Abstract
Autophagy is a highly regulated and evolutionarily conserved process of cellular self-digestion. Recent evidence suggests that this process plays an important role in regulating T cell homeostasis. In this study, we have utilized Rag1−/− blastocyst complementation and in vitro embryonic stem (ES) cell differentiation to address the role of Beclin 1, one of the key autophagic proteins, in lymphocyte development. Beclin 1-deficient Rag 1−/− chimeras displayed a dramatic reduction in thymic cellularity compared to control mice. Using ESC differentiation in vitro, we found that the inability to maintain normal thymic cellularity is likely caused by impaired maintenance of thymocyte progenitors. Interestingly, despite drastically reduced thymocyte numbers, the peripheral T cell compartment of Beclin 1-deficient Rag 1−/− chimeras is largely normal. Peripheral T cells displayed normal in vitro proliferation despite significantly reduced numbers of autophagosomes. In addition, these chimeras had greatly reduced numbers of early B cells in the bone marrow compared to controls. However, the peripheral B cell compartment was not dramatically impacted by Beclin 1 deficiency. Collectively, our results suggest that Beclin 1 is required for maintenance of undifferentiated/early lymphocyte progenitor populations. In contrast, Beclin 1 is largely dispensable for the initial generation and function of the peripheral T and B cell compartments. This indicates that normal lymphocyte development involves Beclin 1-dependent early-stage, and distinct, Beclin 1-independent, late stage processes.
Introduction
Autophagy is essential for maintaining cellular energy homeostasis during nutritional stress, and also appears to play important roles in development and differentiation. During nutrient starvation-induced autophagy, portions of the cytoplasmic material are sequestered within double-membrane vesicles and then delivered to the lysosome for degradation. Beclin 1, the mammalian ortholog of yeast Atg6, is a critical component of a complex containing class III PI3K and other proteins, including UVRAG, Ambra-1, Bif-1, and Atg14L that stimulates autophagy by initiating the isolation membrane formation (1, 2). Beclin 1 also interacts with antiapoptotic Bcl-2 family members, Bcl-2 and Bcl-xL (3, 4), and it is believed that this interaction could represent an important link between autophagy and apoptosis. Several studies have demonstrated that Beclin 1 can affect the apoptotic pathway in different types of eukaryotic cells (5–7). In addition, Bcl-2 has been shown to inhibit Beclin 1-mediated autophagic death induced by nutrient starvation in cultured cells (4).
The analyses of several genetically modified mouse strains and early embryonic stem cell (ESC)-derived mouse embryos deficient in proteins essential for autophagy, including Beclin 1, indicate that autophagy is critical for maintaining cellular energy homeostasis and normal development (8–12). Beclin 1-deficient mice die during early embryonic development (8, 13), which prevents the analysis of Beclin 1 deficiency in specific tissues. In our previous report using Beclin 1-GFP transgenic mice, we provided evidence that Beclin 1 could be involved in T cell development in the thymus (14). In this study, we have used a Rag1 blastocyst complementation approach (15) and in vitro ESC differentiation to T cells to analyze the role of Beclin 1 in T and B cells in more detail. Our studies indicate that Beclin 1 plays an essential role in maintaining normal thymic cellularity and early B cells in the bone marrow. However it appears to be largely dispensable for initial generation and proliferation of peripheral T and B cells. The absence of Beclin 1 does not appear to cause any specific block in the development of T or B lineage cells. Rather, Beclin 1 deficiency results in impaired maintenance of lymphoid progenitors in vivo and in vitro. Our results thus reveal a selectively early role for Beclin 1 in lymphocyte development distinct from that observed for other autophagic proteins, such as Atg5 and Atg7.
Materials and Methods
Generation of Rag 1−/− chimeric mice
Rag 1−/− mice of both sexes were purchased from The Jackson Laboratory. Rag 1−/− blastocysts harvested from pregnant Rag 1−/− females (3.5 post coitum) were injected with 8–15 of either Beclin 1−/− or Beclin 1+/− ESCs and implanted into pseudopregnant foster mothers. Both ESC clones used in this study were described previously (8). All chimeric mice were maintained under specific pathogen-free conditions at either The Mount Sinai Animal Care Facility or The Rockefeller University Animal Care Facility and used at 4–8 weeks of age. All studies and procedures were approved by Animal Care and Use Committees at both institutions.
Antibodies and Flow Cytometry
Fluorescently-labeled monoclonal antibodies against mouse CD4 (clone GK1.5), CD8 (clone 53-6.7), CD43 (clone IM7), IgM (R6-60.2), CD43 (clone S7), B220 (clone RA3-6B2), CD5 (53-7.3), CD229.1 (Ly9.1, 30C7), CD21 (7G6), CD23 (B3B4), and CD117 (c-Kit, 2B8), and APC-conjugated lineage cocktail were purchased from BD Biosciences (San Jose, CA). PE-conjugated anti-mouse IgD (clone 11–26) was from Southern Biotech (Birmingham, AL). Antibodies against mouse CD69 (clone H1.2F3), CD25 (PC61.5), CD44 (IM7), CD127 (IL-7Rα, clone A7R34) and Ly-6A/E (Sca-1, clone D7) were purchased from eBioscience (San Diego, CA). Single-cell suspensions were generated from the spleen, bone marrow, and thymus, and stained for 30 min on ice using saturating concentrations of directly conjugated monoclonal antibodies. Cells were washed three times with PBS containing 2% fetal bovine serum (FBS), and data acquired using either FACScan or LSR II flow cytometers (BD Biosciences, San Jose, CA). Flow cytometry data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
In vitro differentiation of Beclin 1−/− and Beclin 1+/− ESCs into T cells
In vitro differentiation of embryonic stem (ES) cells to T cells was done as previously described (16). Briefly, both ESC clones, Beclin 1−/− and Beclin 1+/−, were maintained in an undifferentiated state on Mitomycin C arrested mouse embryonic fibroblasts (mEFs) (Millipore/Chemicon, Billerica, MA) in ESC media (16) with addition of 103 units/ml of leukemia inhibitory factor (LIF) (ESGRO from Millipore/Chemicon). Co-culture experiments were initiated by seeding 5×104 ESCs on top of a ~80% confluent monolayer of the OP9-R stromal cell line in OP9 media (16). Mesoderm colonies were trypsinized on day 5 of co-culture and 5×105 cells were plated on a new OP9-R monolayer with additional supplement of Flt-3Ligand (R&D Systems, Minneapolis, MN) to a final concentration of 5 ng/ml in the OP9 media. The formation of HSCs was observed by day 8 of co-culture and HSCs were transferred to a OP9-DL1 monolayer of cells that were virally transduced to express the Notch ligand Delta-like 1 (17). From this point, the OP9 medium was supplemented with Flt-3L and IL-7 (PeproTech, Rocky Hill, NJ) to a final concentration of cytokines of 5 ng/ml and 1ng/ml, respectively. Co-cultures were subjected to alternating media change and no-trypsin passages in 2-day intervals. Differentiating lymphocytes were analyzed on days 12 and 19/20 by flow cytometry.
Live cells were gated by excluding dead cell discriminator (DCD) (Invitrogen, Carlsbad, CA) positive cells. OP9 feeders were excluded by staining with rat-anti mouse CD45 Ab conjugated to phycoerythrin (PE) (Caltag/Invitrogen, Carlsbad, CA) and gating on the CD45 positive population of cells. For day 12 analyses, the differentiated lymphocytes were further stained with rat-anti mouse CD44 conjugated to fluorescein (FITC) and rat-anti mouse CD25 conjugated to allophycocyanin (APC) (both from Caltag/Invitrogen Laboratories). On day 19/20, differentiated lymphocytes were analyzed with rat-anti mouse CD4 conjugated to APC (Caltag/Invitrogen Laboratories) and rat-anti mouse CD8α conjugated to FITC (BD/Pharmigen, Franklin Lakes, NJ).
Lymphocyte proliferation assays
For T cell stimulation, flat-bottomed 96-well plates were pre-coated with 10 µg/ml of 2C11 antibody in PBS (BD Biosciences) for 4 hours in a tissue culture incubator at 37°C, or for 12 hours at 4°C. Plates were washed three times with ice-cold PBS, and splenocytes (2×105 cells/well) incubated in RPMI 1640 media supplemented with 10% FBS. For B cell proliferation assays, naïve splenic B cells were purified by depletion-type magnetic separation using CD43 (Ly-48) MACS beads (Miltenyi Biotec, Auburn, CA), and purified B cells (2×105 cells/well) cultured in RPMI 1640 media supplemented with 10% FBS in the presence of 5 ng/ml IL4 (Sigma-Aldrich, St. Louis, MO) and 25 µg/ml LPS (Sigma-Aldrich, St. Louis, MO).
Cultures were incubated in a tissue culture incubator at 37°C in a humidified atmosphere containing 5% CO2 for 24 or 48 hours. Proliferation assays were performed using BrdU-based calorimetric ELISA kit (Roche, Nutley, NJ). Briefly, cells were stimulated in a tissue culture incubator for 48 hours, pulsed overnight with BrdU (10 µM), followed by incubation with peroxidase-labeled anti-BrdU antibody. Development of color following substrate (tetramethyl-benzidine) addition was monitored using an ELISA reader at 370 nm.
For CFSE-based T cell proliferation assays, spleen cells were labeled with 0.5 µM CFSE in PBS (Invitrogen, Carlsbad, CA) for 8 min at room temperature (RT). To quench the reaction, FCS was added immediately to the final concentration of 20%, and cells washed three times in PBS and resuspended in RPMI 1640 media supplemented with 10% FBS. The CFSE-labeled spleen cells were then incubated with plate-bound 2C11 antibody (10 µg/ml) in a tissue culture incubator at 37°C in a humidified atmosphere containing 5% CO2 for 72 hours and analyzed using FACScan flow cytometer.
Confocal Microscopy
Spleen T cells were isolated using Dynal mouse T cell negative isolation kit (Invitrogen, Carlsbad, CA) and stimulated in 24-well plates in the presence of T cell activating magnetic beads coupled with anti-CD3 and anti-CD28 antibodies (Invitrogen, Carlsbad, CA) and IL-2 (0.5 ng/ml) in a CO2 incubator for 7 days. Following incubation stimulating beads were removed with a magnet, and cytospins prepared using Shandon cytospin (Thermo Fisher Scientific, Waltham, MA) with ~1×105 T cell on each microscope slide. T cells were subsequently fixed for 20 min in 4% paraformaldehyde in PBS on ice, washed twice with PBS/1% bovine serum albumin (BSA), and permeabilized 5 min at RT with 0.5 % saponin/0.03 M sucrose/1% BSA in PBS. The cells were washed once with PBS/1% BSA and blocked using 5% normal goat serum (NGS) in PBS/1% BSA for 15 min at room temperature. Following a single wash with PBS/1% BSA, T cells were incubated with polyclonal anti-LC3 antibody PM036 (MBL International Corporation, Woburn, MA) for 60 min at RT. Following this incubation T cells were three times and then blocked with 5 % NGS PBS/1% BSA. Following subsequent wash with PBS/1% BSA, T cells were incubated with FITC -conjugated goat anti-rabbit antibody for 30 min at RT. T cells were finally washed three times in PBS/1% BSA, nuclei counterstained with DRAQ5 (Biostatus Limited, Leicestershire, UK) DNA stain for 5 min at RT, and mounted using SlowFade Gold antifade reagent (Molecular Probes, Eugene, OR). The cells were visualized using an Olympus Fluoview300 confocal microscope (Olympus, Center Valley, PA) at a magnification of 600X.
Statistical analysis
Statistical analysis was performed using two-tailed Student’s t-test. P<0.05 was considered statistically significant.
Results
Loss of thymocytes in the absence of Beclin 1
Targeted disruption of Beclin 1 in mice results in early embryonic lethality prior to generation of a lymphoid system, thus preventing the analysis of in situ lymphoid development (8). In order to circumvent this obstacle, we have generated Beclin 1−/− →Rag 1−/− chimeric mice and control, Beclin 1+/− →Rag 1−/− chimeras using Beclin 1−/− and Beclin 1+/− ESC clones, respectively. Since Rag1−/− mice can not produce mature T and B cells due to deficient V(D)J recombination (18), any mature lymphocytes in chimeric mice must be derived from microinjected ESCs, indicating that they have the potential to complement Rag1 deficiency in lymphopoiesis. The injection of Beclin 1−/− and Beclin 1+/− ESC clones into Rag 1−/− blastocyst mostly produced mice with extensive agouti-black coat-color chimerism (≥ 80%), which confirmed the developmental potential of Beclin 1−/− and Beclin 1+/− cells. Since ESC clones used in this study are of 129/SvJ origin, any lymphocytes derived from these cells can be easily distinguished from Rag 1−/− –derived cells, which are of C57BL/6 origin, based on the expression of the Ly9.1 alloantigen. This alloantigen, present on ESCs but absent on Rag 1−/−– derived cells, is a surface glycoprotein expressed by most thymocytes, peripheral B and T cells, bone marrow lymphoid cells, and hematopoietic progenitors.
To determine T cell development in Beclin 1-deficient chimeric mice we analyzed thymocytes using flow cytometry. Virtually all double positive (DP) and single positive (SP) thymocytes in chimeric mice expressed the Ly9.1 surface marker, indicating that they developed from injected ESCs (data not shown). In 20% of Beclin 1−/−→Rag 1−/− chimeras a complete reconstitution of the major thymocyte populations was observed compared to control Beclin 1+/− →Rag 1−/− chimeras (Fig. 1A). However, in ~80% of chimeras we have detected various degrees of loss of Beclin 1-deficient DP thymocytes (Figs. 1B and 1C). This loss ranged from 20% to almost complete depletion of DP thymocytes in some Beclin 1−/− →Rag 1−/− chimeras (Fig. 1C), but was never observed in control animals. Enumeration of thymocytes and spleen cells by trypan blue exclusion also revealed a dramatic reduction of thymocyte numbers in Beclin 1−/− →Rag 1−/− chimeras (Fig. 1D). Different levels of chimerism in individual mice could potentially explain the high variability of thymocyte numbers observed in control chimeras (Fig. 1D). Nevertheless, the differences between Beclin 1 deficient and control chimeras are significant (17.7 × 106 vs. 72.8 × 106, p=0.0002).
Figure 1.
T cell development in the absence of Beclin 1. Single cell suspensions were generated from thymi and spleens of Beclin 1−/− →Rag 1−/− and control chimeras, stained with antibodies against CD4 and CD8 and analyzed by flow cytometry. The percentage of cells within each T cell subset defined by the corresponding quadrants of the dot plots is indicated. Data from three different experiments (A, B, and C) are shown. (D) Absolute numbers of thymocytes and spleen cells in Beclin 1−/− →Rag 1−/− and control chimeras as determined by trypan blue exclusion test. Each symbol represents an individual mouse. (E) The analysis of DN thymocytes in Beclin 1-deficient chimeras. The expression of CD25 and CD44 on pre-gated Ly9.1+CD4−CD8−CD3− thymocytes is shown. The analysis was repeated three times with similar results. All chimeras were 5–8 weeks of age.
The dramatic loss of thymocytes in Beclin 1−/−→Rag 1−/− chimeras could potentially arise from a specific block during double negative (DN) thymocyte development. To address this possibility, DN thymocytes expressing the Ly9.1 marker were stained with CD44 and CD25 antibodies and analyzed by flow cytometry (Fig. 1E). Compared to control mice, Beclin 1-deficient chimeras displayed perturbed DN thymocyte populations with accumulation of both DN1 cells (CD44+CD25−) as well as the intermediate DN1–DN2 (CD44+CD25lo) cells. Our results indicate that Beclin 1 deficiency results in the loss of thymocytes, and apparent abnormalities in the DN thymocyte compartment. However from these data alone, it is not possible to discern if the abnormalities observed in Beclin 1-deficient DN thymocytes represent a true developmental block, or a result of competition between Beclin 1−/− and Rag1−/−derived DN cells.
Beclin 1−/− ESCs fail to maintain normal T cell development in vitro
To further investigate the thymocyte abnormalities discovered in Beclin 1 −/− chimeric animals, we utilized in vitro ESC differentiation system with OP9 stroma cells expressing the Delta-like-1 (DL1) protein (17) to generate T cells in the absence of Rag1 −/− cell competition. After 12 days of co-culture, both Beclin 1−/− and control ESCs were capable of generating normal DN thymocyte subsets, as indicated by similar CD25 vs. CD44 profiles, as well as similar numbers of c-Kithi DN1 (CD25−CD44+) cells containing early T-lineage progenitors up to day 16 (Fig. 2A and data not shown). We also observed that the rate of DN2/3 cell generation from CD45+ precursors (as indicated by the ratio of CD25:CD45 positive cells) was equivalent in both knockout and control co-cultures (Fig 2B). This would rule out an intrinsic developmental block at this stage of thymocyte development caused by Beclin 1 deficiency. Remarkably, however, day 19/20 co-cultures derived from Beclin 1−/− ESCs displayed sharp declines in DN thymocyte populations compared to control, Beclin 1+/− ESC co-cultures (10.44 ± 3.1 vs. 1.66 ± 0.52, p<0.05, Figs. 2C and 2D). We also observed impaired output of more mature CD8+ T cells that is likely secondary to the loss of their progenitors in the DN compartment (Figs. 2C and 2D). The co-culture-derived Beclin 1-deficient DP thymocytes exhibited normal sensitivity to apoptosis and normal T cell receptor beta chain expression (data not shown). Since earlier co-culture time points, including day 12 (Figure 2A) and day 16 indicated no difference in the ability of Beclin 1−/− and control ESCs to initially generate T cells in vitro (unpublished observation), our results from Rag 1−/− chimeras as well as in this ESC differentiation system, strongly suggest that the failure of Beclin 1−/− ESC co-culture to sustain normal T cell generation in vitro at later time points is caused by impaired maintenance of thymocyte precursors at, or prior to, the DN stage.
Figure 2.
Beclin 1-deficient ESCs fail to maintain normal T cell development in vitro. T cells differentiation from Beclin 1−/− and control ESCs was carried out in the presence of OP9-DL1 cells, as described in Materials and Methods. The co-culture aliquots were taken at days 12 (A) and 19/20 (C), stained with indicated antibodies, and analyzed using flow cytometry. The experiments were repeated three times with similar results. (B) The ratio of CD25:CD45 positive cells in Beclin 1−/− and control co-cultures at day 12 indicating equivalent rates of DN2/3 cell generation from CD45+ precursors in both co-cultures. (D) Distribution of T cell subsets based on the expression of CD4 and CD8 surface markers in Beclin 1−/− and control co-cultures at day 19/20. Data represent average percentages ± SD of cells from three independent experiments.
Normal proliferation of Beclin 1-deficient peripheral T cells
Despite the dramatic loss of thymocytes in Beclin 1−/− →Rag 1−/− chimeras, we did not observe any change in peripheral, spleen CD4+ and CD8+ T cells numbers (Figs 1A, 1B, and 1C, right dot plot panels, and 3A), suggesting that Beclin 1 is not essential for maintaining normal numbers of T cells in the periphery. The analysis of peripheral T cell subsets did not indicate any imbalance among naive CD44loCD62Lhi, effector CD44hiCD62Llo, and memory CD44hiCD62Lhi T cells in Beclin 1-deficient chimeras (Fig. 3B). Surprisingly, peripheral T cells exhibited normal proliferative response in vitro compared to control T cells (Figs. 3C and 3D), as determined by both BrdU incorporation (Fig. 3C) and CFSE (Fig. 3D) assays, and also displayed normal induction of activation markers, CD69 and CD25 (Fig. 3E). Since previous studies have suggested that autophagy is required for peripheral T cell proliferation (19, 20), we analyzed if Beclin 1-deficient T cells contained autophagosomes by anti-LC3 staining. The confocal imaging of LC3-stained, activated T cells revealed a reduced number of fluorescent puncta in Beclin 1-deficient vs. control T cells (Fig. 4). Beclin 1−/− deficient T cells contained on average 0.72 ± 0.09 fluorescent puncta per cell compared to 1.70 ± 0.12 puncta in control Beclin 1+/− T cells (p=0.002). Since the presence of LC3 puncta has been considered a universal marker of autophagosomes (21), our results suggest that Beclin 1-deficient T cells have reduced autophagic activity but normal proliferation.
Figure 3.
Characterization of Beclin 1-deficient peripheral T cells. (A) Absolute numbers of spleen CD4+ and CD8+ T cells from Beclin 1−/− →Rag 1−/− chimeric mice (open bars) and control mice (filled bars). Bars represent means of at least four experiments ± SD. (B) FACS analysis of CD44 and CD62L marker expressions on CD4+ (upper panels) and CD8+ (lower panels) T cells from Beclin 1−/− →Rag 1−/− chimeric mice and control mice. Data are representative of four independent experiments. The percentage of cells in each quadrant is indicated. Proliferative response of Beclin 1-deficient and control spleen T cells to in vitro stimulation with plate-bound 2C11 antibody as measured by colorimetric BrdU-incorporation assay (C) and CFSE (D). Data points in Figure 3C represent means of triplicate cultures ± SD. Data are representative of two independent experiments. CFSE-labeled proliferating CD4+ (upper panels) and CD8+ T cells (lower panels) in Figure 3D are indicated with elliptical gates. (E) Induction of activation markers CD69 and CD25 on Beclin 1-deficient and control CD4+ and CD8+ T cells following activation with plate-bound 2C11 antibody.
Figure 4.
Reduced number of LC3 puncta in activated, Beclin 1-deficient T cells. Confocal microscopy images of immunofluorescent staining with anti-LC3 antibody reveals a punctate localization of LC3 in activated T cells from Beclin 1+/− (A), as well as Beclin 1−/− →Rag 1−/− chimeras (B). Two smaller images in the bottom represent enlarged areas defined by two red rectangles. The average numbers of LC3 puncta in activated T cells from Beclin 1+/− vs. Beclin 1−/− →Rag 1−/− chimeras counted by confocal microscopy from 12 fields, each 52,430 µm2 in area ± SD (C). Original magnification × 400.
Poor reconstitution of the early B cell compartment in Beclin 1−/−→Rag 1−/− chimeras
To determine if Beclin 1 plays a more general role in lymphocyte development, we used flow cytometry to analyze if Beclin 1-deficient ESCs could effectively contribute to the bone marrow, as well as to the bone marrow-derived B cell compartment in chimeric mice. This analysis revealed approximately twofold reduction in the frequency of Ly9.1+ bone marrow cells in Beclin 1−/−→Rag 1−/− chimeras, indicating a somewhat reduced contribution potential of Beclin 1−/− ESCs (Fig. 5A and Fig. 5B, middle panel). In addition, we also observed severely reduced numbers of B220+ IgM+ cells (Fig. 5B, top panel). The analysis of Ly9.1 marker expression during early stages of B cell development as defined by B220 and CD43 expressions indicates that, as opposed to control chimeras, pro-B cells (B220lowCD43+) from Beclin 1−/−→Rag 1−/− chimeras contained only a small number of ESC-derived, Ly9.1+ cells (Fig. 5B, bottom panel). These results indicate that Beclin 1−/− ESCs fail to efficiently contribute to the early B cell compartment of Rag 1−/− bone marrow.
Figure 5.
Compromised reconstitution of B cell development in the bone marrow of Rag 1−/− mice by Beclin 1-deficient ESCs. (A) Frequency of Ly9.1+ cells in the bone marrow of age and sex-matched Beclin 1−/− →Rag 1−/− and control chimeras as determined by flow cytometry. All mice were used between 4 and 8 weeks of age. (B) Bone marrow cells were stained with fluorescently-labeled antibodies against indicated B cell markers as well as Ly9.1 and analyzed by flow cytometry. The bottom histogram represents the expression of Ly9.1 on pro and pre-B cells as defined by CD43 and B220 surface markers. Gate positions are indicated with rectangles. The analysis was repeated three times with similar results.
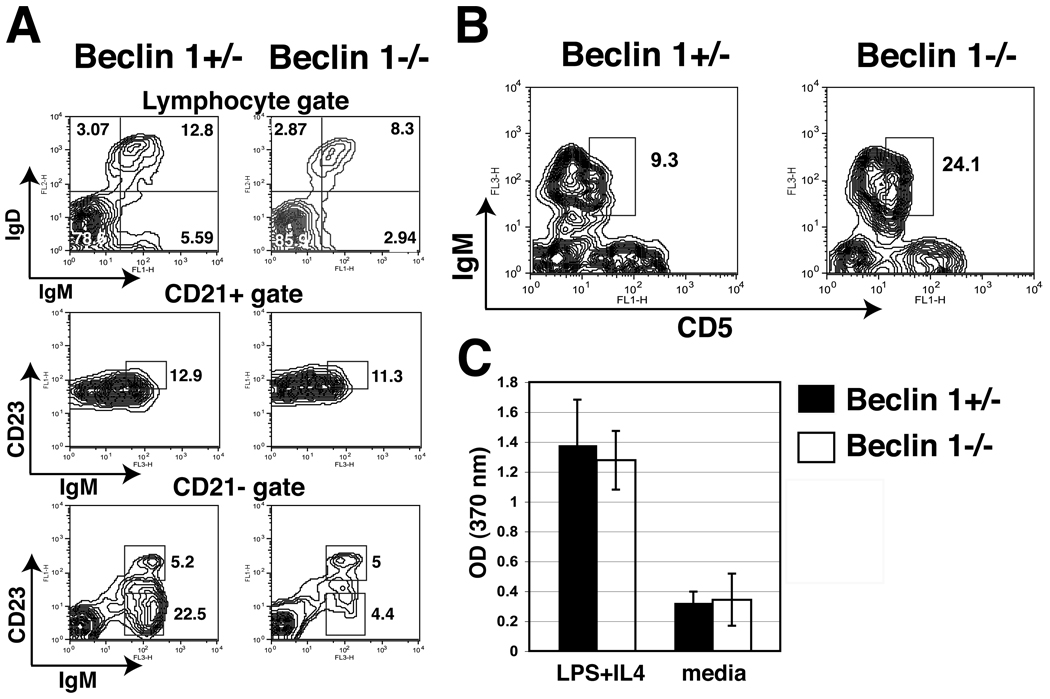
In order to analyze the peripheral B cell compartment in chimeric mice, we stained spleen cell using fluorescently-labeled antibodies and analyzed them by flow cytometry. We found that Beclin 1−/− →Rag 1−/− chimeras had slightly reduced populations of IgM+IgD+ B cells (12.6 ± 4.5 vs. 19.1 ± 5.3, P < 0.05, n=7) (Fig. 6A, top panel). The frequencies of CD21hiCD23+IgM+ T2 as well as CD21+CD23−IgM+ marginal zone (MZ) B cells were generally unchanged (Fig. 6A, middle and bottom panels). However, the most developmentally early population of CD21− CD23−IgM+ (T1) peripheral B cells (representing recent bone marrow emigrants) was significantly diminished in Beclin 1−/− →Rag 1−/− chimeras (5.6 ± 1.7 vs. 19.9 ± 3.6, P < 0.05, n=3) (Fig. 6A, bottom panel). Importantly, the analysis of peritoneal B1 cells using CD5 and IgM marker expression revealed normal development of the B1 cell population in Beclin 1−/− →Rag 1−/− chimeric mice (Fig. 6B). In order to determine if peripheral, Beclin 1-deficient B cells were functional, we analyzed the proliferative response of purified spleen B cells stimulated in vitro with LPS and IL-4. Our result indicate that in vitro proliferative response of Beclin 1−/− B cells is not significantly different from the response of control, Beclin 1+/− B cells (Fig. 6C). In summary, we find that, Beclin 1 deficiency has far more dramatic effects at early stages of B cell development than at later stages. Thus, similar to T cells, Beclin 1 appears to be largely dispensable for normal numbers and proliferation of B cell populations in the periphery.
Figure 6.
Reduced early bone marrow emigrant B2 cell numbers but otherwise preserved peripheral B2 and B1 B cell subsets in the absence of Beclin 1. (A) Spleen cells from chimeric mice were stained with indicated fluorescently-labeled antibodies and analyzed by flow cytometry. The applied gates are indicated above each pair of contour blots. Note the reduction in CD21− CD23−IgM+ cell subset representing the subset of recent bone marrow emigrants (bottom pair of plots). (B) Peritoneal exudate cells from chimeric mice were stained with antibodies against CD5, IgM, and CD23. The expression of CD5 and IgM on CD23− cells is shown. (C) Purified splenic B cells (2×105/well) from chimeric mice were incubated in 96-well plates in the presence of LPS (25 µg/ml) and IL-4 (5 ng/ml), and proliferation assay carried our as described in Materials and Methods. Bars represent means of triplicate cultures ± SD. Data are representative from three independent experiments.
Impaired generation of Beclin 1-deficient lymphoid progenitors
The early stage defects in T and B cell lineage cells of Beclin 1−/− –deficient chimeras prompted an analysis of early lymphoid precursors in the bone marrow. We analyzed Beclin 1−/− →Rag 1−/− chimeric mice for potential alterations in common lymphoid progenitor (CLP) and hematopoietic stem cells (HSC) populations, as previously described (22). We observed that Ly9.1+ CLPs were substantially reduced in Beclin 1−/−→Rag 1−/− chimeras compared to control mice, indicating that Beclin 1-deficient ESCs poorly contributed to the CLP pool in chimeric mice (17.54 ± 11 vs. 55.2 ± 3.3, P =0.03)(Fig. 7A). In addition to the CLP pool reduction, the CD127− Lin−c-Kit+ Sca-1− fraction containing common myeloid progenitors (CMP) (23, 24) was virtually absent in Beclin 1−/−→Rag 1−/− chimeras even as numbers of Ly9.1+ splenic macrophages (CD11b+) appeared normal in these mice (Supplementary Figure 1). These data suggested an upstream defect in earlier hematopoietic progenitors. We therefore further analyzed the cell compartment upstream of the oligolineage progenitors for the presence of HSCs. Beclin 1-deficient ESCs did generate Ly9.1+ HSCs, albeit at much reduced frequency (2.3 ± 3.2 vs. 42.4 ± 2.8, P <0.01) (Fig. 7B). These results suggest that Beclin 1 is required for normal and/or sustained contribution to the pools of progenitors of lymphocytes in the competitive environment of chimeric mouse bone marrow. This may help explain the early stage defects observed in Beclin 1-deficient T and B lineage cells.
Figure 7.
Reduced CLP and HSC compartments in the bone barrow of Beclin 1−/− →Rag 1−/− chimeric mice. Frequencies of Beclin 1-deficient ESC-derived (A) CLPs and (B) HSCs were determined based on the expression of Ly9.1 (32). CLPs are defined as Lin−CD127+C-Kitlo Sca-1lo and HSC as Lin−CD127−c-Kit+ Sca-1+ cells (22, 33). Bone marrow cells were stained with indicated antibodies and analyzed using LSR II flow cytometer. Gate positions were indicated with rectangles. The percentage of positive cells within each subset defined by corresponding gates within contour plots and histograms are indicated with small numbers within each gate. Data are representative from three independent experiments.
Discussion
Several studies have recently addressed the role of autophagy in lymphocytes. First, autophagy has been found to play a role in CD4+ T cell death induced by HIV (25), indicating that this process can be triggered in T cells by extracellular cues different from starvation. In addition, T cell activation in vitro induces increased autophagic activity in CD4+ T cells (26). These reports, in combination with our previous analyses of Beclin 1 gene regulation in thymocytes (14) prompted the present study of the role of the Beclin 1 autophagy gene in T cell development.
Mice with T cell-specific inactivation of Atg5 or Atg7 genes exhibited a variety of intrinsic defects in thymocytes and peripheral T cells. In the case of thymocytes, these defects involve reduction of thymic cellularity in the absence of a specific developmental block or impaired survival. As for peripheral T cells, reduced numbers of CD4+ and CD8+ T cells exhibiting impaired survival and proliferation defects, as well as alterations of activation/memory/homeostatic expansion were observed in the absence of Atg5 or Atg7 (19, 20, 27). These defects are likely caused by impaired autophagy-dependent removal of damaged or aging mitochondria in peripheral T cells (20, 27). It is therefore clear that Atg5 and Atg7 autophagy genes are required at both early and late stages of T cell development.
We found that Rag1 blastocyst chimeras lacking Beclin 1, similarly to Atg5 and Atg7-deficient mice, show reduced thymocyte numbers (Fig. 1). We also detected an altered distribution of DN thymocytes in these chimeras with accumulation of cells in the DN1 population (Fig. 1E). This, however, likely does not reflect a genuine developmental block. Rather, it would seem that Beclin 1-deficient DN thymocytes are outcompeted by Rag1−/− -derived DN cells. This is because, using an in vitro ESC differentiation system for generating T cells, we observed a normal rate of initial DN subset generation and did not detect significant DN subset aberrations at early time points in the co-culture (Figs. 2A and 2B). However, we did observe a dramatic reduction in the DN compartment at later time points (Figs. 2C and 2D). Importantly, we observe this reduction in the absence of competition from Beclin 1 sufficient cells that exist in the chimeric mice. Thus, the defects in thymocyte progenitor maintenance seen in vitro (and likely the related progenitor maintenance defects seen in vivo) are intrinsic to Beclin 1 deficiency. These in vitro data help illuminate the likely basis for the reduction of total thymocyte numbers observed in Beclin 1-deficient chimeras. This could be the result of a decreased frequency (over time) of hematopoietic progenitors seeding the thymus. Collectively, these results point to the impaired maintenance of thymocyte progenitors in the absence of Beclin 1.
In sharp contrast to the peripheral T cell defects previously reported for Atg5 and Atg7-deficient T cells (19), we could not detect a significant impact of Beclin 1-deficiency on peripheral T cell numbers and their proliferative response in vitro (Fig. 3). Considering the essential roles of Atg 5 and Atg7 in autophagy, their apparent requirement for T cell proliferation is commonly regarded as a general requirement for autophagy in peripheral T cells. However, we were able to detect LC3 puncta in Beclin 1-deficient T cells (Fig. 4), suggesting that a non-canonical, Beclin 1-independent autophagy appears to be sufficient to sustain normal peripheral T cell proliferation. Similar examples of Beclin 1-independent autophagy have been observed in other cell types under certain conditions (28–31). The fact that Beclin 1-deficient T cells still retain some autophagic activity could thus potentially explain different outcomes of Beclin 1 and Atg5/Atg7 deficiencies in peripheral T cell phenotype and proliferation. In addition, we did not observe any significant accumulation of mitochondria in Beclin 1−/− T cells (unpublished observation), as seen in Atg5 −/− T cells (20, 27). In summary, our data indicate that the Beclin 1 autophagy gene plays an essential, but selectively early, role during T cell development. This is because, unlike Atg5 and Atg7, Beclin 1 function seems largely dispensable in peripheral T cells.
While previous studies indicate an important role for autophagic protein Atg5 in both early and late stage T cells, the data describing the impact of Atg5 deficiency in B cells are less clear. Atg5-deficient fetal liver chimeric mice display defects that suggest reduced lymphoid progenitor activity (19) including a significant reduction in the number of peripheral B cells. In contrast, a subsequent study in which the Atg5 gene was conditionally targeted in B cells (using CD19-cre) showed partial deficiencies at the pro-B to pre-B cell transition in the bone marrow, but a largely normal peripheral B cell compartment (29). The differences observed in the severity of the Atg5-deficient B cell phenotype undoubtedly stem from the disparate experimental systems used in these two studies. Nevertheless, these prior data suggest at least an early-stage role for Atg5 in the B cell compartment, even if its role in peripheral B cells remains nebulous.
In concordance with the Atg5 knockout fetal liver chimera data, we detect deficiencies in lymphoid progenitors in the absence of Beclin 1. Our data also show that Beclin 1 deficiency causes significant defects at early stages of B cell development in the bone marrow, and a lesser deficit in the earliest peripheral B cell subset representing recent bone marrow emigrants. In contrast Beclin 1 deficient B cells at later stages of development appear largely normal. Taken together, the data indicate that both Beclin 1 and Atg5 proteins are required in lymphoid progenitors and early stage T and B cell development. While Atg5 may be required in peripheral B cells (19), our data suggest that, as in peripheral T cells, Beclin 1 activity is not essential for most peripheral B cells. While peripheral lymphocytes seem largely normal in our Beclin 1 −/− chimeric mice, we cannot rule out the possible emergence of long-term deficits over time. It is conceiva ble that Beclin 1 activity will prove significant in preventing age-related decline in lymphocyte homeostasis and function. Long-term monitoring of Beclin 1 −/− chimeric and control mice will be required to test this hypothesis.
Our findings of an essential role for Beclin 1 at early stages of both T and B cell development suggest that the reduction of early T cell and B cell numbers in Beclin 1-deficient chimeras could result from a reduced pool of lymphoid progenitors in the bone marrow. We examined this possibility by analyzing CLPs and HSC in both control and Beclin 1-deficient chimeras (32). Our results indicate that Beclin 1−/− →Rag 1−/− chimeras have significantly reduced Ly9.1+CLPs, and also a severely reduced population of Ly9.1+HSCs compared to control chimeras (Figure 7). The impaired maintenance of lymphoid progenitors in the bone marrow may therefore explain the defects seen in the early T and B cell compartments in the absence of Beclin 1. It is not yet clear if the problem in progenitor maintenance is selectively at a single stage, (such as the HSC stage) or a more general defect in maintenance of multiple types of undifferentiated progenitors. Nevertheless, using an in vitro ESC differentiation system to generate T cells, we found a depletion of the DN thymocyte compartment over time resulting in reduced maintenance of T cell generation in the co-cultures at later time points. Because this depletion occurs in the absence of competition from Beclin 1 sufficient cells in the chimeras, this defect would be consistent with impaired progenitor activity/maintenance issues intrinsic to cells lacking Beclin 1. In future studies, this in vitro system may enable a comprehensive assessment of the generation, maintenance and function of thymocyte progenitors at multiple stages in order to determine which populations are dependent on Beclin 1 function and why.
In summary, Beclin 1 deficiency results in severe depletion of T and B cell precursors, but has limited impact on peripheral T and B cells. Furthermore, loss of Beclin 1 function has no discernible effect on peripheral T cell proliferation despite reduced autophagy. Together with other recent reports, our results thus indicate distinct roles for various, known autophagic proteins in T cells. While recent studies in Atg5 and Atg7-deficient mice indicate a clear role for autophagy, in general, throughout lymphocyte development, we propose that Beclin 1-dependent processes are selectively required in progenitor cells and early stages of lymphocyte development. It remains to be seen, however, whether these processes reflect the role of Beclin 1 in autophagy or could involve other autophagy-independent function(s) of this protein in cell survival/differentiation. Importantly, our results reveal that non-canonical, Beclin 1 independent autophagy is sufficient to support later stage peripheral T and B cell development and proliferation.
Supplementary Material
Acknowledgments
We would like to thank Kevin Kelley (Mount Sinai School of Medicine) and Rada Norinsky (Rockefeller University) for their expert Rag 1−/− chimeric mice generation, Chingwen Yang (Rockefeller University) for ESC work, Tatyana Leonova (Rockefeller University) for help with B cell assays, Joon Kim (Hunter College) for flow cytometry, and Derek Sant’Angelo (Memorial Sloan-Kettering Cancer Center) for helpful comments on the manuscript. We are indebted to Roxanne Holmes and Juan-Carlos Zúñiga-Pflücker for invaluable discussions and the ongoing advice and reagents that enabled us to establish in vitro T cell differentiation at CUNY.
Abbreviations used in this paper
- CLP
common lymphoid progenitor
- DL1
Delta-like 1
- DN
double negative
- DP
double positive
- ESC
embryonic stem cell
- HSC
hematopoietic stem cell
- Rag1
recombination activating gene 1
- RT
room temperature
Footnotes
The project described was supported by Award Number SC3GM088044 from the National Institute Of General Medical Sciences (to I.A.), National Science Foundation Grant MCB-0236964 (to B.D.O) and bridge funds from the Hunter College Gene Center funded by NIH grant RR03037 (to B.D.O). Grants C023048 and C024302 from the New York State Stem Cell Science program of the New York State Department of Health (to B.D.O) supported training in (and adoption of) in vitro embryonic stem cell differentiation technology at Hunter College. A.A. and J.H. were recipients of the Minority Access to Biomedical Research (MARC) fellowship (T32GM8498) from NIH. M.M. was a recipient of Grant GM 08153 from NIH.
References
- 1.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsunaga K, Noda T, Yoshimori T. Binding Rubicon to cross the Rubicon. Autophagy. 2009;5:876–877. doi: 10.4161/auto.9098. [DOI] [PubMed] [Google Scholar]
- 3.Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Furuya D, Tsuji N, Yagihashi A, Watanabe N. Beclin 1 augmented cis-diamminedichloroplatinum induced apoptosis via enhancing caspase-9 activity. Exp Cell Res. 2005;307:26–40. doi: 10.1016/j.yexcr.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Schiff M, Czymmek K, Talloczy Z, Levine B, Dinesh-Kumar SP. Autophagy regulates programmed cell death during the plant innate immune response. Cell. 2005;121:567–577. doi: 10.1016/j.cell.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Takacs-Vellai K, Vellai T, Puoti A, Passannante M, Wicky C, Streit A, Kovacs AL, Muller F. Inactivation of the Autophagy Gene bec-1 Triggers Apoptotic Cell Death in C. elegans. Curr Biol. 2005;15:1513–1517. doi: 10.1016/j.cub.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 10.Qu X, Zou Z, Sun Q, Luby-Phelps K, Cheng P, Hogan RN, Gilpin C, Levine B. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–946. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 11.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsukamoto S, Kuma A, Murakami M, Kishi C, Yamamoto A, Mizushima N. Autophagy Is Essential for Preimplantation Development of Mouse Embryos. Science. 2008;321:117–120. doi: 10.1126/science.1154822. [DOI] [PubMed] [Google Scholar]
- 13.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arsov I, Li X, Matthews G, Coradin J, Hartmann B, Simon AK, Sealfon SC, Yue Z. BAC-mediated transgenic expression of fluorescent autophagic protein Beclin 1 reveals a role for Beclin 1 in lymphocyte development. Cell Death Differ. 2008;15:1385–1395. doi: 10.1038/cdd.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Lansford R, Stewart V, Young F, Alt FW. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci U S A. 1993;90:4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes R, Zuniga-Pflucker JC. The OP9-DL1 system: generation of T-lymphocytes from embryonic or hematopoietic stem cells in vitro. CSH Protoc. 2009;2009 doi: 10.1101/pdb.prot5156. pdb.prot5156. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 18.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 19.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pua HH, Guo J, Komatsu M, He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J Immunol. 2009;182:4046–4055. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- 21.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WAJ, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovacs AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, Lopez-Otin C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Melendez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Munz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nurnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Talloczy Z, Tanaka K, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcategui NL, van der Klei I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 23.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 24.Nemeth MJ, Curtis DJ, Kirby MR, Garrett-Beal LJ, Seidel NE, Cline AP, Bodine DM. Hmgb3: an HMG-box family member expressed in primitive hematopoietic cells that inhibits myeloid and B-cell differentiation. Blood. 2003;102:1298–1306. doi: 10.1182/blood-2002-11-3541. [DOI] [PubMed] [Google Scholar]
- 25.Espert L, Denizot M, Grimaldi M, Robert-Hebmann V, Gay B, Varbanov M, Codogno P, Biard-Piechaczyk M. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Invest. 2006;116:2161–2172. doi: 10.1172/JCI26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Capan E, Zhao Y, Zhao J, Stolz D, Watkins SC, Jin S, Lu B. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. J Immunol. 2006;177:5163–5168. doi: 10.4049/jimmunol.177.8.5163. [DOI] [PubMed] [Google Scholar]
- 27.Stephenson LM, Miller BC, Ng A, Eisenberg J, Zhao Z, Cadwell K, Graham DB, Mizushima NN, Xavier R, Virgin HW, Swat W. Identification of Atg5-dependent transcriptional changes and increases in mitochondrial mass in Atg5-deficient T lymphocytes. Autophagy. 2009;5:625–635. doi: 10.4161/auto.5.5.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scarlatti F, Maffei R, Beau I, Ghidoni R, Codogno P. Non-canonical autophagy: an exception or an underestimated form of autophagy? Autophagy. 2008;4:1083–1085. doi: 10.4161/auto.7068. [DOI] [PubMed] [Google Scholar]
- 29.Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R. Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 2008;15:1318–1329. doi: 10.1038/cdd.2008.51. [DOI] [PubMed] [Google Scholar]
- 30.Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong CH, Iskandar KB, Yadav SK, Hirpara JL, Loh T, Pervaiz S. Simultaneous induction of non-canonical autophagy and apoptosis in cancer cells by ROS-dependent ERK and JNK activation. PLoS One. 2010;5:e9996. doi: 10.1371/journal.pone.0009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanyal M, Tung JW, Karsunky H, Zeng H, Selleri L, Weissman IL, Herzenberg LA, Cleary ML. B-cell development fails in the absence of the Pbx1 protooncogene. Blood. 2007;109:4191–4199. doi: 10.1182/blood-2006-10-054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai AY, Lin SM, Kondo M. Heterogeneity of Flt3-expressing multipotent progenitors in mouse bone marrow. J Immunol. 2005;175:5016–5023. doi: 10.4049/jimmunol.175.8.5016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.