiology, we compared functional magnetic resonance imaging (fMRI) activation patterns in STC and CTC pathways of PDAR and PDT subjects to Controls and to each other. In the past, numerous fMRI studies have investigated brain activation in PD (Haslinger et al., 2001; Buhmann et al., 2003; Cerasa et al., 2006; Yu et al., 2007; Tinaz et al., 2008; Helmich et al., 2009) that involved PDAR subjects or subjects with mixed symptoms. To our knowledge, no study has either investigated the fMRI activation pattern in PDT subjects compared to Controls, or directly compared PDAR and PDT subjects to each other.
Experimental Procedures
Subjects
Seventeen PD subjects (nine PDT and eight PDAR) were recruited for this study from a tertiary movement disorders clinic (see Table 1 for demographic information). Each PD subject was diagnosed by a movement disorders specialist (XH) based on previously published criteria (Gibb and Lees, 1988). Twelve PD subjects (six PDAR and six PDT) had right side predominant symptoms. Most subjects were early in their disease course (see Table 1), with a mean (±SD) disease duration for PDAR subjects of 16.0±23.8 mon (range 0.23-72.3 mon) an d PDT subjects 4.1±5.2 mon (range 0-12.2 mon). All PD subjects wer e free of other neurological illnesses, psychosis, and cognitive decline. Fourteen Control subjects were recruited from IRB-approved advertisements on campus and in the surrounding community. On-going psychiatric or neurological illnesses were an exclusion criterion for the Controls. All subjects were free of hypothyroidism, vitamin B12 or folate deficiency, and free of kidney or liver disease. In addition, all subjects (PDs and Controls) were strongly right handed as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971).
Table 1.
Subject demographic data
| Controls (n = 14) |
PDAR (n = 8) |
PDT (n = 9) |
P value | |
|---|---|---|---|---|
| Age (mean ± SD, yrs) | 51.4±14.5 | 51.3±10.2 | 59.3±6.6 | >0.05* |
| Disease duration (mean±SD, mon) | NA | 16.0±23.8 | 4.1±5.2 | 0.280** |
| Total UPDRS | NA | 9.5±3.9 | 9.2±3.2 | 0.884** |
| Hoehn & Yahr score | NA | 1.3±0.5 | 1.3±0.4 | 0.657** |
| Levodopa equivalent dosage (LED), mg | NA | 225±192 | 67.5±130 | 0.050** |
| Tremor/AR ratio | NA | 0.20±0.31 | 3.02±2.41 | 0.005** |
| Right side symptom onset | NA | 6 | 6 | NA |
| Handedness | Right | Right | Right | NA |
Summary of demographic information for Control, PDAR, and PDT subjects included in the study. Age, disease duration, total UPDRS, Hoehn & Yahr score, LED, and Tremor/AR ratio were compared using a two-tailed t-test. PDAR – akinetic/rigid predomiant PD, PDT – tremor predominant PD, NA – not applicable, mon – months, tremor/AR ratio – tremor/akinetic-rigidity ratio.
Comparison of age for Controls and PDAR subjects p=0.990, Controls and PDT subjects p=0.139, and for PDAR and PDT subjects p=0.066.
P value reflects the comparison between PDAR and PDT subjects.
Unified Parkinson's Disease Rating part III motor (UPDRS-III) and Hoehn and Yahr scores were obtained for each PD subject after withholding all PD medication overnight (~12 hr) before fMRI scans (Table 1). In addition, levodopa equivalent dosages (LEDs) were calculated for each PD subject. For PDAR subjects, three subjects were not taking any PD-related medications at the time of the study, three were on Sinemet, and two were taking Mirapex. In the PDT group, six subjects were not taking any medications at the time of the study, one was on Sinemet, and one was taking Requip.
PD subjects were classified as either PDAR or PDT using the modified ratio developed by Schiess et al. (2000) based on the UPDRS-III. Namely, a numerical ratio was derived from a patient's mean tremor score and mean akinetic-rigidity score. Tremor was assessed using a nine-item scale that included history of left or right tremor (two items), rest tremor of the face/lips/chin and each limb (five items), as well as postural tremor of the right and left upper extremities (two items). The 12-item akinetic-rigidity scale assessed passive range of motion rigidity of the neck and each extremity (five items), rapid opening/closing of hands (one item), finger tapping (one item), rising from a chair (one item), posture and postural instability (two items), gait (one item), and body bradykinesia (one item). Each item was rated 0-4 with 0 representing absence of the symptom or normal activity and 4 significant presence of the symptom or impairment. The mean of each scale was calculated and then the ratio (tremor/akinetic-rigidity score) determined. Using this method, PDAR subjects will have a ratio <0.8, whereas PDT subjects will have a ratio >1.0. In the current study, the average ratio for PDAR subjects was 0.20±0.31 (range 0-0.71) and for PD T subjects 3.02±2.41 (range 1.07-8.00; Table 1). The study protocol followed the Helsinki principles, and was reviewed and approved by the University of North Carolina Institutional Review Board. Written informed consent was obtained from all subjects who participated in the study.
Functional MRI Acquisition
Images were acquired using a 3.0 Tesla Siemens scanner (Siemens, Erlangen, Germany) with a birdcage type standard quadrature head coil and an advanced nuclear magnetic resonance echoplanar system. PD subjects were scanned in a practically defined ‘off’ state (>12 hr off PD medications). The subject's head was positioned along the canthomeatal line. Foam padding was used to minimize head motion within the coil. High-resolution T1-weighted anatomical images (3D SPGR, TR=14 ms, TE=7.7 ms, flip angle=25°, voxe l dimensions 1.0×1.0×1.0mm, 176×256 voxels, 160 slices) were acquired for co-registration of functional images. A total of 49 co-planar functional images were acquired using a gradient echoplanar sequence (TR=3000ms, TE=30ms, flip angle=80°, NEX=1, voxel dimensions 3.0×3.0×3.0 mm, imaging matrix 64×64 voxels). Functional runs consisted of 200 time points, and two radio frequency excitations were performed prior to image acquisition to achieve steady-state transverse relaxation.
Activation paradigm – sequential finger-tapping movements
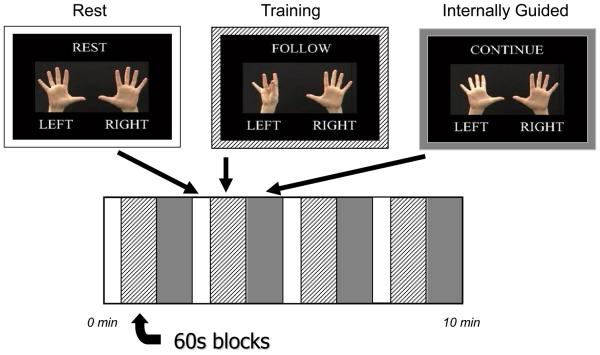
This study used a modified activation paradigm based on previous studies in control and PD subjects (Sabatini et al., 2000; Lewis et al., 2007; Sen et al., 2009). Briefly, the paradigm consists of sequential finger-tapping movements (SFM) at 0.5 tap/sec using either the right or left hands. The sequences were presented with instructions that first asked the subject to follow the finger sequence presented on the screen during an externally guided training session, and then asked the subject to continue the finger-tapping sequence (internally guided task). Each SFM block was 60 seconds long, and each block was preceded and followed by a 30 second rest (R) period. Each run consisted of four blocks of rest, externally guided training, and internally guided tasks (total 10 minutes, Figure 1). The finger-tapping sequences of each block were alternated to prevent memorization from previous blocks (see Table 2). All subjects practiced the task with both hands for about 20 minutes prior to scanning in order to obtain adequate performance on the tasks. For this manuscript, we present the data from the internally guided task performed by the right (dominant) hand, as previous studies have suggested that PD mainly affects internally rather than externally guided tasks (Jahanshahi et al., 1995; Chuma et al., 2006; Nowak et al., 2006), and the non-dominant hand displays a more complicated pattern of neurocircuitry recruitment [(Mattay et al., 1998) and unpublished data from our lab)]. Two runs of fMRI data from each subject were included for analysis.
Figure 1.
Schematic of paradigm.
Table 2.
Finger sequences used in the fMRI paradigm.
| Paradigm | Description of sequences |
|---|---|
| Sequence 1 | Thumb to digit 2 →3→4→5→ open and close fists twice → Repeat → Return to beginning of sequence |
| Sequence 2 | Thumb to digit 3 →5→2→4→ open and close fists twice → Repeat → Return to beginning of sequence |
FMRI Image Pre-processing
The fMRI data was preprocessed using SPM5 software (Wellcome Trust Center for Neuroimaging, London, UK) for spatial realignment and motion correction. The spatial smoothing of functional time series was performed with a Gaussian smoothing kernel with full width at half maximum (FWHM) = 6 mm×6 mm×6 mm.
Generation of first level fMRI activation maps
The spatial transformation into a common coordinate space that is traditionally done for most fMRI studies deemphasizes inherent anatomical variability of the human brain, factors that may be amplified by aging and neurodegenerative processes. In addition, covarying regions are often of great importance, but are not included in the group methods described in traditional SPM analyses. To circumvent these problems, we carried out first level statistical analyses in native space, and generated individual T-maps using SPM5 comparing the activation pattern of the IG task performed by the right hand to rest. The anatomic region of interest (ROI) based-multivariant analysis of fMRI activation pattern then was applied as described below.
Obtaining the fMRI activation level in each ROI constituting the STC and CTC circuits
The anatomical regions of interest (ROIs) were defined on high resolution T1 images using automatic segmentation software [AutoSeg, Neuro Image Research and Analysis Laboratories, University of North Carolina at Chapel Hill, NC; (Joshi et al., 2004; Gouttard et al., 2007)] for each subject. This permits statistical comparisons of similar brain areas across subjects without warping (“normalizing”) the brain. The percent of voxels activated with a t value > 1.96 (corresponding to a p = 0.05) was calculated for each ROI in the bilateral STC [lentiform nucleus (Lent), thalamus (Th), supplementary motor area (SMA), and primary motor cortex (PMC)] and CTC circuits. For the purpose of this study, the CTC pathway was divided into CHTC [cerebellar hemisphere, Th, lateral premotor cortex (PrMC), and somatosensory cortex (SMC)] and CvTC [vermis/paravermis including bilateral dentate nuclei (Vm), Th, PrMC and SMC] circuits. We divided the cerebellum into three segments of two lateral hemispheres and one midline vermis/paravermis because these regions have been suggested to subserve different functions (Afifi and Bergman, 1998).
Statistical comparison of fMRI activation patterns in STC and CTC circuits between groups
A statistical method that compared multiple ROIs together, namely multivariate analysis of variance (MANOVA), was employed to compare the fMRI activation patterns between groups in ROIs of the neurocircuitry described above. ROIs that constitute the bilateral STC, CHTC, and CVTC circuits were treated as co-multivariate response variables. The number of ROIs included in each pathway was limited to four based on the sample size in each PD group (n=8 or 9). Specifically, it is required in the MANOVA procedure that the number of ROIs plus the number of groups will have to be less than the sample size (Johnson and Wichern, 2002). The percent activation in the ROIs of these pathways in each subject was the multivariate dependent variable, whereas the independent variable was PD status (2=Control, 1=PDT, 0=PDAR). Activity in STC and CTC circuits was compared between Controls and PDAR or PDT subjects. PDAR and PDT subjects also were compared directly. All comparisons were conducted by utilizing multiple MANOVAs with adjustment of age using the PROC GLM command with option MANOVA in SAS (System 9.1, SAS Inc., Cary, NC). In addition, when PDAR and PDT subjects were compared we also included side of symptom onset as a factor in the analysis. Significant MANOVA results were probed with a Student's t-test. P values less than 0.05 were considered to represent significant differences between groups.
Results
Demographic data
Although PDT subjects appeared on average to be slightly older than Control and PDAR subjects, there were no significant differences in age among the groups (Table 1). For PD subtypes, there were no significant differences in disease duration, total UPRDS, or Hoehn and Yahr scores. PDAR subjects had a higher LED compared to PDT subjects. As expected, the two subtypes showed significant differences in the tremor/AR ratio (Table 1).
PDAR versus Control subjects
In all ROIs comprising the bilateral STC and CTC circuits, PDAR subjects displayed increased activity relative to Controls (see Figure 2). Direct comparison of PDAR and Controls revealed significant differences in all STC and CTC circuits, with contralateral CTC pathways having p values <0.0001, ipsilateral CTC circuits 0.01, and those for the contralateral and ipsilateral STC pathways 0.0002 and 0.0012, respectively. After adjusting for age, however, only the contralateral CTC circuits remained significant (CHTC p=0.0113, CVTC p=0.0169), with a trend towards significance in the bilateral STC pathways (p values ~0.09, see Table 3). Post-hoc t-tests within the significant CTC pathways revealed no statistical significance for any of the ROIs.
Figure 2.
Comparison of percent activation changes in STC and CTC circuits in Control and PDAR subjects. Control (black bars) and PDAR (gray bars) subjects performed the finger tapping task with their right hand. P values for each circuit were deduced from MANOVA comparison of the collective percent activation changes within ROIs composing each circuit (see Materials and Methods). Bars in the figures represent mean±SEM of percent activation within a given ROI f or simple visualization (not for MANOVA calculation). Circuits are defined as listed in the legend of Table 3.
Table 3.
MANOVA comparisons among Controls, PDAR and PDT subjects.
| Control vs PDAR | Control vs. PDT | PDAR vs. PDT | |
|---|---|---|---|
| Contralateral STC‡ | 0.0909 | 0.0089 | 0.8999 |
| Contralateral ChTC‡‡ | 0.0113 | 0.0404 | 0.8483 |
| Contralateral CvTC‡‡‡ | 0.0169 | 0.0472 | 0.7433 |
| Ipsilateral STC† | 0.0959 | 0.1940 | 0.0048 |
| Ipsilateral ChTC†† | 0.2690 | 0.3085 | 0.0438 |
| Ipsilateral CvTC††† | 0.3518 | 0.4176 | 0.0030 |
The percent of voxels activated with a t value > 1.96 (corresponding to a p = 0.05) were calculated for each ROI in the STC (Lent, Th, SMA, and PMC), CHTC (CB, Th, PreMC, and SMC), and CVTC (Vm, Th, PreMC and SMC) circuits. Network analyses were performed using MANOVA with percent activation of individual ROIs as the dependent variables. All p values have been adjusted for age.
Contralateral STC was defined as contralateral Lent, Th, SMA, and PMC
Contralateral CHTC was defined as ipsilateral CB and contralateral Th, PreMC, and SMC
Contralateral CVTC was defined as Vm and contralateral Th, PreMC, and SMC
Ipsilateral STC was defined as ipsilateral Lent, Th, SMA, and PMC
Ipsilateral CHTC was defined as contralateral CB and ipsilateral Th, PreMC, and SMC
Ipsilateral CVTC was defined as Vm and ipsilateral Th, PreMC, and SMC.
PDT versus Control subjects
In bilateral STC and CTC pathways, PDT subjects displayed increased activity relative to Controls in most ROIs comprising these circuits, although they demonstrated decreased activity in contralateral PMC and bilateral lentiform nucleus. Direct comparison of PDT and Controls demonstrated significant differences in all STC and CTC circuits except the ipsilateral CVTC circuit (p=0.0565). All contralateral pathways had p values <0.0002, whereas those for the ipsilateral STC and CHTC circuits were 0.0094 and 0.0299, respectively. These statistically significant differences remained only in contralateral STC (p=0.0089) and CTC (CHTC p=0.0404 and CVTC p=0.0472) pathways after adjusting for age (Table 3), although none of the ROIs reached significance by post-hoc t-test.
PDAR versus PDT subjects
As shown in Figure 4, PDAR subjects displayed more activity in almost all cortical and subcortical ROIs relative to PDT subjects, whereas PDT subjects showed more activity in the vermis, contralateral cerebellar hemisphere, and ipsilateral thalamus. Direct comparison between PDAR and PDT subject revealed statistically significant difference in all STC and CTC circuits (p values ranging from 0.0031-0.0255) except for the ipsilateral STC (p=0.0582). Most of the statistical differences disappeared after individual adjustment for age or side of symptom onset in PD subjects. Yet there was an even stronger significant difference between the subtypes in ipsilateral STC (p=0.005) and CTC (p=0.043 for CHTC and p=0.003 for CVTC) circuits after adjustment for both factors (see Table 3). None of the individual ROIs, however, reached significance when probed with a t-test.
Figure 4.
Comparison of percent activation changes in STC and CTC circuits in PDAR and PDT subjects. PDAR (black bars) and PDT (gray bars) subjects performed the finger tapping task with their right hand. P values for each circuit were deduced from MANOVA comparison of the collective percent activation within ROIs composing each circuit (see Materials and Methods). Bars in the figures represent mean±SEM of percent activation changes within a given ROI for simple visualization (not for MANOVA calculation). Circuits are defined as listed in the legend of Table 3.
Discussion
In doing the first fMRI study that has compared Control, PDAR, and PDT subjects, we have shown that there is differential involvement of STC and CTC pathways for the two different PD clinical presentations (or subtypes). The results indicate first, that PDAR and PDT subjects both display overall increased recruitment of STC and CTC neurocircuitry compared to Controls during this internally guided motor task. Second, the pattern of the recruitment of STC and CTC circuits was different for PDAR and PDT subjects. Namely, PDAR subjects showed significantly increased activity in contralateral CTC circuits with trends toward significant differences in bilateral STC pathways, whereas PDT subjects displayed significant changes in contralateral STC and CTC pathways. Third, the direct comparison of PDAR and PDT subjects supports significant differences in STC and CTC circuits between these PD subtypes. PDAR subjects displayed increased activity relative to PDT subjects, notably in areas related to the primary pathology in PD (lentiform nucleus of the basal ganglia), whereas PDT subjects demonstrated higher activity in cerebellar and thalamic regions suggested to be involved in tremorgenesis. These results help contribute to understanding the pathophysiology and clinical heterogeneity of PD presentation.
PDAR and PDT subjects displayed an overall increase in activity in bilateral motor areas relative to Controls, consistent with previous functional imaging studies in PD (Rascol et al., 1997; Haslinger et al., 2001; Cerasa et al., 2006; Yu et al., 2007). Although the exact meaning of blood oxygen level dependent (BOLD) changes measured by fMRI is still controversial, the increase in BOLD signal changes during tasks may indirectly reflect increased activity or oxygenation demands in these brain regions (Heeger et al., 2000; Rees et al., 2000; Logothetis et al., 2001). The increased recruitment of STC and CTC motor areas demonstrated in the current fMRI study suggests compensatory activation or an overall decrease in efficiency of these brain regions to drive the same motor task in the pathological state of PD. These results are consistent with previous studies in PD subjects that suggest simple tasks recruit brain regions that are normally associated with more complex tasks (Carbon et al., 2007; Moraschi et al., 2010).
Consistent with previous studies demonstrating increased activity in cerebellum and lateral premotor areas (Rascol et al., 1997; Sabatini et al., 2000; Thobois et al., 2004; Wu and Hallett, 2005), PDAR subjects showed significantly increased activity in CTC pathways. PDAR subjects also displayed a trend toward significant increases in in bilateral STC pathways compared to Controls, suggesting a possible increased recruitment (decreased efficiency) in these circuits as well. These results are consistent with previous studies that demonstrated increased activation in regions comprising the STC circuits in PDAR subjects (Haslinger et al., 2001; Cerasa et al., 2006). Moreover, the current study echoes recent studies in dystonic patients (Neychev et al., 2008; Argyelan et al., 2009), where traditional views have placed responsibility for motor dysfunction on BG circuitry. In each case, there is emerging evidence that in addition to BG circuit dysfunction, cerebellar circuits also are involved (Cerasa et al., 2006; Yu et al., 2007; Neychev et al., 2008; Argyelan et al., 2009; Benninger et al., 2009; Sen et al., 2009).
In comparing PDT subjects to Controls, we found that PDT subjects showed a pattern of activation similar to PDAR subjects in CTC pathways. Within the contralateral STC pathway, however, PDT subjects displayed differential activity relative to Controls. Namely, PDT subjects had increased activity in contralateral SMA and thalamus but decreased activity in PMC and lentiform nucleus, which may imply less compensatory activity (or more efficient activity) in the STC circuits of PDT subjects. The present results are complimentary to studies that have reported pathologically increased oscillations in contralateral STC and CTC circuits in PDT subjects (Timmermann et al., 2003; Pollok et al., 2009).
Relative to PDT subjects, PDAR subjects demonstrated increased recruitment of most ROIs comprising the STC and CTC pathways. This increased recruitment, however, was only significant in the ipsilateral STC and CTC circuits. The exact mechanism for this observation is unclear at this moment. Bilateral recruitment of motor areas with unilateral motor tasks, indeed, has been reported previously (Cincotta et al., 2006; Moraschi et al., 2010), and was suggested to be reflective of underlying neuropathological mechanisms. Mirror movements (MM) in unused hands have been reported in PD (Vidal et al., 2003; Espay et al., 2005; Espay et al., 2006; Li et al., 2007), and are particularly associated with rigidity (Espay et al., 2005). Transcranial magnetic stimulation and PET activation studies have demonstrated enhanced facilitation and increased rCBF in the ipsilateral motor cortex of primary MM syndromes (Kanouchi et al., 1997). Furthermore, recent fMRI studies have indicated significant recruitment of ipsilateral brain regions during a simple motor task in PD (Moraschi et al., 2010). Together, these data lead to the hypothesis that both PDAR and PDT subjects may have maximized the recruitment of contralateral structures that normally are used for performing IG motor tasks (Rascol et al., 1997; Mattay et al., 1998; Elsinger et al., 2003). Conversely, PDAR subjects may require increased recruitment of additional brain structures (even from ipsilateral hemispheres) to compensate for the functions usually carried out by these contralateral structures. These results lend support to the notion that PDAR may represent a more advanced stage of the pathophysiological process occurring during PD (Jellinger, 1991; Jellinger and Paulus, 1992).
Most interestingly, PDAR and PDT subjects demonstrated different patterns of activity in basal ganglia and cerebellar regions, areas linked to the primary pathology of PD and tremorgenesis, respectively. Namely, PDAR subjects displayed higher activity in the lentiform nucleus whereas PDT subjects showed increased activity in vermis, cerebellar hemisphere, and thalamus of the ipsilateral pathways. These results suggest different degrees of pathological changes in these regions in the different subtypes of PD (Zaidel et al., 2009). If the higher activity does imply increased pathology (less efficient brain regions), these results are consistent with previous studies demonstrating increased neuronal loss in the basal ganglia in PDAR subjects (Jellinger, 1991; Jellinger and Paulus, 1992), as well as increased cerebral blood flow in the vermis (Deiber et al., 1993) and decreased gray matter volume in the declive of the cerebellum (Benninger et al., 2009) in PDT subjects. Together, the current results also support the greater involvement of the cerebellum in tremorgenesis in PD (Poirier et al., 1975; Pechadre et al., 1976; Deiber et al., 1993; Duffau et al., 1996; Zaidel et al., 2009).
There are several limitations for our study. First, the sample size limited our ability to study additional regions of interest in a more detailed manner (such as subdiving the lentiform nucleus into its independent components, the putamen and GP); Second, although PD subjects were imaged in the practically defined ‘off drug’ state, we cannot rule out potential residual effects of dopaminergic medications on the neurocircuits. Also, it is well known that tremor in PD generally is less responsive to dopaminergic modulation than bradykinesia and rigidity (Marjama-Lyons and Koller, 2000). The current study did not address the effect of levodopa on STC and CTC circuitry in the different PD subtypes. Third, the behavioral assesments were subjective, relying on the demonstrated proficiency immediately prior to the fMRI session and not reflecting the exact performance during fMRI data collection. Future studies with a larger sample size, drug naïve patients, better behavioral data recording, and subject response to levodopa would be useful.
In the nearly two decades since its description, the classic model of motor control emphasized the role of the BG in influencing cortical function (DeLong et al., 1984; Alexander et al., 1986; Albin et al., 1989), and elegantly posited an explanation for rigidity and bradykinesia. The model, however, was not able to explain resting tremor in PD. In both normal (Kelly et al., 2009; Hoshi et al., 2005; Bostan et al., 2010) and disease conditions (Cerasa et al., 2006; Yu et al., 2007; Neychev et al., 2008; Argyelan et al., 2009; Sen et al., 2009), increasing evidence suggests the necessity of incorporating cerebellar function/dysfunction into a comprehensive view of motor control and tremorgenesis in PD (Deiber et al., 1993; Benninger et al., 2009). The current study provides further evidence that there is differential involvement of striato- and cerebello-thalamo-cortical circuits in PDT and PDAR subtypes.
Collectively, this evidence suggests that the classic model should be modified with integration of cerebellum influences if the clinical heterogeneity of PD (and the “unexplained” tremor) are to be better understood. Elegant work by Strick and colleagues previously demonstrated that the basal ganglia and cerebellum project to and influence similar cortical regions [summarized in (Middleton and Strick, 2000)], but the need for a revised model is accentuated by recent findings that the striatum and cerebellum also may communicate and/or influence each others' functions at the subcortical level through di- and trisynaptic connections (Hoshi et al., 2005; Bostan et al., 2010). Figure 5 provides a hypothetical schematic in which STC and CTC circuits are integrated. In this model, both the striatum and cerebellum influence cortical motor functions through parallel or complementary STC and CTC circuits, with both structures also modulating each other at the subcortical level [Figure 5, long dash and small dotted arrows, respectively; (Hoshi et al., 2005; Bostan et al., 2010)]. The primary dysfunction (decreased efficiency with over activation) in the STC circuit (Figure 5, light gray structures connected with dashed arrows) may lead to bradykinesia and rigidity observed in PDAR subjects. In contrast, primary dysfunction in the CTC circuit (Figure 5, dark gray structures connected with solid arrows), particularly the vermis/paravermis region, may be responsible for the occurrence of resting tremor in PDT. The different degrees of functional deficits of the STC and CTC circuits may assist in understanding the observed clinical heterogeneity of PD motor symptoms. Future studies exploring such hypotheses, with detail understanding role of subnuclies of BG, cerebellums and related structures, may not only shed light on PD pathophysiology, but also help elucidate the function of these circuits in other disorders as well as the healthy brain.
Figure 5.
New model integrating STC and CTC circuits. Neuroanatomical evidence indicates that for the STC circuit (light gray structures connected with dashed arrows) the output nucleus of the striatum, the internal segment of the GP, projects to the ventral lateral anterior nucleus of the thalamus, up to the SMA and then PMC, with reciprocal projections back to the striatum. For the CTC circuit (dark gray structures with solid arrows), the cerebellum projects to the ventral intermediate nucleus of the thalamus, up to the premotor cortex and then over to the SMC. The cortex then sends projections back to the cerebellum. In addition, we hypothesize that the PrMC and SMC also send fibers to the finally motor endpoint, the PMC. Recent evidence suggests that the basal ganglia (Bostan et al., 2010) and cerebellum (Hoshi et al., 2005) communicate and/or influence each other's functions at the subcortical level. These connections are indicated using dashed-arrows (basal ganglia to cerebellum) and small-dotted arrows (cerebellum to basal ganglia). BG: basal ganglia, GP: globus pallidus, PMC: primary motor cortex, PN: pontine nuclei, PrMC: lateral premotor cortex, SMA: supplementary motor area, SMC: somatosensory cortex, STN: subthalamic nucleus, Vla: ventrolateral anterior nucleus of the thalamus, Vim: ventral intermediate nucleus of the thalamus
Research Highlights.
PDAR & PDT subjects show more activity in STC and CTC circuits compared to Controls
PDT subjects show significant changes from Controls in contralateral STC and CTC
PDAR subjects only show significance in contralateral CTC compared to Controls
Comparing PDT & PDAR subjects shows significant differences in ipsilateral STC and CTC
Figure 3.
Comparison of percent activation changes in STC and CTC circuits in Control and PDT subjects. Control (black bars) and PDT (gray bars) subjects performed the finger tapping task with their right hand. P values for each circuit were deduced from MANOVA comparison of the collective percent activation changes within ROIs composing each circuit (see Materials and Methods). Bars in the figures represent mean±SEM of percent activation within a given ROI f or simple visualization (not for MANOVA calculation). Circuits are defined as listed in the legend of Table 3.
Acknowledgements
We thank all those who participated in the study and support of the UNC MRI center. This work was supported in part by NIH grants AG21491 (XH), NS060722 (XH, MML, and GD), and the UNC GCRC (RR00046).
Abbreviations
- BG
Basal ganglia
- CTC
Cerebello-thalamo-cortical
- CHTC
Cerebellar hemisphere-thalamo-cortical
- CVTC
Cerebellar vermis-thalamo-cortical
- fMRI
Functional magnetic resonance imaging
- FWHM
Full width at half maximum
- Lent
Lentiform nucleus
- MANOVA
Multiple analysis of variance
- NEX
Number of excitations
- PD
Parkinson's disease
- PDAR
Akinetic/rigidity predominant Parkinson's disease
- PDT
Tremor predominant Parkinson's disease
- PMC
Primary motor cortex
- PrMC
Lateral premotor cortex
- ROI
Region of interest
- SFM
Sequential finger tapping movements
- SMA
Supplementary motor area
- SMC
Somatosensory cortex
- SNc
Substantia nigra pars compacta
- SPGR
Spoiled gradient recall
- TE
Echo time
- Th
Thalamus
- TR
Repeat time
- Vim
Ventral intermediate nucleus of the thalamus
- Vla
Ventral lateral anterior of the thalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have reported no conflicts of interest.
Statistical Analysis: Performed by Young Truong8, Ph.D., Seonjoo Lee, B.S., and Atsushi Kawaguchi, Ph.D.
Please see Appendix Cover Page for summary of all abbreviations used in this Application.
Specific Contributions of Authors
Mechelle M. Lewis: Data acquisition, analysis, and interpretation; obtaining funding for the study, administrative support for the study; primary drafting and critical revision of the manuscript.
Guangwei Du: Data analysis and interpretation, drafting and critical revision of the manuscript,
Suman Sen: Data acquisition, critical revision of the manuscript.
Atsushi Kawaguchi: Statistical analysis of the data and data interpretation.
Young Truong: Statistical analysis of the data, data interpretation, critical revision of the manuscript.
Seonjoo Lee: Statistical analysis of the data and critical revision of the manuscript.
Richard Mailman: Data interpretation and critical revision of the manuscript.
Xuemei Huang: Conception and design of the study; data acquisition, analysis, and interpretation; drafting and critical revision of the manuscript; obtaining funding for the study; administration and supervision of the project.
References
- Afifi AK, Bergman RA. Functional Neuroanatomy: Text and Altas. McGraw-Hill; New York: 1998. [Google Scholar]
- Albin RL, Young AB, Penney J. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, Dhawan V, Eidelberg D. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29:9740–9747. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger DH, Thees S, Kollias SS, Bassetti CL, Waldvogel D. Morphological differences in Parkinson's disease with and without rest tremor. J Neurol. 2009;256:256–263. doi: 10.1007/s00415-009-0092-2. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhmann C, Glauche V, Sturenburg HJ, Oechsner M, Weiller C, Buchel C. Pharmacologically modulated fMRI--cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain. 2003;126:451–461. doi: 10.1093/brain/awg033. [DOI] [PubMed] [Google Scholar]
- Carbon M, Felice GM, Dhawan V, Eidelberg D. Correlates of movement initiation and velocity in Parkinson's disease: A longitudinal PET study. Neuroimage. 2007;34:361–370. doi: 10.1016/j.neuroimage.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasa A, Hagberg GE, Peppe A, Bianciardi M, Gioia MC, Costa A, Castriota-Scanderbeg A, Caltagirone C, Sabatini U. Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson's disease. Brain Res Bull. 2006;71:259–269. doi: 10.1016/j.brainresbull.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Chuma T, Faruque RM, Ikoma K, Mano Y. Motor learning of hands with auditory cue in patients with Parkinson's disease. J Neural Transm. 2006;113:175–185. doi: 10.1007/s00702-005-0314-4. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Giovannelli F, Borgheresi A, Balestrieri F, Vanni P, Ragazzoni A, Zaccara G, Ziemann U. Surface electromyography shows increased mirroring in Parkinson's disease patients without overt mirror movements. Mov Disord. 2006;21:1461–1465. doi: 10.1002/mds.20972. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Pollak P, Passingham R, Landais P, Gervason C, Cinotti L, Friston K, Frackowiak R, Mauguiere F, Benabid AL. Thalamic stimulation and suppression of parkinsonian tremor. Evidence of a cerebellar deactivation using positron emission tomography. Brain. 1993;116(Pt 1):267–279. doi: 10.1093/brain/116.1.267. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Alexander GE, Georgopoulos AP, Crutcher MD, Mitchell SJ, Richardson RT. Role of basal ganglia in limb movements. Hum Neurobiol. 1984;2:235–244. [PubMed] [Google Scholar]
- Duffau H, Tzourio N, Caparros-Lefebvre D, Parker F, Mazoyer B. Tremor and voluntary repetitive movement in Parkinson's disease: comparison before and after L-dopa with positron emission tomography. Exp Brain Res. 1996;107:453–462. doi: 10.1007/BF00230425. [DOI] [PubMed] [Google Scholar]
- Elsinger CL, Rao SM, Zimbelman JL, Reynolds NC, Blindauer KA, Hoffmann RG. Neural basis for impaired time reproduction in Parkinson's disease: an fMRI study. J Int Neuropsychol Soc. 2003;9:1088–1098. doi: 10.1017/S1355617703970123. [DOI] [PubMed] [Google Scholar]
- Espay AJ, Li JY, Johnston L, Chen R, Lang AE. Mirror movements in parkinsonism: evaluation of a new clinical sign. J Neurol Neurosurg Psychiatry. 2005;76:1355–1358. doi: 10.1136/jnnp.2005.062950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay AJ, Morgante F, Gunraj C, Chen R, Lang AE. Mirror movements in Parkinson's disease: effect of dopaminergic drugs. J Neurol Neurosurg Psychiatry. 2006;77:1194–1195. doi: 10.1136/jnnp.2005.086892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouttard S, Styner M, Joshi S, Smith RG, Hazlett HC, Gerig G. Subcortical structure segmentation using probabilistic atlas priors; Medical Image Computing and Computer Assisted Intervention Workshop; 10-1-2007.pp. 37–46. [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos-Baumann AO. Event-related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain. 2001;124:558–570. doi: 10.1093/brain/124.3.558. [DOI] [PubMed] [Google Scholar]
- Heeger DJ, Huk AC, Geisler WS, Albrecht DG. Spikes versus BOLD: what does neuroimaging tell us about neuronal activity? Nat Neurosci. 2000;3:631–633. doi: 10.1038/76572. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Aarts E, de Lange FP, Bloem BR, Toni I. Increased dependence of action selection on recent motor history in Parkinson's disease. J Neurosci. 2009;29:6105–6113. doi: 10.1523/JNEUROSCI.0704-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain. 1995;118(Pt 4):913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Kapadia AS. Functional decline in Parkinson disease. Arch Neurol. 2001;58:1611–1615. doi: 10.1001/archneur.58.10.1611. [DOI] [PubMed] [Google Scholar]
- Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40:1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Pathology of Parkinson's disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol. 1991;14:153–197. doi: 10.1007/BF03159935. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Paulus W. Clinico-pathological correlations in Parkinson's disease. Clin Neurol Neurosurg. 1992;94(Suppl):S86–S88. doi: 10.1016/0303-8467(92)90033-y. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. 5e Prentice Hall; 2002. [Google Scholar]
- Joshi S, Davis B, Jomier M, Gerig G. Unbiased diffeomorphic atlas construction for computational anatomy. Neuroimage. 2004;23(Suppl 1):S151–S160. doi: 10.1016/j.neuroimage.2004.07.068. [DOI] [PubMed] [Google Scholar]
- Kanouchi T, Yokota T, Isa F, Ishii K, Senda M. Role of the ipsilateral motor cortex in mirror movements. J Neurol Neurosurg Psychiatry. 1997;62:629–632. doi: 10.1136/jnnp.62.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, de ZG, Di MA, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K. L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci. 2009;29:7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MM, Slagle CG, Smith AB, Truong Y, Bai P, McKeown MJ, Mailman RB, Belger A, Huang X. Task specific influences of Parkinson's disease on the striato-thalamo-cortical and cerebello-thalamo-cortical motor circuitries. Neuroscience. 2007;147:224–235. doi: 10.1016/j.neuroscience.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Espay AJ, Gunraj CA, Pal PK, Cunic DI, Lang AE, Chen R. Interhemispheric and ipsilateral connections in Parkinson's disease: relation to mirror movements. Mov Disord. 2007;22:813–821. doi: 10.1002/mds.21386. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Louis ED, Tang MX, Cote L, Alfaro B, Mejia H, Marder K. Progression of parkinsonian signs in Parkinson disease. Arch Neurol. 1999;56:334–337. doi: 10.1001/archneur.56.3.334. [DOI] [PubMed] [Google Scholar]
- Marjama-Lyons J, Koller W. Tremor-predominant Parkinson's disease. Approaches to treatment. Drugs Aging. 2000;16:273–278. doi: 10.2165/00002512-200016040-00003. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Callicott JH, Bertolino A, Santha AK, Van Horn JD, Tallent KA, Frank JA, Weinberger DR. Hemispheric control of motor function: a whole brain echo planar fMRI study. Psychiatry Res. 1998;83:7–22. doi: 10.1016/s0925-4927(98)00023-7. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Moraschi M, Giulietti G, Giove F, Guardati M, Garreffa G, Modugno N, Colonnese C, Maraviglia B. fMRI study of motor cortex activity modulation in early Parkinson's disease. Magn Reson Imaging. 2010 doi: 10.1016/j.mri.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DA, Tisch S, Hariz M, Limousin P, Topka H, Rothwell JC. Sensory timing cues improve akinesia of grasping movements in Parkinson's disease: a comparison to the effects of subthalamic nucleus stimulation. Mov Disord. 2006;21:166–172. doi: 10.1002/mds.20657. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pechadre JC, Larochelle L, Poirier LJ. Parkinsonian akinesia, rigidity and tremor in the monkey. Histopathological and neuropharmacological study. J Neurol Sci. 1976;28:147–157. doi: 10.1016/0022-510x(76)90100-3. [DOI] [PubMed] [Google Scholar]
- Poirier LJ, Pechadre JC, Larochelle L, Dankova J, Boucher R. Stereotaxic lesions and movement disorders in monkeys. Adv Neurol. 1975;10:5–22. [PubMed] [Google Scholar]
- Pollok B, Makhloufi H, Butz M, Gross J, Timmermann L, Wojtecki L, Schnitzler A. Levodopa affects functional brain networks in Parkinsonian resting tremor. Mov Disord. 2009;24:91–98. doi: 10.1002/mds.22318. [DOI] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Fabre N, Brefel C, Loubinoux I, Celsis P, Senard JM, Montastruc JL, Chollet F. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain. 1997;120(Pt 1):103–110. doi: 10.1093/brain/120.1.103. [DOI] [PubMed] [Google Scholar]
- Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat Neurosci. 2000;3:716–723. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O. Cortical motor reorganization in akinetic patients with Parkinson's disease: a functional MRI study. Brain. 2000;123(Pt 2):394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Schiess MD, Zheng H, Soukup VM, Bonnen JG, Nauta HJW. Parkinson's disease subtypes: clinical classification and ventricular cerebrospinal fluid analysis. Parkinsonism and Related Disorders. 2000;6:69–76. doi: 10.1016/s1353-8020(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Sen S, Kawaguchi A, Truong Y, Lewis MM, Huang X. Dynamic changes in cerebellothalamo-cortical motor circuitry during progression of Parkinson's disease. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel J, Hellwig D, Farmakis G, Jost WH, Samnick S, Fassbender K, Kirsch CM, Dillmann U. Myocardial sympathetic degeneration correlates with clinical phenotype of Parkinson's disease. Mov Disord. 2007;22:1004–1008. doi: 10.1002/mds.21499. [DOI] [PubMed] [Google Scholar]
- Thobois S, Jahanshahi M, Pinto S, Frackowiak R, Limousin-Dowsey P. PET and SPECT functional imaging studies in Parkinsonian syndromes: from the lesion to its consequences. Neuroimage. 2004;23:1–16. doi: 10.1016/j.neuroimage.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain. 2003;126:199–212. doi: 10.1093/brain/awg022. [DOI] [PubMed] [Google Scholar]
- Tinaz S, Schendan HE, Stern CE. Fronto-striatal deficit in Parkinson's disease during semantic event sequencing. Neurobiol Aging. 2008;29:397–407. doi: 10.1016/j.neurobiolaging.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal JS, Derkinderen P, Vidailhet M, Thobois S, Broussolle E. Mirror movements of the non-affected hand in hemiparkinsonian patients: a reflection of ipsilateral motor overactivity? J Neurol Neurosurg Psychiatry. 2003;74:1352–1353. doi: 10.1136/jnnp.74.9.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets FJ, Schulzer M, Calne DB, Snow BJ. Which clinical sign of Parkinson's disease best reflects the nigrostriatal lesion? Ann Neurol. 1997;41:58–64. doi: 10.1002/ana.410410111. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson's disease. Brain. 2005;128:2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage. 2007;35:222–233. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel A, Arkadir D, Israel Z, Bergman H. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr Opin Neurol. 2009;22:387–393. doi: 10.1097/WCO.0b013e32832d9d67. [DOI] [PubMed] [Google Scholar]
- Zetusky WJ, Jankovic J, Pirozzolo FJ. The heterogeneity of Parkinson's disease: clinical and prognostic implications. Neurology. 1985;35:522–526. doi: 10.1212/wnl.35.4.522. [DOI] [PubMed] [Google Scholar]