Abstract
Peroxisomes are the cellular location of many antioxidants and are themselves significant producers of reactive oxygen species. In this report we demonstrate the induction of peroxisome biogenesis genes in both plant and animal cells by the universal stress signal molecule hydrogen peroxide. Using PEX1–LUC transgenic plants, rapid local and systemic induction of PEX1–luciferase could be demonstrated in vivo in response to physiological levels of hydrogen peroxide. PEX1–luciferase was also induced in response to wounding and to infection with an avirulent pathogen. We propose a model in which various stress situations that lead to the production of hydrogen peroxide can be ameliorated by elaboration of the peroxisome compartment to assist in restoration of the cellular redox balance.
Keywords: hydrogen peroxide/oxidative stress/PEX genes/plant pathogenesis/wounding
Introduction
Peroxisomes are subcellular respiratory organelles that carry out a wide range of functions in eukaryotic cells, including β-oxidation of fatty acids, glyoxylate metabolism and metabolism of reactive oxygen species (van den Bosch et al., 1992). In recent years >20 genes involved in peroxisomal protein import and peroxisome biogenesis (PEX genes) have been identified (Distel et al., 1996). pex mutants lack peroxisomes or mislocalize peroxisomal proteins. In many cases the function(s) of the corresponding proteins is unknown, as is the mechanism by which peroxisomes import proteins into the organelle (Subramani, 1998).
PEX1 is a peroxisome biogenesis gene that encodes a member of the AAA (ATPases associated with diverse cellular activities) superfamily of ATPases. Proteins of this family are involved in a wide range of cellular functions (Patel and Latterich, 1998). In humans, mutations in PEX1 are responsible for complementation group 1 (CG1), which comprises the most severe of the peroxisome biogenesis disorders, such as Zellweger syndrome. CG1 cells have abnormal peroxisomes that mislocalize peroxisomal proteins (Portsteffan et al., 1997). In yeasts, pex1 null mutants contain small peroxisomes with very reduced matrix content. This suggests that these mutants contain peroxisomes with a competent protein import machinery but are unable to grow (Heyman et al., 1994; Kiel et al., 1999). In Pichia pastoris, Pex1p and its interacting partner, another AAA ATPase, Pex6p, are mainly found associated with vesicles distinct from peroxisomes and only a small amount of these proteins are peroxisome associated (Faber et al., 1998). In contrast, in Hansenula polymorpha most Pex1p and Pex6p co-fractionates with peroxisomes (Kiel et al., 1999). Pex1p and Pex6p have high sequence identity with proteins thought to facilitate membrane fusion events in mammalian and yeast cells, like NSF/Sec18p and p97 /Cdc48p (Patel and Latterich, 1998 and references therein). Consequently, these proteins have been suggested to mediate lipid and/or membrane addition to peroxisome membranes, allowing the peroxisomes to grow (Faber et al., 1998; Kiel et al., 1999).
Peroxisomes are sensitive to external signals and are able to proliferate. In yeasts, where peroxisomes are the sole site of β-oxidation, many peroxisomal enzymes and PEX genes are induced by fatty acids, notably oleate, and repressed by glucose. In Saccharomyces cerevisiae, oleate-inducible genes contain an oleate response element (ORE) within the promoter that binds a transcription factor Oaf1p/Oaf2p (Karpichev et al., 1997). In mammalian cells, expression of a range of genes involved in lipid homeostasis, including all the peroxisomal β-oxidation genes, is controlled by the peroxisome proliferator activator receptor α isoform (PPARα), which binds to the peroxisome proliferator response element (PPRE) in the promoters of these genes (Lemberger et al., 1996). A broad range of medium and long chain fatty acids and a diverse class of compounds, including clofibrate, which act as hypolipidemic drugs are all ligands for PPARα (Isseman and Green, 1990). Administration of clofibrate results in up-regulation of PPARα target genes, including acyl-CoA oxidase, but does not affect PEX gene expression (Okumoto et al., 1998a; Shimuzu et al., 1999). In plants, proliferation of peroxisomes has been reported in early post-germinative growth (Mansfield and Briarty, 1996) in response to herbicides (de Felipe et al., 1988), xenobiotics (Palma et al., 1991) and ozone (Morre et al., 1990), and during senescence (Pastori and del Rio, 1997), but the mechanism is not known.
In recent years reactive oxygen species, particularly hydrogen peroxide, have been implicated in a number of signal transduction pathways. These include activation of the transcription factor NF-κB in mammalian cells (Schreck et al., 1991), gene regulation in bacteria (Demple, 1991) and plant stress. Hydrogen peroxide has been shown to act as a signal in osmotic stress (Guan et al., 2000), ABA-mediated guard cell closure (Pei et al., 2000), stress caused by excess light (Karpinski et al., 1999) and in response to infection by avirulent pathogens leading to a hypersensitive response (Alvarez et al., 1998). Nitric oxide is also required for the hypersensitive response (Delledonne et al., 1998; Durner et al., 1999) and nitric oxide synthase has recently been discovered in plant peroxisomes (Barroso et al., 1999). Peroxisomes contain antioxidant molecules, such as ascorbate and glutathione, and also a battery of antioxidant enzymes, including superoxide dismutase, ascorbate peroxidase, dihydro- and monohydroascorbate reductase, glutathione reductase and the cell’s principal H2O2-degrading enzyme, catalase. Changes in activities of these enzymes are correlated with many situations in which plants experience stress (del Rio et al., 1998 and references therein). Accordingly, peroxisomes have been suggested to play important roles in defence against abiotic and biotic stress in plants (Willekens et al., 1997; del Rio et al., 1998; Barroso et al., 1999) and in altered redox status associated with ageing and a variety of disease states in mammals (Masters and Crane, 1995). However, the signal(s) or substrates that trigger peroxisome proliferation as a result of stress have not been identified. In addition, it is not known whether peroxisomes are induced as a result of the stress or as a consequence of the metabolic events that occur in stress situations such as lipid degradation, protein losses or damage of organelle structures.
In this study we provide the first demonstration that PEX genes in both plant and animal cells are induced by the universal stress signal H2O2. Using Arabidopsis plants transformed with a PEX1 promotor–luciferase reporter (PEX1–LUC) we show induction of PEX1 expression in vivo in naturally occurring stress situations such as pathogen attack and wounding.
Results
PEX1 from Arabidopsis
We cloned a PEX1 cDNA from Arabidopsis. A composite sequence (DDBJ/EMBL/GenBank accession No. AF275382) was assembled from two clones that are identical in 189 bp of overlap. The 5′-end of the cDNA sequence and ∼995 bp of genomic sequence are shown in Figure 1. There are two in-frame ATG motifs (underlined). The more probable start site is the second ATG as it possesses a strong Kozak consensus GTGATGG (Kozak, 1997). Assuming that translation starts at this ATG, the deduced amino acid sequence of Arabidopsis Pex1p comprises 1117 amino acids with a predicted molecular weight of 122 305 Da. Two ATP-binding motifs (P-loops) are predicted between amino acids 604 and 611 and 889 and 896, and an AAA family signature between 985 and 1003. A protein database search with AtPex1p identified human Pex1p as the most similar sequence in the database (E = 1 × 10–79), closely followed by Pex1p from the yeasts Yarrowia lipolytica, H.polymorpha and P.pastoris. AtPex1p shares identity with known Pex1 proteins ranging from 27.6% with S.cerevisiae to 29.3% with P.pastoris. AtPex1p can be immunoprecipitated by antibodies raised against P.pastoris Pex1p (data not shown). The PEX1 promoter does not contain any obvious ORE or PPRE-like elements.
Fig. 1. (A) AtPEX1 promoter and N-terminus of coding region. Italics show the additional 5′ sequence derived from the 215 bp partial cDNA. The two possible ATG start codons are underlined. The amino acid sequence deduced from the cDNA clone is shown below the nucleotide sequence for the first and part of the second exon. The DDWE motif where the fusion to luciferase was made is shown in bold. (B) The fusion junction between PEX1 and luciferase.
AtPEX1 expression in seedlings
Figure 2 shows the expression profile of AtPEX1 and AtPEX5 (Brickner et al., 1998), which encodes Pex5p, the receptor for PTS1-targeted proteins (Dodt et al., 1995). Both genes are expressed at a low level in imbibed seeds and increase rapidly during the first 2 days of post-germinative growth before falling to a lower steady-state level by day 3, which is maintained to day 8. At this time (0–3 days post-imbibition), peroxisome size and number increase considerably in the cotyledon cells of Arabidopsis (Mansfield and Briarty, 1996). Malate synthase, a unique enzyme of the glyoxylate cycle, is maximally expressed at 1–2 days post-imbibition, which coincides with the maximal rate of lipid breakdown (Mansfield and Briarty, 1996), but thereafter declines to almost undetectable levels (Eastmond et al., 2000). The up-regulation of PEX1 and PEX5 expression at days 1–2 is consistent with their involvement in peroxisome biogenesis, and their continued expression at a lower level after day 2 presumably reflects the requirement for continued peroxisome biogenesis as cells divide and organelles turn over.
Fig. 2. Expression profile of AtPEX1 and AtPEX5 in seedlings. RNA from seedlings at the days indicated was hybridized with probes for AtPEX1, AtPEX5 and At malate synthase.
PEX1 induction by hydrogen peroxide
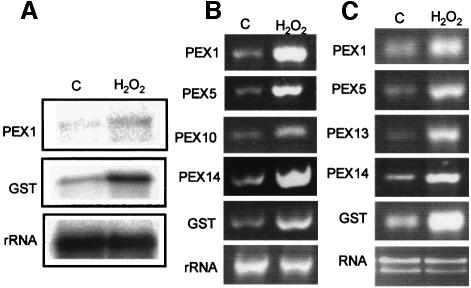
Different stress situations yield hydrogen peroxide as a signal molecule that triggers gene expression of various cellular protectants (Levine et al., 1994; Mittler and Zilinkas, 1994; Willekens et al., 1994a; Karpinski et al., 1999; Guan et al., 2000). As proliferation of peroxisomes and induction of some peroxisomal genes in response to oxidative stress has been reported, the effect of the stress signal molecule H2O2 on the expression of PEX genes was investigated. Northern blots showed that AtPEX1 was induced ∼10-fold in detached leaves by H2O2 (Figure 3A), similar to the level of induction of glutathione S-tranferase (GST), a well-characterized H2O2-responsive gene (Levine et al., 1994). The same result was obtained by semi-quantitative RT–PCR (Figure 3B) with gene-specific primers against AtPEX1, GST, AtPEX5, AtPEX14 (J.Oh, E.Lopez-Huertas, W.Charlton and A.Baker, in preparation) and AtPEX10 (Schuman et al., 1999). As peroxisomes may also be important in protecting animal cells against oxidative stress, the effect of H2O2 treatment on PEX gene expression in CHO cells was investigated (Figure 3C). Semi-quantitative RT–PCR showed that PEX1, PEX5, PEX13 and PEX14 are all induced to a comparable or greater extent than GST in cells treated with 1 mM H2O2 for 1 h.
Fig. 3. H2O2 induces plant and animal peroxisome biogenesis genes. RNA used in (A) (northern blot) and (B) (RT–PCR) was obtained from control and 1 mM H2O2-treated Arabidopsis leaves, whereas in (C) (RT–PCR) the RNA is from control and 1 mM H2O2-treated chinese hamster ovary cells.
Response of AtPEX1 to stress in whole plants
The PEX1 promoter was linked to the luciferase (LUC) gene to create transgenic plants (PEX1–LUC) that would report upon activation of PEX1 in response to stress. Figure 1 shows the fragment used. A translational fusion was made at the sequence DDWE, which is conserved in all known PEX1 genes. Depending on which start codon is used in vivo, a translational fusion with either 107 or 113 amino acids of the N-terminus of Pex1p fused to luciferase will be expressed.
All experiments were carried out on T2 progeny from two lines, 5ci and 1ci, which were recovered from independent transformations. Because the T2 plants of the two lines are segregating for the transgene, the absolute level of expression varies between plants and quantitative comparisons cannot be made. However, qualitative results are extremely consistent both within and between lines. No luciferase expression was seen in plants transformed with a promoter-less luciferase construct (data not shown). In untreated PEX1–LUC plants, PEX1–luciferase is mainly expressed in the meristems and young developing leaves, whereas there is little expression in mature leaves (controls in Figures 4A–C, 5B and 6B). These results suggest that PEX1 expression and peroxisome biogenesis are highest in actively dividing tissue and much lower in tissues where organelle maintenance (rather than biogenesis) is required. When PEX1–LUC plants were sprayed with 1 mM H2O2, PEX1–luciferase expression increased rapidly and dramatically throughout the plants (Figure 4A). This is consistent with the northern and RT–PCR results obtained with H2O2-treated wild-type plants (Figure 3A and B). To verify that increased PEX1 expression reflects increased peroxisome biogenesis, plants were subjected to two stress conditions in which peroxisomes have previously been described to proliferate, clofibrate treatment (Palma et al., 1991) and natural senescence (Pastori and del Rio, 1997). Incubation of PEX1–LUC plants with clofibrate resulted in a significant increase in the expression of PEX1–luciferase (Figure 4B). Compared with a young (20-day-old) plant, naturally senescent plants (60-day-old) also showed increased expression of PEX1–luciferase, which was no longer restricted to meristems and young tissue but was high in light yellow senescent leaves (Figure 4C).
Fig. 4. H2O2 rapidly induces AtPEX1–LUC gene expression. (A) Control plants (left) were sprayed with 1 mM H2O2 and light emission measured after 30 min (right). Clofibrate treatment (B) and senescence (C) were used as positive controls for peroxisome proliferation.
Fig. 5. H2O2 induces PEX1–LUC systemically. (A) Opposite leaves (arrows) were incubated with either 1 mM H2O2 (bottom leaf) or 1 mM H2O2 plus catalase (upper leaf) for 15 min. (B) The bottom leaf was incubated with the H2O2-regenerating system G/GO. Light emission was measured at the indicated times.
Fig. 6. PEX1–LUC is induced by pathogen attack and wounding. (A) Pseudomonas syringae pv. tomato was inoculated (I) into the indicated leaf and the effect on PEX1–LUC expression compared with a control leaf inoculated with buffer only. Light emission was measured at the indicated times. (B–D) Wounded plants. (B) Whole plant before (left) and 90 min after wounding (right). (C) Close up of a leaf of the plant shown in (B). (D) Close up of leaf before and after wounding; different plant.
Hydrogen peroxide induces PEX1 systemically
Hydrogen peroxide acts as a mobile signal (Alvarez et al., 1998; Karpinski et al., 1999). Opposite leaves of PEX1–LUC plants (Figure 5A, arrows) were incubated with a 1 mM H2O2 solution or a 1 mM H2O2 solution containing catalase (control) (Figure 5A). After 10 min incubation, a strong induction of PEX1–luciferase was detected throughout the treated leaf, but no photon emission was detected in the control leaf. By 30 min, induction of PEX1–luciferase in distant leaves, including the opposite (control) leaf, was observed.
Plant tissues rapidly break down exogenously applied H2O2 and it is difficult to estimate the resulting intracellular concentration. However, treatment of cell suspensions with the H2O2-regenerating system glucose (2.5 mM) and glucose oxidase (2.5 U/ml) (G/GO) initiates a steady and prolonged production of >5 µM H2O2. This mimics the level of H2O2 generated by the oxidative burst during pathogen attack (Delledonne et al., 1998). One leaf of a young PEX1–LUC plant was incubated with the G/GO H2O2-regenerating system (Figure 5B). PEX1–luciferase expression was induced locally and systemically within minutes of the start of the treatment, especially in vascular tissue. Note that photon emission from the treated leaf is much reduced, as it is submerged in G/GO solution.
The pathogen-induced hypersensitive response rapidly results in PEX1 induction
The ability to reproduce PEX1–luciferase induction with an authentic avirulent pathogen was tested. PEX1–LUC plants were inoculated in one leaf with Pseudomonas syringae pv. tomato, which induces an oxidative burst and hypersensitive response. An adjacent (control) leaf in the same plant was mock infiltrated. Photon emission in the inoculated and control leaves was monitored for 4 h immediately after inoculation (Figure 6A). Soon after infiltration of the pathogen, increased PEX1–luciferase expression was clearly seen in the treated leaf compared with expression in the control leaf and with the treated leaf before inoculation. Expression in the inoculated leaf was maximal at ∼2 h. Although the pathogen was inoculated at the tip of the leaf, gene expression was mainly localized around the vascular tissue, similar to when a leaf was incubated with 10 µM H2O2-regenerating solution (Figure 5B); however, the response to pathogen was localized rather than systemic.
PEX1 is induced by wounding
Plants respond to wounding and pathogen attack by distinct signalling mechanisms, although these pathways interact in a complex and incompletely understood manner (Malek and Dietrich, 1999). Photon emission was measured before wounding and then after excision of one or two leaves at the indicated times (Figure 6B–D). PEX1–luciferase expression was induced in the wounded leaf, but, as with pathogen treatment, the response remained localized. In the wounded leaves, PEX1– luciferase expression was observed mainly around the vascular tissues in the veins and the periphery of the leaf. In five independent experiments (two of which are shown) expression typically peaked between 45 and 120 min and then declined. Expression of PEX1 in the detached portion of the leaf continued to increase throughout the time course of the experiment, with expression increasing from the cut surface towards the tip of the leaf (data not shown). This may be the combined result of wounding and senescence responses.
Discussion
Up-regulation of PEX genes by H2O2
In this paper we provide the first demonstration that peroxisome biogenesis is directly responsive to the major cellular stress signal H2O2. Peroxisomes are significant sources of active oxygen species as well as the site of important antioxidant enzymes and molecules. Consequently, peroxisomes are likely to play an important role in cellular redox balance. Steady-state mRNA levels of both plant and animal peroxisome biogenesis genes increase rapidly in response to 1 mM exogenous H2O2 treatment (Figure 3). PEX5 encodes the import receptor for PTS1-targeted proteins (Dodt et al., 1995), including catalase. PEX13 and PEX14 comprise the docking site for PTS1 and PTS2 import receptors and as such are central components of the peroxisomal import machinery (Gould et al., 1996; Albertini et al., 1997). PEX10 is a peroxisome membrane protein with a RING type zinc finger, which when mutated severely compromises the import of peroxisomal matrix proteins (Okumoto et al., 1998b).
AtPEX1 gene expression in whole plants in response to stress
Promoter–luciferase fusions are ideal for detecting rapid real time changes in gene expression with fidelity and high sensitivity (Millar et al., 1992). Using transgenic plants containing a PEX1–LUC transgene, exogenous application of 1 mM H2O2 resulted in the appearance of luciferase activity in the treated leaf within 10 min and the PEX1 promoter was activated systemically within 30 min (Figure 5A). This very rapid response argues strongly for activation of transcription of PEX1 being a direct consequence of the application of H2O2. The systemic induction is consistent with the known properties of H2O2 as a signal molecule (Levine et al., 1994). Sustained production of low micromolar concentrations of H2O2 by G/GO resulted in similar kinetics of induction of the PEX1 promoter (Figure 5B), demonstrating that this is a response to physiological levels of H2O2.
Infection with an avirulent pathogen results in the well-characterized production of H2O2 in vivo (Levine et al., 1994; Alvarez et al., 1998) and the induction of PEX1 by pathogen treatment is consistent with this (Figure 6A). This result demonstrates that peroxisome biogenesis is up-regulated when H2O2 is produced in a natural situation, thus it is a biologically meaningful response. However, the response is slower and not systemic. This may be because in plants time is required to generate the H2O2 and other mechanisms come into play to modulate the response.
Hydrogen peroxide is cytotoxic to the pathogen and to plant cells in the immediate vicinity, leading to a hypersensitive response. Protection of adjacent cells is achieved by induction of antioxidant defences, but as many of these are located in peroxisomes, elaboration of the peroxisome compartment might also be required early on to permit the uptake of newly synthesized antioxidant enzymes and molecules. The physiological importance of peroxisomal catalase as a sink for H2O2 has been demonstrated (Willekens et al., 1997). Of the three catalase genes in tobacco, Cat 2, which is preferentially expressed in vascular tissue (Willekens et al., 1994b), shows the highest induction by stress (Willekens et al., 1994a). Our results (Figures 4–6) show that PEX1– luciferase expression in response to various stresses is highest around vascular tissue. It is tempting to speculate that Cat 2 represents a stress-induced catalase that is imported into peroxisomes that are themselves elaborated in response to oxidative stress. In addition to protecting surrounding cells, peroxisomal antioxidants could also be responsible for turning off the H2O2 signal.
Wounding also produced a transient and localized response (Figure 6B). Wounding results in the induction of specific sets of genes, including those required for synthesis of jasmonates (Creelman and Mullett, 1997). Synthesis of jasmonic acid requires three cycles of (pre sumably) peroxisomal β-oxidation. Wounding also strongly induces one of the four acyl-CoA oxidases of Arabidopsis, AtACX1 (>10-fold induction within 1 h; Hooks et al., 1998). Either the fatty acids released from the phospholipids, jasmonic acid itself or the H2O2 produced by the wound-induced acyl-CoA oxidase could be responsible for induction of PEX1 upon wounding. The PEX1–LUC plants should be useful in testing these models.
A model for the control of peroxisome biogenesis by oxidative stress
Our results suggest a model whereby diverse stresses that generate H2O2 as a signalling molecule result in peroxisome proliferation via the up-regulation of components (PEX genes) required for biogenesis of the organelle and import of proteins. Previous work (Palma et al., 1991) has demonstrated that clofibrate treatment of pea plants results in elevated H2O2 and increased peroxisome number. The data presented here indicate that there is a causal relation ship between these two events and suggest a mechanism for it. Additionally, two other stresses, wounding and pathogen attack, which also generate H2O2 as a signalling molecule, were demonstrated to induce PEX1 expression. Our model would predict that other stress conditions which elevate H2O2, such as drought (Pei et al., 2000), osmotic stress (Guan et al., 2000) and excess light (Karpinski et al., 1999), would also result in PEX gene induction and peroxisome proliferation. Peroxisome number and morphology has been reported to increase as a function of light intensity (Ferreira et al., 1989).
Induction of PEX genes by H2O2 is not confined to plants. Expression of all mammalian PEX genes tested was significantly increased by exposure to H2O2, suggesting that this may be a common mechanism for dealing with oxidative stress. Induction of peroxisome biogenesis by this general oxidative stress signal fits nicely with the presence of so many antioxidant enzymes within peroxisomes and appears to be an evolutionarily ancient response of cells to stress.
Materials and methods
Biological material
Arabidopsis thaliana ecotype Colombia was used for all experiments. Wild-type seeds were sown on 1/2 MS, 0.7% agar plates containing 500 µg/ml carbenicillin (Melford Laboratories), and 2.5 µg/ml amphotericin B (Sigma) and incubated in 16 h light, 8 h dark at 18°C. Transgenic seeds were sown in pots containing a 9:1 compost:sand mixture and grown in a Sanyo growth chamber at 18°C with 9 h light (240 µmol/m2/s), 15 h dark. Experiments with transgenic plants were carried out with T2 seeds from two independent transgenic lines (5ci and 1ci) and were repeated between two and six times. CHO cells were obtained from the European Collection of Cell Cultures (Salisbury, Wiltshire) and were cultured in Ham’s F12 nutrient mix (Gibco BRL, Paisley, UK) supplemented with 2 mM l-glutamine, 10% (v/v) fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C with 5% CO2.
Isolation of a PEX1 cDNA clone
A tBLASTn search with S.cerevisiae Pex1p identified EST T43478 as a possible Arabidopsis orthologue (Kaplan, 1996) and a BAC clone MAH20 (DDBJ/EMBL/GenBank accession No. AB006697) from chromosome 5 was found that showed a highly significant identity (P = 5.6 × 10–160) to the EST. An 899 bp genomic DNA probe was amplified by PCR and used to screen a fractionated Arabidopsis 3 day seedling hypocotyl cDNA library (CD4-16, size range 3–6 kb; ABRC DNA Stock Centre, Ohio State University). The longest clone (3.5 kb) was sequenced commercially (MWG Biotech). A partial cDNA clone of 215 bp, which extends the sequence 5′, was isolated from a 2 day seedling library.
Isolation of the PEX1 promoter
A fragment of 1436 bp comprising 441 bp of coding sequence and 995 bp of 5′ sequence was amplified by PCR (Figure 1). Clones from three independent PCRs were sequenced and verified against the genomic sequence. The fragment was cloned as a PstI–NcoI fragment into pGRT10 (pUC18 into which the luciferase gene had previously been inserted) to produce pPEX1-GRT. The PEX1–luciferase cassette was excised with NotI (blunted) and SacI and inserted into SmaI–SacI-digested pBI101. To produce a promoterless control, luciferase was excised from pGRT10 with HindIII and SacI and ligated with HindIII–SacI-digested pBI101. Transformation of Arabidopsis was according to the protocol of A.Bent (University of Illinois at Urbana, http://www.cropsci.uiuc.edu/~a-bent/protocol.htm).
RNA analysis
For H2O2 treatments, leaves from young plants were detached and immediately submerged in 1 mM H2O2 ± excess catalase (Sigma) for 1 h at room temperature, then frozen in liquid nitrogen and stored at –80°C. CHO cells were treated with 1 mM H2O2 solution in situ for 1 h at room temperature with gentle shaking. RNA was extracted using the Qiagen RNeasy Midi kit (catalogue no. 75142). Total RNA (10 µg) was separated in a formaldehyde gel and transferred to Hybond NX membrane (Amersham Pharmacia Biotech UK). Probes were amplified by PCR from the corresponding cDNA clone (PEX1); from a 2 day seedling cDNA library and sequenced (PEX5); and from an EST (GST, clone ATTS1553; ABRC, Ohio State University). Probes were labelled using a Rediprime kit and unincorporated label removed using Nick columns (both from Amersham Pharmacia Biotech UK). Hybridization was as described by Hooks et al. (1999). RT–PCRs were carried out using the Reverse-iT One Step kit from the AB gene following the manufacturer’s instructions with equal amounts of RNA from control and H2O2-treated samples.
Plant treatments
For H2O2 treatments of whole plants a solution of H2O2 (1 mM in 0.9% NaCl, 10 mM Tris–HCl pH 7.5) was sprayed from above. For leaf incubations, one leaf was submerged either in 50 ml of 1 mM H2O2 in 0.9% NaCl, 10 mM Tris–HCl pH 7.5 for 15 min or in a 5–10 µM H2O2 solution and an H2O2 regenerating system consisting of glucose (2.5 mM) and glucose oxidase (2.5 U/ml). For clofibrate treatment, 25 µl of a 100 mM solution were placed on opposite leaves of the plant. For pathogen inoculation and wounding experiments, 3- to 5-week-old plants that had not initiated flowering were used. Pseudomonas syringae pv. tomato (Pst) strain DC3000 carrying the avrRpm1 gene was grown overnight from a plate in King’s B Medium containing rifampicin (50 µg/ml) and kanamycin (25 µg/ml), washed twice with 10 mM MgCl2, and ∼5–10 µl of a 106–107 c.f.u./ml suspension in 10 mM MgCl2 were gently inoculated into the underside of an intact leaf using a 1 ml sterile plastic syringe without a needle. Control inoculations were carried out with 10 mM MgCl2. For wounding experiments, one or two leaves were cut with scissors. The wounding and pathogen experiments were conducted with plants that had been kept in the dark overnight. Wounding and G/GO treatments were carried out in the camera to avoid exposing the plants to light between recording of the background image and data capture.
In vivo imaging of luciferase bioluminescence
An intensifier camera (VIM) and photon counting image processor (ARGUS-50) (Hamamatsu Photonics UK) were used for all experiments. Arabidopsis plants were sprayed with 5 mM d-luciferin solution (Analytical Bioluminescence Laboratories, San Diego, CA) for 12 h (Millar et al., 1992) and then again 20 min before photon emission measurements were taken. Plants were left within the camera case for at least 5 min in complete darkness to allow auto-luminescence to decay before measurements were taken. A reference measurement (control) was always taken prior to treatments.
Acknowledgments
Acknowledgements
We thank C.Kaplan for identifying EST T43478, Jacky Pallas for P.syringae strain Pst and much valuable advice, Sarah Tipnis for the CHO cells and Phil Gilmartin for pGRT10. Thanks are due to Andrew Millar, Phil Gilmartin, Javier Corpas and Marc Knight for helpful discussions, to Malcolm Willis for maintaining the imaging equipment and to the Arabidopsis Biological Resource Centre, Ohio State University for the GST clone and CD4-16 library. This work was supported by grant 24/GAT01940 from the Biotechnology and Biology Research Council to A.B. and I.A.G., and an EU Marie Curie Training Fellowship Contract no. BIO4-CT98-5088 to E.L.-H.
References
- Albertini M., Rehling,P., Erdmann,R., Girzalsky,W., Kiel,J.A.K.W., Veenhuis,M. and Kunau,W.-H. (1997) Pex14p, a peroxisomal membrane protein binding both receptors of the two PTS-dependent protein import pathways. Cell, 89, 83–92. [DOI] [PubMed] [Google Scholar]
- Alvarez M.E., Pennell,R.I., Meier, P.-J., Ishikawa,A., Dixon,R.A. and Lamb,C. (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell, 92, 773–784. [DOI] [PubMed] [Google Scholar]
- Barroso J.B., Corpas,F.J., Carreras,A., Sandalio,L.M., Valderrama,R., Palma,J.M., Lupianez,J.A. and del Rio,L.A. (1999) Localization of nitric-oxide synthase in plant peroxisomes. J. Biol. Chem., 274, 36729–36733. [DOI] [PubMed] [Google Scholar]
- Brickner D.G., Brickner,J.H. and Olsen,L.J. (1998) Sequence analysis of a cDNA encoding Pex5p, a peroxisomal targeting signal type 1 receptor from Arabidopsis thaliana (accession no. AF074843). Plant Physiol., 118, 330. [Google Scholar]
- Creelman R.A and Mullet,J.E (1997) Biosynthesis and action of jasmonates in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol., 48, 355–381. [DOI] [PubMed] [Google Scholar]
- de Felipe M.R., Lucas,M.M. and Pozuelo,J.M. (1988) Cytochemical study of catalase and peroxidase in the mesophyll of lolium-rigidum plants treated with isoproturon. J. Plant Physiol., 132, 67–73. [Google Scholar]
- Delledonne M., Xia,Y., Dixon,R.A. and Lamb,C. (1998) Nitric oxide functions as a signal in plant disease resistance. Nature, 394, 585–588. [DOI] [PubMed] [Google Scholar]
- del Rio L.A., Pastori,G.M., Palma,J.M., Sandalio,L.M., Sevilla,F., Corpas,F.J., Jimenez,A., Lopez Huertas,E. and Hernandez,J. (1998) The activated oxygen role of peroxisomes in senescence. Plant Physiol., 116, 1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B. (1991) Regulation of bacterial oxidative stress genes. Annu. Rev. Genet., 25, 315–337. [DOI] [PubMed] [Google Scholar]
- Distel B. et al. (1996) A unified nomenclature for peroxisome biogenesis factors. J. Cell Biol., 135, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt G. Braverman,N., Wong,C., Moser,A., Moser,H., Watkins,P., Valle,D. and Gould,S.J. (1995) Mutations in the PTS1 receptor gene PXR1 define complementation group 2 of the peroxisome biogenesis disorders. Nature Genet., 9, 115–125. [DOI] [PubMed] [Google Scholar]
- Durner J., Gow,A.J., Stamler,J.S. and Glazebrook,J. (1999) Ancient origins of nitric oxide signalling in biological systems. Proc. Natl Acad. Sci. USA, 96, 14206–14207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond P.J. Germain,V., Lange,P., Bryce,J., Smith,S.M. and Graham,I.A. (2000) Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle. Proc. Natl Acad. Sci. USA, 97, 5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber K.N., Heyman,J.A. and Subramani,S. (1998) Two AAA family peroxins, PpPex1p and PpPex6p, interact with each other in an ATP-dependent manner and are associated with different subcellular membranous structures distinct from peroxisomes. Mol. Cell. Biol., 18, 936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R.M.B., Bird,B. and Davies,D.D. (1989) The effect of light on the ultrastructure and organisation of Lemna peroxisomes. J. Exp. Bot., 40, 1029–1035. [Google Scholar]
- Gould S.J., Kalish,J.E., Morrell,J.C., Bjorkman,J., Urquhart,A.J. and Crane,D.I. (1996) Pex13p is an SH3 protein of the peroxisome membrane and a docking factor for the predominantly cytoplasmic PTS1 receptor. J. Cell Biol., 135, 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan L.M., Zhao,J. and Scandalios,J. (2000) Cis elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signalling molecule for the response. Plant J., 22, 87–95. [DOI] [PubMed] [Google Scholar]
- Heyman J.A., Monosov,E. and Subramani,S. (1994) Role of the PAS1 gene of Pichia pastoris in peroxisome biogenesis. J. Cell Biol., 127, 1259–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks M.A., Gallagher L. and Graham I.A (1998) Specific induction of an H2O2 generating acyl-CoA oxidase by cytosolic acidification. In Sanchez,J., Cerda-Olmedo,E. and Martinez-Force,E. (eds), Advances in Plant Lipid Research. Secretariado de Publicaciones, Universidad de Sevilla, Sevilla, Spain, pp. 346–349. [Google Scholar]
- Hooks M.A., Kellas,F. and Graham,I.A. (1999) Long chain acyl-CoA oxidases of Arabidopsis. Plant J., 20, 1–13. [DOI] [PubMed] [Google Scholar]
- Issemann I. and Green,S. (1990) Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature, 347, 645–649. [DOI] [PubMed] [Google Scholar]
- Kaplan C.P. (1996) Isolation and characterisation of genes involved in plant peroxisome biogenesis. PhD thesis, University of Cambridge, UK.
- Karpichev I.V., Marians,R.C and Small,G.M. (1997) A complex containing two transcription factors regulates peroxisome prolifer ation and the co-ordinate induction of β-oxidation enzymes in Saccharomyces cerevisiae. Mol. Cell. Biol., 17, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S., Reynolds,H., Karpinska,B., Wingsle,G., Creissen,G. and Mullineaux,P. (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science, 284, 654–657. [DOI] [PubMed] [Google Scholar]
- Kiel J.A.K.W., Hilbrands,R.E., VanderKlei,I.J., Rasmussen,S.W., Salomons,F.A., VanderHeide,M., Faber,K.N., Cregg,J.M. and Veenhuis,M. (1999) Hansenula polymorpha Pex1p and Pex6p are peroxisome-associated AAA proteins that functionally and physically interact. Yeast, 15, 1059–1078. [DOI] [PubMed] [Google Scholar]
- Kozak M. (1997) Recognition of AUG and alternative initiator codons is augmented by G in position +4 but is not generally affected by the nucleotides in position +5 and +6. EMBO J., 16, 2482–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberger T., Desvergne,B. and Wahli,W. (1996) Peroxisome proliferator activated receptors: a nuclear receptor signalling pathway in lipid physiology. Annu. Rev. Cell Dev. Biol., 12, 335–363. [DOI] [PubMed] [Google Scholar]
- Levine A., Tenhaken,R., Dixon,R. and Lamb,C. (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell, 79, 583–593. [DOI] [PubMed] [Google Scholar]
- Malek K. and Dietrich,R.A. (1999) Defense on multiple fronts: how do plants cope with diverse enemies? Trends Plant Sci., 4, 215–219. [DOI] [PubMed] [Google Scholar]
- Mansfield S.G. and Briarty,L.G. (1996) The dynamics of seedling and cotyledon cell development in Arabidopsis thaliana during reserve mobilisation. Int. J. Plant Sci., 157, 280–295. [Google Scholar]
- Masters C.J. and Crane,D.I. (1995) On the role of the peroxisome in ontogeny, ageing and degenerative disease. Mech. Ageing Dev., 80, 69–83. [DOI] [PubMed] [Google Scholar]
- Millar A.J., Short,S.R., Chua,N.-H. and Kay,S.A. (1992) A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell, 4, 1075–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. and Zilinskas,B.A. (1994) Regulation of pea cytosolic ascorbate peroxidase and other antioxidant enzymes during the progression of drought stress and following recovery from drought. Plant J., 5, 397–405. [DOI] [PubMed] [Google Scholar]
- Morre D.J., Sellden,G., Ojanpera,K., Sandelius,A.S., Egger,A., Morre,D.M., Chalko,C.M. and Chalco,R.A. (1990) Peroxisome proliferation in Norway spruce induced by ozone. Protoplasma, 155, 58–65. [Google Scholar]
- Okumoto K. et al. (1998a) PEX12, the pathogenic gene of group III Zellweger syndrome: cDNA cloning by functional complementation on a CHO cell mutant, patient analysis and characterisation of Pex12p. Mol. Cell. Biol., 18, 4324–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumoto K., Itoh,R., Shimozawa,N., Suzuki,Y., Tamura,S., Kondo,N. and Fujiki,Y. (1998b) Mutations in PEX10 is the cause of Zellweger peroxisome deficiency syndrome of complementation group B. Hum. Mol. Genet., 7, 1399–1405. [DOI] [PubMed] [Google Scholar]
- Palma J.M., Garrido,M., Rodriguez-Garcia,M.I. and del Rio,L.A. (1991) Peroxisome proliferation and oxidative stress mediated by activated oxygen species in plant peroxisomes. Arch. Biochem. Biophys., 287, 68–74. [DOI] [PubMed] [Google Scholar]
- Pastori G.M. and del Rio,L.A. (1997) Natural senescence of pea leaves—an activated oxygen-mediated function for peroxisomes. Plant Physiol., 113, 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. and Latterich,M. (1998) The AAA team: related ATPases with diverse functions. Trends Cell Biol., 8, 65–71. [PubMed] [Google Scholar]
- Pei Z.-M., Murata,Y., Benning,G., Thiomine,S., Klüsener,B., Allen,G.J., Grill,E. and Schroeder,J.I. (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature, 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Portsteffan H., Beyer,A., Becker,E., Epplen,C., Pawlak,A., Kunau,W.-H. and Dodt,G. (1997) Human PEX1 is mutated in complementation group 1 of the peroxisome biogenesis disorders. Nature Genet., 17, 449–452. [DOI] [PubMed] [Google Scholar]
- Schreck R., Rieber,P. and Baeuerle,P.A. (1991) Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J., 10, 2247–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman U., Gietl,C. and Schmid,M. (1999) Sequence analysis of a cDNA encoding Pex10p a zinc binding peroxisomal integral membrane protein from Arabidopsis thaliana. Plant Physiol., 119, 1147. [Google Scholar]
- Shimuzu N. et al. (1999) The peroxin Pex14p. cDNA cloning by functional complementation on a chinese hamster ovary cell mutant, characterisation and functional analysis. J. Biol. Chem., 274, 12593–12604. [DOI] [PubMed] [Google Scholar]
- Subramani S. (1998) Components involved in peroxisome import, biogenesis, proliferation, turnover and movement. Physiol. Rev., 78, 171–188. [DOI] [PubMed] [Google Scholar]
- van den Bosch H., Schutgens,R.B., Wanders,R.J. and Tager,J.M. (1992) Biochemistry of peroxisomes. Annu. Rev. Biochem., 61, 157–197. [DOI] [PubMed] [Google Scholar]
- Willekens H., Van Camp,W., Van Montagu,M., Inze,D., Lagebartels,C. and Sandermann,H.,Jr (1994a) Ozone, sulfur dioxide and ultraviolet B have similar effects on mRNA accumulation of antioxidant genes in Nicotiana plumbagnifolia L. Plant Physiol., 106, 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willekens H., Langebartels,C., Tire,C., Van Montagu,M., Inze,D. and van Camp,W. (1994b) Differential expression of catalase genes in Nicotiana plumbagnifolia L. Proc. Natl Acad. Sci. USA, 91, 10450–10454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willekens H., Chamnongpol,S., Davey,M., Schraudner,M., Langebartels,C., Van Montagu,M., Inze,D. and Van Camp,W. (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C-3 plants. EMBO J., 16, 4806–4816. [DOI] [PMC free article] [PubMed] [Google Scholar]