Abstract
Platelets are dynamic cells with activities that extend beyond thrombosis including an important role in initiating and sustaining vascular inflammation. A role for platelets has been described in many physiologic and pathophysiologic processes such as atherosclerosis, stem cell trafficking, tumor metastasis, and arthritis. Platelet activation at sites of an intact inflamed endothelium contributes to vascular inflammation and vascular wall remodeling. Platelets secrete a wide array of preformed and synthesized inflammatory mediators upon activation that can exert significant local and systemic effects. This review will focus on the role of platelet derived mediators in vascular inflammation and vascular wall remodeling.
Introduction
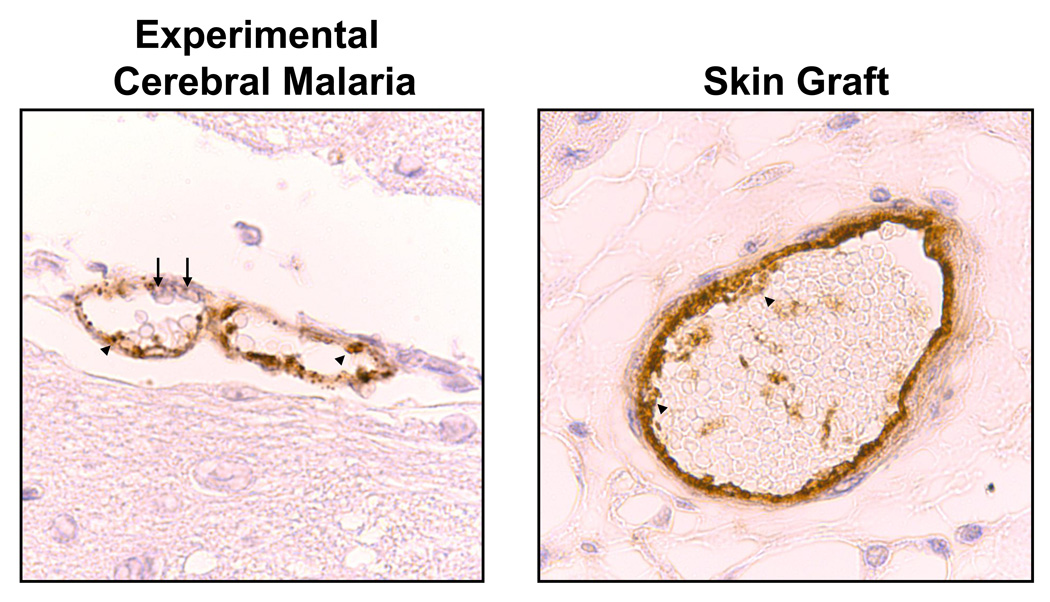
Hemostasis is critical to survival and platelets have many redundant pathways to ensure that activation occurs only when necessary. Platelets can be activated by injury to the vessel wall, activation of the coagulation cascade or by activating factors released from stimulated endothelial cells and platelets (e.g. ADP, thromboxane, von Willebrand Factor). Activation of resting platelets triggers a variety of intra-platelet events including exocytosis of granules, secretion of vasoactive mediators, and conformational changes in receptors (e.g. GP IIb/IIIa). The release of vasoactive mediators also leads to the elaboration of a pro-inflammatory environment within a developing thrombus that can impact local changes in the vessel wall. Platelet functions are most commonly associated with a vessel that has lost its endothelial cell layer, but platelets also interact with an intact inflamed endothelium in either a stable or transient manner leading to the secretion of platelet derived vasoactive mediators. Platelets have been found forming non-occlusive thrombi in many models of vascular inflammation including transplant rejection and cerebral malaria (1, 2) (Figure 1) and platelet derived mediators have also been found in the subendothelial vessel wall (3).
Figure 1.
Non-Occlusive thrombi are present within cerebral vessels of mice with experimental cerebral malaria and within vessels of skin grafts during rejection (immunohistochemical staining for vWF). Arrowheads indicate vWF rich thrombi and arrows enmeshed leukocytes.
This review will focus on platelet derived inflammatory mediator interactions with the vessel wall and how these mediators can lead to changes in the vessel wall. Platelet secreted immune mediators can be broadly classified as either preformed or produced upon stimulation. Platelets have 3 types of granules released in a regulated manner upon stimulation that contain stored proteins and small molecules, many of which have major roles in vascular inflammation (4). Dense granules store small molecules such as ATP, ADP, serotonin, glutamate, and polyphosphates. Alpha granules contain an extensive list of proteins that have important roles in both thrombosis and inflammation. A short list includes Platelet Factor 4 (PF4/CXCL4), β-thromboglobulin (NAP-2/CXCL7 as the active breakdown product), RANTES (CCL5), IL-1α and IL-1β, TGF-β and TNF-α. Each has been shown to have important effects on their own in vascular inflammation, but because they are released in combination at the site of injury or endothelial inflammation, even greater effects then described experimentally are likely to occur in vivo.
To discuss the impact of a few platelet derived mediators on the vessel wall we will divide them into small molecules, prostaglandins, and chemokines/cytokines.
Small Molecules
Small vasoactive molecules are primarily found in dense granules. Serotonin (5-HT) has been recognized as a platelet derived product for many decades with numerous studies in the 1950s noting its role as a platelet derived vasoconstrictor (5, 6). Platelets are the source of circulating serotonin, yet megakaryocytes do not have the enzyme necessary to produce serotonin (tryptophan hydroxylase, Tph). Instead platelets take up and store serotonin from its synthesis site in the duodenum (7). There are 2 isoforms of Thp, Tph-1 and Tph-2, with only Tph-1 expressed outside the brain. Thp-1−/− mice are viable and specifically lack serotonin in the periphery (8). Serotonin has little effect on platelet aggregation and activation on its own, but it can amplify platelet activation when combined with other weak agonists (8). Serotonin’s main peripheral effects may be in its interactions with the vessel wall. Serotonin has well described mitogenic effects on vascular smooth muscle cells (VSMC), best described in pulmonary arterial hypertension (PAH) (9). Vascular smooth muscle cells express multiple receptor subtypes for serotonin (5-HT1DB, 5-HT2A, 5-HT2B, 5-HT4, 5-HT7) and serotonin mediated VSMC proliferation may be driven by G protein signaling and reactive oxygen species (ROS) signal transduction pathways (9). In addition to proliferative effects, 5-HT has also been shown to be pro-inflammatory to VSMC by driving IL-6 production (10).
The release of nucleotides such as ATP and ADP also has important roles in vascular remodeling. Prolonged exposure to ADP and ATP has been linked to vascular changes associated with accelerated graft arteriosclerosis (AGA), atherosclerosis, and hypertension (11, 12). VSMCs express the ATP responsive P2X1 receptor and several P2Y subtypes (P2Y2,4,6) (13). P2Y receptor signaling mediates an increase in VSMC constriction, proliferation and inflammatory responses (14). Treatment with receptor blockers such as clopidogrel can reduce atherosclerotic lesion progression (15). This may be the result of a combined effect of clopidogrel on both platelets and VSMC.
Thromboxane and Prostaglandins
Aspirin is one of the most commonly used drugs in the world and is a first line drug in the treatment of patients at risk for cardiovascular events. Aspirin irreversibly acetylates cyclooxgenase (COX) and blocks its activity. COX is expressed in many inflammatory cells, but platelets are a major source of COX products in the vasculature. Platelets constitutively express COX-1 and during times of prolonged inflammation can express COX-2 (16). Thromboxane is the major platelet derived COX product with lesser amounts of prostaglandins such as PGE2 and PGI2 also produced. Platelets are the central source of TxA2 in myocardial ischemia with little contribution from leukocytes and the vasculature (17). Platelet deposition at the vessel wall results in the local elaboration of high concentrations of thromboxane that can have effects on VSMC. VSMC express thromboxane receptors (TP) and thromboxane induces VSMC contraction, ROS production, cell proliferation, and hypertrophy (18, 19). PGE2 has similar effects and COX-1 products accelerate atherogenesis in mice (20). Our work has demonstrated that platelet derived glutamate can induce low level COX activation through platelet kainate receptors contributing to amplified platelet activation (21). Studies such as these indicate that platelet COX-1 activation may have a potent role in vascular remodeling. However, without the use of platelet specific COX-1 knockout mice this is difficult to directly demonstrate.
Platelet Chemokines/Cytokines
The activation of platelets leads to the release of more than a dozen chemokines, most of which belong to the CC (e.g. CCL5, regulated upon activation, normal T-cell expressed, and secreted, RANTES) or CXC (e.g. CXCL4/platelet factor 4/PF4) chemokine families. In particular, platelet derived PF4, CXCL7, and IL-1 have important effects in vascular inflammation that have received recent attention.
CXCL7 and CXCL4 are found in very high concentrations in platelet releasates. There are several molecular variants of CXCL7 all of which are derived from the proteolysis from a single 128aa precursor called pre-platelet basic protein (PPBP) (22). The cleavage of the 34-residue leader sequence produces PBP (94aa) and further truncation results in connective tissue activating protein III (CTAP-III, 85aa), β-thromboglobulin (81aa), and neutrophil activating peptide 2 (NAP-2/CXCL7, 70aa) (23). CXCL7 binds to CXCR1 and CXCR2 and induces neutrophil adhesion and migration in vitro (24). CXCL7 may also be responsible for neutrophil accumulation around vascular tissues in combination with activated mast cells (25). However, a more broad appreciation of potentially important roles for this abundant platelet chemokine in vascular inflammation is currently lacking.
PF4 was the first described CXC class chemokine (23). The identity of PF4 receptors and its signaling is still not completely resolved or understood. The addition of free GAG chains or the removal of cell surface GAG abolishes PF4 monocyte binding and signaling (26). Proteoglycans may serve as a functional receptor for PF4, or similar to other chemokines, GAGs may allow PF4 to become localized and facilitate its binding to a chemokine receptor. An alternative splice variant of CXCR3, CXCR3B, has been reported to be a receptor for PF4 on endothelial cells and activated T lymphocytes (27). However, a more recent study demonstrated that PF4 may signal through both CXCR3A and CXCR3B (28). The described functions of PF4 can be equally confusing including both pro and anti- inflammatory and pro and anti-proliferation (29, 30). In vitro, PF4 inhibits T-cell proliferation and cytokine release (31) and PF4 has reported anti-proliferation and angiogenesis activity on endothelial cells (32). Using an in vivo mouse model of cerebral malaria we have shown that PF4 is pro-inflammatory in part by driving increased T-cell CXCR3 expression and trafficking to the brain and INF-γ production, but had no effect on T-cell proliferation (1). Using this model we have also demonstrated that PF4 induced monocyte activation and trafficking in a Kruppel Like Factor 4 (KLF4) transcription factor dependent manner (33). Others have shown that PF4−/− mice crossed with ApoE−/− mice have reduced atherosclerotic lesions compared to control ApoE−/− mice and PF4 is found within the vessel wall at lesion sites (3). PF4-RANTES complexes also appear to be very important to this process (29). These seemingly disparate effects of PF4, pro and anti inflammatory/proliferation, are likely the result of the different model systems used and in vitro versus in vivo effects, reflecting the complex nature of PF4 interactions with other molecules and cells in the vasculature. Direct effects of PF4 on VSMC has not been explored, but may also be important in atherosclerosis progression, particularly in humans where there is more VSMC proliferation and inflammation compared to mouse models.
Platelet derived IL-1 has recently received significant attention in the literature. Platelets store both IL-1α and IL-1β and mild stimulation leads to the translation of more platelet IL-1β (34). Platelet derived microparticles have been identified as mediators of joint inflammation associated with arthritis in a platelet IL-1α dependent manner (35) and platelet IL-1α also drives cerebrovascular inflammation (36). IL-1 and its receptors are increased in atherosclerotic tissue and increased plasma IL-1 is associated with increased risk of atherosclerosis (37). Platelet interactions at the site of lesion development may be an important source of IL-1. IL-1 can drive vascular smooth muscle proliferation, endothelial adhesion molecule expression and cell trafficking all of which are important steps in the pathogenesis of atherosclerosis (38, 39).
Summary
Platelets are dynamic cells with many important functions ‘beyond the clot’. Platelets may exert great effects on the vessel wall in acute and chronic inflammatory diseases. A more complete understanding of these functions is a work in progress.
Acknowledgments
Funding Sources: R01HL093179, R01HL093179-02S109 and R01HL094547 to CNM
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Srivastava K, Cockburn IA, Swaim A, Thompson LE, Tripathi A, Fletcher CA, et al. Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe. 2008 Aug 14;4(2):179–187. doi: 10.1016/j.chom.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrell CN, Murata K, Swaim AM, Mason E, Martin TV, Thompson LE, et al. In vivo platelet-endothelial cell interactions in response to major histocompatibility complex alloantibody. Circ Res. 2008 Apr 11;102(7):777–785. doi: 10.1161/CIRCRESAHA.107.170332. [DOI] [PubMed] [Google Scholar]
- 3.Sachais BS, Turrentine T, Dawicki McKenna JM, Rux AH, Rader D, Kowalska MA. Elimination of platelet factor 4 (PF4) from platelets reduces atherosclerosis in C57Bl/6 and apoE−/− mice. Thromb Haemost. 2007 Nov;98(5):1108–1113. [PubMed] [Google Scholar]
- 4.Morrell CN, Matsushita K, Chiles K, Scharpf RB, Yamakuchi M, Mason RJ, et al. Regulation of platelet granule exocytosis by S-nitrosylation. Proc Natl Acad Sci U S A. 2005 Mar 8;102(10):3782–3787. doi: 10.1073/pnas.0408310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bracco M, Curti PC. The vasoconstrictor factor of platelets. Experientia. 1954 Feb 15;10(2):71–72. doi: 10.1007/BF02161461. [DOI] [PubMed] [Google Scholar]
- 6.Zucker MB, Friedman BK, Rapport MM. Identification and quantitative determination of serotonin (5-hydroxytryptamine) in blood platelets. Proc Soc Exp Biol Med. 1954 Feb;85(2):282–285. doi: 10.3181/00379727-85-20855. [DOI] [PubMed] [Google Scholar]
- 7.Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008 Nov 28;135(5):825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003 Dec 26;115(7):851–862. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- 9.Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, et al. Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension. Circ Res. 2004 May 14;94(9):1263–1270. doi: 10.1161/01.RES.0000126847.27660.69. [DOI] [PubMed] [Google Scholar]
- 10.Ito T, Ikeda U, Shimpo M, Yamamoto K, Shimada K. Serotonin increases interleukin-6 synthesis in human vascular smooth muscle cells. Circulation. 2000 Nov 14;102(20):2522–2527. doi: 10.1161/01.cir.102.20.2522. [DOI] [PubMed] [Google Scholar]
- 11.Hechler B, Freund M, Ravanat C, Magnenat S, Cazenave JP, Gachet C. Reduced atherosclerotic lesions in P2Y1/apolipoprotein E double-knockout mice: the contribution of non-hematopoietic-derived P2Y1 receptors. Circulation. 2008 Aug 12;118(7):754–763. doi: 10.1161/CIRCULATIONAHA.108.788927. [DOI] [PubMed] [Google Scholar]
- 12.Davies MG, Ramkumar V, Gettys TW, Hagen PO. The expression and function of G-proteins in experimental intimal hyperplasia. J Clin Invest. 1994 Oct;94(4):1680–1689. doi: 10.1172/JCI117513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris GE, Nelson CP, Everitt D, Brighton PJ, Standen NB, Challiss RA, et al. G protein-coupled receptor kinase 2 and arrestin2 regulate arterial smooth muscle P2Y-purinoceptor signalling. Cardiovasc Res. 2010 Aug 21; doi: 10.1093/cvr/cvq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wihlborg AK, Wang L, Braun OO, Eyjolfsson A, Gustafsson R, Gudbjartsson T, et al. ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler Thromb Vasc Biol. 2004 Oct;24(10):1810–1815. doi: 10.1161/01.ATV.0000142376.30582.ed. [DOI] [PubMed] [Google Scholar]
- 15.Hermann A, Weber AA, Schror K. Clopidogrel inhibits platelet adhesion and platelet-dependent mitogenesis in vascular smooth muscle cells. Thromb Res. 2002 Jan 15;105(2):173–175. doi: 10.1016/s0049-3848(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 16.Belton OA, Duffy A, Toomey S, Fitzgerald DJ. Cyclooxygenase isoforms and platelet vessel wall interactions in the apolipoprotein E knockout mouse model of atherosclerosis. Circulation. 2003 Dec 16;108(24):3017–3023. doi: 10.1161/01.CIR.0000104565.78013.AD. [DOI] [PubMed] [Google Scholar]
- 17.Schror K. The effect of prostaglandins and thromboxane A2 on coronary vessel tone--mechanisms of action and therapeutic implications. Eur Heart J. 1993 Nov;14 Suppl I:34–41. [PubMed] [Google Scholar]
- 18.Ali S, Davis MG, Becker MW, Dorn GW., 2nd Thromboxane A2 stimulates vascular smooth muscle hypertrophy by up-regulating the synthesis and release of endogenous basic fibroblast growth factor. J Biol Chem. 1993 Aug 15;268(23):17397–17403. [PubMed] [Google Scholar]
- 19.Zhang M, Dong Y, Xu J, Xie Z, Wu Y, Song P, et al. Thromboxane receptor activates the AMP-activated protein kinase in vascular smooth muscle cells via hydrogen peroxide. Circ Res. 2008 Feb 15;102(3):328–337. doi: 10.1161/CIRCRESAHA.107.163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pratico D, Tillmann C, Zhang ZB, Li H, FitzGerald GA. Acceleration of atherogenesis by COX-1-dependent prostanoid formation in low density lipoprotein receptor knockout mice. Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):3358–3363. doi: 10.1073/pnas.061607398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun H, Swaim A, Herrera JE, Becker D, Becker L, Srivastava K, et al. Platelet Kainate Receptor Signaling Promotes Thrombosis by Stimulating Cyclooxygenase Activation. Circ Res. 2009 Aug 13; doi: 10.1161/CIRCRESAHA.109.198861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt JC, Rabellino EM, Gewirtz AM, Gunkel LM, Rucinski B, Niewiarowski S. Occurrence of platelet basic protein, a precursor of low affinity platelet factor 4 and beta-thromboglobulin, in human platelets and megakaryocytes. Exp Hematol. 1988 May;16(4):302–306. [PubMed] [Google Scholar]
- 23.Brandt E, Petersen F, Ludwig A, Ehlert JE, Bock L, Flad HD. The beta-thromboglobulins and platelet factor 4: blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J Leukoc Biol. 2000 Apr;67(4):471–478. doi: 10.1002/jlb.67.4.471. [DOI] [PubMed] [Google Scholar]
- 24.Schenk BI, Petersen F, Flad HD, Brandt E. Platelet-derived chemokines CXC chemokine ligand (CXCL)7, connective tissue-activating peptide III, and CXCL4 differentially affect and cross-regulate neutrophil adhesion and transendothelial migration. J Immunol. 2002 Sep 1;169(5):2602–2610. doi: 10.4049/jimmunol.169.5.2602. [DOI] [PubMed] [Google Scholar]
- 25.Schiemann F, Grimm TA, Hoch J, Gross R, Lindner B, Petersen F, et al. Mast cells and neutrophils proteolytically activate chemokine precursor CTAP-III and are subject to counterregulation by PF-4 through inhibition of chymase and cathepsin G. Blood. 2006 Mar 15;107(6):2234–2242. doi: 10.1182/blood-2005-06-2424. [DOI] [PubMed] [Google Scholar]
- 26.Kasper B, Brandt E, Ernst M, Petersen F. Neutrophil adhesion to endothelial cells induced by platelet factor 4 requires sequential activation of Ras, Syk, and JNK MAP kinases. Blood. 2006 Mar 1;107(5):1768–1775. doi: 10.1182/blood-2005-06-2501. [DOI] [PubMed] [Google Scholar]
- 27.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003 Jun 2;197(11):1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller A, Meiser A, McDonagh EM, Fox JM, Petit SJ, Xanthou G, et al. CXCL4-induced migration of activated T lymphocytes is mediated by the chemokine receptor CXCR3. J Leukoc Biol. 2008 Apr;83(4):875–882. doi: 10.1189/jlb.1006645. [DOI] [PubMed] [Google Scholar]
- 29.Koenen RR, von Hundelshausen P, Nesmelova IV, Zernecke A, Liehn EA, Sarabi A, et al. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat Med. 2009 Jan;15(1):97–103. doi: 10.1038/nm.1898. [DOI] [PubMed] [Google Scholar]
- 30.von Hundelshausen P, Petersen F, Brandt E. Platelet-derived chemokines in vascular biology. Thromb Haemost. 2007 May;97(5):704–713. doi: 10.1160/th07-01-0066. [DOI] [PubMed] [Google Scholar]
- 31.Romagnani P, Maggi L, Mazzinghi B, Cosmi L, Lasagni L, Liotta F, et al. CXCR3-mediated opposite effects of CXCL10 and CXCL4 on TH1 or TH2 cytokine production. J Allergy Clin Immunol. 2005 Dec;116(6):1372–1379. doi: 10.1016/j.jaci.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 32.Maione TE, Gray GS, Petro J, Hunt AJ, Donner AL, Bauer SI, et al. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science. 1990 Jan 5;247(4938):77–79. doi: 10.1126/science.1688470. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava K, Field DJ, Aggrey A, Yamakuchi M, Morrell CN. Platelet factor 4 regulation of monocyte KLF4 in experimental cerebral malaria. PLoS One. 2010;5(5):e10413. doi: 10.1371/journal.pone.0010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, et al. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005 Aug 12;122(3):379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. Jan 29;327(5965):580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thornton P, McColl BW, Greenhalgh A, Denes A, Allan SM, Rothwell NJ. Platelet interleukin-1alpha drives cerebrovascular inflammation. Blood. 2010 Apr 29;115(17):3632–3639. doi: 10.1182/blood-2009-11-252643. [DOI] [PubMed] [Google Scholar]
- 37.Dewberry R, Holden H, Crossman D, Francis S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arterioscler Thromb Vasc Biol. 2000 Nov;20(11):2394–2400. doi: 10.1161/01.atv.20.11.2394. [DOI] [PubMed] [Google Scholar]
- 38.Libby P, Warner SJ, Friedman GB. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988 Feb;81(2):487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshizumi M, Kurihara H, Morita T, Yamashita T, Oh-hashi Y, Sugiyama T, et al. Interleukin 1 increases the production of endothelin-1 by cultured endothelial cells. Biochem Biophys Res Commun. 1990 Jan 15;166(1):324–329. doi: 10.1016/0006-291x(90)91948-r. [DOI] [PubMed] [Google Scholar]