Abstract
The present study evaluated the influence and mechanism of action of dietary protein intake in Dahl SS hypertension and renal disease. Rats were fed isocaloric diets with low (6%), normal (18%), or high (30%) amounts of protein and 0.4% NaCl from 5 to 12 weeks of age; the NaCl content of the diets was then increased to 4.0% NaCl from 12 to 15 weeks of age. Rats fed the high protein diet developed the highest MAP and urine albumin to creatinine ratio when fed the 4.0% NaCl diet (153±7 mmHg and 8.0±2.4, respectively) compared to rats fed normal (132±3 mmHg, 1.2±0.3) or low protein diets (132±6mmHg, 0.3±0.1). Significantly greater numbers of infiltrating T-lymphocytes were observed in kidneys of SS rats fed the high protein diet (18.9 ± 3 × 105 cells) than in rats fed the low protein diet (9.1 ± 3 × 105 cells). Furthermore, treatment of SS rats fed the high protein diet with the immunosuppressant agent mycophenolate mofetil (20mg/kg/day, ip) significantly reduced the number of infiltrating T cells in the kidneys (from 18.9 ± 2.7 to 10.6 ± 2.0 × 105 cells) while decreasing blood pressure (from 133 ± 3 to 113 ± 4 mmHg) and the albumin/creatinine ratio (from 10.9 ± 2.3 to 5.4 ± 1.2). These results demonstrate that restriction of protein intake protects the Dahl SS from hypertension and kidney disease and indicates that infiltrating immune cells play a pathological role in Dahl SS rats fed a high protein diet. Moreover, the results show that hypertension in Dahl SS rats is sensitive to both NaCl and protein intake.
Keywords: hypertension, kidney disease, albuminuria, T-lymphocytes, immunosuppressive agents
INTRODUCTION
Dietary nutrients have a significant influence on arterial blood pressure in humans and experimental animals. Consumption of cholesterol, saturated fats, or carbohydrates is associated with elevated arterial blood pressure, while high protein diets are linked to decreased blood pressure in the general human population.1,2,3,4,5 Evidence in patients with renal insufficiency, however, indicates that elevated protein intake may accelerate the decline in renal function.6,7,8,9 The effects of changes in dietary fat, protein, and carbohydrate on blood pressure have also been examined in experimental animal models. The development of hypertension is accelerated in genetic models of hypertension depending on the protein,10 carbohydrate11,12,13,14 and fat.14,15 composition of the diet. These clinical and experimental results indicate that various dietary components can influence arterial blood pressure and kidney damage independently of sodium intake.
Recent studies from our laboratory have demonstrated that the source of protein in the chow fed to Dahl SS rats modified the degree of sodium-sensitive hypertension and the associated damage to the kidney observed with elevated NaCl intake.16,17 Further experiments demonstrated that the renal damage in Dahl SS rats is associated with infiltration of macrophages and T-lymphocytes and that suppression of immune cell infiltration attenuates sodium-sensitive hypertension and kidney damage.18,19 Since characteristics of salt-dependent hypertension and kidney damage in the SS rat strain are similar to those observed in human populations,20 an understanding of environmental effects that modify disease phenotypes can provide insight into human disease. The present study was specifically designed to test the hypothesis that the amount of protein in the diet can modify sodium-sensitive hypertension and kidney damage in Dahl SS rats. Further experiments were designed to examine the infiltration of immune cells into the kidneys of rats fed elevated NaCl and to determine the importance of these infiltrating cells in the development of these protein- and sodium-sensitive disease phenotypes.
To address these questions, rats were fed custom diets containing different amounts of protein. The custom diets consisted of simple modifications of the AIN-76A diet formulation with low (6%), normal (18%), or high (30%) amounts of protein. The rats were fed the different diets containing 0.4% NaCl from approximately 4 to 12 weeks of age; the amount of salt in the diet was then increased to 4.0% NaCl for the final three weeks of the study. The influence of the dietary protein content on the development of sodium-sensitive hypertension and kidney damage and the potential role of infiltrating immune cells in this process was assessed in the final week of the high NaCl intake period.
METHODS
Experimental Animals
Experiments were performed on inbred Dahl SS rats obtained from Charles River Laboratories (SS/JrHsdMcwiCrl) and outbred Sprague-Dawley (SD) rats obtained from Harlan Sprague Dawley (Madison, WI). The SS rats were received at approximately 4–5 weeks of age and randomly placed and maintained on one of the three low, normal, or high protein diets described below. The salt content of each diet was 0.4% NaCl from 4–12 weeks of age. At 12 weeks of age, the salt content of the chow was increased to 4.0% NaCl, and the rats were maintained on this diet for an additional 3 weeks. A subset of SS rats fed the high protein/high salt diet were treated with the immunosuppressant agent mycophenolate mofetil (20 mg/kg/day, ip) or vehicle daily throughout the period of elevated salt intake. The SD rats, which served as a normotensive control strain in this experiment, were fed either normal or high protein diet from 4–15 weeks of age. Both the normal and high protein diet contained 0.4% NaCl from 4–12 weeks for all SD rats; the NaCl content of the diet was then either maintained at 0.4% NaCl in a group fed the normal protein chow or increased to 4.0% NaCl in groups fed the normal or high protein chow from 12–15 weeks of age. The MCW Institutional Animal Care and Use Committee approved all experimental protocols.
Diets
The diets were obtained from Dyets Inc. (Bethlehem, PA). The standard AIN-76A chow containing 18% casein served as normal protein chow. Custom formulations were also obtained containing 6% and 30% casein and served as low and high protein diets, respectively. The different percentages of protein in each diet were substituted for or replaced with carbohydrate (sucrose) and were isocaloric.
Surgical Procedure
Surgical procedures were performed on the first day of the two-week experimental protocol and occurred two weeks following the transition from low (0.4%) to high (4.0%) NaCl chow. The rats were deeply anesthetized with an intraperitoneal injection of ketamine (35 mg/kg), xylazine (10 mg/kg), and acepromazine (0.5 mg/kg) with supplemental anesthesia administered when needed. Using aseptic technique, polyvinyl catheters were implanted in the femoral artery, tunneled subcutaneously, and exteriorized at the back of the neck in a lightweight tethering spring. Both antibiotic and analgesic agents were administered post-surgically, and the rats were allowed to fully awaken from anesthesia on a temperature-controlled pad. Following recovery from anesthesia, all rats were placed in individual stainless steel cages that permit daily measurement of arterial blood pressure and overnight urine collection.
Experimental Procedures
The rats were permitted to recover for a week following surgery. During this time they were maintained on the individual diets containing 4.0% NaCl. After the recovery period, high salt blood pressure measurements were obtained from 9:00 am to 12:00 pm on three consecutive days as previously described.16,17 Following the second day of blood pressure measurement, an overnight urine collection (from 4:00 pm to 8:00 am) was obtained for measurement of urinary sodium, creatinine, and albumin excretion rates. The data are normalized to a 24 hour period and may therefore provide an overestimate of excretory parameters in these nocturnal animals. On the final day, arterial plasma samples were obtained for measurement of plasma creatinine concentration while the rats were maintained on the high NaCl diet.
Urine electrolytes were measured by flame photometry (IL-943, Instrumentation Laboratories, Lexington, MA). Plasma and urine creatinine values were measured with an assay based on the Jaffé Reaction by autoanalyzer (ACE, Alfa Wasserman, Fairfield, NJ). Urine albumin was quantified with a fluorescent assay that utilized Albumin Blue 580 dye (Molecular Probes, Eugene, OR) and a fluorescent plate reader (FL600, Bio-Tek, Winooski, VT).
Histological and Immunohistochemical Analysis
Kidneys were obtained for histological and immunohistochemical analysis using methods we have previously described.17,18,19 The rats were anesthetized with sodium pentobarbital (50 mg/kg, ip); the kidneys were then removed and placed in a 10% formaldehyde solution. The tissue was paraffin embedded (Microm HMP 300), cut in 3 µm sections (Microm HM355S), mounted, and stained with Gomori’s One-Step Trichrome. Individual glomeruli (average of 37 per rat) were evaluated with the method of Raij et al.21 For immunohistochemistry, slides were deparaffinized and incubated with Proteinase K for antigen retrieval. The primary monoclonal antibody used to detect T cells was anti-CD43 (Abcam). A biotinylated horse anti-mouse secondary antibody was used for development with avidin-biotinylated horseradish peroxidase complex (Vectastain ABC kits; Vector Laboratory, Burlingame, CA). The slides were lightly counterstained with aniline blue dye and photographed.
T-Cell Isolation
Isolation of infiltrating cells was performed using methods we previously described.19 Rats were anesthetized with sodium pentobarbital (50 mg/kg, ip), the abdominal aorta was isolated and cannulated, and the kidneys were perfused with a solution containing: 154 mM NaCl and 100 Units/ml heparin. The kidneys were removed and cut into 1–2 mm-thick sections; the sections were incubated at 37° C for 60 min in dissection solution containing: 135 mM NaCl, 3 mM KCl, 2 mM KH2PO4, 5.5 mM Glucose, 20 mM HEPES (pH 7.2) and 0.85 mg/ml collagenase (Sigma 573 units/mg). During incubation, the samples were gently shaken and bubbled with 95% O2/5% CO2. The digested solution was then filtered through 100 µm and 70 µm filters and centrifuged at 300 × g for 10 minutes. The pellet was resuspended in PBS containing DNase 1 (20 units/ml). The isolated cells were layered over 5 ml of Histopaque (Sigma), centrifuged at 400 × g for 30 minutes at room temperature, and the mononuclear cell layer was collected. The separated mononuclear cells were resuspended, washed, and incubated with a rat Pan T cell antibody coupled to magnetic microbeads (MACS Rat Pan T Cell Microbeads, Miltenyi Biotec) for 15 minutes at 4–8°C. The cells were then washed, resuspended in labeling buffer, and the cell suspension was applied to a magnetic column (MACS Separation Columns, Miltenyi Biotec) to isolate T-lymphocytes.
Statistical Analysis
Data are presented as the mean ± standard error. Experiments were performed on 5–10 rats/group. A one-way analysis of variance was utilized to determine the differences in parameters between the rats maintained on the different diets. A Tukey post-hoc test was used when appropriate. An unpaired t-test was used to evaluate differences between two groups. The 95% confidence interval was considered significant.
RESULTS
High salt mean arterial blood pressure was significantly elevated in SS rats fed the high protein diet (153±7 mmHg) compared to those fed the low or normal protein diet (Figure 1-top). No differences were detected between the rats fed the low and normal protein diet. The heart rate in rats fed the high NaCl diet was not altered in the low or high protein diet groups from the value measured in the normal protein rats (389±14 beats/minute). As an index of kidney disease, it was observed that urinary albumin excretion rate in the high salt rats was directly related to the protein content of the diet. The ratio of albumin to creatinine in urine was approximately 10-fold greater in the rats fed the high protein diet than that observed in rats fed the low protein diet (Figure 1-bottom). Albumin and protein excretion rate averaged 114±37 mg/day and 220±45 mg/day, respectively, in the high protein rats. Steady state sodium excretion rate was not different between the groups, and averaged 10±2 mEq/day in the normal protein rats fed the high NaCl chow.
Figure 1.
Mean arterial blood pressure (MAP, top) and urine albumin to creatinine excretion ratio (bottom) in SS/Mcw rats maintained on high, normal or low protein diets for 14 weeks. The measurements were made following 3 weeks on of a high NaCl (4.0%) diet. * indicates p<0.05 vs. the low protein diet group; † indicates p<.05 vs. the normal protein diet group.
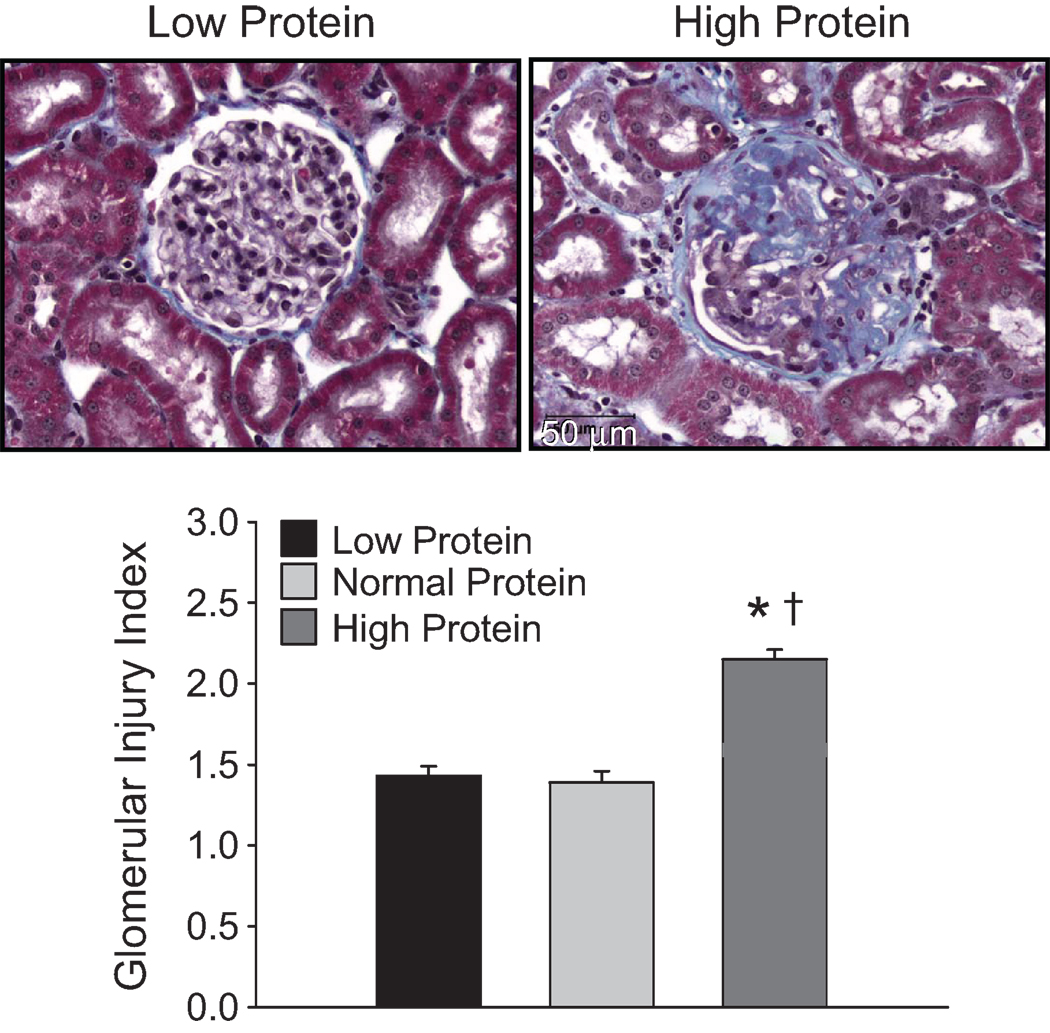
Representative examples of histological differences observed between the kidneys of rats fed the low and high protein diet are illustrated in Figure 2. Consistent with previous reports,18,19 the presence of large amounts of blue fibrotic tissue with collapsed glomerular capillaries are apparent in the kidneys of SS/Mcw fed the high protein diet. Visibly less glomerular damage is evident in the kidneys of rats fed the low protein diet, despite similar levels of sodium intake. The glomerular injury index (Figure 2-bottom) was significantly greater in the Dahl SS/Mcw rats fed the high protein diet (2.2±0.1) than in the rats fed the normal (1.4±0.2) or low protein diet (1.5±0.3). Despite the influence of dietary protein content on glomerular histological damage and on albumin and protein excretion, no differences were detected in creatinine clearance. Though the conscious creatinine clearance rate on high salt tended to decrease with the increased protein intake, it was not different in the groups fed the high (0.48±0.12 ml/min/gkwt) or low (0.66±0.10 ml/min/gkwt) protein diet compared to the average of 0.62±0.06 ml/min/gkwt measured in the Dahl SS rats fed the normal protein diet.
Figure 2.
Light microscopy images of representative glomeruli (40X original magnification, bottom panels) from kidneys obtained from male Dahl SS/Mcw rats fed the low protein or high protein diets. The lower panel illustrates changes in the glomerular injury score in rats fed the low, normal, or high protein diet. The NaCl content of each diet was increased from 0.4% to 4.0% for the final 3 weeks of the experiment. * indicates p<0.05 vs. the low protein diet group; † indicates p<.05 vs. the normal protein diet group.
Body weight was not different between rats fed the high (379±5 g) and the normal (374±9 g) protein diet; rats fed the low protein diet were significantly smaller (313±13 g) than rats fed the normal or high protein diets. Kidney weight was directly related to the protein intake; total kidney mass of rats fed the high protein diet (4.8±0.2 g) was significantly greater than that observed with rats fed the normal (3.4±0.2 g) or low protein diet (2.7±0.2 g). No differences were detected in plasma protein or albumin concentration between groups. Plasma protein and albumin concentration averaged 5.6±0.1 and 2.9±0.1 mg/dl in rats fed the high protein diet; 5.5±0.1 and 2.9±0.1 in rats fed the normal protein diet; and 5.7±0.1 and 3.0±0.1 mg/dl in rats fed the low protein diet.
The high protein/4% NaCl diet had no influence on blood pressure or renal albumin excretion in normotensive SD rats compared to values obtained from rats fed normal protein/4% NaCl or normal protein/0.4% NaCl chow. Mean arterial pressure (111±3 mmHg, n=5) and urine albumin to creatinine excretion ratio (1.2±0.3) in SD rats fed the high protein diet containing 4.0% NaCl was not different from the values obtained in the SD rats fed the normal protein diet containing 0.4% (108±2 mmHg and 0.8±0.2, respectively) or the rats fed the normal protein/4.0% NaCl diet (109±2 mmHg and 0.5±0.2, respectively).
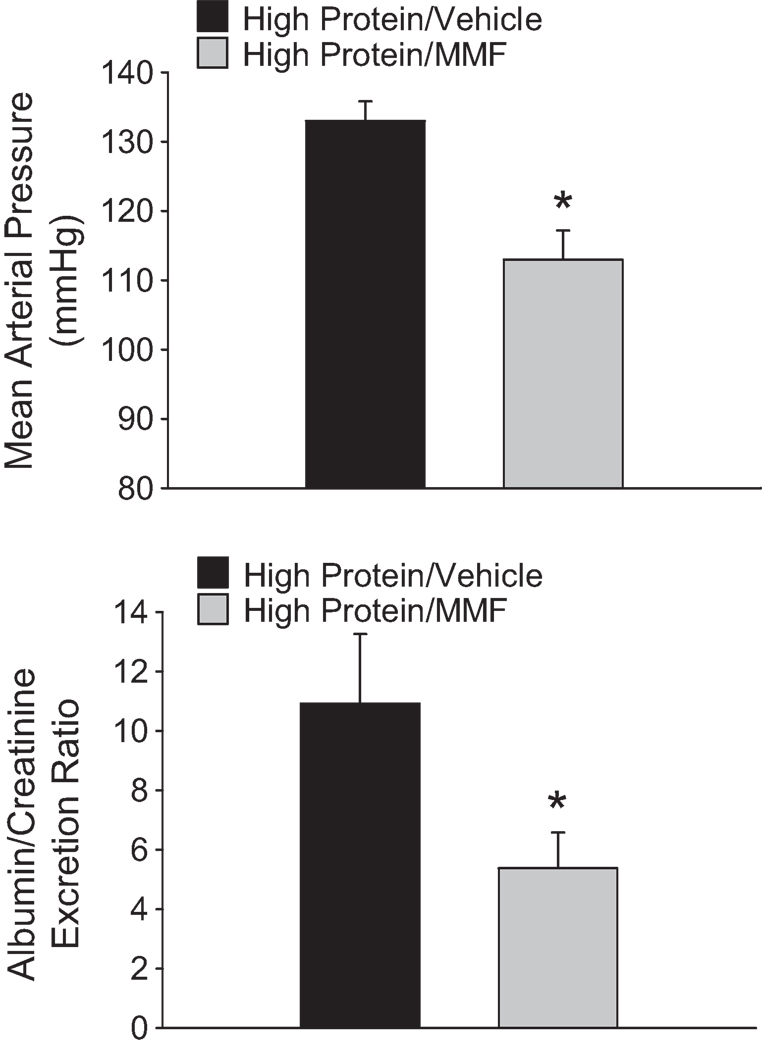
Results of further studies to examine the infiltration of T-lymphocytes into the kidneys of SS rats fed the low or high protein diet are summarized in Figure 3. The top panels provide an illustration of the localization of infiltrating CD-43 positive cells in the tissue surrounding damaged glomeruli in the renal cortex (left) and damaged tubules and vasa recta bundles (right) in the renal outer medulla. Significantly greater numbers of infiltrating T-lymphocytes were observed in the kidneys of SS rats fed the high protein diet following three weeks of elevated NaCl intake (18.9 ± 3 × 105 cells/ two kidneys) than in rats fed the low protein/high NaCl diet (9.1 ± 3 × 105 cells/ two kidneys) (Figure 3-bottom left). To elucidate a role for the infiltrating T cells in the hypertension and renal disease, separate groups of rats fed the high protein diet were treated with the immunosuppressive agent mycophenolate mofetil (20 mg/kg/day) or vehicle during the period of elevated NaCl intake. Treatment of SS rats fed the high protein diet with MMF significantly reduced the number of infiltrating T cells in the kidneys by approximately 44% (Figure 3-bottom right). Moreover, the MMF treatment significantly attenuated the development of hypertension and albuminuria in Dahl SS rats fed high salt and maintained on the high protein diet. The high salt MAP value was reduced by 20 mmHg while the albumin/creatinine excretion ratio was reduced by 50% in rats treated with MMF (Figure 4).
Figure 3.
Immunohistochemical localization of T cells in the renal cortex (Top Left) and medulla (Top Right) of Dahl SS rats fed 4.0% NaCl chow. T cells were localized in the renal tissue with antibodies directed against the cell surface marker CD43 (original magnification 20X). Bottom Left: Infiltrating T lymphocytes in the kidneys of Dahl SS/Mcw rats maintained on high or low protein diets for 14 weeks with 4.0% NaCl for the final three weeks. Bottom Right: Influence of vehicle or mycophenolate mofetil treatment (20 mg/kg/day, ip) during the high salt period on infiltrating T lymphocytes in the kidneys of Dahl SS/Mcw rats maintained on a high protein diet for 14 weeks with 4.0% NaCl for the final three weeks. * indicates p<0.05 vs. other group.
Figure 4.
Influence of mycophenolate mofetil (20 mg/kg/day, ip) during the high salt period on mean arterial blood pressure (MAP, top) and urine albumin to creatinine excretion ratio (bottom) in SS/Mcw rats maintained on high low protein diets for 14 weeks with 4.0% NaCl administered for the final 3 weeks. * indicates p<0.05 vs. other group.
DISCUSSION
The present experiments demonstrate that hypertension and renal disease phenotypes in Dahl SS/Mcw rats are sensitive to elevated dietary protein intake. Rats fed the high protein diet developed higher blood pressure, had greater albumin and protein excretion rates, and demonstrated greater renal hypertrophy and histological damage when compared to rats fed diets containing low or normal protein. The results of this study therefore indicate that the hypertension and kidney disease phenotypes are sensitive to both NaCl and protein intake in the Dahl SS/Mcw rat.
A mechanism for the increased development of disease in rats fed an elevated protein diet is unclear. The present studies, however, provide some insight into the development of disease in this genetic model. In addition to the kidney damage observed following three weeks of the high NaCl intake, rats fed the high protein diet demonstrated an increased renal infiltration of T lymphocytes when compared to rats maintained on the low protein diet. Renal infiltration of immune cells has been demonstrated in nonimmune models of hypertension and kidney disease.22,23,24,25,26,27 Lymphocytes and macrophages infiltrate the kidney in experimental and genetic models of hypertension disease,22,23,24,25,26,27,28 and immunosuppression through genetic or pharmacological means has been demonstrated to attenuate hypertension and renal disease.29,30,31,32 The mechanisms utilized by infiltrating immune cells to increase blood pressure and renal disease are unclear. It has been proposed that infiltrating immune cells can participate in the disease phenotype by releasing free radicals, cytokines, or other vasoactive factors. It is possible that the infiltrating immune cells are exerting their deleterious effects in the kidney; alternatively, infiltration of T cells into systemic blood vessels, the brain, or other organs may mediate the deleterious effects of immune cells in hypertension. Moreover, the factors mediating infiltration of immune cells in salt-sensitive hypertension remain to be elucidated.
The present data may reveal mechanisms whereby a high protein diet can exacerbate renal disease and hypertension. Though a high protein diet has not been convincingly demonstrated to induce renal disease in normal individuals, epidemiological and experimental data indicate that an elevated protein diet is deleterious in subjects with pre-existing kidney disease.6,7,8,9 This observation is consistent with the hypothesis posed by Brenner and colleagues in which it was postulated that an elevated protein intake can lead to renal vasodilation and elevated glomerular capillary hydrostatic pressure with resultant glomerular damage.33 Protein-induced renal dilation in the Dahl SS rat, which is susceptible to glomerular damage and hypertension, could accelerate the disease process.
The blood pressure effects of varying protein intake have also not been consistently noted.34 A high protein diet has been demonstrated to increase blood pressure in a genetic model of hypertension.10 Clinical data indicate that high protein diets may also increase arterial blood pressure in patients35,36 although other human data indicate an inverse association between blood pressure and protein intake.37,38 Of note, a previous study in rats demonstrated that protein-overloading of normal Lewis rats by intraperitoneal injection of large amounts of Bovine Serum Albumin led to infiltration of immune cells, renal damage, and salt-sensitive hypertension.39 Of interest, the description of the histological changes in the renal medulla of these rats was similar to that observed in Dahl SS rats fed high salt, though the glomerular damage in the protein-overload rats appeared to be less severe than observed in the Dahl SS. The present data support the concept that elevated dietary protein intake enhances hypertension in a genetic animal disease model.
The Dahl SS rats fed the normal protein/high salt diet in the present experiment have attenuated hypertensive and renal disease phenotypes when compared to previous data we have published with Dahl SS/Mcw rats fed the same AIN-76A diet.16,17,18,19 Though the Dahl SS rats used in the present study are genetically identical to those previously studied, the animals in the present study were obtained from a commercial vendor. Based upon our previous work in this area that indicated that the diet fed to the mothers during pregnancy and weaning can have a marked impact on the final disease phenotype,16,17 we speculate that the difference in the degree of the final disease phenotype may be attributable to the diet fed to the breeding stock during pregnancy and nursing at Charles River (Purina 5L79, a grain-based diet) in comparison to the AIN-76A diet fed to the breeding colony at MCW.
PERSPECTIVES
The present experiments illustrate the harmful effects of a high protein diet in an animal model of salt-sensitive hypertension. The results indicate that the deleterious effects of high NaCl intake are exacerbated in Dahl SS/Mcw rats when superimposed on the background of a high protein intake. One potential mechanism identified in this study may be related to factors promoting the infiltration of immune cells, particularly T lymphocytes. Further study will be necessary to elucidate the mechanisms leading to the infiltration of T cells in the kidneys of these hypertensive animal model as well as the potential mechanisms that these cells may use to alter disease phenotypes.
Acknowledgments
SOURCES OF FUNDING
This work was partially supported by National Institutes of Health Grants HL-29587 and DK-62803.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST/DISCLOSURE
None
BIBLIOGRAPHY
- 1.Hajjar I, Kotchen T. Regional variations of blood pressure in the United States are associated with regional variations in dietary intakes: the NHANES-III data. J Nutr. 2003;133:211–214. doi: 10.1093/jn/133.1.211. [DOI] [PubMed] [Google Scholar]
- 2.Hajjar IM, Grim CE, George V, Kotchen TA. Impact of diet on blood pressure and age-related changes in blood pressure in the US population. Arch Int Med. 2001;161:589–593. doi: 10.1001/archinte.161.4.589. [DOI] [PubMed] [Google Scholar]
- 3.Kesteloot H, Joossens JV. Relationship of serum sodium, potassium, calcium, and phosphorus with blood pressure. Belgian Interuniversity Research on Nutrition and Health. Hypertension. 1988;12:589–593. doi: 10.1161/01.hyp.12.6.589. [DOI] [PubMed] [Google Scholar]
- 4.Stamler J, Caggiula A, Grandits GA, Kjelsberg M, Cutler JA for the MRFIT Research Group. Relationship to blood pressure of combination of dietary macronutrients. Circulation. 1996;94:2417–2423. doi: 10.1161/01.cir.94.10.2417. [DOI] [PubMed] [Google Scholar]
- 5.Elliott P, Stamler J, Dyer AR, Appel L, Dennis B, Kesteloot H, Ueshima H, Okayama A, Chan Q, Garside DB, Zhou B for the INTERMAP Cooperative Research Group. Association between protein intake and blood pressure: The INTERMAP Study. Arch Intern Med. 2006;166:79–87. doi: 10.1001/archinte.166.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fouque D, Laville M. The Cochrane Collaboration. Vol. 3. John Wiley and Sons, Ltd; 2009. Low protein diets for chronic renal disease in non diabetic adults (Review) pp. 1–28. [Google Scholar]
- 7.Martin WF, Armstrong LE, Rodriguez NR. Dietary protein intake and renal function. Nutrition & Metabolism. 2005;2:25. doi: 10.1186/1743-7075-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460–467. doi: 10.7326/0003-4819-138-6-200303180-00009. [DOI] [PubMed] [Google Scholar]
- 9.Pedrini MT, Levey AS, Lau J, Chalmers TC, Wang PH. The effect of dietary protein restriction on the progression of diabetic and nondiabetic renal diseases: a meta-analysis. Ann Int Med. 1996;124:627–632. doi: 10.7326/0003-4819-124-7-199604010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Nevala R, Vaskonen T, Vehniainen J, Korpela R, Vapaatalo H. Soy based diet attenuates the development of hypertension when compared to casein based diet in spontaneously hypertensive rat. Life Sci. 2000;66:115–124. doi: 10.1016/s0024-3205(99)00569-x. [DOI] [PubMed] [Google Scholar]
- 11.Mori Y, Murakawa Y, Yokoyama J, Tajima N, Ikeda Y, Nobukata H, Ishikawa T, Shibutani Y. Effect of highly purified eicosapentaenoic acid ethyl ester on insulin resistance and hypertension in Dahl salt-sensitive rats. Metabolism. 1999;48:1089–1095. doi: 10.1016/s0026-0495(99)90120-8. [DOI] [PubMed] [Google Scholar]
- 12.Young JB, Landsberg L. Effect of oral sucrose on blood pressure in the spontaneously hypertensive rat. Metab Clin Exp. 1981;30:421–424. doi: 10.1016/0026-0495(81)90173-6. [DOI] [PubMed] [Google Scholar]
- 13.Preuss HG, Knapka JJ, MacArthy P, Yousufi AK, Sabnis SG, Antonovych TT. High sucrose diets increase blood pressure of both salt-sensitive and salt-resistant rats. Am J Hypertens. 1992;5:585–591. doi: 10.1093/ajh/5.9.585. [DOI] [PubMed] [Google Scholar]
- 14.Zhang HY, Reddy S, Kotchen TA. A high sucrose, high linoleic acid diet potentiates hypertension in the Dahl salt sensitive rat. Am J Hypertens. 1999;12:183–187. doi: 10.1016/s0895-7061(98)00238-6. [DOI] [PubMed] [Google Scholar]
- 15.Shimokawa T, Moriuchi A, Hori T, Saito M, Naito Y, Kabasawa H, Nagae Y, Matsubara M, Okuyama H. Effect of dietary alpha-linolenate/linoleate balance on mean survival time, incidence of stroke and blood pressure of spontaneously hypertensive rats. Life Sci. 1988;43:2067–2075. doi: 10.1016/0024-3205(88)90356-6. [DOI] [PubMed] [Google Scholar]
- 16.Mattson DL, Meister CJ, Marcelle M. Dietary protein source determines the degree of hypertension in renal disease in the Dahl salt-sensitive rat. Hypertension. 2005;45:736–741. doi: 10.1161/01.HYP.0000153318.74544.cc. [DOI] [PubMed] [Google Scholar]
- 17.Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, Cowley AW., Jr Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiol Genomics. 2004;16:194–203. doi: 10.1152/physiolgenomics.00151.2003. [DOI] [PubMed] [Google Scholar]
- 18.Mattson DL, James L, Berdan EA, Meister CJ. Immune Suppression Attenuates Hypertension and Renal Disease in the Dahl Salt-Sensitive Rat. Hypertension. 2006;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 19.De Miguel C, Das S, Lund H, Mattson DL. T-lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol. 2010;298:R1136–R1142. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grim CE, Wilson TW, Nicholson GD, Hassell TA, Fraser HS, Grim CM, Wilson DM. Blood pressure in blacks. Hypertension. 1990;15:803–809. doi: 10.1161/01.hyp.15.6.803. [DOI] [PubMed] [Google Scholar]
- 21.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984;26:137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- 22.Mai M, Geiger H, Hilgens KF, Veelken R, Mann JFE, Daemmrich J, Luft FC. Early changes in hypertension induced renal injury. Hypertension. 1993;22:754–765. doi: 10.1161/01.hyp.22.5.754. [DOI] [PubMed] [Google Scholar]
- 23.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol. 2002;161:1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic Angiotensin II infusions. Am J Physiol. 2007;292:F330–F339. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chávez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol. 2002;282:F191–F201. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol. 2004;286:F606–F616. doi: 10.1152/ajprenal.00269.2003. [DOI] [PubMed] [Google Scholar]
- 27.Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int. 2005;68:2180–2188. doi: 10.1111/j.1523-1755.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 28.Lombardi D, Gordon K, Polinksy P, Suga S, Schwartz S, Johnson R. Salt sensitive hypertension develops after short-term exposure to angiotensin II. Hypertension. 1999;33:1013–1019. doi: 10.1161/01.hyp.33.4.1013. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez V, Quiroz Y, Nava M, Pons H, Rodríguez-Iturbe B. Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am J Physiol. 2002;283:F1132–F1141. doi: 10.1152/ajprenal.00199.2002. [DOI] [PubMed] [Google Scholar]
- 30.Bendich A, Belisle EH, Strausser HR. Immune system modulation and its effect on the blood pressure of the spontaneously hypertensive male and female rat. Biochem Biophys Res Comm. 1981;99:600–607. doi: 10.1016/0006-291x(81)91787-3. [DOI] [PubMed] [Google Scholar]
- 31.Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol. 2007;293:F616–F623. doi: 10.1152/ajprenal.00507.2006. [DOI] [PubMed] [Google Scholar]
- 32.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982;307:652–659. doi: 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- 34.Farnsworth E, Luscombe ND, Noakes M, Wittert G, Argiou E, Clifton PM. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am J Clin Nutrition. 2003;78:31–39. doi: 10.1093/ajcn/78.1.31. [DOI] [PubMed] [Google Scholar]
- 35.Havlik RJ, Fabsitz RR, Kalousdian S, Borhani NO, Christian JC. Dietary protein and blood pressure in monozygotic twins. Prev Med. 1990;19:31–39. doi: 10.1016/0091-7435(90)90004-4. [DOI] [PubMed] [Google Scholar]
- 36.Sacks FM, Donner A, Castelli WP, Gronemeyer J, Pletka P, Margolius HS, Landsberg L, Kass EH. Effect of ingestion of meat on plasma cholesterol of vegetarians. JAMA. 1981;246:640–644. [PubMed] [Google Scholar]
- 37.Liu L, Ikeda K, Yamori Y. Inverse relationship between urinary markers of animal protein intake and blood pressure in Chinese: results from the WHO Cardiovascular Diseases and Alimentary Comparison (CARDIAC) Study. Int J Epidemiol. 2000;31:227–233. doi: 10.1093/ije/31.1.227. [DOI] [PubMed] [Google Scholar]
- 38.Cirillo M, Lombardi C, Laurenzi M, De Santo NG. Relation of urinary urea to blood pressure: interaction with urinary sodium. J Hum Hypertens. 2002;16:205–212. doi: 10.1038/sj.jhh.1001323. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez V, Quiroz Y, Nava M, Pons H, Rodríguez-Iturbe B. Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am J Physiol. 2002;283:F1132–F1141. doi: 10.1152/ajprenal.00199.2002. [DOI] [PubMed] [Google Scholar]