Abstract
Objective
We investigated the effect of ischemia and reperfusion on the vasoactive function of penetrating brain parenchymal arterioles under pressurized conditions.
Methods
Parenchymal arterioles (<50μm diameter) from within the middle cerebral artery territory were dissected from male Wistar rats that were either non-ischemic control (n=16) or ischemic for 1 hour and reperfused for 24 hours (n=16) by temporary filament occlusion of the middle cerebral artery. Arterioles were mounted on glass cannulas within an arteriograph chamber that allowed for measurement of lumen diameter and control over intravascular pressure.
Results
After one hour of equilibration at 10 mmHg, spontaneous myogenic tone developed in both groups of animals, constricting control arterioles from 69±9μm to 49±11μm (29.5±10.2%) and ischemic arterioles from 66±9μm to 45±11μm (33.1±14.1%); p>0.05. Contraction to the nitric oxide synthase inhibitor nitro-L-arginine (10−4M) was significantly diminished in ischemic arterioles, constricting only 3.2±3.3% vs. 15.6±12.5% in control arterioles (p=0.017). Both groups dilated to nifedipine, however, the response was significantly diminished after ischemia. The EC50 for nifedipine in control arterioles was 3.54 ± 0.11nM vs. 9.90 ± 0.71nM for ischemic arterioles (p=0.024).
Conclusions
These findings demonstrate that functional changes occur in brain parenchymal arterioles after ischemia and reperfusion, a result that may significantly influence stroke outcome by altering blood flow to an ischemic region.
Keywords: focal ischemia, brain arterioles, myogenic tone, reactivity
Introduction
The middle cerebral artery (MCA) territory is the most commonly occluded brain region with MCA stroke accounting for ~40% of all ischemic strokes [16]. The MCA is the largest of the major branches of the internal carotid artery, the architecture of which consists of a large stem that gives rise to smaller penetrating arterioles, the lenticulostriates, that branch off the MCA at right angles [16,20]. The lenticulostriates are long and largely unbranched vessels that supply the basal ganglia and connect the MCA to the microcirculatory network [16]. These penetrating arterioles are unique vessels in the brain in that they have more pressure-induced myogenic tone than pial arteries and arterioles [4,19], are closely associated with other cell types such as pericytes and astrocytes [6], and lose their extrinsic innervation upon entering the brain parenchyma [4]. Both arteries and arterioles in the brain contribute to proper cerebral perfusion and vascular resistance [7,9]. In addition, communication between these different segments of the vasculature in the brain has been shown to occur via retrograde dilation [10,18], making both artery and arteriole components integrated and important regulators of cerebral blood flow (CBF).
Previous studies have demonstrated vascular dysfunction of the MCA after focal ischemia and reperfusion (I/R) [1–3,12–14]. Two hours of ischemia followed by 24 hours of reperfusion caused loss of pressure-induced contraction in the MCA and diminished dilation to acetylcholine [1]. Other studies also found significant effects of I/R on vascular smooth muscle (VSM) function, including altered VSM inward rectifying potassium channels and diminished vasodilation to nitric oxide (NO) [12,13]. In addition, elegant studies by Marrelli et. al. found that I/R augmented endothelium-derived hyperpolarizing factor (EDHF) production in the MCA that was shown to be due to altered endothelial cell calcium handling [13,14]. Together, these studies demonstrate significant functional abnormalities of the MCA after I/R.
Unlike most peripheral vascular beds, in the cerebral circulation, large vessels such as the MCA contribute ~50% to cerebrovascular resistance and therefore alterations in large vessel function after I/R could significantly impact control of CBF and worsen stroke outcome [7]. However, proper brain perfusion depends on coordinated vasomotor responses of the penetrating arterioles with upstream pial vessels [9,10,19]. The importance of this communication is demonstrated by the existence of retrograde dilation of pial arterioles during activation of focal brain activity [10,19]. In addition, parenchymal arterioles contribute ~40% to cerebrovascular resistance and have autoregulatory capacity similar to larger upstream vessels [4,8,9]. It therefore seems important to understand how I/R affects the function of penetrating arterioles in addition to alterations in larger upstream vessels. Interestingly, Ngai et al. demonstrated that conducted vasodilation in brain parenchymal arterioles was enhanced after I/R [19]; however, the effect of I/R on other functional responses of penetrating brain arterioles is largely unknown. The purpose of the present study was to determine how I/R affects VSM and endothelial cell function in isolated and pressurized lenticulastriate arterioles. We determined myogenic reactivity as well as NO and endothelial-derived hyperpolarizing factor (EDHF) production after 1 hour of ischemia followed by 24 hours of reperfusion and compared these responses to nonischemic controls.
Materials and Methods
Middle cerebral artery occlusion (MCAO) and reperfusion
I/R was accomplished by temporary filament occlusion of the MCA, as previously described [1–3]. Briefly, male Wistar rats (280–300g) were anesthetized via inhalation mask with halothane and oxygen. With the aid of a dissecting microscope, the right carotid bifurcation was exposed and the external carotid artery coagulated distal to the bifurcation. After temporary ligature of the common carotid artery, a 5-0 nylon monofilament coated with silicone was inserted through the external carotid artery stump and carefully advanced to occlude the origin of the MCA. Successful occlusion and reperfusion of the MCA was confirmed using laser Doppler flowmetry, as previously described [1–3]. Ischemic animals were exposed to 1 hour of ischemia, after which the suture was removed to allow for reperfusion for 24 hours. Control animals underwent parallel anesthesia, but were not exposed to I/R. All procedures were approved by the Institutional Animal Care and Use Committee and complies with the NIH guidelines for the use and care of animals.
Preparation of arteriole segments and pressurized arteriograph system
Penetrating brain arterioles were obtained either after the MCAO procedure (I/R, n=16), or from non-ischemic control (CTL, n=16) animals. Animals were anesthetized with halothane and oxygen and decapitated. The brain was quickly removed and placed in cold HEPES solution, pH=7.4±0.05. Arterioles were identified as branches off the MCA that penetrate at right angles into the brain parenchyma. Once identified, surrounding brain tissue was carefully cleared and the vessel removed, placed in the arteriograph chamber and mounted on glass cannulas.
The proximal cannula of the arteriograph was connected to an in-line pressure transducer and a servo mechanism that continually measured and adjusted intravascular pressure. The servo system consisted of a miniature peristaltic pump and controller that permitted intravascular pressure to be either maintained at a constant pressure (static) or increased at a variable rate. The distal cannula was closed off so there was no flow through the arterioles. The entire chamber was placed on an inverted microscope with an attached video camera and monitor. An optical window on the bottom of the chamber allowed lumen diameter to be measured using video dimensional analysis, as described previously [1–3].
Experimental protocol
Parenchymal arterioles were equilibrated for 1 hour at 10 mmHg and 37±0.5°C during which time spontaneous myogenic tone developed. Diameter was recorded prior to the start of the experiment (i.e., prior to equilibration) as well as after equilibration. Intravascular pressure was then increased to 150 mmHg in 10 mmHg increments and diameter recorded at each pressure once stable, approximately 10 minutes. Once active pressure-diameter curves were obtained, pressure was lowered to 40 mmHg for the rest of the experiment. This pressure was chosen based on previous studies approximating this as the pressure at which these arterioles operate in vivo [7]. The nitric oxide (NO) synthase inhibitor nitro-L-arginine (L-NNA, 0.1 mM) was then added to the bath and diameter recorded. With L-NNA still in the bath, the L-type calcium channel blocker nifedipine (1.0–100 nM) was then cumulatively added and diameter recorded at each concentration. Once the concentration-response curves were complete, the phosphodiesterase inhibitor papaverine (0.1 mM) was then added to the bath to fully relax the vessels and obtain passive measurements. In the presence of papaverine, pressure was increased to 150 mmHg, then lowered in 10 mmHg increments to obtain passive diameter measurements at each pressure and to calculate passive distensibility.
Separate sets of CTL (n=8) and ISC (n=8) arterioles were used to determine the response of arterioles to calcium ionophore A23187 or sodium nitroprusside (SNP) in the presence of L-NNA and the cyclooxygenase inhibitor indomethacin. Briefly, arterioles were equilibrated for 1 hour at 40 mmHg during which time spontaneous tone developed. L-NNA (0.1 mM) and indomethacin (10 μM) were added to the bath. Increasing concentrations of either A23187 (1–20μM) or SNP (10−8 − 3×10−5 M) were cumulatively added to the bath and diameter recorded at each concentration.
Drugs and solutions
HEPES, nifedipine, L-NNA, A23187, SNP and papaverine were all purchased from Sigma. L-NNA, SNP and papaverine were made fresh each week as stock solutions of 10−2M and stored at 4°C. A23187 was mixed fresh each day. Vessel reactivity experiments were conducted in a physiologic saline solution, the composition of which was (mM): NaCl (142), KCl (4.7), MgSO4 (1.71), EDTA (0.50), CaCl2 (2.8), HEPES (1.0), KH2PO4 (1.2), and glucose (5.0).
Data calculations
Percent tone was calculated as a percent decrease in diameter from the fully relaxed diameter in papaverine at each intravascular pressure by the equation: (1−(ϕtone/ϕpapav))*100%; where ϕtone=diameter of vessels with tone and ϕpapav=diameter in papaverine.
Contraction to L-NNA was calculated as a percent decrease in diameter from baseline.
Reactivity to SNP and nifedipine was calculated as a percent dilation from baseline.
The EC50 for nifedipine i.e., the amount of agonist necessary to dilate the arterioles 50% of maximum, was calculated for each arteriole by first plotting the concentration-response curves (sensitivity) on a logarithmic scale and extrapolating the value from a best- fit line between 20% and 80% dilation.
Distensibility was calculated at each pressure, fully relaxed in papaverine, by determining diameter changes as a function of pressure and calculated by the following equation:([ϕpressure/ϕ5mmHg] −1)×100; where ϕpressure is the diameter at that particular pressure and ϕ5mmHg is the diameter at 5 mmHg. Distensibility for each arteriole was normalized to the diameter at 1 mmHg because arterioles often collapse at 0 mmHg.
Statistical analysis
Results are presented as mean ± SEM. Differences between non-ischemic control and ischemic arterioles were determined using one-way analysis of variance (ANOVA) with p<0.05 considered significant. The animal number was used as the n-value; only one arteriole was taken per animal.
Results
Arterial diameters were measured over the pressure range from 10–150 mmHg, both actively to assess changes in myogenic activity, and passively to assess differences in size and distensibility. Under active conditions, both CTL and ISC arterioles developed significant myogenic tone during equilibration at 10 mmHg that was maintained over a large pressure range. CTL and ISC arterioles had initial diameters of 69±9μm and 67±9μm and constricted to 49±11 μm and 45±11 μm at 10 mmHg, respectively. Figure 1 shows the active and passive diameters of parenchymal arterioles at the start of the experiment, after one hour of equilibration at 10 mmHg and at each pressure within the studied range. There was no difference in the active or passive diameters between CTL vs. ISC arterioles at any pressure studied. In addition, both CTL and ISC arterioles responded myogenically to increased pressure and maintained significant tone even at pressures as high as 140 mmHg. When myogenic tone was calculated as a percent decrease in diameter from fully relaxed in papaverine, there was no significant difference in the amount of pressure-induced tone between the two groups of arterioles (data not shown). In addition, I/R had no effect on passive distensibility (data not shown).
Figure 1.
Graph showing active (circles) and passive (triangles) lumen diameters of parenchymal arterioles from control (CTL, closed symbols) and ischemic (ISC, open symbols) rats in response to increased intravascular pressure. Both groups of arterioles developed significant myogenic tone and responded myogenically to increased pressure. There was no difference in active or passive lumen diameters between groups.
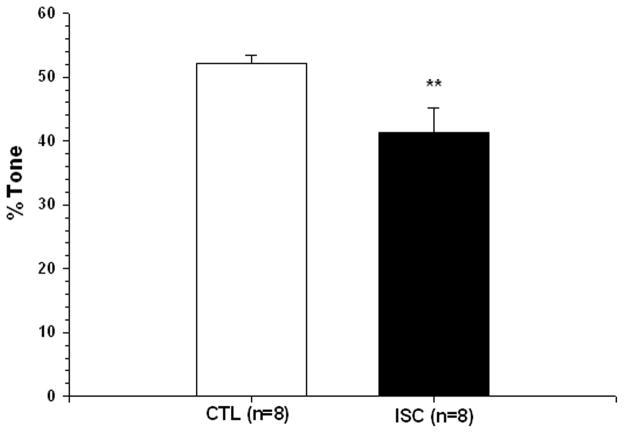
Because cerebral arteries and arterioles have considerable basal NO production that mitigates resting tone, elimination of basal NO with L-NNA causes constriction. Constriction to a large, single concentration of L-NNA (0.1mM) was used as an indirect measure of basal NO production, as has been done in other studies [13]. Figure 2 shows that the percent constriction produced by elimination of basal NO by L-NNA was significantly reduced in ischemic arterioles compared to controls: 3.2±3.3% vs.15.6±12.5% (p=0.017). When percent tone was recalculated in the presence of L-NNA, ISC arterioles had significantly less tone than CTL arterioles (Figure 3).
Figure 2.
Graph showing percent constriction to addition of 0.1 mM of the nitric oxide synthase (NOS) inhibitor nitro-L-arginine (L-NNA) in arterioles from control (CTL, open bar) and ischemic (ISC, closed bar) rats. Both types of arterioles constricted to NOS inhibition demonstrating basal NO release in these vessels. However, arterioles from ISC animals had significantly diminished constriction to L-NNA suggesting that ischemia and reperfusion diminishes basal NO production in these arterioles. **p<0.01 vs. CTL.
Figure 3.
Graph showing percent tone at 40 mmHg in the presence of the nitric oxide synthase (NOS) inhibitor nitro-L-arginine (L-NNA) in arterioles from control (CTL, open bar) and ischemic (ISC, closed bar) rats. Although basal tone was not different between groups, ISC arterioles has significantly diminished tone in the presence of L-NNA. **p<0.01 vs. CTL.
Pressure-induced contraction in cerebral arteries has been shown to be dependent on depolarization and opening of L-type calcium channels in vascular smooth muscle [11]. We used the dihydropyridine calcium channel blocker nifedipine to assess how I/R affected L-type calcium channel in smooth muscle. Addition of nifedipine to arterioles in the presence of L-NNA caused a concentration-dependent dilation in each group of parenchymal arteriole that was significantly diminished in the ISC group (Figure 4). The EC50 for nifedipine for CTL vs. ISC arterioles was: 3.54 ± 0.11nM vs. 9.90 ± 0.71nM (p=0.024).
Figure 4.
Graph showing the concentration-response curves to nifedipine in arterioles from control (CTL, closed circles) and ischemic (ISC, open circles) rats in the presence of the nitric oxide synthase (NOS) inhibitor nitro-L-arginine (L-NNA, 0.1 mM) and the cyclooxygenase inhibitor indomethacin (10 μM). Arterioles from ISC rats were significantly less sensitive to nifedipine compared to arterioles from control rats.*p<0.05 vs. CTL;**p<0.01 vs. CTL.
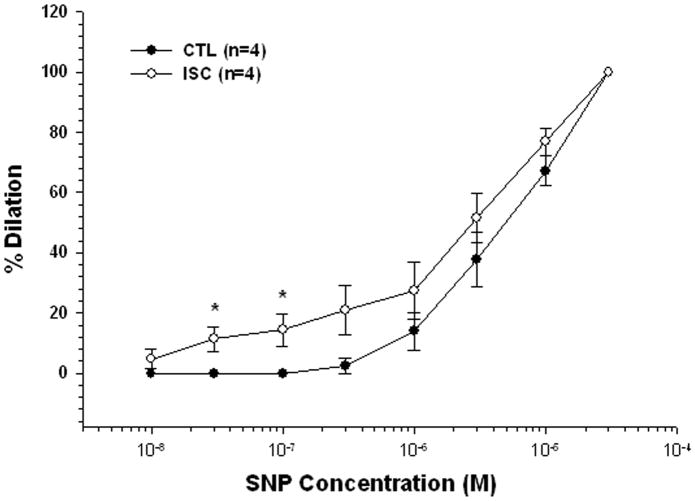
Because there was a difference in basal NO production between CTL and ISC groups, we compared the sensitivity to SNP in separate groups of arterioles to determine if the difference in constriction to L-NNA was due to a difference in sensitivity to NO. SNP caused dilation of both groups of arterioles that was increased in the ISC arterioles only at lower concentrations (<1.0 μM; Figure 5).
Figure 5.
Graph showing the concentration-response curves to sodium nitroprusside (SNP) in arterioles from control (CTL, closed circles) and ischemic (ISC, open circles) rats in the presence of the nitric oxide synthase (NOS) inhibitor nitro-L-arginine (L-NNA, 0.1 mM) and the cyclooxygenase inhibitor indomethacin (10 μM). Arterioles from ISC rats were more sensitive to the effects of SNP compared to arterioles from CTL rats only at lower concentrations.*p<0.05 vs. CTL.
Lastly, because myogenic tone was similar between groups, but basal NO production was decreased, there appeared to be another vasodilator being produced that mitigated tone similar to NO. We therefore measured EDHF production by the calcium ionophore A23187 in the presence of NOS and cyclooxygenase inhibition. A23187 caused modest dilation in CTL arterioles that was significantly enhanced in ISC arterioles (Figure 6), suggesting that similar to other studies [13,14], I/R caused increased EDHF production.
Figure 6.
Graph showing the concentration-response curves to the calcium ionophore A23817 in arterioles from control (CTL, closed circles) and ischemic (ISC, open circles) rats in the presence of the nitric oxide synthase (NOS) inhibitor nitro-L-arginine (L-NNA, 0.1 mM) and the cyclooxygenase inhibitor indomethacin (10 μM). Arterioles from ISC rats were more sensitive to the effects of calcium ionophore and dilated to a greater extent compared to arterioles from CTL rats.*p<0.05 vs. CTL;**p<0.01 vs. CTL.
Discussion
The purpose of the present study was to investigate how I/R affect the function of penetrating brain parenchymal arterioles. Under normal conditions, these small arterioles have considerable myogenic tone and contribute ~40% to cerebrovascular resistance [4,8,9,19]. We found that under basal conditions, parenchymal arterioles from animals exposed to 1 hour of ischemia and 24 hours of reperfusion appeared to have myogenic tone and reactivity to pressure that was similar to nonischemic controls. However, after I/R, elimination of basal NO production by L-NNA produced considerably less constriction, but enhanced EDHF-mediated dilations, suggesting that these arterioles maintain resting tone via NO-independent mechanisms.. Together, these results suggest that during I/R, parenchymal arterioles have altered endothelial vasodilator production, a compensation that results in similar basal tone as nonischemic controls.
Previous studies have demonstrated a significant effect of I/R on MCA myogenic tone and reactivity to pressure [1–3]. Loss of tone in the MCA was evident as early as 30 minutes of ischemia with 24 hours of reperfusion [2]. In contrast, other studies found no effect of I/R on basal tone of the MCA [12–14]. The difference between these studies may be due to presence or absence or luminal flow during the experiments or the degree of ischemia. In the present study, we found no apparent effect of I/R on basal myogenic tone of parenchymal arterioles. This result is similar to a study by Ngai et al. who also saw no effect of either 1 or 2 hours of ischemia with 24 hours of reperfusion on myogenic tone of parenchymal arterioles [19]. Interestingly, in that study, conducted vasodilation, but not constriction, was significantly enhanced in arterioles after I/R.
Although basal tone did not appear to be affected by I/R, there was a significant effect on endothelial cell vasodilator production. Arterioles exposed to I/R constricted significantly less to elimination of basal NO production. When tone was then graphed in the presence of L-NNA, arterioles had significantly less tone after I/R. These findings are similar to what was found by Marrelli et al. in the MCA after 2 hours of ischemia and 24 hours of reperfusion [13]. In that study, further examination determined that the diminished tone in the presence of NOS inhibition was associated with diminished sensitivity of the VSM to S-nitroso-N-acetylpenacillamine (SNAP) and hyperpolarization. In the present study, we tested the response of the VSM to SNP, a similar endothelium-independent vasodilator and NO donor, but did not see any decrease in sensitivity with I/R. In fact, at low concentrations of SNP, I/R caused a modest, but significant increase in sensitivity. However, it is possible that arteriolar VSM was hyperpolarized after I/R in the presence of L-NNA because the sensitivity to the dihydropyridine L-type calcium channel blocker was significantly diminished. This type of calcium channel blocker is highly sensitive to membrane potential and would have decreased sensitivity in arterioles that were hyperpolarized [17]. Alternatively, the expression and/or function of L-type calcium channels could be affected by I/R, resulting in decreased sensitivity to nifedipine. Further studies regarding the role of calcium and L-type channels function and/or expression after I/R is needed to determine the underlying cause of the altered sensitivity to nifedipine.
In addition to decreased NO production after I/R, we found that EDHF production was significantly enhanced in parenchymal arterioles. This was shown by greater dilation of ISC arterioles to the calcium ionophore A23187 in the presence of cyclooxygenase and NOS inhibition. Enhanced EDHF production in the MCA was also demonstrated in previous studies [13,14]. While the mechanism by which I/R enhances agonist-induced EDHF production is not clear, Marrelli showed that I/R caused elevated endothelial cell calcium in response to agonist stimulation to levels that are known to produce EDHF [14]. While we did not measure endothelial cell calcium in the present study, it is likely that a similar effect is occurring in parenchymal arterioles.
Lastly, in contrast to the MCA [1,5], we did not find any effect of I/R on passive distensibility of parenchymal arterioles. Passive distensibility is an indirect measure of vessel structure and elastin:collagen ratio. In a previous study, 2 hours of ischemia and 24 hours of reperfusion was shown to affect structural properties of the MCA, including increased distensibility [1,5]. However, there was no effect of I/R on parenchymal arterioles. The difference between the MCA and the parenchymal arterioles may be a reflection of the different time periods for I/R in the two studies, or the clear structural difference between the two vessel types.
In summary, the present study demonstrates parenchymal arterioles have considerable myogenic tone and reactivity to pressure that was preserved after 1 hour of ischemia and 24 hours of reperfusion. The preservation of myogenic tone appeared to be due to diminished basal NO production that was significantly less in arterioles after I/R. Together, these results suggest that the cerebral endothelium of parenchymal arterioles compensates in response to I/R to prevent the loss of basal myogenic tone, including diminished basal NO production and enhance EDHF production. In addition, smooth muscle sensitivity to L-type calcium channel blockade was significantly diminished after I/R, demonstrating an effect on both the endothelium and smooth muscle. Understanding the underlying mechanisms by which I/R alters arteriolar structure and function may provide new therapeutic targets for vascular protection.
Acknowledgments
Grant support: NINDS grant RO1 NS40071, RO1 NS043316 to MJC and the Totman Medical Research Trust.
This work was supported by the National Institutes of Health, National Institute of Neurologic Disorders and Stroke grant NS40071 (MJC) and NS043316 (MJC). We would also like to acknowledge the generous support of the Totman Medical Research Trust.
References
- 1.Cipolla M, McCall A, Lessov N, Porter J. Reperfusion decreases myogenic reactivity and alters middle cerebral artery function after focal cerebral ischemia in rats. Stroke. 1997;28:176–180. doi: 10.1161/01.str.28.1.176. [DOI] [PubMed] [Google Scholar]
- 2.Cipolla M, Lessov N, Hammer ES, Curry AB. The threshold duration of ischemia for myogenic tone in middle cerebral arteries: Effect on vascular smooth muscle actin. Stroke. 2001;32:1658–1664. doi: 10.1161/01.str.32.7.1658. [DOI] [PubMed] [Google Scholar]
- 3.Cipolla M, Curry AB. Middle Cerebral Artery Function After Stroke: The Threshold Duration of Reperfusion for Myogenic Activity. Stroke. 2002;33:2094–9. doi: 10.1161/01.str.0000020712.84444.8d. [DOI] [PubMed] [Google Scholar]
- 4.Cipolla MJ, Li R, Vitullo L. Perivascular innervation of penetrating brain parenchymal arterioles. J Cardiovasc Pharm. 2004;44:1–8. doi: 10.1097/00005344-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Coulson RJ, Chesler NC, Vitullo L, Cipolla MJ. Effects of ischemia and myogenic activity on active and passive mechanical properties of rat cerebral arteries. Am J Physiol. 2002;283:H2268–H2275. doi: 10.1152/ajpheart.00542.2002. [DOI] [PubMed] [Google Scholar]
- 6.Dirnagl U, Lindauer U. Microcirculatory disturbances in cerebral ischemia. In: Ginsberg MD, Bogousslavsky J, editors. Cerebrovascular Disease: Pathophysiology, Diagnosis and Management. Blackwell Science; Malden, MA: 1998. [Google Scholar]
- 7.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- 8.Heistad DD, Kontos HA. The Cardiovascular System III. In: Berne RM, Sperelakis N, editors. Handbook of Physiology. American Physiological Society; Bethesda, MD: 1979. pp. 137–182. [Google Scholar]
- 9.Hurn PD, Traystman RJ. Overview of cerebrovascular hemodynamics. In: Welch KMA, Caplan LR, Reis DJ, Siesjo BK, Weir B, editors. Primer on Cerebrovascular Diseases. Chapter 11. Academic Press; San Diego: 1997. pp. 42–44. [Google Scholar]
- 10.Iadecola C, Yang G, Ebner TJ, Chen G. Local and propogated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol. 1997;78:651–659. doi: 10.1152/jn.1997.78.2.651. [DOI] [PubMed] [Google Scholar]
- 11.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508(Pt. 1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marrelli SP, Johnson TD, Khorovets A, Childres WF, Bryan RM., Jr Altered function of inward rectifier potassium channels in cerebrovascular smooth muscle after Ischemia/Reperfusion. Stroke. 1998;29:1469–1474. doi: 10.1161/01.str.29.7.1469. [DOI] [PubMed] [Google Scholar]
- 13.Marrelli SP, Khorovets A, Johnson TD, Childres WF, Bryan RM., Jr P2 purinoceptor-mediated dilations in the rat middle cerebral artery after ischemia-reperfusion. Am J Physiol. 1999;276:H33–41. doi: 10.1152/ajpheart.1999.276.1.H33. [DOI] [PubMed] [Google Scholar]
- 14.Marrelli SP. Altered endothelial Ca2+ regulation after ischemia/reperfusion produces potentiated endothelium-derived hyperpolarizing factor-mediated dilations. Stroke. 2002;33(9):2285–91. doi: 10.1161/01.str.0000027439.61501.39. [DOI] [PubMed] [Google Scholar]
- 15.Mayhan WG, Faraci FM, Heistad DD. Mechanisms of protection of the blood-brain barrier during acute hypertension in chronically hypertensive rats. Hypertension. 1987;9(Suppl III):III-101–III-105. doi: 10.1161/01.hyp.9.6_pt_2.iii101. [DOI] [PubMed] [Google Scholar]
- 16.Mohr JP, Lazar RM, Marshall RS, Gautier JC, Hier DB. Middle cerebral artery disease. In: Barnett HJM, Mohr JP, Stein BM, Yatsu FM, editors. Stroke. Pathophysiology Diagnosis and Management. Churchll Livingstone; Philadelphia, PA: 1998. pp. 427–479. [Google Scholar]
- 17.Nelson MT, Worley JF. Dihydropyridine inhibition of single calcium channels and contraction in rabbit mesenteric artery depends on voltage. J Physiol. 1989;412:65–91. doi: 10.1113/jphysiol.1989.sp017604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ngai AC, Winn HR. Estimation of shear and flow rates in pial artrerioles during somatosensory stimulation. Am J Physiol. 2002;270:H1712–H1717. doi: 10.1152/ajpheart.1996.270.5.H1712. [DOI] [PubMed] [Google Scholar]
- 19.Ngai AC, Nguyen T-S, Meno JR, Britz GW. Postischemic augmentation of conducted dilation in cerebral arterioles. Stroke. 2007;38:124–130. doi: 10.1161/01.STR.0000252157.93998.47. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are the bottleneck in the perfusion of neocortex. Proc Nat’l Acad Sci USA. 2007;104:365–370. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]