Abstract
Adoptive T-cell therapy (ACT) using expanded tumor-infiltrating lymphocytes (TIL) with high-dose IL-2 is a promising form of immunotherapy for Stage IV melanoma having clinical response rates of 50% or more. One of the major problems preventing further success of this therapy is that the current protocols used to highly expand TIL for infusion drive CD8+ T cells to differentiate into effector cells losing key co-stimulatory molecules such as CD28 and CD27. This has been associated with a lack of persistence in vivo for reasons not entirely clear. In this study, we demonstrate that while human melanoma CD8+ TIL lost CD27 and CD28 expression during the rapid expansion for ACT, they gained expression of the alternative co-stimulatory molecule CD137/4-1BB, and to a lesser extent CD134/OX40. Post-REP TIL were found to be highly sensitive to activation-induced cell death (AICD) when re-activated through the TCR with low levels of OKT3 antibody. However, co-ligation of 4-1BB using two different agonistic anti-4-1BB antibodies potently prevented AICD of post-REP CD8+ TIL, including those specific for MART-1, and facilitated even further cell expansion. This was correlated with increased levels of bcl-2 and bcl-xL together with decreased bim expression. 4-1BB-co-stimulated post-REP TIL also expressed increased levels of the cytolytic granule proteins and exhibited enhanced CTL activity against melanoma cells. Lastly, post-REP CD8+ TIL were protected from cell death by anti-4-1BB ligation when exposed to HLA-matched melanoma cells. Our results indicate that 4-1BB co-stimulation may significantly improve TIL survival during melanoma ACT and boost anti-tumor cytolytic activity.
Keywords: 4-1BB/CD137, TNF-receptor family, co-stimulation, tumor-infiltrating lymphocytes, adoptive T cell therapy, melanoma
Introduction
Adoptive T-cell therapy (ACT) involving the transfer of autologous tumor infiltrating lymphocytes (TIL) is currently the most effective immunotherapy for metastatic melanoma patients that have failed other first and second line therapies. ACT used for melanoma treatment involves the expansion of TIL over a three- to five-week period with IL-2, followed by a “rapid expansion protocol” (REP) involving the large-scale expansion of the TIL with IL-2 over 14 days after stimulation with anti-CD3 (OKT3) and autologous or allogeneic PBMC feeder cells.1,2 The activated TIL are infused into the prior lymphodepleted patients to facilitate TIL survival and expansion in vivo through the removal of cytokine sinks and T-regulatory cells.1–3
Once the TIL are re-infused into the patient, they encounter antigen, resulting in the activation of the TIL, but the TIL are ultimately short-lived while. Re-stimulation of the TIL through antigen contact together with exposure to IL-2 during ACT may result in TIL proliferation and tumor control or may lead to deletion through apoptosis (activation-induced cell death) or induction of a non-proliferative (anergic) state due to lack of appropriate co-stimulation.
The majority of post-REP CD8+ T cells lose the expression of the co-stimulatory molecule CD28.4 The loss of this potential critical co-stimulatory signaling pathway on CD8+ TIL has emerged as a significant setback in ACT.4,5 Furthermore, the concomitant loss of CD27 on CD8+ TIL also reduces the possibility of co-stimulation through the CD27−CD70 axis that can further sensitize the cells to apoptosis or anergy 6. Given this loss of CD28 and CD27 co-stimulation in highly expanded CD8+ TIL, the role of alternative co-stimulation pathways may become critical at this stage. A potentially powerful source of alternative co-stimulation for expanded TIL used in ACT is through the TNFR superfamily members, especially 4-1BB, that has emerged as a regulator of T-cell survival signaling, expansion, and function, especially during memory T-cell responses.7–9 The effects of co-stimulation through TNFR family members in human melanoma TIL especially in context with adoptive T-cell therapy has not been studied yet.
In our study here, we focused on two key members of the TNFR family, OX40 (CD134) and 4-1BB (CD137). 4-1BB co-stimulation has been shown to boost CD8+ T-cell responses against viral and tumor antigens and has been found to facilitate the generation of CTL responses killing tumor cells in vivo.7,10 OX40 signaling can enhance cytokine production and proliferation of CD4+ T cells.7,10 4-1BB signaling has been shown to co-stimulate more highly differentiated CD8+ T cells, such as effector-memory T cells and anti-viral T cells that have lost CD28 expression.8,10,11 Highly differentiated CD8+ populations that have acquired potent CTL function show down-regulated CD28 expression.4 These CD8+CD28− T cells are more prone to anergy and/or AICD4,12 It has been reported that 4-1BB co-stimulation can compensate for the loss of CD28 in some circumstances and drive CD8+ T-cell proliferation and facilitate effector function.9,13–15
We analyzed the changes in 4-1BB and OX40 expression in TIL during the REP and following TCR re-stimulation of post-REP TIL using low levels of anti-CD3 monoclonal antibody (mAb) to mimic conditions in vivo in the host where TIL encounter tumor antigen together with IL-2 after adoptive transfer. We focused on CD8+ TIL and the expression of 4-1BB (predominantly expressed on CD8+ T cells) because these cells have been found to be one of the key effector cells capable of directly killing tumor cell targets during immunotherapy. Since autologous tumor lines were scarce due to the difficulty in recovering lines from many patients, the use of anti-CD3 mAb allowed us to thoroughly investigate the effect of TCR stimulation on post-REP TIL and the effects of 4-1BB costimulation on a large panel of melanoma TIL samples. We found that CD8+ TIL progressively up-regulated 4-1BB, but also became prone to anti-CD3-mediated apoptosis. OX40 was also induced on CD8+ TIL, but to a lesser extent than 4-1BB. We then tested the effects of 4-1BB co-stimulation in post-REP TIL using two different agonistic anti-4-1BB antibodies (Ab). The first Ab was a commercially-available affinity-purified goat polyclonal anti-4-1BB Ab and the second was a fully human GMP-grade anti-4-1BB mAb from Bristol Myers Squibb (BMS) currently being tested in clinical trials16,17. We found that the commercially available and fully-human anti-4-1BB Abs potently inhibited AICD following TCR re-activation of CD8+CD28− post-REP TIL and drove further post-REP T-cell expansion. The effects were seen in both the bulk CD8+ and MART-1-specific CD8+ TIL populations. In addition, we found that 4-1BB co-stimulation on post-REP TIL also enhanced the killing of melanoma cells and increased antigen-specific IFN-γ secretion. Our data support the use of anti-4-1BB mAb as a surrogate therapy to improve TIL persistence and anti-tumor effector function during ACT for metastatic melanoma.
Materials and Methods
Monoclonal antibodies for TIL stimulation
TIL were stimulated using an anti-CD3 mAb (clone OKT3), with or without the addition of an agonistic anti-4-1BB antibody, as indicated in the different experiments described. Two agonistic anti-4-1BB Ab were used. The first antibody was a goat anti-4-1BB Ab obtained from R&D Systems (Catalog Number AF838; Lot Number CCO01). The Ab was affinity-purified using chromatography in 4-1BB protein columns and was reconstituted with 1 ml of sterile Dulbecco’s PBS (D-PBS), aliquotted at 0.1 mg/ml and stored at −80°C for later use. Each vial of the Ab was thawed and used once for each experiment. This method was used for all of our experiments. This Ab was designated as “4-1BB/1”. The second Ab was a fully human agonistic IgG4 mAb against human 4-1BB (BMS-663513; Lot 6A20377) obtained from Bristol Myers Squibb (BMS; Princeton, NJ) through a Materials Transfer Agreement. BMS-663513 was at 14.9 mg/ml and was stored at 4°C. This Ab was designated as “4-1BB/2”. Both Abs had <0.5 EU/mg endotoxin. Anti-CD3 (clone OKT3) was purchased from Abbott Labs (Abbott Park, IL) and stored at 4°C, as stipulated by the manufacturer. Purified, preservative-free agonistic anti-human CD28 mAb (Clone 28.2) was purchased from eBioscience (La Jolla, CA).
Initial expansion of TIL from human melanoma patient tumors
Tumor samples from Stage IV metastatic melanoma patients were cut into 3–5 mm2 pieces and cultured in TIL culture medium (TIL-CM) containing 6,000 IU/ml recombinant human IL-2 (Proleukin™; Novartis, East Hanover, NJ). It should be noted that originally Novartis (East Hanover, NJ) supplied recombinant human IL-2, but as of September 2009, the manufacturing and marketing of Proleukin™ was taken over by Prometheus Laboratories Inc. (San Diego, CA). The TIL-CM consisted of RPMI 1640 with Glutamax, 1 mM pyruvate, 20 µg/ml Gentamicin, 50 µM 2-mercaptoethanol, 1X Pen-Strep, and 10% heat inactivated human AB Serum (Sigma-Aldrich, St. Louis, MO). All culture medium components were from Invitrogen (Carlsbad, CA). TIL-CM was used for all experiments. The TIL expanded from the tumor fragments over a 4–5 week period (usually >40 × 106 total viable lymphocytes) were harvested and analyzed for CD8 expression along with other T-cell differentiation markers after staining using fluorescent-mAb using flow cytometry, as described below. These TIL were designated as “pre-rapid expansion TIL” (pre-REP TIL). A proportion of the pre-REP TIL were expanded after anti-CD3 activation in a REP for use in the experiments on 4-1BB co-stimulation. T cells not used immediately for the REP were cryopreserved in 10% DMSO, 90% human AB serum and stored in liquid nitrogen for later use.
Rapid expansion of TIL cultures
Pre-REP TIL were activated with OKT3, IL-2, and irradiated feeder cells using the same protocol currently used in ACT clinical trials for melanoma.2 This was done to mimic the conditions used in the clinic in these experiments. Briefly, 1.3 × 105 pre-REP TIL in 10 ml TIL-CM and 10 ml of AIM-V (Invitrogen) were added to upright T-25 flasks containing 30 ng/ml anti-CD3 and 26 × 106 allogeneic irradiated (5,000 cGy) peripheral blood mononuclear cell (PBMC) feeder cells obtained from 6 pooled normal donor buffy coats (Gulf Coast Regional Blood Center, Houston, TX). After the PBMC were isolated from the buffy coats, the PBMC were cryopreserved in freezing media, which contained 90% human Ab serum and 10% dimethyl sulfoxide (DMSO). Aliquots of these PBMC feeder cells were thawed, washed, irradiated as described above and immediately used in the REP. On day 2 of the REP, 6,000 IU/ml IL-2 (final concentration) was added to each flask. The TIL were expanded for another 12 days and diluted as needed with 1:1 TIL-CM and AIM-V keeping the concentration of IL-2 at 6,000 IU/ml. Routinely, 25–50 × 106 post-REP TIL were isolated from each REP after 14 days.
Flow cytometric sorting of post-REP CD8+CD28− TIL
The expanded post-REP TIL were re-suspended in 2 ml of sterile (0.22 µm filtered) FACS Wash Buffer (D-PBS, 1% BSA) and centrifuged at 365 × g for 5 minutes. The cells were then re-suspended at 20 × 106/ml in sterile, cold (4°C) FACS Stain Buffer (FSB) consisting of D-PBS, 1% BSA, 5% goat serum. The cells were then stained for CD8 and CD28 for 20 minutes on ice and washed in cold (4°C), sterile FACS Wash Buffer (FWB) to avoid capping and internalization of antibody-labeled, cell-surface determinants. The cells were re-suspended at 20 × 106/ml in sterile FACS Wash Buffer containing D-PBS, 1% BSA and immediately subjected to cell sorting for the CD8+CD28− subpopulation using a FACSAria sorter (BD Biosciences, San Jose, CA) at the M.D. Anderson Cancer Center South Campus FACS Core Facility. The positive TIL population was sorted into sterile tubes containing TIL-CM and 200 IU/ml IL-2, washed in TIL-CM and then used for experiments after resting for 3 h in TIL-CM with 200 IU/ml IL-2.
Re-stimulation of post-REP TIL
24 well plates (Nunc) were coated with the indicated concentrations of anti-CD3 (OKT3) with or without the different sources of anti-4-1BB agonistic mAb (see above) at 4°C overnight. Two different types of anti-4-1BB Abs were used; a commercially available agonistic anti-4-1BB monoclonal antibody (R&D Systems), designated as 4-1BB/1, and a fully human GMP- grade anti-4-1BB mAb (663513) obtained from BMS, designated as 4-1BB/2. After initial experiments, a concentration of 30 ng/ml of anti-4-1BB/1 and a concentration of 10 ng/ml of anti-4-1BB/2 was found to have optimal activity, respectively. These concentrations of Ab were then used in further experiments. After coating the plates with the Ab at 4°C overnight, the plates were washed 2 times with D-PBS and unbound sites were blocked with 1% BSA, D-PBS for 1 hour at room temperature. The plates were then washed with D-PBS. Bulk post-REP TIL or sorted CD8+CD28− TIL in TIL-CM (1 ml; 200,000 cells total) were added to the Ab-coated 24-well plates containing 1 ml of TIL-CM (2 ml total). IL-2 (200 IU/ml final) was added to all wells. The TIL were incubated over a 7-day period and analyzed for cell expansion, apoptosis, and effector function.
Flow cytometry staining and analysis
Pre-REP cells were cryopreserved, thawed, and cultured for 2 days in TIL-CM and 200 IU/ml IL-2 and then analyzed using flow cytometry. The post-REP and re-stimulated cells used for flow cytometry were taken directly from culture. The post-REP and re-stimulated TIL were stained for 20 min on ice in 0.1 ml FSB (6×106/ml) using fluorochrome-conjugated monoclonal antibodies recognizing surface markers CD4, CD8, CD27, CD28, CD56, CD57, OX40, 4-1BB in the indicated combinations (BD Biosciences, BD Pharmingen, eBioscience). An unstained control was used to verify the positive staining of the cell-surface determinants. The cells were washed in FWB and either fixed immediately for analysis or stained for granzyme B (GB) and perforin (Perf) expression using anti-GB-Alexa Fluor 647 (BD Biosciences) or anti-Perf-APC (BioLegend) after permeabilization. A mouse IgG2b, k isoptype control was used for the intracellular staining (Biolegend). The final stained cells were then washed with FWB and re-suspended in 0.3 ml D-PBS, 1% para-formaldehyde, 0.25% ethanol. The stained cells were analyzed using the BD FACScanto II flow cytometry analyzer using FACSDiva software. The gating of the TIL was done on live CD8+ cells using forward scatter and side scatter profiles. The data was later analyzed using FlowJo software (TreeStar).
Measurement of apoptosis
Apoptosis of the TIL was measured using 7-AAD and Annexin V staining using a kit from BD Biosciences (San Jose, CA). The TIL were harvested (usually 3 days following TCR re-stimulation when maximal apoptosis was found) and washed in cold D-PBS. The cells were re-suspended in 100 µl Binding Buffer supplied with the kit and centrifuged at 365 × g for 5 minutes. The cells were then stained with anti-CD8-Pacific Blue, Annexin V-PE, and 7-AAD for 20 min at 4°C. The cells were washed with 2 ml Binding Buffer, re-suspended in Binding Buffer and analyzed by flow cytometry within 1 hour.
Cytotoxic T-cell assay
Analysis of antigen-specific and melanoma tumor cell-specific cytotoxic T-cell (CTL) activity was done according to a flow cytometric method measuring the cleavage of caspase-3 in target cells.18 TIL from HLA-A2.1+ patients, found to recognize the HLA-A2.1-restricted altered MART-1 epitope ELAGIGILTV, as determined by staining with an HLA-A2.1-peptide tetramer, were used in CTL assays19. The target cells were either T2 cells (TAP1/2-deficient HLA-A2.1+ human lymphoma cells) pulsed with the ELAGIGILTV peptide, or the HLA-A2.1+ melanoma cell line 624 targets endogenously expressing MART-1. The T2 or 624 cells were first labeled with a far-red fluorescent marker dye, DDAO-SE (Invitrogen Carlsbad, CA). The T2 targets were also pulsed with MART-1 peptide (ELAGIGILTV) or a control HLA-A2.1-binding HIV rev peptide at 5 µg/ml for 1 h. The targets were then incubated with TIL at different E:T ratios for 3–4 h. The cells were then fixed, permeabilized, and stained with a PE-conjugated anti-cleaved caspase-3 rabbit mAb (BD Biosciences, San Jose, CA). The stained cells were analyzed within 24 h using a FACScanto II flow cytometer.
IFN-γ ELISA
IFN-γ production was measured in culture supernatants collected 7 days after anti-CD3 re-stimulation with or without anti-4-1BB using a human IFN-γ ELISA kit (Thermo Scientific KB132422). The IFN-γ ELISA protocol was performed according to the manufacturer’s instructions after a 1:1 dilution of the culture supernatants. The samples were added in triplicates of 50 µl to each well. The plates were developed using TMB substrate for 10 min and the reaction stopped by adding 100 µl of Stop Solution (1 M H2SO4) to each well. The plate was read in a 96-well ELISA plate reader (ELx808, Bio-Tek Instruments Inc., Houston, TX) using Gen5 software with a 450 nm filter. The absorbance values obtained from the standards were plotted against concentration (pg/ml) and unknown values were calculated and graphed using Gen5 software.
Quantitative real-time PCR
Quantitative RT-PCR (qRT-PCR) was used to measure the expression of the anti-apoptotic genes bcl-2 and bcl-xL and pro-apoptotic molecule bim from RNA isolated two to three days after anti-CD3 re-stimulation with or without 4-1BB co-stimulation. RNA from 3 × 106 cells was isolated using the Qiagen RNeasy Mini Kit according to the manufacturer’s instructions (Qiagen, Duesseldorf, Germany). The purity and RNA concentration was determined using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE). The RNA was converted to cDNA using the SuperScript III First-Strand Synthesis System according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA), and qRT-PCR reactions were done using iQ™ SYBR Green Supermix (BioRad, Santa Clara, CA). Triplicate reactions were set up for each gene in a 96-well Optical Reaction Plate (BioRad). Each well contained 12.5 µl IQ Buffer, 0.5 µl Primer Forward, 0.5 µl Primer Reverse, 1 µl cDNA, and 10.5 µl DNAase, RNAase free distilled water. The following sequences were used for the primers. bcl2: Forward primer: 5’-CAGAAGGGACTGAATCGGAG-3’, Reverse primer: 5’-TGGGATGTCAGGTCA CTGAA-3; bcl-xL Forward primer: 5'-TGAGTCGGATCG CAGCTTGG-3’, Reverse primer: 5'-TGGATG GTCAGTGTCTGGTC-3’; Bim: Forward primer: 5’-ACAGGA GCCCAGCACCCATG-3’, Reverse primer: 5'-ACGCCGCAACTCTTGGGCGA-3’; and β-actin: Forward primer: 5'-TTGCCGACAGGATGCAGAA-3’, Reverse primer: 5' GCCGATCCACACGGAGT ACT-3’. The PCR reactions were run in an iCycler (BioRad) using the following program: 95°C for 30 seconds (denaturation); 60°C for 30 seconds (annealing); 72°C for 30 seconds (elongation).
Statistical analysis
Microsoft Excel was used for graphing and statistical analysis. The Student’s t-test (two-tailed with unequal variance) was used in order to analyze the significance of the results. A p-value of less than or equal to 0.05 was deemed to be statistically significant.
Results
Post-REP TIL induce the expression of TNF-R family members
We first examined the status of 4-1BB and OX40 expression in TIL isolated after the REP and following re-stimulation of post-REP TIL with coated OKT3 mAb for 24 h in TIL-CM with 200 IU/ml IL-2. Fig. 1 shows a representative experiment with two TIL lines (TIL 1007 and TIL 1008). Twenty four hours after the incubation with media and IL-2, the post-REP TIL and post-REP TIL plus OKT3 were analyzed by flow cytometry for their cell surface phenotype after gating on live cells using forward scatter versus side scatter profiles. Almost all post-REP had a low to intermediate frequency of CD8+ TIL expressing 4-1BB. When post-REP TIL were re-stimulated with OKT3 and IL-2, the frequencies of CD8+ TIL expressing 4-1BB markedly increased compared to post-REP TIL (Fig.1A). As shown in Fig. 1A, TCR re-stimulation of post-REP TIL induced high levels of 4-1BB on the majority of the CD8+CD27−CD28− TIL (Fig. 1A). The few CD27+ and CD28+ cells in these TIL also exhibited an increase in 4-1BB expression (Fig. 1A). In TIL 1007, the percentage of 4-1BB after TCR re-stimulation in the CD8+ population was 73%, 4-1BB in the CD28− population was 72% after re-stimulation and 4-1BB in the CD27− population was 70% after re-stimulation (Fig. 1A). The 4-1BB frequencies in TIL 1007 in the CD27+ population increased from 0.4% to 3% in re-stimulated TIL and increased from 0% to 0.7% in the CD28+ population (Fig. 1A). In TIL 1008, the percentage of 4-1BB after TCR re-stimulation in the CD8+ population was 97%, 4-1BB in the CD28− population was 91% and 4-1BB in the CD27− population was 87% after TCR re-stimulation (Fig. 1A). The frequency of 4-1BB increased after OKT3 re-stimulation from 0% to 10.5% in the CD27+ population and from 0% to 6.5% in the CD28+ population (Fig. 1A). Screening a larger number of patient TIL lines (n=19) found that 4-1BB expression, as observed by the increase in the percentage of 4-1BB positive cells in the CD8+ subset, successively increased from the pre-REP culture stage through the REP and following TCR re-stimulation after the REP using 10 ng/ml of OKT3 found to maximally stimulate post-REP TIL (Fig. 1B). On average, over 60% of the CD8+ post-REP TIL had cell surface 4-1BB expression following TCR re-stimulation suggesting that a high proportion of the population may be sensitive to 4-1BB co-stimulation. OX40 was also expressed on CD8+ TIL, but only on a smaller fraction of cells throughout the stages of TIL culture, and over the whole sample population this change was not statistically significant (Fig. 1B). We also analyzed the expression of 4-1BB and OX40 in HLA-A0201+ patients containing MART-1 tetramer+ cells by staining pre-REP, post-REP and 24 hour after re-stimulation with OKT3 in a similar fashion. We gated on live CD8+MART-1+ TIL and found that the frequency of these expressing 4-1BB markedly increased after the REP and also after OKT3 re-stimulation (Fig. 1C). Analysis of OX40 expression on these MART-1 tetramer+ cells pre-REP, post-REP, and 24 hour after re-stimulation with OKT3 found that frequency of OX40+ cells increased slightly post-REP and after OKT3 re-stimulation (Fig. 1C).
Fig. 1. Post-REP and re-activated TIL exhibit up-regulation of 4-1BB.
TIL were stained for CD8, CD27, CD28, 4-1BB, or OX40 expression before and following the REP, or 24 h after TCR re-stimulation of post-REP TIL and post-REP TIL with OKT3. A, Staining of post-REP TIL from two representative patients showing changes in 4-1BB expression in the total CD8+ TIL population or in the CD8+CD27−, CD8+CD27+, CD8+CD28−, and CD8+CD28+ subsets. In each case, live cells were gated based on forward scatter and side scatter profiles. B, TIL from different patients were stained for CD8 and 4-1BB (n=26) or CD8 and OX40 (n=19) expression at the pre-REP, post-REP, and 24 h after re-stimulation of post-REP TIL with OKT3. The percentage of 4-1BB+ or OX40+ cells in the gated CD8+ population is shown for each TIL line. C, TIL from HLA-A0201+ patients were stained pre-REP, post-REP and after re-stimulation with CD8, 4-1BB, MART-1 or CD8, OX40 and MART-1. The live cells were gated on CD8+MART-1+ and analyzed for the different positive percentages of 4-1BB or OX40. The black bars indicate the average for all of the TIL lines for each marker. Statistical analysis was done using the Student’s t-test.
4-1BB co-stimulation prevents AICD and increases the recovery of post-REP CD8+ TIL following TCR ligation
It has been shown that the persistence of the TIL in vivo correlates with cancer regression.20,21 As shown above, one of the major changes in large-scale expanded TIL is the loss of CD28 and CD27 co-stimulatory molecules and an increase in the expression of 4-1BB, especially when post-REP TIL are re-stimulated through the TCR, a scenario they will encounter after infusion into patients. We thus subjected isolated post-REP TIL to TCR re-stimulation with or without anti-CD3 mAb (OKT3) to determine what effects this treatment had on TIL survival. IL-2 (200 IU/ml) was added to all cultures as before. As shown in Fig. 2A, post-REP CD8+ TIL were highly susceptible to AICD, with many of the cells undergoing apoptosis after 3 days with low doses of plate-bound OKT3 (maximal apoptosis at 10 ng/ml), as determined by staining with 7-AAD and Annexin V. When post-REP TIL were re-plated with IL-2 alone or IL-2 with 10 ng/ml plate-bound OKT3, the recovery of CD8+ T cells after a 7-day incubation period also declined (Fig. 2B). We then tested the effects of OKT3, with or without anti-4-1BB Ab. Co-ligation of post-REP TIL with OKT3 (10 ng/ml) and an affinity-purified polyclonal anti-4-1BB (4-1BB/1) potently inhibited apoptosis in a dose-dependent manner with maximal protection at 30 ng/ml of plate-bound Ab; at this dose the percentage of 7-AAD+ and Annexin V+ CD8+ TIL was down to the levels seen with IL-2 alone. Interestingly, an increased level of apoptosis was again seen at the 100 ng/ml dose (Fig. 3A) suggesting that over-co-stimulation with anti-4-1BB may reverse these protective effects. A similar protection of post-REP from AICD was observed using the BMS fully human anti-4-1BB mAb (4-1BB/2), except that this mAb was even more potent at protecting the CD8+ TIL from apoptosis, with maximal protection found at 3–10 ng/ml of plate-bound mAb (Fig. 3B). The over-co-stimulation effect with 4-1BB/2 was also observed with a reversal of the protective effect seen at the 30 and 100 ng/ml coated mAb (Fig. 3B). In order to further determine the reproducibility of the protective effect of 4-1BB Ab on reducing AICD, we screened a panel of post-REP TIL from 13 patients using 4-1BB/1 (30 ng/ml) and 7 patients with 4-1BB/2 (10 ng/ml). As shown in Fig. 3C and 3D, co-ligation with both types of anti-4-1BB Ab with OKT3 markedly inhibited induction of apoptosis in the CD8+ TIL.
Fig. 2. Induction of apoptosis and loss of viable cell recovery of post-REP CD8+ TIL after re-stimulation with OKT3 (OKT3).
Post-REP TIL were isolated and re-stimulated with plate-bound OKT3 and analyzed for apoptosis and viable cell recovery in the CD8+ TIL population. A, TIL were analyzed for apoptosis by staining with 7-AAD and Annexin V three days after re-stimulation with the indicated concentrations of plate-bound OKT3; this time interval was when maximal apoptosis was found in previous experiments (data not shown). OKT3 at 10 ng/ml was found to induce maximal apoptosis and was used in all subsequent experiments. B, Post-REP TIL (2 × 106 cells) from the indicated TIL lines were re-stimulated with 10 ng/ml OKT3 and recovery of viable CD8+ TIL determined after 7 days.
Fig. 3. 4-1BB co-ligation during post-REP TIL re-stimulation with OKT3 inhibits induction of apoptosis.
Multiple post-REP TIL lines were re-stimulated with coated OKT3 with or without anti-4-1BB antibody. On day 3 of the re-stimulation, apoptosis was measured using Annexin V and 7AAD staining in the CD8+ subset. A, Flow cytometry profiles of 7-AAD versus Annexin V of post-REP TIL lines re-stimulated for 3 days with or without the indicated concentrations of anti-4-1BB (4-1BB/1). Results with two representative TIL lines shown. Optimal protection from apoptosis was found at 30 ng/ml for 4-1BB/1. B, The experiment was repeated with the same TIL lines using 4-1BB/2 with optimal protection found at 10 ng/ml 4-1BB/2. C. Inhibition of OKT3-induced apoptosis by 4-1BB/1 (30 ng/ml) co-ligation in multiple patient post-REP TIL (n=13). D. Inhibition of OKT3-induced apoptosis by 4-1BB/2 (10 ng/ml) co-ligation in multiple patient post-REP TIL (n=7). The black bars indicate the average for all of the TIL lines for each marker. Statistical analysis was performed using the Student t-test.
We also tracked the recovery of total viable CD8+ TIL 7 days after TCR re-stimulation by performing viable cell counts coupled with staining for CD8+ T cells. Wells were set up in replicates of 4 wells each and cell counts were done for each well with average and standard deviation for each treatment group determined. Here, co-stimulation with both anti-4-1BB Abs not only prevented the loss CD8+ TIL induced by TCR re-stimulation, but also increased the total number of recovered cells than that seen by incubation with IL-2 alone indicating that 4-1BB co-stimulation with TCR ligation also facilitated the further expansion of the post-REP TIL (Fig. 4A and 4B). An anti-CD28 mAb or a non-specific goat IgG, coated along with OKT3, did not affect the level of TCR-induced apoptosis or the recovery of CD8+ post-REP TIL after the re-stimulation period (data not shown). As before, we screened a large number of patients tracking the fold change in CD8+ TIL 7 days after re-stimulation with OKT3 alone or with OKT3 plus anti-4-1BB. Using 4-1BB/1 (30 ng/ml optimal concentration), we screened 20 different patient TIL lines and found that 4-1BB co-stimulation induced an average of 6.5-fold expansion over 7 days of the post-REP CD8+ TIL, while treatment with OKT3 alone had a 1-fold increase or no expansion (p<0.001) (Fig. 4C). Similar results were found with 4-1BB/2, which stimulated an even greater expansion of CD8+ TIL after TCR re-stimulation using the optimal 10 ng/ml coating concentration (average of 10-fold increase in CD8+ cells) in 10 different patient TIL lines tested (Fig. 4D).
Fig, 4. Continued expansion of CD8+ post-REP TIL re-stimulated with 4-1BB co-stimulation.
Post-REP TIL (3 × 106 per condition) were re-activated using OKT3 as before with or without anti-4-1BB antibody in 4 replicate wells. Viable cell numbers were determined on day 7 by hemocytometer counts after Trypan Blue staining of each well and flow cytometry analysis was performed to determine percentage of CD8+ T cells in the viable lymphocyte gate. CD8+ T-cell recovery was determined by multiplying the percentage of CD8+ T-cell found by flow cytometry by the viable cell count. CD8+ TIL recovery after re-stimulation with OKT3 together with different doses of 4-1BB/1 in TIL 2055A (A) or 4-1BB/2 in TIL 2060 (B) is shown. In each case an average of 2 cell counts per condition is shown. C and D, The experiment was repeated with 20 different patient TIL lines using 4-1BB/1 (C) and 10 different patient TIL lines using 4-1BB/2 (D). The black bars indicate the average for all of the TIL lines for each condition shown. Statistical analysis was performed using the Student t-test with biological relevance occuring when P<0.05.
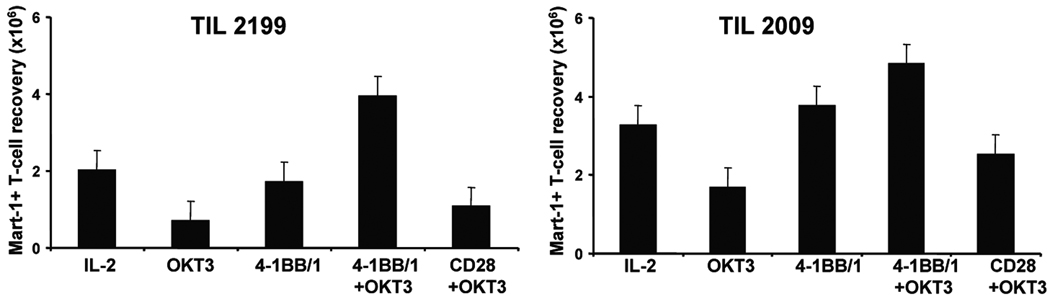
We also determined whether 4-1BB co-stimulation could protect melanoma antigen-specific post-REP CD8+ TIL from AICD following TCR ligation. Using TIL from HLA-A0201+ patients, we tracked the fate of MART-1-specific post-REP CD8+ T cells using tetramer staining and measurement of viable cell recovery 7 days following TCR stimulation. Again, wells were set up in replicates of 4 wells each and cell counts were done for each well. With IL-2 treatment alone, the TIL retained the same MART-1+ T-cell recovery as Day 0 of the re-stimulation. OKT3 treatment with IL-2 reduced the recovery of CD8+MART-1 tetramer+ TIL versus IL-2 alone. In contrast, treatment with OKT3 together with 4-1BB/1 (30 ng/ml) prevented this loss and increased the yield of antigen-specific TIL over that seen with IL-2 alone (Fig. 5). This increased yield of CD8+MART-1 tetramer+ TIL was not seen when OKT3 was combined with anti-CD28 (Fig. 5), indicating that the protective effect was specific for 4-1BB in this case. Similar results were obtained with these TIL lines when the bulk CD8+ TIL were monitored.
Fig. 5. Increased expansion and recovery of MART-1-specific CD8+ post-REP TIL following re-stimulation with 4-1BB co-stimulation.
TIL from HLA-A0201+ patients that had a significant population of CD8+MART-1 tetramer+ T cells were subjected to the REP. The post-REP TIL were re-stimulated with or without OKT3, with or without anti-4-1BB (30 ng/ml 4-1BB/1) for 7 days in 4 replicate wells. Post-REP TIL re-stimulated with anti-CD28 was used as a control. On day 7 of the re-stimulation, viable cell counts were done using hemocytometer counts of each well and the recovered TIL were also stained and analyzed for MART-1 and CD8 using flow cytometry. The recovery of CD8+MART-1 tetramer+ T cells was then calculated for each of the re-stimulation conditions indicated. The results of two separate experiments with post-REP TIL lines from two patients is shown.
4-1BB co-stimulation modulates apoptosis-regulatory genes in post-REP TIL
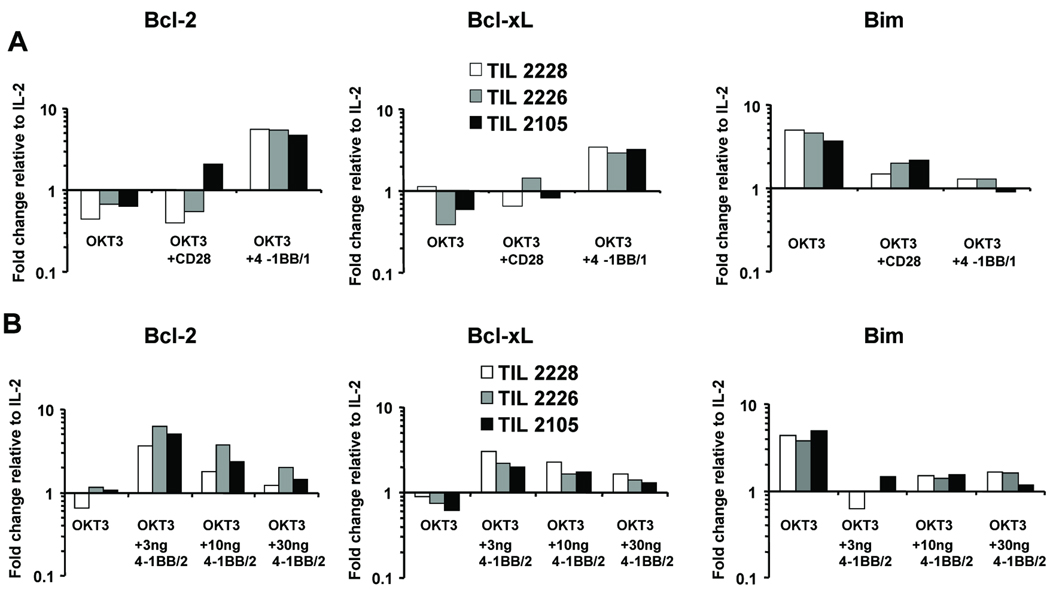
In the results above, we showed that 4-1BB co-stimulation prevents AICD and prevents the loss of both bulk and MART-1-reactive post-REP CD8+ TIL. We next tested whether this protection from apoptosis was associated with the modulation of anti-apopotic and pro-apoptotic genes. Post-REP TIL were re-cultured with IL-2 and OKT3 with or without the optimal concentrations of 4-1BB/1 or 4-1BB/2 as before. TIL re-cultured with IL-2 alone was used as control. The levels of bcl-1, bcl-xL (anti-apoptotic) and bim (pro-apoptotic) was determined using real-time qRT-PCR from each condition in RNA isolated 2 days after re-plating. As shown in Fig. 6A, co-stimulation with 4-1BB/1 induced a significant modulation of these genes in the indicated treatment groups relative to IL-2 alone. OKT3 reduced relative bcl-2 and bcl-xL expression, while increasing bim. Similar results were found in Ab control cultures treated with OKT3 and anti-CD28 (Fig. 6A). In contrast, co-stimulation with 4-1BB/1 increased relative bcl-2 and bcl-xL expression and led to significantly lower increases in bim expression (Fig. 6A). Similarly, 4-1BB/2 was tested in a dose-response experiment and found to maximally induce bcl-2 and bcl-xL expression at 3 and 10 ng/ml, while suppressing bim expression in comparison to OKT3 alone (Fig. 6B). Interestingly, relative bcl-2 and bcl-xL expression was somewhat lower at the 30 ng/ml dose. This correlates with the increased apoptosis seen at these higher 4/1BB/2 doses, suggesting again that an over-co-stimulation effect can occur with these Abs under the conditions used here in vitro.
Fig. 6. 4-1BB co-stimulation increases the relative expression of anti-apoptotic genes in post-REP TIL.
Post-REP TIL lines from three separate patients were re-stimulated with OKT3 with or without anti-4-1BB antibody. On day 2, RNA was isolated and the expression of bcl-2, bcl-xL, and bim was determined using qRT-PCR. All qRT-PCR data for the different treatments indicated were normalized to the level of expression in post-REP TIL re-cultured with IL-2 alone (relative expression of 1). Each PCR reaction was ran in triplicate and the coefficient of variance (CV) was found to be <5%. A, Post-REP TIL from the indicated patients were re-stimulated with OKT3 (10 ng/ml) alone or with 30 ng/ml 4-1BB/1 (optimal concentration) or anti-CD28 (negative control). B, The same experiment was repeated with different doses of 4-1BB/2.
4-1BB ligation co-stimulation of post-REP CD8+ TIL increases anti-tumor effector function
4-1BB co-stimulation has been reported to not only improve activated T-cell survival, but also increase the cytolytic activity of CD8+ T cells10,22. This would also have important ramifications on improving ACT for melanoma. Thus, we went on to determine whether post-REP TIL re-stimulated with OKT3 mAb together with anti-4-1BB exhibited any changes in effector cell activity and phenotype.
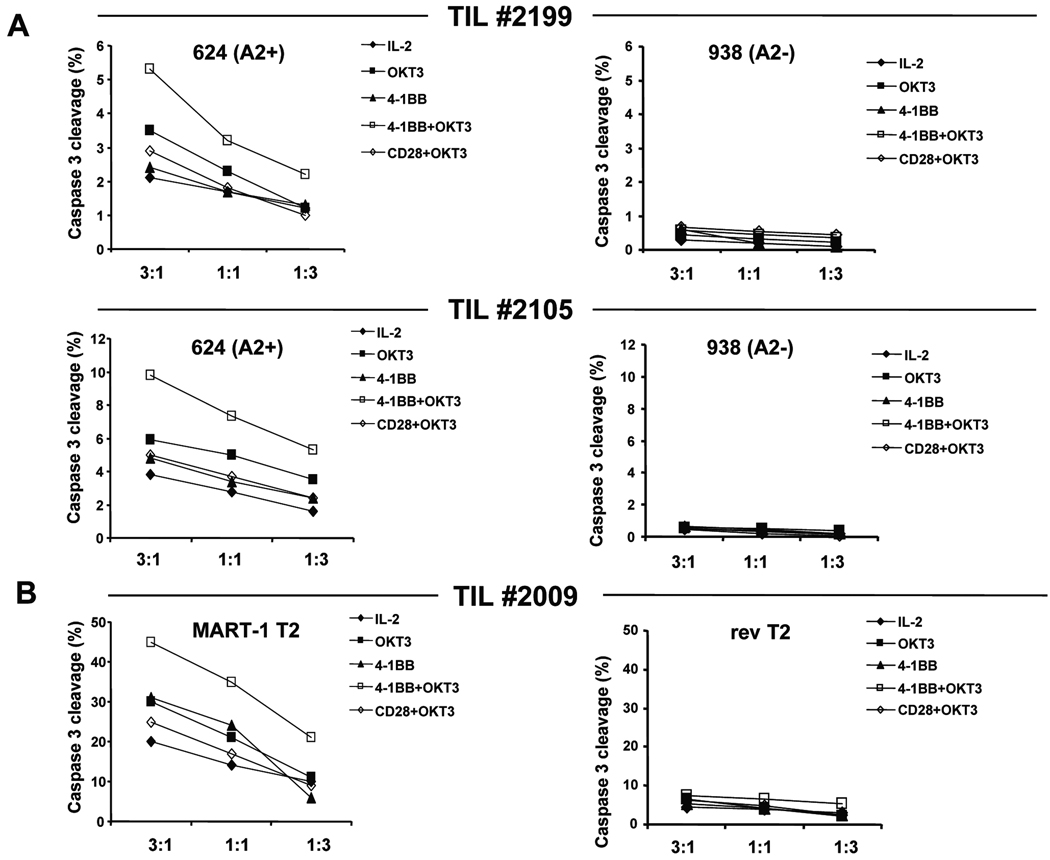
We first examined the effects of 4-1BB co-stimulation on antigen-specific CTL activity after re-stimulation of post-REP TIL. In this case we used TIL from HLA-A0201+ patients containing MART-1 tetramer-reactive CD8+ T cells to measure CTL activity against HLA-A0201+ 624 melanoma cells endogenously expressing the MART-1 tumor antigen.23 Post-REP TIL were stimulated as before with plate-bound OKT3 (10 ng/ml) and IL-2 with or without 4-1BB/1 at the optimal dose of 30 ng/ml. Post-REP TIL re-stimulated with OKT3 and anti-CD28 was used as control. On day 7 after re-stimulation, CTL activity was measured using a caspase-3 cleavage CTL assay18 using MART-1 peptide-pulsed T2 cell targets and 624 melanoma cell targets. HIV rev peptide-pulsed T2 cells and HLA unmatched 938 melanoma cells were used as controls. Re-stimulation of post-REP TIL with 4-1BB/1 and OKT3 for 7 days yielded cells with an increased specific killing of 624 melanoma cells compared to post-REP TIL re-stimulated with IL-2 alone, while co-stimulation with anti-CD28 as control Ab had no effect (Fig.7A). Similar results were obtained with MART-1 peptide pulsed T2 target cells with another post-REP TIL sample (Fig. 7B). We also analyzed the re-stimulated post-REP TIL isolated from the different re-stimulation cultures for the frequency of CD8+MART-1 tetramer+ T cells from all treatments and found that in each case the MART-1-specific CD8+ T-cell frequency was similar. Similarly, we determined the overall frequency of CD8+ T cells in the different groups and found that no significant differences between the groups were evident (see Fig. S2 in Supplementary Data online for this data corresponding to the CTL data in Fig. 7). Thus, the higher CTL activity was not due to an increased frequency of CD8+ T cells or MART-1-specific T cells (as expected due to the polyclonal OKT3 re-stimulation), but due to a higher intrinsic capacity for CTL activity. When the fully human 4-1BB/2 mAb was tested in these same experiments, a similar increase in killing capacity of 624 cells and MART-1 peptide-pulsed T2 cells was found (data not shown).
Fig. 7. Increased antigen-specific CTL activity in post-REP TIL re-stimulated with anti-4-1BB co-stimulation without a change in antigen-specific T-cell frequency.
Post-REP TIL from HLA-A0201+ patients were re-stimulated as before with or without 4-1BB/1 antibody or with anti-CD28 (negative control). After 7 days, CTL activity was monitored using a flow cytometry-based assay measuring the cleavage of caspase-3 in target cells as readout, as described in the Materials and Methods. A, CTL activity was determined in two separate patient TIL lines (#2199 and #2105) against HLA-A0201+ 624 melanoma targets (graphs on left hand side), or against an HLA unmatched melanoma cell line 938 as negative controls (graphs on right hand side). B, CTL activity from TIL line #2009 was assayed using MART-1 peptide-pulsed T2 target cells (graphs on left hand side), or T2 cells pulsed with HIV rev peptide as the negative control (graphs on right hand side). Fig. S2 (Supplementary Data online) is accompanying data to this figure showing that the frequency of CD8+ and CD8+MART-1 tetramer+ T cells isolated from all the treatment groups was similar.
To further investigate the mechanism behind the increased melanoma-specific killing capacity of post-REP TIL re-activated with 4-1BB co-stimulation, we analyzed the levels of GB and Perf expression in CD8+ TIL. As shown in Fig. 8, re-stimulation of post-REP TIL with OKT3 and 4-1BB/1 increased both the percentage of and mean fluorescence intensity (MFI) of both GB (Fig. 8A) and Perf (Fig. 8B) expression in comparison to IL-2 alone or IL-2 and OKT3. The MFI was analyzed on the gated GB+ and Perf+ populations. Thus, the increased CTL activity of post-REP TIL after co-stimulation through 4-1BB was correlated with an increased GB and Perf expression.
Fig. 8. GB and Perf expression in re-stimulated post-REP CD8+ TIL is increased by 4-1BB co-stimulation.
Post-REP TIL were re-stimulated with OKT3 with or without anti-4-1BB antibody as before. On day 7 after the re-stimulation, phenotypic analysis of the live CD8+ T cells for Perf expression and GB expression was determined by flow cytometry analysis. Results of post-REP TIL from three different patients (#2199, #2198, and #2206) are shown. A, The frequency (left hand panels) and MFI (right hand panels) of GB+ expression in the TIL lines following re-stimulation with OKT3 with or without 4-1BB/1 (30 ng/ml). B, An analysis of Perf+ expression was similarly performed on the three TIL lines. In each case, 4-1BB co-stimulation markedly increased both the percentage of CD8+ TIL expressing Granzyme B and Perforin as well as the MFI for both proteins.
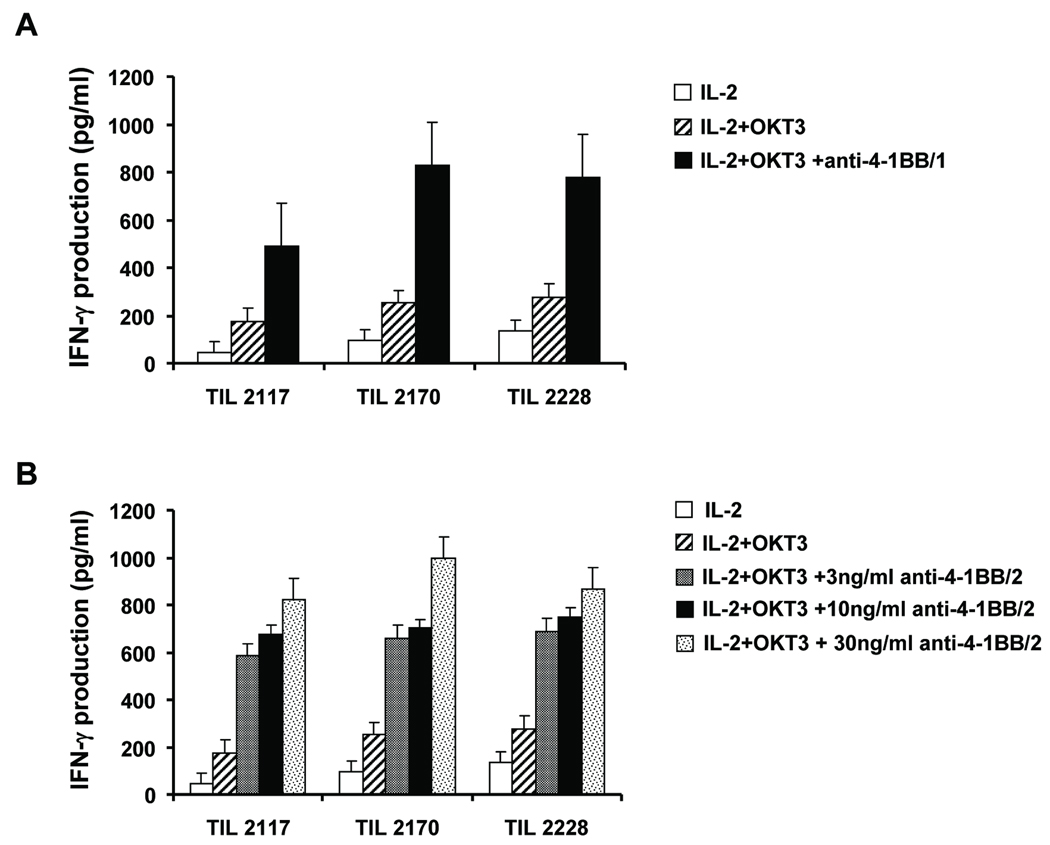
In addition to measuring changes in CTL activity and cytolytic molecule expression, we also measured the effect of 4-1BB co-stimulation on IFN-γ secretion after post-REP TIL were re-stimulated with OKT3. Culture supernatants were collected from each condition on day 7 after post-REP TIL re-stimulation with OKT3 and IL-2 with or without anti-4-1BB, and IFN-γ levels were determined by ELISA. Post-REP TIL re-stimulated with 4-1BB/1 (30 ng/ml) or 4-1BB/2 (10 ng/ml) induced markedly more IFN-γ secretion than with IL-2 alone or IL-2 plus OKT3 (Fig. 9).
Fig. 9. Increased IFN-γ production by post-REP TIL re-stimulated together with co-stimulation through 4-1BB.
Post-REP TIL were re-activated with IL-2 alone, or with OKT3 with or without anti-4-1BB/1 antibody or 4-1BB/2. On day 7, the culture supernatants were collected and the concentration of IFN-γ determined using an ELISA. Results with post-REP TIL from 3 different patients (#2117, #2170 and #2228) are shown for co-stimulation with 4-1BB/1 at 30 ng/ml (A) or with the indicated concentrations of 4-1BB/2 (B). TILs re-stimulated with 4-1BB produced more IFN-γ than TILs stimulated with OKT3 (A). All assays were done in triplicate with averages and standard deviation shown.
4-1BB ligation co-stimulates sorted CD8+CD28− TIL inhibiting AICD and increasing cytotoxic T-cell function
Although most post-REP CD8+ TIL have lost CD28 and CD27 expression (see Fig. 1) there is still nevertheless a significant fraction of remaining CD8+CD28+ T cells that varies from patient to patient. Thus, we wanted to make sure that 4-1BB co-stimulation can act on the CD28− subset in the absence of any remaining CD28+ T cells. To address this issue, post-REP TIL from HLA-A2.1+ patients having MART-1 tetramer+ CD8+ T cells were stained with anti-CD8 and anti-CD28 mAb and the CD8+CD28− T cells sorted out and re-stimulated with plate-bound OKT3 mAb with or without anti-4-1BB together. We then followed the re-stimulation cultures over a 7-day period determining the levels of apoptosis, expansion of CD8+ MART-1 tetramer+ T cells, and the resulting CTL activity against MART-1 peptide-loaded T2 target cells. In each case, co-stimulation with 4-1BB/1 (30 ng/ml) resulted in protection from apoptosis, further expansion of MART-1-specific TIL, and increased CTL activity after TCR re-stimulation in the two sorted post-REP TIL shown (Fig. S1 in Supplementary Data online). Thus, the protective effects of 4-1BB ligation from AICD during TCR re-stimulation of post-REP TIL and enhancement of CTL effector activity was found to occur specifically in the most highly-differentiated CD8+CD28− T-cell subset.
Reduced apoptosis of antigen-specific CD8+ TIL co-incubated with melanoma cells expressing 4-1BB ligand
Lastly, we went on to test the role of 4-1BB co-stimulation when post-REP TIL were exposed to tumor cells presenting melanoma antigens triggering the TCR. This is a scenario likely to happen in vivo after TIL infusion during TIL contact with melanoma cells. Here, we took two HLA-A0201+ post-REP TIL samples containing MART-1 tetramer+ CD8+ TIL and co-incubated the cells with vector control-transduced 624 melanoma tumor cells or 624 melanoma cells transduced with 4-1BB ligand. Post-REP TIL were incubated with these 624 melanoma cells at a 1:1 ratio and apoptosis (7-ADD and Annexin V positive cells) was enumerated by flow cytometry after 3 days in the gated CD8+MART-1 tetramer+ T-cell population. A marked induction of apoptosis was found with vector control-transduced 624 cells, the 4-1BB ligand-transduced 624 cells induced significantly less apoptosis (Fig. 10). In addition, addition of 3 or 10 ng/ml 4-1BB/2 also protected the antigen-specific TIL from apoptosis (note that the vector control 624 cells served as the 0 ng/ml 4-1BB/2 control in this case) (Fig. 10).
Fig. 10. Reduced apoptosis of antigen-specific CD8+ TIL co-incubated with melanoma cells expressing 4-1BB ligand.
Post-REP TIL from two HLA-A0201+ patients containing MART-1-specific CD8+ T cells were co-incubated with vector control-transduced 624 melanoma cells (MART-1 expressing) or 624 melanoma cells transduced with 4-1BBL at a 1:1 ratio. After 3 days, the cells were stained and gated on CD8+ and MART-1+ tetramer, and apoptosis was measured using Annexin V plus 7-AAD staining. The same TIL were also co-incubated in parallel with vector-transduced 624 cells with 3 or 10 ng/ml of 4-1BB/2.
Discussion
Adoptive T-cell therapy using expanded TIL is currently one of the leading experimental treatments for metastatic melanoma.1,3,24,25 There are a number of factors that play a role in determining the therapeutic efficacy of ACT. First and foremost is the issue of the length of TIL persistence in vivo after adoptive transfer into the patient. The length of TIL persistence in vivo has been shown to correlate with extent and duration of melanoma tumor regression.20,25 A key finding made in our lab was that post-REP TIL after extensive ex vivo expansion lost the expression of the critical co-stimulatory molecule, CD28, with also loss of CD27, another important T-cell co-stimulatory molecule.4 Although this loss of CD28 expression is associated with a gain of anti-tumor effector (CTL) function, it also causes the cells to become hypo-responsive to further stimulation and susceptible to AICD.4 4-1BB ligation was previously found to markedly improve CD8+ T-cell proliferation and anti-tumor effector function in cancer vaccine and anti-viral vaccine models10,26,27, and has been found to be an additional regulator of T-cell survival and expansion when CD28 is lost.9,10,28 It has also bee shown to be superior to CD28 co-stimulation in expanding functional anti-tumor CD8+ T cells using both artifical APC or tumor cells expressing 4-1BB ligand.29,30 This prompted us to look at the expression and role 4-1BB has on melanoma TIL. We wanted to determine whether the tumor necrosis factor receptor (TNFR) superfamily member 4-1BB could serve as a co-stimulatory pathway and prevent the hypo-responsiveness of post-REP TIL to TCR triggering and associated AICD. We also asked whether 4-1BB co-stimulation could enhance anti-tumor CTL function in post-REP TIL.
One of the main problems ACT with expanded TIL is a lack of persistence in vivo following adoptive transfer into patients. Although TIL may survive for long periods of time undetected in tissues outside the systemic circulation, a number of clinical studies have found that a rapid disappearance of major TCR clonotypes in the peripheral blood within a few weeks after treatment that were in the original TIL correlates to a more limited tumor control in many patients during melanoma ACT and lack of clinical responses.20 A number of factors, such as lack of cytokines and telomere loss have been associated with lack of TIL persistence, as detected in the blood. However, since many TIL are melanoma antigen-specific, it is also highly possible that lack of adequate co-stimulation during contact with tumor antigen in the blood and tissues leading to AICD is may also be a critical mechanism contributing to TIL loss in vivo. This reasoning, together with the loss of other critical co-stimulatory molecules during TIL expansion (CD27 and CD28) prompted us to determine whether members of the TNF-R family, 4-1BB in particular, could serve as a potent source of alternative co-stimulatory signals in post-REP TIL. We used a commercially available agonistic anti-4-1BB antibody, as well as a fully human anti-4-1BB antibody (BMS-663513), provided by Bristol-Myers Squibb that is currently being tested in a number of Phase I and Phase II clinical trials for multiple types of solid cancers, including melanoma.22 We wanted to test whether this clinical-grade anti-4-1BB mAb was also active in our experimental systems.
We first looked at changes in 4-1BB expression during melanoma TIL expansion and found that the frequency of CD8+ post-REP TIL expressing 4-1BB at the cell surface markedly increased to high levels when the cells were re-stimulated through the TCR-CD3 complex. OX40 was also increased in a more limited fashion. This increase in 4-1BB expression was not induced by IL-2 alone. Overall, 4-1BB expression increased somewhat during the REP in the CD8+ TIL population, we found that re-stimulation through the TCR-CD3 complex was required for an acute induction of the molecule within 24 h. Although the high doses of IL-2 in the REP have been shown in some cases to transiently down-modulate the cell surface expression of costimulatory molecules such as CD276, we have not found that IL-2, even at high doses (6,000 IU/ml), significantly modulates cell surface 4-1BB expression (Hernandez-Chacon, unpublished observations). In multiple TIL lines, we found that anti-4-1BB co-ligation could prevent AICD in post-REP TIL and also induced considerable further expansion of the CD8+ T cells in the TIL product. We also found that anti-apoptotic genes bcl-2 and bcl-xL had a higher relative expression in 4-1BB co-stimulated TIL compared to OKT3 alone. Thus, 4-1BB signaling can serve as an alternative co-stimulatory pathway in post-REP melanoma TIL used for ACT by facilitating the continued proliferation after TCR-CD3 stimulation and preventing AICD most likely by regulating bcl-2, bcl-xL, and bim. These results suggest that provision of anti-4-1BB during ACT may improve TIL persistence in vivo, where continuous contact with melanoma antigens, especially in the tumor microenvironment, may otherwise induce AICD and TIL deletion.
Another aspect of 4-1BB co-stimulation is its ability to boost CTL activity against tumor antigens.10,28,31 We therefore also sought to determine whether 4-1BB co-stimulation during TCR restimulation of post-REP TIL resulted in a surviving T-cell population with enhanced anti-tumor CTL effector function against melanoma cells. Together with the increased TIL expansion, we also found that 4-1BB co-stimulation induced the further expansion of CD8+ TIL with enhanced killing activity against HLA-matched tumor cells and peptide-pulsed target cells. This increased CTL activity was most likely due to the marked increase in cells expressing GB and Perf which was found in all post-REP CD8+ TIL costimulated with 4-1BB. However, addition of anti-4-1BB directly into the CTL assay did not affect target cell killing activity (Hernandez-Chacon, unpublished observations) indicating that 4-1BB costimulation induces a program of gene expression changes enhancing the expression of cytolytic molecules in the CD8+ T cells. Thus, the positive effect of 4-1BB co-stimulation on the survival of post-REP CD8+ TIL, their improved proliferative ability, and better CTL activity, suggests that activating the 4-1BB pathway during ACT using an agonistic mAb, such as the BMS-663513, may significantly improve TIL persistence and facilitate stronger and longer-lasting tumor regressions by increasing their cytotoxic potential while helping to prevent AICD. This may greatly improve progression-free survival and long-term overall survival in patients receiving ACT.
A note of caution however is the possibility that over-co-stimulation or “hyper-co-stimulation” can occur by applying too much anti-4-1BB mAb. We noticed in our experiments that each anti-4-1BB Ab tested had an effective dose range (0–30 ng/ml) in which protection from AICD was found, but when higher doses of the Abs were used, a reversal of the protective effect was seen. This suggests that too much signaling through these TNF-R pathways can trigger apoptosis. This conclusion is supported by work in mouse model systems with anti-4-1BB as well as over-stimulation or chronic stimulation of other TNF-R family members such as CD27 where abnormal immune events take place. For example, Croft and colleagues recently demonstrated hyper-co-stimulation through 4-1BB by repeated agonistic anti-4-1BB Ab induced a large loss of B cells and toxicity related to high pro-inflammatory cytokine production, such as IFN-γ.32 Chronic CD27 stimulation in mice also perturbs the immune system.33–35 For example, CD70 transgenic mice showed a progressive conversion of naive T cells into effector-memory cells, which culminated in the depletion of naive T cells from lymph nodes and spleen. This was associated with susceptibility rather than protection against opportunistic infections.35 These results indicate that over-stimulation of CD27 in T cells can chronically activate and exhaust the T-cell pool. Using a novel superagonist anti-CD28 monoclonal antibody in clinical trials also resulted in a disastrous cytokine storm.36 The patients exhibited headache, nausea, vomiting and other severe side effects within 90 minutes of receiving a single intravenous injection of the superagonist anti-CD28 monoclonal antibody.36 Currently, we are awaiting the results of clinical testing of the BMS-663513 fully human anti-4-1BB mAb in metastatic cancer patients.
In our experiments with melanoma TIL, the mechanism behind why higher doses of anti-4-1BB triggered increased apoptosis again is not known. In our experience, we have found that post-REP TIL especially are highly susceptible to activation signals and have low thresholds of activation that can easily be surpassed leading to AICD. It is conceivable that at higher anti-4-1BB levels (100 ng/ml), even higher IFN-γ levels secreted by the TIL acted directly on them facilitating apoptosis. High IFN-γ levels have been shown to facilitate CD8+ T-cell apoptosis and deletion and alleviation of inflammation.37–39 This may be regulated through death receptor (e.g., fas and fas-ligand expression).40,41 Future experiments will need to determine the exact mechanism behind this over-co-stimulation effect by monitoring the expression apoptosis-regulating genes, the levels of IFN-γ and other pro-inflammatory cytokines, as well as whether the fas death receptor system may be triggered. One possibility is that bcl-2 and bcl-xL expression is inhibited and bim increases, or that an alternative apoptosis-promoting pathway is activated.
It is important to note however that we have observed the over-co-stimulation effect in vitro and do not know whether this effect would be seen in vivo. Nevertheless, our data indicates that the 4-1BB signaling system is very potent with receptor cross-linking with relatively low levels of Ab having maximal effects. Thus, careful dosing and the use of low dose ranges of anti-4-1BB may be efficacious in improving TIL persistence and function in vitro.
In summary, for the first time we have tested the effects of an alternative costimulatory pathway through the TNF-R family member 4-1BB on the survival and function of melanoma post-REP TIL similar to those used in the treatment of metastatic melanoma patients receiving ACT. Post-REP CD8+ TIL losing CD28 and CD27 are active CTL anti-tumor effector cells, but are also highly sensitive to AICD. Co-stimulation through the TNF-R family member 4-1BB can potently protect CD8+ TIL from AICD leading to the continued expansion with IL-2 of more potent anti-tumor effector cells expressing increased anti-apoptotic molecules. Provision of 4-1BB co-stimulation during ACT should be considered in future clinical trials.
Supplementary Material
Acknowledgements
The authors like to thank the members of the “TIL Lab” (Rahmatu Mansaray, Orenthial Fulbright, Renjith Ramachandran, Chris Toth, and Seth Wardell) at M.D. Anderson Cancer Center for help with processing of melanoma tumors and initial culturing of TIL for experiments. We also thank our clinical team, including Priscilla Miller, Michelle Glass, and the other melanoma medical oncologists at M.D. Anderson for identifying and accruing patients for our TIL therapy trials and studies. Lastly, we thank the melanoma surgeons at M.D. Anderson (including Jeff Lee, Jeff Gershenwald, Merrick Ross, Paul Mansfield, Janice Cormier, Amy Heimberger, and Anthony Lucci) and Victor Prieto (Dept. of Pathology at M.D. Anderson) for their help in obtaining tumor tissues for this study.
Grant Support: This work was supported by the NCI SPORE grant 5 P50 CA093459-05-DRP21, a Team Science Award from the Melanoma Research Alliance (MRA), and the Miriam and Sheldon Adelson Medical Research Foundation (AMRF).
Abbreviations
- ACT
adoptive T cell therapy
- TIL
tumor-infiltrating lymphocyte
- 7-AAD
7-amino-actinomycin D
- REP
rapid expansion protocol
- GB
Granzyme B
- Perf
Perforin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
References
- 1.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nature reviews. 2003;3(9):666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26(4):332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Current opinion in immunology. 2009;21(2):233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, et al. MART-1--specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro. J Immunol. 184(1):452–465. doi: 10.4049/jimmunol.0901101. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, et al. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175(10):7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176(12):7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9(4):271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shuford WW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. The Journal of experimental medicine. 1997;186(1):47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, et al. 4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J Immunol. 2007;179(7):4910–4918. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annual review of immunology. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 11.Laderach D, Movassagh M, Johnson A, Mittler RS, Galy A. 4-1BB co-stimulation enhances human CD8(+) T cell priming by augmenting the proliferation and survival of effector CD8(+) T cells. International immunology. 2002;14(10):1155–1167. doi: 10.1093/intimm/dxf080. [DOI] [PubMed] [Google Scholar]
- 12.Radvanyi LG, et al. CD28 costimulation inhibits TCR-induced apoptosis during a primary T cell response. J Immunol. 1996;156(5):1788–1798. [PubMed] [Google Scholar]
- 13.Bukczynski J, Wen T, Watts TH. Costimulation of human CD28− T cells by 4-1BB ligand. European journal of immunology. 2003;33(2):446–454. doi: 10.1002/immu.200310020. [DOI] [PubMed] [Google Scholar]
- 14.Cannons JL, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol. 2001;167(3):1313–1324. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 15.DeBenedette MA, Shahinian A, Mak TW, Watts TH. Costimulation of CD28− T lymphocytes by 4-1BB ligand. J Immunol. 1997;158(2):551–559. [PubMed] [Google Scholar]
- 16.Lin GH, et al. Evaluating the cellular targets of anti-4-1BB agonist antibody during immunotherapy of a pre-established tumor in mice. PloS one. 5(6):e11003. doi: 10.1371/journal.pone.0011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nature reviews. 2007;7(2):95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 18.He L, et al. A sensitive flow cytometry-based cytotoxic T-lymphocyte assay through detection of cleaved caspase 3 in target cells. Journal of immunological methods. 2005;304(1–2):43–59. doi: 10.1016/j.jim.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Valmori D, et al. Diversity of the fine specificity displayed by HLA-A*0201-restricted CTL specific for the immunodominant Melan-A/MART-1 antigenic peptide. J Immunol. 1998;161(12):6956–6962. [PubMed] [Google Scholar]
- 20.Huang J, et al. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005;28(3):258–267. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbins PF, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173(12):7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SW, Croft M. 4-1BB as a Therapeutic Target for Human Disease. Advances in experimental medicine and biology. 2009;647:120–129. doi: 10.1007/978-0-387-89520-8_8. [DOI] [PubMed] [Google Scholar]
- 23.Rivoltini L, et al. Quantitative correlation between HLA class I allele expression and recognition of melanoma cells by antigen-specific cytotoxic T lymphocytes. Cancer research. 1995;55(14):3149–3157. [PMC free article] [PubMed] [Google Scholar]
- 24.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straten P, Becker JC. Adoptive cell transfer in the treatment of metastatic melanoma. The Journal of investigative dermatology. 2009;129(12):2743–2745. doi: 10.1038/jid.2009.204. [DOI] [PubMed] [Google Scholar]
- 26.Bertram EM, Dawicki W, Watts TH. Role of T cell costimulation in anti-viral immunity. Seminars in immunology. 2004;16(3):185–196. doi: 10.1016/j.smim.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Bukczynski J, Wen T, Ellefsen K, Gauldie J, Watts TH. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(5):1291–1296. doi: 10.1073/pnas.0306567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunological reviews. 2009;229(1):192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnson BD, et al. Neuroblastoma cells transiently transfected to simultaneously express the co-stimulatory molecules CD54, CD80, CD86, and CD137L generate antitumor immunity in mice. J Immunother. 2005;28(5):449–460. doi: 10.1097/01.cji.0000171313.93299.74. [DOI] [PubMed] [Google Scholar]
- 30.Yan X, Johnson BD, Orentas RJ. Murine CD8 lymphocyte expansion in vitro by artificial antigen-presenting cells expressing CD137L (4-1BBL) is superior to CD28, and CD137L expressed on neuroblastoma expands CD8 tumour-reactive effector cells in vivo. Immunology. 2004;112(1):105–116. doi: 10.1111/j.1365-2567.2004.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheuk AT, Mufti GJ, Guinn BA. Role of 4-1BB:4-1BB ligand in cancer immunotherapy. Cancer gene therapy. 2004;11(3):215–226. doi: 10.1038/sj.cgt.7700670. [DOI] [PubMed] [Google Scholar]
- 32.Lee SW, Salek-Ardakani S, Mittler RS, Croft M. Hypercostimulation through 4-1BB distorts homeostasis of immune cells. J Immunol. 2009;182(11):6753–6762. doi: 10.4049/jimmunol.0803241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Colvenaer V, et al. Continuous CD27 triggering in vivo strongly reduces NK cell numbers. Eur J Immunol. 40(4):1107–1117. doi: 10.1002/eji.200939251. [DOI] [PubMed] [Google Scholar]
- 34.Nolte MA, van Olffen RW, van Gisbergen KP, van Lier RA. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol Rev. 2009;229(1):216–231. doi: 10.1111/j.1600-065X.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 35.Tesselaar K, et al. Lethal T cell immunodeficiency induced by chronic costimulation via CD27-CD70 interactions. Nat Immunol. 2003;4(1):49–54. doi: 10.1038/ni869. [DOI] [PubMed] [Google Scholar]
- 36.Suntharalingam G, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. The New England journal of medicine. 2006;355(10):1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Janeway CA., Jr Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990;172(6):1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asavaroengchai W, et al. An essential role for IFN-gamma in regulation of alloreactive CD8 T cells following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13(1):46–55. doi: 10.1016/j.bbmt.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groux H, Monte D, Plouvier B, Capron A, Ameisen JC. CD3-mediated apoptosis of human medullary thymocytes and activated peripheral T cells: respective roles of interleukin-1, interleukin-2, interferon-gamma and accessory cells. Eur J Immunol. 1993;23(7):1623–1629. doi: 10.1002/eji.1830230734. [DOI] [PubMed] [Google Scholar]
- 40.Boselli D, et al. IFN-gamma regulates Fas ligand expression in human CD4+ T lymphocytes and controls their anti-mycobacterial cytotoxic functions. Eur J Immunol. 2007;37(8):2196–2204. doi: 10.1002/eji.200636541. [DOI] [PubMed] [Google Scholar]
- 41.Zarozinski CC, McNally JM, Lohman BL, Daniels KA, Welsh RM. Bystander sensitization to activation-induced cell death as a mechanism of virus-induced immune suppression. J Virol. 2000;74(8):3650–3658. doi: 10.1128/jvi.74.8.3650-3658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.