Abstract
Speech and language disorders are some of the most common referral reasons to child development centers accounting for approximately 40% of cases. Stuttering is a disorder in which involuntary repetition, prolongation or cessation of the sound precludes the flow of speech. About 5% of individuals in the general population have a stuttering problem, and about 80% of the affected children recover naturally. The causal factors of stuttering remain uncertain in most cases; studies suggest that genetic factors are responsible for 70% of the variance in liability for stuttering, whereas the remaining 30% is due to environmental effects supporting a complex cause of the disorder. The use of high-resolution genome wide array comparative genomic hybridization (array-CGH) has proven to be a powerful strategy to narrow down candidate regions for complex disorders. We report on a case with a complex set of speech and language difficulties including stuttering who presented with a 10 Mb deletion of chromosome region 7q33-35 causing the deletion of several genes and the disruption of CNTNAP2 by deleting the first 3 exons of the gene. CNTNAP2 is known to be involved in the cause of language and speech disorders and Autism Spectrum Disorder (ASD) and is in the same pathway as FOXP2, another important language gene, which makes it a candidate gene for causal studies speech and language disorders such as stuttering.
Keywords: microdeletion, CNTNAP2, stuttering
Introduction
Fluent speech production is a process that involves three major steps: conceptualization, formulation and articulation [Levelt 1999] and disturbances of this balanced cascade can lead to speech disorders such as stuttering. Stuttering is a common worldwide communication disorder characterized by syllable repetitions and/or prolongations, and disturbances in the ability to produce smooth flow of speech [Conture and Kelly 1991; Wingate 1964]. The disorder is first noted during early childhood; approximately 80% of affected children improve naturally within 1 to 4 years of onset [Andrews 1964; Yairi and Ambrose 1999]. Several studies reported females recover more often than males, resulting in a male-female ratio of 4:1 in older children and adults [Buchel and Sommer 2004; Felsenfeld 2002; Yairi and Ambrose 1999].
The primary cause of stuttering remains unknown in the majority of cases. Andrews et al. [1991] and Felsenfeld et al. [2000] reported that genetic effects contribute to approximately 70% of the variance in liability for stuttering whereas the remaining 30% are due to environmental effects, supporting a complex cause of the disorder. Familial aggregation of stuttering has been studied extensively, with an increase of approximately 15% in first-degree relatives of propositus, compared to 5% in the general population [Ambrose et al., 1993].
Speech disorders have a broad clinical phenotype usually of unknown cause and pathogenesis; one of the biggest challenges is to define homogeneous subgroups for causal studies. Stuttering can occur as an isolated event or as part of a more complex phenotype or syndrome. The identification of important genetic factors will increase the knowledge of the causes of stuttering, along with diagnosis and treatment. Recently, several genome wide studies have reported susceptibility loci for stuttering at 18p11 [Shugart et al., 2004];12q23 [Riaz et al., 2005]; on chromosomes 2, 7, 9 and 15 [Suresh et al., 2006]; chromosome 3, 13 and 15 [Raza et al., 2010; Wittke-Thompson et al., 2007] but the results remain controversial and, to date, no strong candidate genes have been reported. A study with Pakistani families and unrelated individuals from Pakistan and North America identified mutations in GNPTAB, GNPTG and NAGPA genes that were associated with stuttering [Kang et al., 2010].
Different strategies can be use for gene identification, among those, the recent use of DNA microarrays (array-CGH) has helped to identify a new form of submicroscopic genomic imbalance named copy number variants (CNV) that are the result of common gains or losses of genomic regions and contribute substantially to human genetic diversity; they may cover important genes responsible for human diseases and their identification will contribute to increased knowledge of genetic factors in complex conditions such as stuttering.
We report one case with a complex set of speech, language and learning difficulties with notable problems in speech fluency characterized by stuttering and cluttering, developmental delay, and some minor phenotypic signs such as broad nasal root, mild thoracic asymmetry, broad halluces, and distal toe phalanges with a 10 Mb deletion involving chromosome region 7q33-35 identified by array-CGH. The region is a candidate for extended analysis and contains at least one strong candidate gene, CNTNAP2, which is known to be involved in language and speech problems and Autism Spectrum Disorder (ASD) [Alarcon et al., 2008; Poot et al., 2009; Rossi et al., 2008; Stromswold 2008; Vernes et al., 2006].
Material and Methods
The propositus, male, born in 1988, is the second child of a normal 23-year-old mother and her normal, non-consanguineous 25-year-old husband. Pregnancy was normal; no toxic, infectious, or traumatic incidents or X-ray exposure was reported Delivery was normal. Birth weight was 3,150 g (>25th centile), length was 49 cm (25th centile). Neonatal development was normal. Neuropsychological development and language acquisition were delayed (first words at 3 years age) and stuttering was noticed early when phrasal constructions began. Examination at age 19 showed a weight of 59 kg (<25 th centile), height of 168 cm (75th centile), OFC of 575 cm (<50th centile). He presented with severe fluency disorder characterized by stuttering and cluttering, minor phenotypic signs such as broad nasal root, mild thoracic asymmetry, and broad halluces and distal toe phalanges. Brain MRI showed mild cerebral and cerebellar atrophy and a large callosal splenium. High resolution GTG banding (850 bands) and Fluorescent in Situ Hybridization (FISH): WCP 7- Spectrum Green-VYSISR) were performed.
An audiological evaluation was performed by tonal audiometry, tympanometry, acoustic immittance and ABR. Video, audio and recording of speech were evaluated by ABFW Test. Cognitive assessments were performed using Wechsler Intelligence Scale for Adults, Wisconsin Cards Sorting Test, Token Test, and the Illinois Test of Psycholinguistic Abilities.
The study was approved by the Ethical Committee at the Hospital de Reabilitacão de Anomalias Craniofaciais - USP - Bauru, SP, Brazil and signed consents were obtained prior to sample collection.
DNA Microarray Analysis
Genomic DNA was isolated from a peripheral blood sample from the propositus and parents and the concentration was measured by a NanoDrop spectrophotometer(ND-1000 V.3.1.2). The microdeletion was detected by a genome wide copy number scan using the Affymetrix Genome Wide Human SNP Array 6.0 (Affymetrix, CA, USA). These arrays offers genome wide coverage with almost 2 million SNPs and CNV probes and measure DNA copy number differences between a reference genome and the sample genome. The results were analyzed using three different software packages: Affymetrix Genotyping Console, Partek Genomics Suite and Nexus Copy Number.
Quantitative Real Time PCR
The quantitative real time PCR was performed with DNA from the propositus, father, mother and a normal control using DNA-binding dye SYBR Green I. This method enables both detection and quantification as absolute number of copies of a specific sequence in a DNA sample. We designed primers for the first 5 exons of the CNTNAP2 gene; all the samples were analyzed in duplicates.
The fold changes per sample were calculated based on methods previously described [Weksberg et al., 2005]. We compared the target sample (propositus) with normal reference controls to obtain the fold changes.
Where ΔKCt – represents copy number gain or loss per sample (fold changes).
ΔKCt values of 0 (± 0.35) indicate an equal ratio of the test and reference, which corresponds to no loss and therefore no genetic abnormality. A ΔKCt value of -1 (± 0.35) indicates loss of one copy (microdeletion), for the affected samples while a value of +1, (± 0.35) would indicate copy gain consistent with microduplication [Weksberg et al., 2005].
Results
High-resolution GTG banding (at 850 bands) showed a 7q33-q34 deletion. Fluorescent in situ hybridization (FISH): WCP 7- Spectrum Green-VYSISR) ruled out translocation of the deleted region. Parents had normal chromosomes (data not shown). Audiological evaluation assessed by tonal audiometry, tympanometry, acoustic immittance, and ABR showed normal parameters. Video and audio recordings of speech samples were evaluated through the ABFW Test and showed high frequency of breakdowns of speech that included stutter disruptions such as repetitions and prolongation of sounds and syllables, involuntary pauses, reiteration, and associated movements (blinking). Cognitive assessment using Wechsler Intelligence Scale for Adults, Wisconsin Cards Sorting Test, Token Test, and the Illinois Test of Psycholinguistic Abilities revealed the patient’s mental performance has normal intellectual level VIQ (86), PIQ (96), FSIQ (90), working memory index (87) and speed processing index (103). The Wisconsin test also showed a perseverative response indicating a difficulty in flexibility strategies. Speech and language evaluation showed moderate difficulty in resolution of complex verbal commands mainly for comprehension of syntactic structures or poor verbal communication. Receptive oral language was better than expressive language. His global academic performance in arithmetic, reading, and writing evaluated through TDE showed low scores in comparison with the normal range.
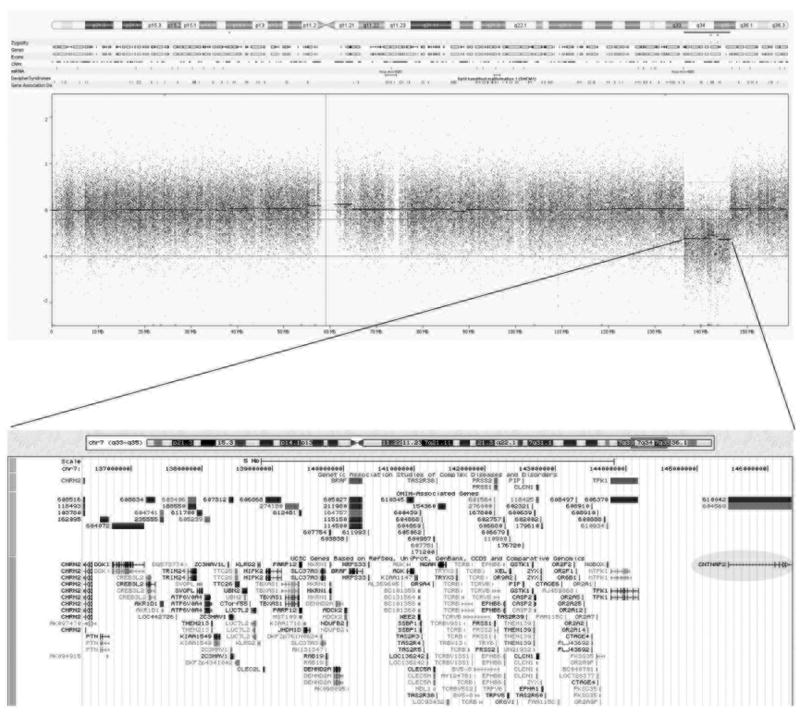
The genome wide copy number scan analysis using microarray showed a 10 Mb deletion encompassing the region 7q33-q35 that includes several genes and disrupts the gene CNTNAP2 by deleting the 3 first exons. Fig. 1 shows details of the deleted region.
Figure 1.
Details of the deleted region – Top: heat map showing the deletion at 7q33-35; Bottom: a expanded view of the deleted region based on UCSC Genome Browser with CNTNAP2 highlighted by a gray circle
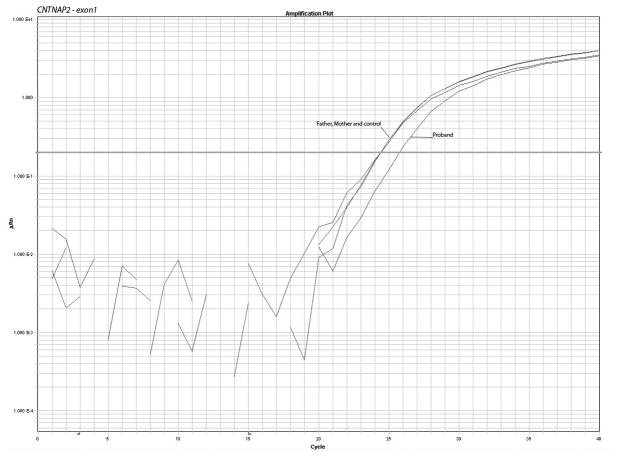
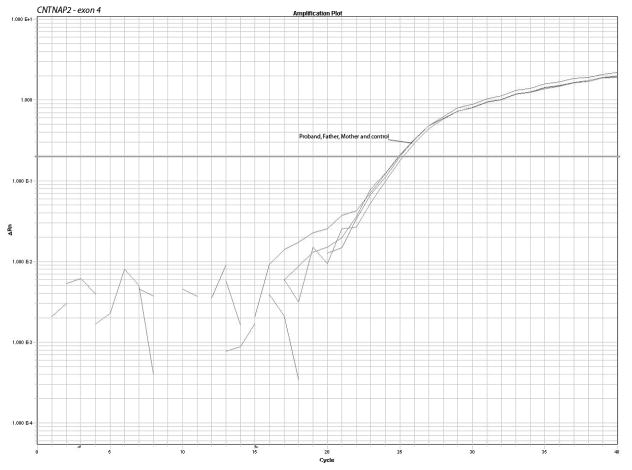
In order to confirm the breakpoints of the deletion and the disruption of the gene, we performed a quantitative PCR using Sybr Green I dye. We designed primers for the first 5 exons of the gene and the results show a pattern compatible with deletion of one copy for the exons one to three and normal values for exons 4 and 5, confirming the microarray results. The quantitative PCR plots and calculations are shown in fig. 2 and Table I.
Figure 2.
Confirmation of the microarrays results and detection of the deletion of the first 3 exons of CNTNAP2 by quantitative PCR using 2 sets of primers for each of exons 1 to 5. The plots show the pattern of amplification in parents, control and from propositus samples. The parents and control samples amplify normally while the patient sample presents a delay of amplification due to the deletion.
Table I.
Copy Number calculations based on qPCR with SybrGreen
| Sample | Primer | Ct | KCt | ΔKCt | |
|---|---|---|---|---|---|
| PROPOSITUS | primer 1_1 | 25.4 25.8 |
25.6 | −1.2 | Loss of 1 copy |
| primer 1_2 | 24.3 24.0 |
24.2 | −1.0 | ||
| primer 2_1 | 24.5 24.8 |
24.7 | −1.3 | ||
| primer2_2 | 25.6 25.0 |
25.3 | −0.7 | ||
| primer 3_1 | 25.1 24.7 |
24.9 | −1.2 | ||
| primer 3_2 | 24.6 24.3 |
24.5 | −1.0 | ||
| primer 4_1 | 23.6 23.5 |
23.6 | 0.7 | No copy number loss | |
| primer 4_2 | 25.0 25.5 |
25.2 | 0.5 | ||
| primer 5_1 | 24.4 24.7 |
24.5 | 0.3 | ||
| primer 5_2 | 24.2 23.9 |
24.1 | 0.1 |
Copy number calculation where: Ct represents the point at which the fluorescence crosses the threshold; KCt is the corrected Ct value and ΔKCt represents copy number gain or loss per sample (fold changes). The analysis show ΔKCt values consistent with loss of 1 copy of exons 1, 2 and 3 and normal values for exons 4 and 5 in the propositus.
The deletion of the first three exons of the CNTNAP2 gene also results in deletion of FOXP2 binding sites present in intron one of CNTNAP2. Since this patient is hemizygous for the FOXP2 protein binding sites in the CNTNAP2 gene there may be a disruption of the molecular mechanism between free and bound FOXP2 protein.
Discussion
Stuttering is a complex disorder that involves genetic and environmental causes. It is a common and sometimes severe disorder that is distributed worldwide and has variable phenotypic severity, it can be syndromic or occur as an isolated event. Several studies have reported important evidence of genetic factors that may be involved in its cause [Ambrose et al., 1993; Andrews et al., 1991; Felsenfeld et al., 2000; Riaz et al., 2005; Suresh et al., 2006] although the wide phenotypic spectrum of language and speech disorders makes it difficult to identify homogenous subgroups for causal studies that could lead to the identification of major, causative genomic variants.
The use of high-resolution microarrays has enhanced discovery of loci and genes for human diseases, especially complex diseases. The current generation of DNA microarrays such as Affymetrix array SNP 6.0 offers excellent genome-wide coverage with almost 2 million SNPs and CNV probes and measures DNA copy number differences between a reference genome and the sample genome. The use of ultrahigh resolution makes it possible to find with precision the microdeletion boundaries.
For a long time, it was thought that stuttering arose from abnormalities of the tongue, larynx, peripheral muscles of the speech, and psychological response to the environment. However, recent studies suggest that stuttering dysfunction resides in the brain [Canhetti-Oliveira and Richieri-Costa 2006].
Our study reports a Brazilian case with complex neurodevelopmental phenotype including stuttering that presents with a 10 Mb deletion at 7q33-q35 involving several genes. Some of the deleted genes are of particular interest due to expression in the brain including: CNTNAP2, CHRM2, HIPK2, DGKI, EPHB6, PTN and SVOPL. Here we summarize the importance of some of the deleted genes in this case.
CHRM2 is one of 5 human genes encoding muscarinic acetylcholine receptors and is involved in neuronal excitability, synaptic plasticity and feedback regulation of acetylcholine release [Gosso et al., 2007]. Muscarinic receptors are involved in many processes in the brain, including attention, learning, memory and cognition [Baxter and Chiba 1999]. CHRM2 is a functionally important membrane-bound protein that is negatively coupled to adenylyl cyclase and is widely expressed in a range of tissues including brain, cardiac, prejunctional cholinergic nerve endings, and smooth muscle [Fenech et al., 2004]. In 2004 Wang et al. reported an association between SNPs in CHRM2 and alcoholism and depressive syndrome [Wang et al., 2004]. Behavioral and pharmacological animal studies involving muscarinic receptors have shown their importance for acquisition and retrieval of several learning tasks [Carey et al., 2001; Gautam et al., 2006; Orsetti et al., 1996; Seeger et al., 2004] but there are no data about speech disorders and CHRM2.
Other deleted genes show association with disease, including HIPK2 with leukemia, DGKI with schizophrenia and bipolar disorder, EPHB6 with neuroblastoma, and CNTNAP2 with ASD susceptibility [Alarcon et al., 2008; Poot et al., 2009; Rossi et al., 2008] and Gilles de la Tourette syndrome which includes complex involuntary motor and vocal tics [Belloso et al., 2007].
Among all these CNTNAP2 is probably one of the most promising candidates for the speech problem based on previous published data although the hypothesis needs confirmatory studies. This gene was previously related to language disorders [Abrahams et al., 2007; Alarcon et al., 2008; Belloso et al., 2007; Poot et al., 2009; Rossi et al., 2008; Verkerk et al., 2003; Vernes et al., 2008], is highly expressed in the cerebral cortex in areas involved with language function [Abrahams et al., 2007; Vernes et al., 2006] and is in the same pathway of FOXP2, another important language gene. A recent study reports the disruption of CNTNAP2 in a case with ASD and speech delay and compares different rearrangements of CNTNAP2 with different phenotypes [Poot et al., 2010].
The CNTNAP2 gene belongs to the superfamily of the neurexins, a group of transmembrane proteins that mediate cell–cell interactions in the nervous system. A recent expression study showed that CNTNAP2 is consistently expressed in circuits involved in higher cortical functions, including language [Abrahams et al., 2007; Bellen et al., 1998; Peles et al., 1997; Poliak et al., 1999]. Another recent study showed that the transcription factor FOXP2 regulates the expression of CNTNAP2 by binding to a regulatory sequence in intron 1 of the gene; they also showed complimentary patterns of expression of FOXP2 and CNTNAP2 in cortical lamination at 18 to 22 weeks of gestation and concluded that these 2 genes form a shared neurogenetic pathway that is involved in language and speech disorders [Vernes et al., 2008].
Vertek et al. [2003] showed that CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome (GTS) involving complex involuntary motor and vocal tics; they discuss that disruption or decreased expression of CNTNAP2 could lead to a disturbed distribution of the K+_ channels in the nervous system, thereby influencing conduction and/or repolarization of action potentials, causing unwanted actions or movements in GTS [Verkerk et al., 2003]. Lu et al. [2009] showed that stuttering cases have an altered connectivity in the basal ganglia compared with non-stuttering controls that may be involved in the difficulties related to speech control [Lu et al., 2009a; Lu et al., 2009b]. As well as the unwanted actions seen in GTS patients, stuttering is a motor problem that impairs normal speech ability. The deletion found in our case supports the causal role of CNTNAP2 in patients with stuttering and similar motor speech problems.
Our study shows evidence that one or more genes in the 7q33-q35 region may play a causal role in speech motor problems as stuttering with a special focus on a partial deletion of CNTNAP2 including a deletion of the binding site for the transcription factor FOXP2.
Acknowledgments
We gratefully acknowledge the family participant in this study and all the professionals involved. This work was supported by the NIH grant DE08559
References
- Abrahams BS, Tentler D, Perederiy JV, Oldham MC, Coppola G, Geschwind DH. Genome-wide analyses of human perisylvian cerebral cortical patterning. Proc Natl Acad Sci U S A. 2007;104:17849–54. doi: 10.1073/pnas.0706128104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, Nelson SF, Cantor RM, Geschwind DH. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–9. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose NG, Yairi E, Cox N. Genetic aspects of early childhood stuttering. J Speech Hear Res. 1993;36:701–6. doi: 10.1044/jshr.3604.701. [DOI] [PubMed] [Google Scholar]
- Andrews G, Morris-Yates A, Howie P, Martin NG. Genetic factors in stuttering confirmed. Arch Gen Psychiatry. 1991;48:1034–5. doi: 10.1001/archpsyc.1991.01810350074012. [DOI] [PubMed] [Google Scholar]
- Andrews JG. The Nature of Stuttering. Med J Aust. 1964;2:919–24. [PubMed] [Google Scholar]
- Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9:178–83. doi: 10.1016/s0959-4388(99)80024-5. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Lu Y, Beckstead R, Bhat MA. Neurexin IV, caspr and paranodin--novelmembers of the neurexin family: encounters of axons and glia. Trends Neurosci. 1998;21:444–9. doi: 10.1016/s0166-2236(98)01267-3. [DOI] [PubMed] [Google Scholar]
- Belloso JM, Bache I, Guitart M, Caballin MR, Halgren C, Kirchhoff M, Ropers HH, Tommerup N, Tumer Z. Disruption of the CNTNAP2 gene in a t(7;15) translocation family without symptoms of Gilles de la Tourette syndrome. Eur J Hum Genet. 2007;15:711–3. doi: 10.1038/sj.ejhg.5201824. [DOI] [PubMed] [Google Scholar]
- Buchel C, Sommer M. What causes stuttering? PLoS Biol. 2004;2:E46. doi: 10.1371/journal.pbio.0020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canhetti-Oliveira CM, Richieri-Costa A. A study of familial stuttering. Am J Med Genet. 2006;A140:2139–41. doi: 10.1002/ajmg.a.31320. [DOI] [PubMed] [Google Scholar]
- Carey GJ, Billard W, Binch H, 3rd, Cohen-Williams M, Crosby G, Grzelak M, Guzik H, Kozlowski JA, Lowe DB, Pond AJ, Tedesco RP, Watkins RW, Coffin VL. SCH 57790, a selective muscarinic M(2) receptor antagonist, releases acetylcholineand produces cognitive enhancement in laboratory animals. Eur J Pharmacol. 2001;431:189–200. doi: 10.1016/s0014-2999(01)01440-6. [DOI] [PubMed] [Google Scholar]
- Conture EG, Kelly EM. Young stutterers' nonspeech behaviors during stuttering. J Speech Hear Res. 1991;34:1041–56. doi: 10.1044/jshr.3405.1041. [DOI] [PubMed] [Google Scholar]
- Felsenfeld S. Finding susceptibility genesfor developmental disorders of speech: the long and winding road. J Commun Disord. 2002;35:329–45. doi: 10.1016/s0021-9924(02)00088-6. [DOI] [PubMed] [Google Scholar]
- Felsenfeld S, Kirk KM, Zhu G, Statham DJ, Neale MC, Martin NG. A study of the genetic and environmental etiology of stuttering in a selected twin sample. Behav Genet. 2000;30:359–66. doi: 10.1023/a:1002765620208. [DOI] [PubMed] [Google Scholar]
- Fenech AG, Billington CK, Swan C, Richards S, Hunter T, Ebejer MJ, Felice AE, Ellul-Micallef R, Hall IP. Novel polymorphisms influencing transcription of the human CHRM2 gene in airway smooth muscle. Am J Respir Cell Mol Biol. 2004;30:678–86. doi: 10.1165/rcmb.2003-0011OC. [DOI] [PubMed] [Google Scholar]
- Gautam D, Duttaroy A, Cui Y, Han SJ, Deng C, Seeger T, Alzheimer C, Wess J. M1–M3 muscarinic acetylcholine receptor-deficient mice: novel phenotypes. J Mol Neurosci. 2006;30:157–60. doi: 10.1385/JMN:30:1:157. [DOI] [PubMed] [Google Scholar]
- Gosso FM, de Geus EJ, Polderman TJ, Boomsma DI, Posthuma D, Heutink P. Exploring the functional role of the CHRM2 gene in human cognition: results from a dense genotyping and brain expression study. BMC Med Genet. 2007;8:66. doi: 10.1186/1471-2350-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Riazuddin S, Mundorff J, Krasnewich D, Friedman P, Mullikin JC, Drayna D. Mutations in the lysosomal enzyme-targeting pathway and persistent stuttering. N Engl J Med. 2010;362:677–85. doi: 10.1056/NEJMoa0902630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJ. Models of word production. Trends Cogn Sci. 1999;3:223–232. doi: 10.1016/s1364-6613(99)01319-4. [DOI] [PubMed] [Google Scholar]
- Lu C, Ning N, Peng D, Ding G, Li K, Yang Y, Lin C. The role of large-scale neural interactions for developmental stuttering. Neuroscience. 2009a;161:1008–26. doi: 10.1016/j.neuroscience.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Lu C, Peng D, Chen C, Ning N, Ding G, Li K, Yang Y, Lin C. Altered effective connectivity and anomalous anatomy in the basal ganglia-thalamocortical circuit of stuttering speakers. Cortex. 2009b doi: 10.1016/j.cortex.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Orsetti M, Casamenti F, Pepeu G. Enhanced acetylcholine release in the hippocampus and cortex during acquisition of an operant behavior. Brain Res. 1996;724:89–96. doi: 10.1016/0006-8993(96)00292-2. [DOI] [PubMed] [Google Scholar]
- Peles E, Nativ M, Lustig M, Grumet M, Schilling J, Martinez R, Plowman GD, Schlessinger J. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J. 1997;16:978–88. doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 1999;24:1037–47. doi: 10.1016/s0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- Poot M, Beyer V, Schwaab I, Damatova N, Van't Slot R, Prothero J, Holder SE, Haaf T. Disruption of CNTNAP2 and additional structural genome changes in a boy with speech delay and autism spectrum disorder. Neurogenetics. 2009 doi: 10.1007/s10048-009-0205-1. [DOI] [PubMed] [Google Scholar]
- Poot M, Beyer V, Schwaab I, Damatova N, Van't Slot R, Prothero J, Holder SE, Haaf T. Disruption of CNTNAP2 and additional structural genome changes in a boy with speech delay and autism spectrum disorder. Neurogenetics. 2010;11:81–9. doi: 10.1007/s10048-009-0205-1. [DOI] [PubMed] [Google Scholar]
- Raza MH, Riazuddin S, Drayna D. Identification of an autosomal recessive stuttering locus on chromosome 3q13.2–3q13.33. Hum Genet. 2010 doi: 10.1007/s00439-010-0871-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz N, Steinberg S, Ahmad J, Pluzhnikov A, Riazuddin S, Cox NJ, Drayna D. Genomewide significant linkage to stuttering on chromosome 12. Am J Hum Genet. 2005;76:647–51. doi: 10.1086/429226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi E, Verri AP, Patricelli MG, Destefani V, Ricca I, Vetro A, Ciccone R, Giorda R, Toniolo D, Maraschio P, Zuffardi O. A 12Mb deletion at 7q33-q35 associated with autism spectrum disorders and primary amenorrhea. Eur J Med Genet. 2008;51:631–8. doi: 10.1016/j.ejmg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C, Wess J. M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci. 2004;24:10117–27. doi: 10.1523/JNEUROSCI.3581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugart YY, Mundorff J, Kilshaw J, Doheny K, Doan B, Wanyee J, Green ED, Drayna D. Results of a genome-wide linkage scan for stuttering. Am J Med Genet A. 2004;124A:133–5. doi: 10.1002/ajmg.a.20347. [DOI] [PubMed] [Google Scholar]
- Stromswold K. The genetics of speech and language impairments. N Engl J Med. 2008;359:2381–3. doi: 10.1056/NEJMe0807813. [DOI] [PubMed] [Google Scholar]
- Suresh R, Ambrose N, Roe C, Pluzhnikov A, Wittke-Thompson JK, Ng MC, Wu X, Cook EH, Lundstrom C, Garsten M, Ezrati R, Yairi E, Cox NJ. New complexities in the genetics of stuttering: significant sex-specific linkage signals. Am J Hum Genet. 2006;78:554–63. doi: 10.1086/501370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AJ, Mathews CA, Joosse M, Eussen BH, Heutink P, Oostra BA. CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder. Genomics. 2003;82:1–9. doi: 10.1016/s0888-7543(03)00097-1. [DOI] [PubMed] [Google Scholar]
- Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, Alarcon M, Oliver PL, Davies KE, Geschwind DH, Monaco AP, Fisher SE. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359:2337–45. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernes SC, Nicod J, Elahi FM, Coventry JA, Kenny N, Coupe AM, Bird LE, Davies KE, Fisher SE. Functional genetic analysis of mutations implicated in a human speech and language disorder. Hum Mol Genet. 2006;15:3154–67. doi: 10.1093/hmg/ddl392. [DOI] [PubMed] [Google Scholar]
- Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, Kwon JM, Wu W, Dick DM, Rice J, Jones K, Nurnberger JI, Jr, Tischfield J, Porjesz B, Edenberg HJ, Hesselbrock V, Crowe R, Schuckit M, Begleiter H, Reich T, Goate AM, Bierut LJ. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13:1903–11. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- Weksberg R, Hughes S, Moldovan L, Bassett AS, Chow EW, Squire JA. A method for accurate detection of genomic microdeletions using real-time quantitative PCR. BMC Genomics. 2005;6:180. doi: 10.1186/1471-2164-6-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingate ME. A Standard Definition of Stuttering. J Speech Hear Disord. 1964;29:484–9. doi: 10.1044/jshd.2904.484. [DOI] [PubMed] [Google Scholar]
- Wittke-Thompson JK, Ambrose N, Yairi E, Roe C, Cook EH, Ober C, Cox NJ. Genetic studies of stuttering in a founder population. J Fluency Disord. 2007;32:33–50. doi: 10.1016/j.jfludis.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yairi E, Ambrose NG. Early childhood stuttering I: persistency and recovery rates. J Speech Lang Hear Res. 1999;42:1097–112. doi: 10.1044/jslhr.4205.1097. [DOI] [PubMed] [Google Scholar]