Abstract
Background
Research on brain activity in schizophrenia has shown that changes in the function of any single region cannot explain the range of cognitive and affective impairments in this illness. Rather, neural circuits that support sensory, cognitive and emotional processes are now being investigated as substrates for cognitive and affective impairments in schizophrenia, a shift in focus consistent with long-standing hypotheses about schizophrenia as a “dysconnection” syndrome. Our goal was to further examine alterations in functional connectivity within and between the default mode network, and three cognitive control networks (frontal-parietal, cingulo-opercular and cerebellar) as a basis for such impairments.
Methods
Resting state fMRI was collected from 40 individuals with DSM-IV-TR schizophrenia, 31 siblings of individuals with schizophrenia, 15 healthy controls, and 18 siblings of healthy controls while they rested quietly with their eyes closed. Connectivity metrics were compared between patients and controls for both within and between network connections, and were used to predict clinical symptoms and cognitive function.
Results
Individuals with schizophrenia showed reduced distal and somewhat enhanced local connectivity between the cognitive control networks as compared to controls. Additionally, greater connectivity between the frontal-parietal and cerebellar regions was robustly predictive of better cognitive performance across groups, and predictive of fewer disorganization symptoms among patients.
Conclusions
These results are consistent with the hypothesis that impairments of executive function and cognitive control result from disruption in the coordination of activity across brain networks, and additionally suggest that these might reflect impairments in normal pattern of brain connectivity development.
Keywords: Schizophrenia, Functional Connectivity, Cognitive Control, Cerebellum, Risk
Introduction
Research focused on elucidating the neural systems that contribute to cognitive impairments among individuals with schizophrenia (1–4) suggests that changes in the function of a single region cannot explain the range of impairments seen in this illness. As such, research has increasingly focused on understanding the integrity of neural circuits that work together to support sensory, cognitive and emotional processes (5). This shift in focus is consistent with long-standing hypotheses about schizophrenia as a “dysconnection” syndrome (6,7).
The efforts to understand altered brain connectivity in schizophrenia have been aided by recent work identifying core networks in the brains of healthy individuals. For example, a “default mode” network (DMN) has been identified (8,9), which consists of a set of brain regions that reliably reduce their activity during active cognitive demands (10), and which may be involved in processes such as attention to internal emotional states (11), self-referential processing (12), or task-independent thought (13). Other work has identified networks that are activated by a variety of cognitive tasks, including a dorsal fronto-parietal network (FP), a cingulo-opercular network (CO), and a cerebellar network (CER) (14–16). The FP network is engaged by a wide range of higher level cognitive tasks and is thought to be involved in adaptive task control (14–16). The CO network is also engaged in a variety of tasks, but is thought to be involved in stable task-set maintenance and error processing (14–16). The CER network also shows error-related activity in a range of tasks, and its activity often covaries with the activity of the dorsal fronto-parietal and cingulo-opercular networks (14–17). Some of the work identifying these networks has focused on examining connectivity among and between networks during resting state. This work compliments research on task related activation of networks by examining the functional correlations between regions that may not be simply a result of deterministic task demands and which may help shape the ability of networks to respond to task demands.
A number of studies have identified abnormalities in the function of the DMN in schizophrenia, with the interpretation that impaired connectivity in this network may contribute to difficulties in disengaging attention to internal states (18). However, the nature of these abnormalities has been variable across studies. For example, work by Whitfield-Gabrieli and colleagues identified abnormally enhanced connectivity within the DMN among individuals with schizophrenia and their first-degree relatives as compared to controls, as well as reduced task-related deactivation (18). Somewhat consistent with this finding, Salvador et al found that a medial prefrontal region of the DMN showed hyper-connectivity among individuals with schizophrenia in an overall brain connectivity analysis (19). In contrast, a number of other studies have found either reduced connectivity in the DMN in schizophrenia (20–23), or a mixed pattern of increased and decreased connections within the DMN (24).
Other studies have identified abnormalities in the connectivity of regions that play a part in the FP and CO networks. For example, Welsh and colleagues found reduced functional connectivity between the medial dorsal nucleus of the thalamus and the anterior cingulate and caudate (25). Zhou and colleagues found reduced functional connectivity between bilateral dorsolateral prefrontal cortex and regions of the parietal cortex, thalamus and striatum in first episode patients with schizophrenia (26).
Although functional connectivity within a given network is clearly important to understanding how network impairments may contribute to disease states, relationships among these networks may be equally important. Changes in how networks integrate and segregate from one another are an important feature of cognitive development (16,27,28). For example, impaired neurodevelopmental processes in schizophrenia (e.g., impaired synaptic plasticity, white matter development or neural migration (6)) could lead to disruptions in the interactions across as well as within brain networks. However, to date, only a few studies have provided data relevant to this hypothesis.
Shen and colleagues found that reduced connectivity between a range of frontal/cingulate regions and the cerebellum contributed most strongly to discriminating individuals with schizophrenia from controls in an unsupervised-learning classifier (29). Zhou et all found a reduced negative correlation between dorsolateral prefrontal cortex and the precuneus (part of the DMN) among the individuals with schizophrenia compared to controls (26). Similarly, Zhou et al found altered connectivity between a task-negative network (overlapping with the DMN) and a task positive network (both FP and CO regions) in schizophrenia (30). Jafri (31) used independent component analysis to identify seven networks in resting state data. One of these networks corresponded to the DMN, and several of the other networks engaged regions involved in the FP and CO networks, as well as additional temporal and subcortical regions. Examination of the relationships between these networks revealed altered connectivity between the default network and two of the additional networks that strongly overlapped with the FP and CO networks in individuals with schizophrenia.
The goal of the current study was to examine alterations in functional connectivity within and between the DMN, FP, CO and CER networks to provide further evidence that schizophrenia reflects – at least in part – a dysconnection syndrome. We hypothesized that individuals with schizophrenia and their siblings would show evidence of impaired connectivity between networks involved in cognitive control, as well as potentially between cognitive control networks and either or both the DMN and CER networks. We studied both individuals with schizophrenia and their currently non-affected siblings. We included non-affected siblings both to address potential confounds associated with medication status (since siblings have never been exposed to antipsychotic medications) and to determine the degree to which changes in connectivity may reflect an endophenotypic marker of risk for psychosis versus a marker of manifest illness.
Methods
Participants
The participants (Table 1) for this study were recruited through the Conte Center for the Neuroscience of Mental Disorders (CCNMD) at Washington University School of Medicine in St. Louis, and included1: (1) probands who were individuals with DSM-IV Schizophrenia (SCZ; N=25); 2) the non-psychotic siblings of individuals with schizophrenia (SCZ-SIB; N=31); (3) healthy controls (CON; N=15); and (4) the siblings of healthy controls (CON-SIB; N=18). Siblings were full siblings, based on self-report. All participants gave written informed consent for participation.
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Measure | Group |
|||||||
|---|---|---|---|---|---|---|---|---|
| Healthy Controls (CON) | Siblings of Controls (CON- SIB) | Individuals with Schizophrenia (SCZ) | Siblings of Schizophrenia (SCZ-SIB) | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age | 23.4 | 2.9 | 22.2 | 2.9 | 24.4 | 3.1 | 24.3 | 3.7 |
| Gender (% male) | 60% | 61% | 72% | 58% | ||||
| Education | 14.2 | 1.9 | 13.7 | 1.8 | 12.2 | 1.8 | 13.5 | 2.5 |
| Parental Education | 13.9 | 1.8 | 14.5 | 1.5 | 14.3 | 2.2 | 15.0 | 2.4 |
| History of Substance Dependence | 0% | 6% | 24% | 16% | ||||
| History of Substance Abuse | 0% | 22% | 44% | 23% | ||||
| Negative Symptoms* | −.39 | 0.21 | −.31 | 0.41 | 1.17a | 0.76 | −.11 | 0.54 |
| Positive Symptoms* | −.48 | 0.13 | −.31 | 0.40 | 1.02 a | 1.07 | −.28 | 0.28 |
| Disorganization Symptoms* | −.27 | 0.38 | −.24 | 0.26 | 0.88 a | 1.08 | −.16 | −.34 |
| IQ^ | −.12 | 0.80 | −.49 | .78 | −1.05 a | .79 | −.37 | .78 |
| Working Memory^ | 0.67 | 0.64 | 0.43 | 0.70 | 0.28 a | 0.74 | −.63 | 0.75 |
| Episodic Memory^ | 0.53 | 0.79 | 0.30 | 0.78 | 0.07 a | 0.56 | −.85 c | 0.51 |
| Executive Function^ | 0.61 | 0.44 | 0.50 | 0.44 | 0.16 a | 0.47 | −.47 b | 0.90 |
Note: The four groups did not differ significantly in age (F(3,85) = 2.0, p>0.10), parental education (F(3,85) = 0.66, p>0.50, or gender (X2=1.3, p>0.5) or race (X2=3.05, p>0.55). The groups did differ in personal education (F(3,85) = 3.68, p<.05), with the individuals with schizophrenia having significantly fewer years of education than controls.
The Ns for individuals with schizophrenia and their siblings and controls and their siblings are not identical given that some participants were excluded for failure to complete the entire protocol or excessive movement during the functional connectivity runs. Of the 101 participants aged 18 or older from whom we collected resting state data, there were 11 who had to be excluded for poor quality imaging data (3 SCZ, 4 SCZ-SIB, 1 CON and 3 SCN).
Symptom scores are reported in Z scores relative to the mean of the entire sample. See Methods for details. One-way ANOVAs comparing the four groups on each of these three symptom domains indicated significant group differences for positive (F(3,85) = 29.16, p<.001), negative (F(3,85) = 39.77, p<.001), and disorganization (F(3,85) = 17.68, p<.001) symptoms.
Post-hoc contrasts using Tukey’s HSD indicated that the SCZ participants had higher scores on all three symptom domains, with no significant differences among the remaining groups.
Cognitive scores are reported in Z scores relative to the mean of the entire sample. See Methods for details. One-way ANOVAs comparing the four groups on each of these four cognitive domains indicated significant group differences for IQ (F(3,85) = 5.47, p<.001), working memory (F(3,85) = 13.57, p<.001), episodic memory (F(3,85) = 18.57, p<.001) and executive function (F(3,85) = 13.24, p<.001).
Post-hoc contrasts using Tukey’s HSD indicated that the SCZ participants had worse performance in all four cognitive domains than CON and CON-SIB.
SCZ-SIB performed worse than CON and CON-SIB on executive function, and showed a
trend for reduced performance on episodic memory (p<.10).
All subjects were diagnosed on the basis of a consensus between a research psychiatrist who conducted a semi-structured interview and a trained research assistant who used the Structured Clinical Interview for DSM-IV Axis I Disorders (32). Participants were excluded if they: (a) met DSM-IV criteria for substance dependence or severe/moderate abuse during the prior 6 months; (b) had a clinically unstable or severe medical disorder; (c) had a history of head injury with documented neurological sequelae or loss of consciousness; or (d) met DSM-IV criteria for mental retardation.
The individuals with schizophrenia were all outpatients, and were stabilized on antipsychotic medication for at least two weeks. Controls were required to have no lifetime history of Axis I psychotic or mood disorders and no first-degree relatives with a psychotic disorder. Potential SCZ-SIB subjects were excluded if they had a lifetime history of any DSM-IV Axis I psychotic disorder, but not other DSM-IV Axis I disorders. CON-SIB subjects were enrolled in an identical manner to SCZ-SIB subjects, and met the same general and specific inclusion and exclusion criteria.
Clinical and Cognitive Assessments
Psychopathology and cognitive function were assessed as previously described (33,34) and as described in the Supplement. Scores for each symptom domain and each cognitive domain are shown in Table 1.
fMRI Scanning
All scanning occurred on a 3T Tim TRIO Scanner at Washington University Medical School. Functional images (BOLD) were acquired using an asymmetric spin-echo, echo-planar sequence (T2*) (repetition time [TR] = 2500 ms, echo time [TE] = 27 ms, field of view [FOV] = 256 mm, flip=90°, voxel size=4× 4× 4 mm). Data were acquired from each participant for two BOLD runs in which participants rested quietly with their eyes closed. Each run contained 164 images, for a total of 328 images and 13.7 minutes of resting state activity. In addition, a T1 structural image was acquired using a sagittal MP-RAGE 3D sequence (TR=2400 ms, TE=3.16 ms, flip=8°; voxel size=1 × 1 × 1 mm).
fcMRI Data Preprocessing
Data preprocessing included: 1) Compensation for slice-dependent time shifts; 2) Removal of first 5 images from each run during which BOLD signal was allowed to reach steady state; 3) Elimination of odd/even slice intensity differences due to interpolated acquisition; 4) Realignment of data within and across runs to compensate for rigid body motion (35); 5) Intensity normalization to a whole brain mode value of 1,000; 6) Registration of the 3D structural volume (T1) to the atlas representative template in the Talairach coordinate system (36) using a 12-parameter affine transform; and 7) Co-registration of the 3D fMRI volume to the structural image and transformation to atlas space using a single affine 12-parameter transform that included a re-sampling to a 3 mm cubic representation. In addition, prior to performing fcMRI analyses, all raw time series BOLD images were further preprocessed to remove baseline and possible sources of spurious correlations, as outlined in the Supplement. Each of the two BOLD runs was preprocessed independently, the two runs were then concatenated into a single timeseries before fcMRI analyses. The initial BOLD preprocessing was accomplished using in-house software, fcMRI preprocessing and analyses described below were performed using custom Matlab code. See Supplement for SNR analyses.
Network Region Definition
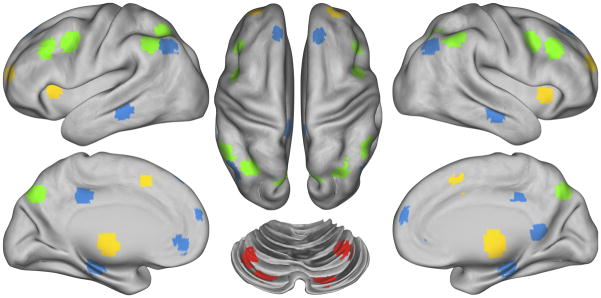
We examined regions included in the DMN as defined by Fox (9), and regions included in the FP, CO and CER networks as defined by Dosenbach (14). To control for individual anatomical variability, regions of interest were defined for each individual in two steps. First, we created spherical ROIs in standard Talairach space centered on the reported coordinates for each region (Figure 1, and Table S1) and 15mm in diameter. Second, we masked the resulting group ROIs with the individual FreeSurfer segmentation of high-resolution structural image that was previously registered to standard Talairach space, excluding any voxels within the group defined ROIs that did not represent the relevant gray matter in the specific individual. We extracted the time series for each of these ROIs and computed the ROI-ROI correlation matrix for all ROIs for each participant. We estimated group-level statistical significance by converting individual correlations to Fisher z values using Fisher r-to-Z transform and used these as the dependent measure.
Figure 1.
Figure illustrating the location of regions within each of the four networks. Regions of the Frontal-Parietal network (FP) are marked in green, the Cingulo-Opercular network (CO) in yellow, the Default Mode Network (DMN) in blue and the Cerebellar network (CER) in red.
Data Analysis
We computed the average connectivity (mean Fisher z value) across all ROI-ROI connections within each of the four networks, and computed the average connectivity across all ROI-ROI connections between each network. We denoted within network averages as wDMN, wFP, wCO, & wCER, and between network connectivity averages as bDMN-FP, bDMN-CO, bDMN-CER, bFP-CO, bFP-CER, and bCO-CER. We used separate repeated measures ANOVAs to compare the groups on within and between network connectivity using these overall measures. We then conducted secondary analyses, using False Discovery Rate to control for multiple comparisons, examining connectivity of each region within a network to its own network and to the other three networks. For the sake of brevity, we do not report main effects or interactions that do not include group. We also conducted analyses that included hemisphere as a factor. This did not change the results reported below, and are presented in the Supplement.
Results
Within Network Connectivity
The within-network ANOVA included diagnostic group and sibling type as between-subject factors, and network as a within subject factor. This ANOVA revealed a trend level main effect of diagnostic group (F(1,85) = 3.34, p=.07), which reflected slightly lower within network connectivity among SCZ and SCZ-SIB as compared to CON and CON-SIB (Figure 2). We next examined whether specific regions within any of the networks showed reduced connectivity with its own network. To do so, we computed, for each region in a network, the average connectivity between it and all other regions in the network. We then used a FDR correction for multiple comparisons within each network. None of the individual regions within any of the networks showed significantly reduced connectivity among SCZ and/or SCZ-SIB.
Figure 2.
Graph illustrating within network connectivity in each of the four groups. SCZ = individuals with schizophrenia; SCZ-SIB = siblings of individuals with schizophrenia; CON = healthy controls; CON-SIB = siblings of healthy controls: DMN = Default Mode Network; FP = Frontal Parietal Network; CO = Cingulo-Opercular Network; CER = Cerebellar Network; w = within. Segments marked in blue indicate networks for which group differences did not reach statistical significance.
Between Network Connectivity
Next we examined between-network connectivity using the same analysis approach. This ANOVA revealed a highly significant main effect of diagnostic group (F(1,85) = 14.58, p<.001) and network (F(5,425) = 32.23, p<.001), as well as a significant interaction between diagnostic group and network (F(5,425) = 5.76, p<.001), but no significant three-way interaction between network, diagnostic group and sibling type (F(5,425) = 0.57, p>.70). As shown in Figure 3, the interaction was driven by the fact that the SCZ and SCZ-SIB showed significantly reduced connectivity between the CO and FP, the CO and CER, and the FP and CER networks, but did not show reduced connectivity between the DMN and any other network.
Figure 3.
Graph illustrating between network connectivity in each of the four groups. SCZ = individuals with schizophrenia; SCZ-SIB = siblings of individuals with schizophrenia; CON = healthy controls; CON-SIB = siblings of healthy controls: DMN = Default Mode Network; FP = Frontal Parietal Network; CO = Cingulo-Opercular Network; CER = Cerebellar Network; b= between. Segments marked in red indicate networks for which connectivity measures showed significant diagnostic group differences. For those networks, the shaded boxes delineate the diagnostic groups (pink = individuals with schizophrenia and their siblings; purple = healthy controls and their siblings). Segments marked in blue indicate networks for which group differences did not reach statistical significance.
We were interested in examining whether the reduced connectivity between the CO, FP and CER networks was a general property of all regions within these networks, or specific to some regions. To address this question, we computed the average connectivity of each region within a network with all of the regions in another network (e.g., the average connectivity of the RdlPFC within the FP with all regions of the CO, or with all regions of the CER). We again used FDR to correct for multiple comparisons.
Frontal-Parietal Network
Within the FP network (Figures S1a and S1b), the right IPS region showed significantly reduced connectivity with the CO network among SCZ and SCZ-SIB (p=.001). In addition, bilateral IPS and IPL regions, as well as the left dlPFC all showed reduced connectivity with the CER network (all ps<.008). No FP regions showed significantly reduced connectivity with the DMN network among SCZ and SCZ-SIB (Figure S2a).
Cingulo-Opercular Network
Several regions (Figures S1a and S1b) within the CO network showed reduced connectivity with the FP network in SCZ and SCZ-SIB, including bilateral fO (p=.003) and left aTH (p=.008). In addition, several regions showed reduced connectivity with the CER network among SCZ and SCZ-SIB, including dACC (p=.009), bilateral aTh (both p<.001), and left aPFC (p=.002). Again, no regions within the CO network showed altered connectivity with the DMN network among SCZ or SCZ-SIB (Figure S2b).
Cerebellar Network
All but one CER region (right IC, see Figure S3) showed reduced connectivity with the FP network in SCZ and SCZ-SIB (all p<.007), and all four CER regions showed reduced connectivity with the CO network in SCZ and SCZ-SIB (all p<.002). In contrast, none of the CER regions showed reduced connectivity with the DMN among SCZ or SCZ-SIB.
Default Mode Network
Only two DMN regions (CT and pCIN) showed reduced connectivity with the CO network among SCZ and SCZ-SIB (p<.003; see Figure S4). No DMN regions showed reduced connectivity with either the FP or the CER networks among SCZ or SCZ-SIB
Local Versus Distal Connections
The analyses presented above focused on within and between network connectivity. However, one might argue that this distinction could be biased by the distance between regions within networks versus between networks. Thus, we divided connections between all the regions in all four networks into five categories: 1) between homologous regions; 2) within the same network and within the same lobule (within-local); 3) between different networks, but within the same lobule (between-local); 4) within the same network but between different lobules (within-distal); and 5) between different networks and different lobules (between-distal).
We analyzed these data using diagnostic group and sibling type as between subject factors and network type (between, within) and lobule (local, distal) as within subject factors, ignoring the homologous connections. This analysis revealed a significant three-way interaction between diagnostic group, network type, and lobule (F(1,85) = 12.92, p<.001). To parse this interaction, we conducted ANOVAs with diagnostic group and sibling type as factors for each of the four connection types. These analyses indicated a significant main effect of diagnostic group for both between-local (F(1,85) = 4.18, p<.05) and between-distal (F(1,85) = 13.75, p<.001) connections, but no significant effects of diagnostic group for either within-local (F(1,85) = 0.74, p>.35) or within-distal (F(1,85) = 2.28, p>.10) connections. Interestingly, the source of these main effects of diagnostic group differed for between-local versus between-distal connections. SCZ and SCZ-SIB (see Figure S5) showed reduced connectivity for between-distal connections, but increased connectivity for between-local connections.
Relationship to Clinical and Cognitive Variables
We conducted hierarchical regressions for the three network connectivity metrics that differed between groups (bFP-CO, bFP-CER, bCO-CER), predicting our cognitive and clinical measures (Table 1). In step 1, we entered categorical variables for group status. In Step 2, we entered the connectivity measures. In Step 3, we entered interaction terms between group status and the connectivity measures, to determine if there were group differences in the relationship between that connectivity measure and the dependent variable. To protect against false-positives, we used p<.01. For bFP-CO and bCO-CER connectivity, there were no significant increases in variance accounted for by adding the connectivity measures in Step 2 (Table 2). However, for bFP-CER, the increase in variance accounted for in step 2 was significant for all four cognitive measures, with greater connectivity predicting better performance (Table 2). Step 3 was not significant for any of the cognitive measures, with the scatter plots visually confirming a similar relationship between bFP-CER and cognitive performance in all groups (Figure S6). In addition, bFP-CER connectivity accounted for a significant increase in explained variance in disorganization symptoms in Step 2, with greater connectivity associated with less disorganization (Table 3). However, Step 3 was also significant (p<.01), indicating a significant group difference in the relationship between bFP-CER connectivity and disorganization symptoms. Follow-up correlations conducted for each group revealed a significant negative correlation in the SCZ (r = −.48, p<.05), but not in SCZ-SIB (r=−.07, p>.2), CON (r=−.35, p>.2), or CON-SIB (r=−.32, p>.19). See Supplement for additional mediation analyses.
Table 2.
Beta Coefficients Describing the Relationship Between Connectivity Measures and Clinical and Cognitive Variables
| Connectivity Type |
|||
|---|---|---|---|
| FP to CO Connectivity | FP to CER Connectivity | CO to CER Connectivity | |
| Cognitive Domains | β | β | β |
| IQ | −.07 | .28** | .14 |
| Working Memory | −.10 | .35*** | .16 |
| Episodic Memory | −.11 | .25** | .11 |
| Executive Function | −.04 | .32*** | .24 |
| Clinical Domains | |||
| Positive Symptoms | −.01 | −.04 | −.07 |
| Negative Symptoms | .03 | −.09 | −.05 |
| Disorganization | −.01 | −.26** | −.21 |
p<.01;
p<.001
Discussion
The goal of the current study was to examine differences in functional connectivity within and between known brain networks in patients with schizophrenia and their unaffected siblings to test the hypothesis that schizophrenia involves disruptions in the coordinated activity of brain regions. Our results suggest that both individuals with schizophrenia and their siblings have impaired brain connectivity, and that these impairments are most prominent between networks as compared to within networks. The presence of these abnormalities in the siblings of individuals suggests that they are not due to treatment and other secondary environmental factors. Importantly, the strength of connectivity between the FP and CER networks was associated with better cognitive function across all domains in all groups, and was associated with fewer disorganization symptoms among the patients with schizophrenia. These findings suggest that the observed differences in connectivity have important functional implications for patients with schizophrenia.
The FP, CO and CER network are thought to be key networks involved in cognitive control, task set maintenance and error processing (14,15,17). We did not find that individuals with schizophrenia and their siblings showed impaired connectivity within these networks, but did show impairments in the connectivity across networks. These results are consistent with a number of prior studies that also found reduced connectivity between regions in CO, FP and CER networks (26,29), they though differ from those studies that also found reduced connectivity between DMN and regions in CO or FP networks (30,31). The fact that impairments in the connectivity between the FP and CER networks consistently predicted cognitive performance across domains and across groups further speaks to the importance of these networks for a range of cognitive processes and the need to evaluate the functionality of the networks as a whole and not just individual regions. Further, the finding that the CER network was involved in these disruptions is consistent with previous suggestions that cognitive impairments in schizophrenia reflect deficits in cortical-subcortical-cerebellar circuits (7). Although the precise contribution of the CER to higher level cognition is not yet clear, it has been speculated that the CER may play a key role in learning from errors, and in the timing and sequencing of a range of cognitive functions (37–42). Thus, disruptions in the coordination of CER activity with other networks may have major implications for impairments in cognitive adaptation and coordination in schizophrenia.
Interestingly, although we found evidence for decreases in average between-network connectivity for some of the networks, these decreases were primarily driven by reductions in between-network connectivity for more distal (across lobule) connections, with contrasting evidence of increased connectivity for more local connections. This combination resulted in decreased average between-network connectivity due to the higher number of distal versus local connections in these analyses.. These results are intriguing from the perspective of hypotheses about the cellular mechanisms that may underlie disrupted connectivity in schizophrenia. For example, Stephan and colleagues argued that such dysconnection in schizophrenia could arise from disruptions in the “wiring” of association fibers during the course of brain development (43–46); and/or impaired synaptic plasticity. There is now a growing body of research on the normative development of the FP, CO, and CER networks, which suggests that development proceeds from what has been described as a “local to distributed” fashion (16,27). In other words, normative development is characterized by a decrease in correlation strength among spatially close brain regions (local) and an increase in correlation strength between spatially distant brain regions (distal). As such, the patterns of altered connectivity in schizophrenia could reflect – at least in part – alterations in the normative development of connectivity among these networks that results in stronger local, but reduced distal, connectivity. However, we should note that these patterns of altered local and distal connectivity in schizophrenia were only present for between network connections. In the developmental literature, the progression of “local to distributed” changes occurred both within and between networks. This suggests that additional processes or mechanisms that specifically influence the coordination of activity across networks may be altered in schizophrenia. For example, one speculative hypothesis is that the interactions among networks may develop later than connectivity within networks, potentially occurring during the pubertal period. If so, connectivity between networks could potentially be susceptible to greater disruption by the processes or mechanisms that may be contributing to the increased risk for schizophrenia that seems to arise through the course of puberty.
Surprisingly, we did not find evidence for disrupted connectivity within or with the DMN. While there is evidence for altered DMN connectivity in schizophrenia, prior findings has been mixed (18–24). It is possible that factors such as stage of illness may influence these variable results. Our patients were relatively young and early in the course of illness as compared to patients in a number of the studies that did find altered DMN connectivity (18,20,21,31). This raises the possibility that altered DMN connectivity may evolve as a function of extended experience with altered internal experiences. However, we should note that the findings of Whitfield-Gabrieli (18) (enhanced DMN connectivity in schizophrenia) are not consistent with this suggestion, given the young age of their sample.
The current study had several limitations. First, the individuals with schizophrenia were medicated, and there is some evidence that antipsychotic medications might reduce resting state functional connectivity across neural networks (47). However, the fact that the majority of our results were also present in the siblings of the individuals with schizophrenia – who were not taking any antipsychotic medications -- argues against this interpretation. Second, while we suggest that our results may reflect the outcome of disrupted developmental processes, our data are cross-sectional and cannot directly address developmental issues. Third, we could not control for the arousal level of our participants during the resting state scans, and it is possible that arousal levels differed across groups. However, given our focal pattern of connectivity differences across groups, global changes in arousal as a factor contributing to group differences is less likely to be a confounding factor.
In summary, the current study contributes important new information to the body of the literature on the functional significance of brain network connectivity and its impairment in schizophrenia. We found that connections among brain networks thought to be critical for cognitive control were reduced among individuals with schizophrenia and their unaffected siblings, and that these reductions were associated with both cognitive impairments and clinical symptoms. These alterations may reflect impairments in the neurodevelopment of these key brain circuits, a hypothesis that remains to be directly tested in longitudinal studies of brain maturation in individuals at risk for the development of the illness.
Supplementary Material
Acknowledgments
We thank the staff of the Administrative/Assessment and Biostatistical Cores of the CCNMD at Washington University School of Medicine for collection of the clinical and imaging data and data management. This research was supported by NIH grants P50 MH071616 and R01 MH56584.
Footnotes
Financial Disclosures
Dr. Barch has received grants from the NIMH, NIA, NARSAD, Allon, Novartis, and the McDonnell Center for Systems Neuroscience Dr. Csernansky has received research grants from the NIMH and NIA, and receives honoraria for serving on data monitoring committees for Eli Lilly and Sanofi-Aventis. Dr. Repovs is a consultant on NIMH grants.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology. 2006;20:497–510. doi: 10.1037/0894-4105.20.5.497. [DOI] [PubMed] [Google Scholar]
- 2.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: Meta-analysis. British Journal of Psychiatry. 2005;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- 4.Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry. 2009;166:863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calhoun VD, Eichele T, Pearlson G. Functional brain networks in schizophrenia: a review. Front Hum Neurosci. 2009;3:17. doi: 10.3389/neuro.09.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 8.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Perterson SE. Common blood flow changes across visual tasks: II. decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 11.Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 12.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGuire PK, Paulesu E, Frackowiak RS, Frith CD. Brain activity during stimulus independent thought. Neuroreport. 1996;7:2095–2099. [PubMed] [Google Scholar]
- 14.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008 doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvador R, Sarro S, Gomar JJ, Ortiz-Gil J, Vila F, Capdevila A, et al. Overall brain connectivity maps show cortico-subcortical abnormalities in schizophrenia. Hum Brain Mapp. 2010 doi: 10.1002/hbm.20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117:21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Camchong J, Macdonald AW, 3rd, Bell C, Mueller BA, Lim KO. Altered Functional and Anatomical Connectivity in Schizophrenia. Schizophr Bull. doi: 10.1093/schbul/sbp131. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, et al. Retrosplenial cortex connectivity in schizophrenia. Psychiatry Res. 2009;174:17–23. doi: 10.1016/j.pscychresns.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mannell MV, Franco AR, Calhoun VD, Canive JM, Thoma RJ, Mayer AR. Resting state and task-induced deactivation: A methodological comparison in patients with schizophrenia and healthy controls. Hum Brain Mapp. 2009;31:424–437. doi: 10.1002/hbm.20876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welsh RC, Chen AC, Taylor SF. Low-Frequency BOLD Fluctuations Demonstrate Altered Thalamocortical Connectivity in Schizophrenia. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- 27.Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fair DA, Cohen AL, Church JA, Miezin FM, Barch D, Raichle ME, et al. The maturing architecture of the brain’s default network. Proceedings of the National Academy of Sciences. 2008;105:4028–4035. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen H, Wang L, Liu Y, Hu D. Discriminative analysis of resting-state functional connectivity patterns of schizophrenia using low dimensional embedding of fMRI. Neuroimage. 2010;49:3110–3121. doi: 10.1016/j.neuroimage.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 31.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for the DSM-IV-TR Axis I disorders. Washington, D. C: American Psychiatric Press; 2001. [Google Scholar]
- 33.Delawalla Z, Barch DM, Fisher Eastep JL, Thomason ES, Hanewinkel MJ, Thompson PA, Csernansky JG. Factors mediating cognitive deficits and psychopathology among siblings of individuals with schizophrenia. Schizophrenia Bulletin. 2006;32:525–537. doi: 10.1093/schbul/sbj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harms MP, Wang L, Mamah D, Barch DM, Thompson PA, Csernansky JG. Thalamic shape abnormalities in individuals with schizophrenia and their nonpsychotic siblings. J Neurosci. 2007;27:13835–13842. doi: 10.1523/JNEUROSCI.2571-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ojemann J, Akbudak E, Snyder A, McKinstry R, Raichle M, Conturo T. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- 36.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 37.Durisko C, Fiez JA. Functional activation in the cerebellum during working memory and simple speech tasks. Cortex. 2010;46:896–906. doi: 10.1016/j.cortex.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 39.Ben-Yehudah G, Guediche S, Fiez JA. Cerebellar contributions to verbal working memory: beyond cognitive theory. Cerebellum. 2007;6:193–201. doi: 10.1080/14734220701286195. [DOI] [PubMed] [Google Scholar]
- 40.Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB, Fiez JA. Cerebellar damage produces selective deficits in verbal working memory. Brain. 2006;129:306–320. doi: 10.1093/brain/awh685. [DOI] [PubMed] [Google Scholar]
- 41.Fiez JA. Cerebellar contributions to cognition. Neuron. 1996;16:13–15. doi: 10.1016/s0896-6273(00)80018-5. [DOI] [PubMed] [Google Scholar]
- 42.Fiez JA, Petersen SE, Cheney MK, Raichle ME. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain. 1992;115(Pt 1):155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- 43.Bullmore ET, Frangou S, Murray RM. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28:143–156. doi: 10.1016/s0920-9964(97)00114-x. [DOI] [PubMed] [Google Scholar]
- 44.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 45.Feinberg I. Cortical pruning and the development of schizophrenia. Schizophr Bull. 1990;16:567–570. doi: 10.1093/schbul/16.4.567. [DOI] [PubMed] [Google Scholar]
- 46.Pantelis C, Yucel M, Wood SJ, Velakoulis D, Sun D, Berger G, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 47.Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.