Abstract
Siderophores are low molecular weight, high-affinity iron(III) ligands, produced by bacteria to solubilize and promote iron uptake under low iron conditions. Two prominent structural features characterize the majority of the marine siderophores discovered so far: (1) a predominance of suites of amphiphilic siderophores composed of an iron(III)-binding headgroup that is appended by one or two of a series of fatty acids and (2) a prevalence of siderophores that contain α-hydroxycarboxylic acid moieties (e.g., β-hydroxyaspartic acid or citric acid) which are photoreactive when coordinated to Fe(III). Variation of the fatty acid chain length affects the relative amphiphilicity within a suite of siderophores. Catecholate sulfonation is another structural variation that would affect the hydrophilicity of a siderophore. In addition to a review of the marine amphiphilic siderophores, we report the production of petrobactin disulfonate by Marinobacter aquaeolei VT8.
Keywords: Marine siderophores, Petrobactins, Bacillibactins, Sulfonated siderophores
Introduction
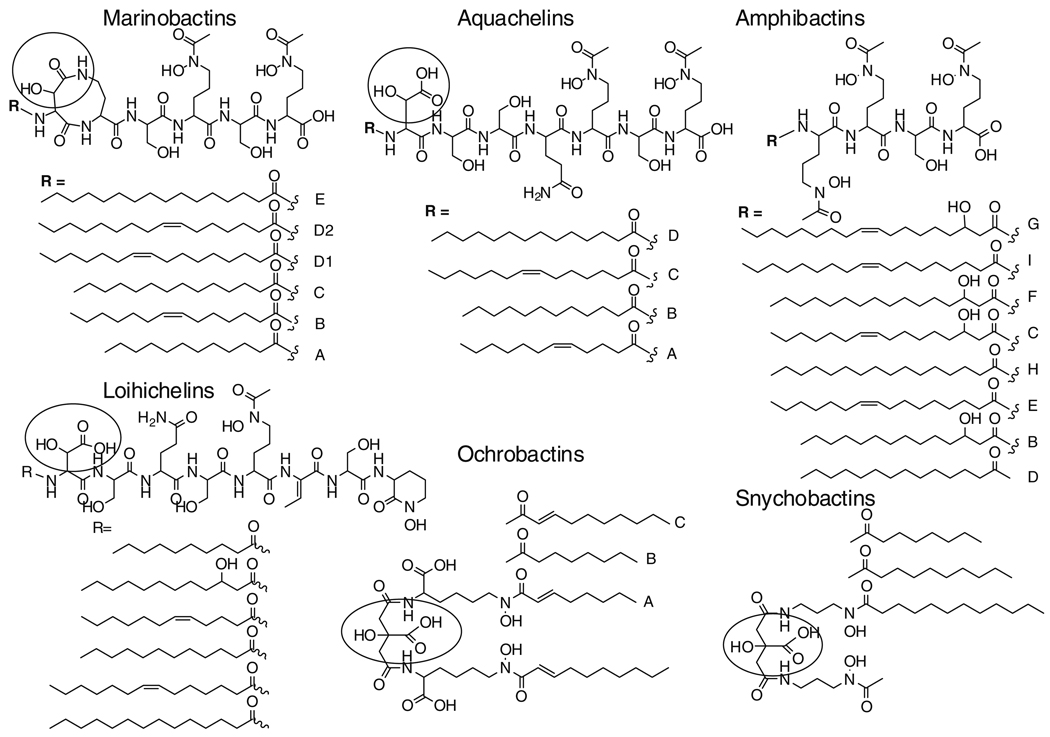
Siderophores are low molecular weight, high-affinity iron(III) ligands, produced by bacteria to solubilize and promote iron uptake under low iron conditions. While relatively few siderophore structures from marine bacteria are known compared to those from terrestrial bacteria, two prominent structural features characterize the majority of the marine siderophores discovered so far. One structural characteristic is the predominance of suites of amphiphilic siderophores composed of an iron(III)-binding headgroup that is appended by one or two of a series of fatty acids (Butler 2005; Martinez et al. 2000, 2003; Ito and Butler 2005; Martin et al. 2006; Homann et al. 2009) (Fig. 1). The other structural characteristic is the prevalence of siderophores that contain α-hydroxycarboxylic acid moieties (e.g., β-hydroxyaspartic acid or citric acid) which are photoreactive when coordinated to Fe(III) (Küpper et al. 2006; Barbeau et al. 2001–2003; Hickford et al. 2004) (circled portions in Figs. 1, 2). Within the amphiphilic siderophores, the amphibactins, aquachelins, loihichelins, and marinobactins form a class of peptide amphiphiles, whereas the ochrobactins and synechobactins are based on a citrate-containing head group. Many of the marine siderophores contain both distinctive structural features, in that they are both produced as suites of amphiphiles and the Fe(III) complexes are photoreactive (e.g., aquachelins, marinobactins, loihichelins, ochrobactins and synechobactins).
Fig. 1.
Structures of amphiphilic marine siderophores: Marinobactins and Aquachelins (Martinez et al. 2000), Amphibactins (Martinez et al. 2003), Loihichelins (Homann et al. 2009), Ohrobactins (Martine et al. 2006), Synechobactins (Ito and Butler 2005). The circled moieties are the α-hydroxycarboxylic acids or a α-hydroxycarboxyamide that is photoreactive when coordinated to Fe(III)
Fig. 2.
Examples of non amphiphilic marine peptide and citrate siderophores: Alterobactins (Reid et al. 1993), Pseudoalterobactins (Kanoh et al. 2003), Aerobactin (Haygood et al. 1993), Vibrioferrin (Amin et al. 2007)
These marine siderophores have been isolated from distinct genera of marine bacteria (e.g., species of Vibrio, Marinobacter, Halomonas, Ochrobactrum and the cyanobacterium, Synechococcus PCC7002) (Martinez et al. 2000, 2003; Ito and Butler 2005; Martin et al. 2006; Homann et al. 2009). The production of amphiphilic siderophores across many genera of marine bacteria, with relatively fewer examples in terrestrial microbes, suggests that the structural trait of amphiphilicity is advantageous for marine microbes. One possibility is that the fatty acid appendage has evolved as a means to prevent or lessen siderophore diffusion. Another possibility is that the fatty acid carbon chain length tunes the relative degree of hydrophobic or hydrophilic character within the amphiphilic spectrum. We have previously investigated the membrane partitioning of the marinobactins (Xu et al. 2002) and the amphibactins (Martinez et al. 2003). The partition coefficients for the suite of marinobactins A–E (36–5,818 M−1) correlates with the length of the fatty acid, as well as the extent of unsaturation in the fatty acid (Xu et al. 2002). For example, marinobactin E containing hexadecanoic acid (C16:0) partitioned about an order of magnitude more than Marinobactins D1 and D2 (with C16:1 ω-7 and ω-9 fatty acids) or marinobactin C with tetradecanoic acid (C14:0), a shorter fatty acid. Likewise, the partitioning of marinobactin B (C12:1 ω-7) and marinobactin A (C12:0) partition overall about two orders of magnitude less than marinobactin E. Thus this suite of siderophores could create a concentration gradient of marinobactins emanating out from the bacterium, which has been proposed as a possible bucket brigade approach to the transport of Fe(III) (Martinez et al. 2003).
Other types of structural modifications may also tune the relative hydrophobic or hydrophilic character of a siderophore. Aromatic sulfonation would be particularly effective for catechol-containing siderophores, as well as aromatic glycosylation. Previously we reported monosulfonated petrobactin as a siderophore produced by Marinobacter hydrocarbonoclasticus (Hickford et al. 2004). We now report herein isolation of petrobactin disulfonate (petrobactin-(SO3)2) from Marinobacter aquaeolei strain VT8, along with petrobactin and petrobactin monosulfonate. M. aquaeolei strain VT8 (Huu et al. 1999) is a facultative mixotrophic iron oxidizer (K. J. Edwards, unpublished data), as are other members of this genera (Antunes et al. 2007). Marinobacter and related genera occur widely throughout the water column and in the deep ocean, and are commonly associated with hydrothermal plumes (Kaye et al. 2004) and marine show (Balzano et al. 2008).
Materials and methods
Siderophore isolation from Marinobacter aquaeolei
M. aquaeolei VT8 was purchased from the American Type Culture Collection (ATCC). Strain purity, before and after siderophore growth experiments was confirmed by multiple streaking to isolation on plates, followed by 16S rDNA sequencing to genotype the strain. For siderophore isolation, the strain was cultured in an artificial seawater medium (ASG-Fe) for approximately 2–4 days on a rotary shaker (200 rpm) (Martin et al. 2006). The cultures were harvested by centrifugation at 6,000 rpm for 30 min at 4°C. Amberlite XAD-2 resin (Aldrich) was added to the cell-free supernatant to adsorb the siderophores, after which the XAD-2 slurry was washed with doubly deionized water, and packed in a column; the siderophores were then eluted with 100% methanol. Siderophore-containing fractions were pooled and concentrated via rotary vacuum evaporation. The concentrated solution was further purified by reversed-phase high-pressure liquid chromatography (RP-HPLC) using a preparative Vydac C4 column (250 mm length × 22 mm diameter). Compounds were eluted with a linear gradient from 100% solvent A (0.05% trifluoroacetic acid (TFA) in doubly deionized water (Barnstead Nanopure II)) to 75% solvent A—25% B (0.05% TFA in acetonitrile) over 25 min. The eluent was monitored at 215 nm and peaks were collected by hand. Collected fractions were concentrated under reduced pressure and lyophilized.
To separate petrobactin-(SO3)2 from petrobactin-SO3, the concentrated sample solution was applied to a semi-preparative Vydac C18 monomeric column. Compounds were eluted with a linear gradient from 100% solvent A (0.05% trifluoroacetic acid in dd H2O (Barnstead Nanopure II)) to 77% solvent A—23% B (0.05% trifluoroacetic acid in methanol) over 23 min. All collected fractions were concentrated under reduced pressure and lyophilized.
Mass spectrometry
Electrospray ionization mass spectrometry (ESI-MS) and tandem mass spectrometry using argon as a collision gas were carried out on a Micromass Q-TOF2 (Waters Corp.). The same instrument, calibrated with peptide standards, was used to determine the exact mass of these siderophores.
Nuclear-magnetic resonance (NMR) spectroscopy
1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Varian Unity INOVA 500 MHz instrument using either dimethylsulfoxide (d6-DMSO, 99.9% Cambridge Isotopes, Inc.) or methanol-D4 (D, 99.8%, Cambridge Isotopes, Inc.) as the solvent. NMR spectra of petrobactin, and petrobactin sulfonate, isolated from M. aquaeolei VT8 were compared to previously published spectra of these siderophores (Barbeau et al. 2002; Bergeron et al. 2003; Hickford et al. 2004).
Results
M. aquaeolei VT8 produces three siderophores with the exact mass values of m/z 879.2758 (M + H)+, m/z 799.3183 (M + H)+, and m/z 719.3640 (M + H)+. The masses are consistent with the siderophores petrobactin disulfonate (petrobactin-(SO3)2; C34H50N6O17S2; Δ 1.3 ppm), which is a new siderophore that has not been previously reported, petrobactin sulfonate (petrobactin-SO3; C34H50N6O14S; Δ 0.6 ppm), and petrobactin; C34H50N6O11; Δ 3.4 ppm), respectively.
Two of the petrobactin siderophores, petrobactin (1) and petrobactin-SO3 (2) were isolated previously from Marinobacter hydrocarbonoclasticus (Hickford et al. 2004; Bergeron et al. 2003). Petrobactin and petrobactin-SO3 isolated here from M. aquaeolei were established by 1H NMR (Table 1) and tandem mass spectrometry (see Supplementary Information) and compared to previously published data (Hickford et al. 2004); (Bergeron et al. 2003).
Table 1.
1H- and 13C-NMR chemical shifts for petrobactin-SO3 and petrobactin from M. aquaeolei VT8 in d6-DMSO
 | ||||
|---|---|---|---|---|
| Position | Petrobactin-SO3 (this work) δH (J(Hz)) |
Petrobactin-SO3 (Hickford et al. 2004) δH (J(Hz)) | Petrobactin (this work) δH (J(Hz)) |
Petrobactin (Bergeron et al. 2003) δH (J(Hz)) |
| 1 | na | na | na | na |
| 1′ | na | na | (1 = 1′) | (1 = 1′) |
| 2 | 7.26 d (2.0) | 7.27 d (2.0) | 7.27d (2.0) | 7.28 d (2.1) |
| 2′ | na | na | (2 = 2′) | (2 = 2′) |
| 3 | na | na | na | na |
| 3′ | na | na | (3 = 3′) | (3 = 3′) |
| 4 | na | na | na | na |
| 4′ | na | na | (4 = 4′) | (4 = 4′) |
| 5 | 6.76 d (8.5) | 6.76 d (8.5) | 6.76 d (8.0) | 6.76 d (8.3) |
| 5′ | 6.76 d (8.5) | 6.76 d (8.5) | (5 = 5′) | (5 = 5′) |
| 6 | 7.17 dd (2.0, 8.0) | 7.18 dd (2.0, 8.5) | 7.18 dd (2.0, 8.0) | 7.18 |
| 6′ | 6.71 d (8.0) | 6.72 d (8.5) | (6 = 6′) | (6 = 6′) |
| 7 | na | na | na | na |
| 7′ | na | na | (7 = 7′) | (7 = 7′) |
| 8, 8′ | 3.27 | 3.27 | 3.28 | 3.28 |
| 9 | 1.79 | 1.80 | 1.80 | 1.80 |
| 9′ | 1.79 | 1.80 | (9 = 9′) | (9 = 9′) |
| 10 | 2.89 | 2.89 | 2.89 | 2.90 |
| 10′ | 3.12 | 3.12 | (10 = 10′) | (10 = 10′) |
| 11, 11′ | 2.89 | 2.89 | 2.89 | 2.90 |
| 12, 12′ | 1.56 | 1.56 | 1.42 | 1.42 |
| 13, 13′ | 1.43 | 1.43 | 1.55 | 1.55 |
| 14, 14′ | 3.03 | 3.04 | 3.04 | 3.04 |
| 15, 15′ | na | na | na | na |
| 16, 16′ | 2.50 | 2.50 d (15) | 2.50/ 2.59 | 2.50/ 2.59 |
| 2.57 dd (4.5, 15) | 2.58 dd (4, 15) | d (15) | d (14.3) | |
| 17 | na | na | na | na |
| 18 | na | na | na | na |
| N1 | 8.30 t (6) | 8.31 t (6) | 8.31 t (6.0) | 8.30 t (5.7) |
| N1′ | 8.18 t (6) | 8.19 t (6) | (N1 = N1′) | (N1 = N1′) |
| N2 | 8.21 | 8.27 | 8.37 | 8.37 |
| N2′ | 8.19 | 8.23 | (N2 = N2′) | (N2 = N2′) |
| N3, N3′ | 7.99 t (5.5) | 7.99 t (5) | 7.99 t (6.0) | 7.89 t (5.6) |
| OH(3) | 9.12 | 9.12 | 9.12 | 9.10 |
| OH (3′) | 11.22 s | 11.23 s | (OH3 = OH3′) | (OH3 = OH3′) |
| OH (4, 4′) | 9.16, 9.53 | 9.16, 9.54 | 9.55 | 9.53 |
Atom numbering is shown in (1) and (2) above
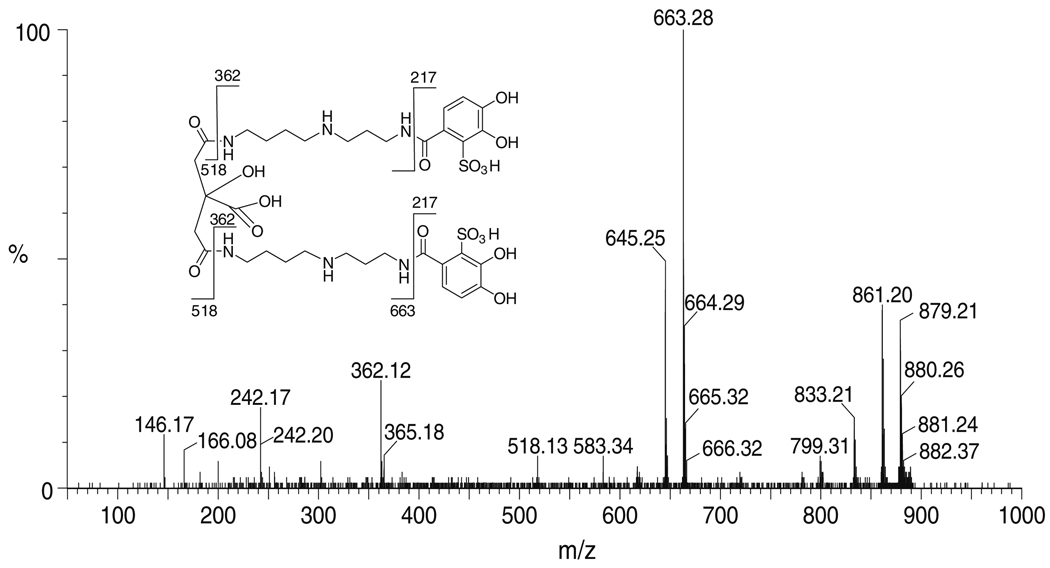
The siderophore with the m/z of 879, which is consistent with disulfonated petrobactin (3), is produced in very small quantities and was characterized by tandem mass spectrometry (Fig. 3). The expected daughter ions for petrobactin-(SO3)2 are m/z 663, m/z 518, m/z 362, and m/z 217). Most of these fragments are observed for this siderophore. The fragment with m/z 217 is not observed, probably due to the negative charge that the sulfonic acid group carries. A loss of 18 mass units is commonly seen in the citrate-containing fragments, such as m/z 663 and m/z 645, as well as in petrobactin-(SO3)2 at m/z 879 and m/z 861. This loss can be attributed to the formation of a five-membered ring between the carboxylic acid of the citrate moiety and the adjacent amide (Bergeron et al. 2003). Further experiments are in progress to obtain more petrobactin-(SO3)2 for further characterization and determination of the stability constants. Additionally the time-dependence of mono- and di-sulfonation during the growth of the culture is of interest, as is the biosynthetic pathway for sulfonation.
Fig. 3.
Tandem mass spectrum of petrobactin-(SO3)2. Inset: Structure of petrobactin-(SO3)2 showing the “y” and “b” fragmentation (Roepstorff and Fohlmann 1984) from the tandem mass spectrum
Discussion
In summary, M. aquaeolei strain VT8 produces three siderophores, petrobactin-(SO3)2, petrobactin-SO3, and petrobactin. To our knowledge, this is the first report of petrobactin-(SO3)2. The production of petrobactin and petrobactin-SO3 had previously been found in another Marinobacter species, M. hydrocarbonoclasticus (Barbeau et al. 2002; Hickford et al. 2004). Petrobactin production along with bacillibactin has been reported for Bacillus anthracis str. Stern (Koppisch et al. 2005). In the case of Bacillus anthracis, neither petrobactin-SO3, petrobactin-(SO3)2 nor glycosylated bacillibactin have been reported. The biosynthetic genes of petrobactin are well described (Wilson et al. 2006; Abergel et al. 2006), and the predicted genes for petrobactin synthesis in the marinobacter genome are present. Further genetic and biochemical investigations are in progress to investigate whether Marinobacter aquaeolei is making other siderophores in addition to the suite of petrobactins, as well as the biosynthetic pathway for sulfonation.
The sulfonate functionality in an aromatic ring is rarely found in microbial natural products. In most cases O-sulfonation of the oxygenated benzene ring is observed (Strott 2002). Aromatic C-sulfonation in natural products has previously been reported in the dihydropyroverdins produced by Pseudomonas sp. 267 (Budzikiewicz et al. 1998), pseudoalterobactin A and B produced by Pseudoalteromonas sp. KP20-4 (Kanoh et al. 2003), aeruginosin B produced by Pseudomonas aeruginosa (Herbert and Holliman 1969); (Bentley and Holliman 1970), as well as petrobactin sulfonate produced by M. hydrocarbonoclasticus (Hickford et al. 2004). Except for aeruginosin B, the sulfonate group in the natural products mentioned above, is found adjacent to a hydroxyl group. By analogy to the first step in the Bucherer reaction, Budzikiewicz has postulated the formation of these products via attack of hydrogen sulfite on the aromatic ring (Budzikiewicz 2006). However, to our knowledge, bacterial enzymes that catalyze C-sulfonation have not been reported. Sulfonation of the aromatic ring would increase the water solubility of aromatic compounds. Sulfonation might also lead to stabilization of the catechol ring against oxidation, and affect the Fe(III) stability constant.
Supplementary Material
Acknowledgments
Support from the National Institutes of Health GM38130 is gratefully acknowledged.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10534-009-9237-0) contains supplementary material, which is available to authorized users.
Contributor Information
Vanessa V. Homann, Department of Chemistry and Biochemistry, University of California, Santa Barbara, CA 93106-9510, USA
Katrina J. Edwards, Department of Biological Sciences, Marine Environmental Biology Section, University of Southern California, Los Angeles, CA 90089-0371, USA Department of Earth Sciences, University of Southern California, Los Angeles, CA 90089-0371, USA.
Eric A. Webb, Department of Biological Sciences, Marine Environmental Biology Section, University of Southern California, Los Angeles, CA 90089-0371, USA Department of Earth Sciences, University of Southern California, Los Angeles, CA 90089-0371, USA.
Alison Butler, Email: butler@chem.ucsb.edu, Department of Chemistry and Biochemistry, University of California, Santa Barbara, CA 93106-9510, USA.
References
- Abergel RJ, Wilson MK, Arceneaux JEL, Hoette TM, Strong RK, Byers BR, Raymond KN. Anthrax pathogen evades the mammalian immune system through stealth siderophore production. Proc Natl Acad Sci USA. 2006;103:18499–18503. doi: 10.1073/pnas.0607055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin SA, Küpper FC, Green DH, Harris WR, Carrano CJ. Boron binding by a siderophore isolated from marine bacteria associated with the toxic dinoflagellate G. catenatum. J Am Chem Soc. 2007;129:478–479. doi: 10.1021/ja067369u. [DOI] [PubMed] [Google Scholar]
- Antunes A, França L, Rainey FA, Huber R, Nobre MF, Edwards KJ, da Costa MS. Marinobacter halosydne sp. nov., a novel species from the brine-seawater interface of the Shaban Deep, Red Sea. Int J Syst Evol Microbiol. 2007;57:1035–1040. doi: 10.1099/ijs.0.64862-0. [DOI] [PubMed] [Google Scholar]
- Balzano S, Statham PJ, Lloyd JR, Pancost RD. Role of microbial populations in the release of reduced iron to the water column from marine aggregates. Abstracts of the 12th international symposium on microbial ecology, Cairns; Australia. 2008. [Google Scholar]
- Barbeau K, Rue EL, Bruland KW, Butler A. Photochemical cycling of iron in the surface ocean mediated by microbial iron(III)-binding ligands. Nature. 2001;413:409–413. doi: 10.1038/35096545. [DOI] [PubMed] [Google Scholar]
- Barbeau K, Zhang G, Live DH, Butler A. Petrobactin, a photoreactive siderophore produced by the oil-degrading marine bacterium Marinobacter hydrocarbonoclasticus. J Am Chem Soc. 2002;124:378–379. doi: 10.1021/ja0119088. [DOI] [PubMed] [Google Scholar]
- Barbeau K, Rue EL, Trick CG, Bruland KW, Butler A. The photochemical reactivity of siderophores produced by marine heterotrophic bacteria and cyanobacteria based on characteristic iron(III)-binding groups. Limnol Oceanogr. 2003;48:1069–1078. [Google Scholar]
- Bentley RK, Holliman FG. Pigments of Pseudomonas species. 3. Synthesis of demethylaeruginosin-B and aeruginosin-B. J Chem Soc C-Org. 1970;18:2447. doi: 10.1039/j39700002447. [DOI] [PubMed] [Google Scholar]
- Bergeron RJ, Huang G, Smith RE, Bharti N, McManis JS, Butler A. Total synthesis and structure revision of petrobactin. Tetrahedron. 2003;59:2007–2014. [Google Scholar]
- Bister B, Bischoff D, Nicholson GJ, Valdebenito M, Schneider K, Winkelmann G, Hantke K, Süssmuth RD. The structure of salmochelins: C-glucosylated enterobactins of Salmonella enterica. Biometals. 2004;17:471–481. doi: 10.1023/b:biom.0000029432.69418.6a. [DOI] [PubMed] [Google Scholar]
- Budzikiewicz H. Bacterial aromatic sulfonates-a Bucherer reaction in nature? Mini-Rev Org Chem. 2006;3:93–97. [Google Scholar]
- Budzikiewicz H, Fuchs R, Taraz K, Marek-Kozuczuk M, Slorupska A. Dihydropyoverdin-7-sulfonic acids—unusual bacterial metabolites. Nat Prod Lett. 1998;12:125–130. [Google Scholar]
- Butler A. Marine siderophores and microbial iron mobilization. Biometals. 2005;18:369–374. doi: 10.1007/s10534-005-3711-0. [DOI] [PubMed] [Google Scholar]
- Haygood MG, Holt PD, Butler A. Aerobactin production by a planktonic marine Vibrio sp. Limnol Oceanogr. 1993;38:1091–1097. [Google Scholar]
- Herbert RB, Holliman FG. Pigments of Pseudomonas species. 2. Structure of aeruginosin B. J Chem Soc C-Org. 1969;18:2517–2520. doi: 10.1039/j39690002517. [DOI] [PubMed] [Google Scholar]
- Hickford SJH, Küpper FC, Zhang G, Carrano CJ, Blunt JW, Butler A. Petrobactin sulfonate, a new siderophore produced by the marine bacterium Marinobacter hydrocarbonoclasticus. J Nat Prod. 2004;67:1897–1899. doi: 10.1021/np049823i. [DOI] [PubMed] [Google Scholar]
- Homann VV, Sandy M, Tincu A, Templeton A, Tebo B, Butler A. Loihichelins A–F, a suite of amphiphilic siderophores produced by the marine bacterium halomonas LOB-5. J Nat Prod. 2009;72 doi: 10.1021/np800640h. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu NB, Denner EBM, Ha DTC, Wanner G, Stan-Lotter H. Marinobacter aquaeolei sp. nov., a halophilic bacterium isolated from a Vietnamese oil producing well. Int J Syst Bacteriol. 1999;49:367–375. doi: 10.1099/00207713-49-2-367. [DOI] [PubMed] [Google Scholar]
- Ito Y, Butler A. Structure of synechobactins, new siderophores of the marine cyanobacterium Synechococcus sp. PCC 7002. Limnol Oceanogr. 2005;50:1918–1923. [Google Scholar]
- Kanoh K, Kamino K, Leleo G, Adachi K, Shizuri YJ. Pseudoalterobactin A and B, new siderophores excreted by marine bacterium Pseudoalteromonas sp KP20–4. J Antibiot. 2003;56:871–875. doi: 10.7164/antibiotics.56.871. [DOI] [PubMed] [Google Scholar]
- Kaye JZ, Marquez MC, Ventosa A, Baross JA. Halomonas neptunia sp. nov., Halomonas sulfidaeris sp. nov., Halomonas axialensis sp. nov. and Halomonas hydrothermalis sp. nov.: halophilic bacteria isolated from deep-sea hydrothermal-vent environments. Int J Syst Evol Microbiol. 2004;54:499–511. doi: 10.1099/ijs.0.02799-0. [DOI] [PubMed] [Google Scholar]
- Koppisch AT, Browder CC, Moe AL, Shelley JT, Kinkel BA, Hersman LE, Iyer S, Ruggiero CE. Petrobactin is the primary siderophore synthesized by Bacillus anthracis str. Sterne under conditions of iron starvation. Biometals. 2005;18:577–585. doi: 10.1007/s10534-005-1782-6. [DOI] [PubMed] [Google Scholar]
- Küpper FC, Carrano CJ, Kuhn J-U, Butler A. Photoreactivity of iron(III)-aerobactin: photoproduct structure and iron(III) coordination. Inorg Chem. 2006;45:6026–6033. doi: 10.1021/ic0604967. [DOI] [PubMed] [Google Scholar]
- Martin JD, Ito Y, Homann VV, Haygood MG, Butler A. Structure and membrane affinity of new amphiphilic siderophores produced by Ochrobactrum sp. SP18. J Biol Inorg Chem. 2006;11:633–641. doi: 10.1007/s00775-006-0112-y. [DOI] [PubMed] [Google Scholar]
- Martinez JS, Zhang GP, Holt PD, Jung H-T, Carrano CJ, Haygood MG, Butler A. Self-assembling amphiphilic siderophores from marine bacteria. Science. 2000;287:1245–1247. doi: 10.1126/science.287.5456.1245. [DOI] [PubMed] [Google Scholar]
- Martinez JS, Carter-Franklin JN, Mann EL, Martin JD, Haygood MG, Butler A. Structure and membrane affinity of a suite of amphiphilic siderophores produced by a marine bacterium. Proc Natl Acad Sci USA. 2003;100:3754–3759. doi: 10.1073/pnas.0637444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RT, Live DH, Faulkner DJ, Butler A. A siderophore from a marine bacterium with an exceptional ferric ion affinity constant. Nature. 1993;366:455–458. doi: 10.1038/366455a0. [DOI] [PubMed] [Google Scholar]
- Roepstorff P, Fohlmann J. Proposal for a common nomenclature for sequence ions in mass-spectra of peptides. Biomed Mass Spectrom. 1984;11:601. doi: 10.1002/bms.1200111109. [DOI] [PubMed] [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical-assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Strott CA. Sulfonation and molecular action. Endocr Rev. 2002;23:703–732. doi: 10.1210/er.2001-0040. [DOI] [PubMed] [Google Scholar]
- Wilson MK, Abergel RJ, Raymond KN, Arceneaux JEL, Byers BR. Siderophores of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. Biochem Biophys Res Comm. 2006;348:320–325. doi: 10.1016/j.bbrc.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Xu G, Martinez JS, Groves JT, Butler A. Membrane affinity of the amphiphilic marinobactin siderophores. J Am Chem Soc. 2002;124:13408–13415. doi: 10.1021/ja026768w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.