Abstract
Background
Behavioral inflexibility is a feature of schizophrenia, attention deficit-hyperactivity disorder, and behavior addictions that likely results from heritable deficits in the inhibitory control over behavior. Here, we investigate the genetic basis of individual differences in flexibility, measured using an operant reversal learning task.
Methods
We quantified discrimination acquisition and subsequent reversal learning in a cohort of 51 BXD strains of mice (2–5 mice/strain, N = 176) for which we have matched data on sequence, gene expression in key CNS regions, and neuroreceptor levels.
Results
Strain variation in trials to criterion on acquisition and reversal was high, with moderate heritability (~0.3). Acquisition and reversal learning phenotypes did not covary at the strain level, suggesting that these traits are effectively under independent genetic control. Reversal performance did covary with dopamine D2 receptor levels in the ventral midbrain, consistent with a similar observed relationship between impulsivity and D2 receptors in humans. Reversal, but not acquisition, is linked to a locus on mouse chromosome 10 with a peak LRS at 86.2Mb (p <.05 genome-wide). Variance in mRNA levels of select transcripts expressed in neocortex, hippocampus, and striatum correlated with the reversal learning phenotype, including Syn3, Nt5dc3 and Hcfc2.
Conclusions
This work demonstrates the clear trait independence between, and genetic control of, discrimination acquisition and reversal and illustrates how globally coherent data sets for a single panel of highly-related strains can be interrogated and integrated to uncover genetic sources and molecular and neuropharmacological candidates of complex behavioral traits relevant to human psychopathology.
Keywords: cognitive flexibility, impulsivity, response inhibition, genetics, quantitative trait loci, recombinant inbred mice
Introduction
Several neuropsychiatric disorders – attention deficit hyperactivity disorder and behavior addictions, amongst them - involve difficulty with suppressing pre-potent behavior and behavioral inflexibility (1–4). These phenotypes, which relate to poor inhibitory response control, may depend on heritable variation in dopamine system dynamics (5, 6) and are likely linked to variation in cognition and executive function (6–16). Laboratory tests of inhibitory control, including the ability to stop or change a response during reversal learning reveal complex relationships between dopamine synthesis capacity, D2 receptor activity, and reversal performance (5, 17), and also expose deficits in subjects diagnosed with attention-deficit hyperactivity disorder and behavior addictions (18–23). Reversal learning studies reveal a robust pattern of findings across species and research paradigms, consistently relating task performance to orbitofrontal cortex in rodents (24, 25), monkeys (26, 27) and humans (28, 29). Correspondingly, structural/functional pathology within the orbitofrontal cortex is associated with deficits of inhibitory control and behavioral flexibility in ADHD and addictions (23, 30).
Impaired behavioral inhibition is thought to have a heritable component (31–33), but sequence variants or single nucleotide polymorphisms that account for substantial variation in humans have been difficult to identify (7, 34–36). Operant procedures that capture aspects of inhibitory control and behavioral flexibility can be mastered with reasonable efficiency by most strains of mice (37), making it practical to measure relevant trait variation across large panels of inbred mice that vary phenotypically across the natural range, and for which well-defined, extensively curated genotypes exist. These improvements make it possible to efficiently map genetic causes of, and relations between, flexibility-related phenotypes.
Genetic reference populations such as the BXD panel of recombinant inbred mice (38) have been used for over two decades to study the genetic basis of variation in behavioral traits. The BXD strains (~80 in number) trace their descent from fully-sequenced parental strains, so sequence variation throughout the panel is exceptionally well-defined, each strain representing a unique mosaic of B and D alleles, preserved in perpetuity, so that phenotypic data collected by different researchers at different times can be archived and compared with new data at any time. The BXD strains have been typed for a wide range of behavioral, anatomical, and neuropharmacological traits potentially relevant to inhibitory control, making it practical to test specific hypotheses regarding the covariance and genetic linkage between complex networks of genes and phenotypes associated with traits of interest.
We applied an operant training procedure to measure the ability to learn and reverse spatial discriminations in 51 BXD strains, leveraging the translational value of the reversal learning phenotype and the resources available for the BXD panel to dissect phenotypes relevant to inhibitory control, and to build a multi-scale model that explained genetic variation, associated alterations in neurotransmitter signaling, and consequent influences on flexibility-related behaviors.
Materials and Methods
Subjects
A total of 176 adult male mice (2–5 mice per strain), selected from 51 BXD recombinant inbred strains, including both older and newer strains (39), plus the relevant founder strains (C57BL/6 and DBA/2), were obtained from the University of Tennessee Health Science Center (UTHSC). The sample size per strain is justified because the value used in mapping is the mean performance of all mice that have inherited either the C57BL6 or DBA2 alleles (all mice from all strains that inherit the B allele are compared that to all mice from all strains that inherit the D allele). Thus, mapping relies on the mean of these genotype-grouped means, which will be very accurate across 51 strains, being based on about 25 strain means per genotype mean (40).
Subjects were habituated to the UCLA facility for 4–6 wks after importation, group-housed by strain in standard cages on a 14/10 light/dark schedule, initially with food and water ad libitum. Mean age at onset of training was 121+2 days. Three to five days prior to the onset of training, mice received restricted access to food in their home cage, sufficient to achieve a ~10–15% decline of their free-feeding weights at the onset of testing, adjusted up to a 20% decline during testing.
Two BXD strains have accumulated spontaneous mutations that could have influenced performance. BXD24 (JAX stock 005243) has a known mutation in the Cep20 gene that drastically impairs vision, but the BXD24 animals we studied do not carry the mutation and have normal vision. BXD29-Tlr4<lps-2J> (henceforth simply BXD29) has a known mutation in Tlr4 (JAX stock 000029). This strain also has a second mutation associated with a cortical heterotopia in the caudal neocortex (41). Despite this strain’s marked cortical abnormality, learning and reversal scores were near average (Fig 1A), and we therefore opted to include this strain in all statistical analyses and mapping.
Figure 1.

Trait distributions for (A) acquisition and (B) reversal. From left to right, strain means are ordered from best performance (fewest trials to criterion) to worst performance. Note different strain distributions across the two traits. Error bars depict standard error of the mean. The parental strains are included here for reference purposes, but were not included in subsequent correlational and genetic analyses.
Procedures were consistent with the Public Health Service’s Guide for the Care and Use of Laboratory Animals and were approved by the Chancellor’s Animal Research Committee at UCLA.
Training/Testing
Mice were trained and tested daily in individual Med Associates (St Albans VT) operant conditioning chambers fitted with a horizontal array of 5 nose poke apertures on one side of the box and a photocell-equipped food-delivery magazine on the other. The subjects were trained to initiate trials by nose poking into the central aperture, triggering illumination of flanking apertures. Responding in one of the two illuminated apertures was reinforced (1 food pellet; termed a correct response), while responses at the other aperture earned a time-out (all lights extinguished for 5 seconds; termed an incorrect response). The aperture reinforced during initial training was randomized across subjects and strains. All animals were tested in daily sessions that ended after: 1) one hour, 2) 126 trials, or 3) the performance criterion (16 out of 20 correctly-completed trials, computed as a moving window) was reached. Computing the performance criterion as a moving window of 20 trials ensured that mice were consistently sampling from the reinforced hole, ruling out generic hyperactivity or disinhibited responding as a factor contributing to successful stage completion. Once a performance criterion was met within a session, testing on that day was stopped and the reinforcement contingencies were reversed beginning in the following session. All mice completed the initial discrimination phase and a reversal phase.
Genetic Correlations and QTL analyses
The primary outcome measures were the number of trials required to reach performance criterion (TTC) during the initial acquisition and reversal conditions. It is customary in QTL studies, particularly of recombinant inbred lines, to control for the potential influence of potential non-genetic factors on the dependent variable(s) by a regression strategy; typically each factor is entered into a regression model and factors showing a significant relationship are entered into a multiple regression model, with the resulting residual scores submitted to genomic analysis as the phenotype. Individual subject performance data was therefore assessed for variation due to age at onset of testing, free-feeding weight, average percentage of weight decline during testing at each phase, transport batch, and operant chamber assignment. The categorical variables (i.e. chamber assignment and transport batch) were analyzed by “dummy coding” each variable. None of the known non-genetic factors accounted for a significant portion of the variation in acquisition of the initial choice or reversal (see Table S1 in the Supplement); therefore, the unadjusted TTC measures were assumed to represent the acquisition and reversal learning phenotypes. Each phenotype was then submitted to one-way ANOVA by strain to estimate a broad-sense heritability, calculated as the amount of variance explained by strain membership, reported as R2 (sum of squared errors between strains divided by the overall sum or squared errors in the dataset) using SPSS v. 17.0 software. Mean TTC values were computed for each strain and used for all subsequent analyses.
The phenotypes associated with this paper are available at GeneNetwork.org (www.genenetwork.org) in the BXD Phenotype database under the record ID numbers 12730, 12731, and 12762. A comprehensive list of BXD genotypes, phenotypes, and BXD gene expression data gathered by other researchers is also available at GeneNetwork.org (select Species = Mouse, Group = BXD). These multiscalar data were used to perform both hypothesis-driven testing and global exploratory analysis. The percentage of variance explained by identified QTL was estimated using R (software version 2.12.0) and the R/qtl package (42); the locus specific heritability is calculated as 1 – 10−2LOD/N.
Strain means for performance during the acquisition and reversal phases were submitted to separate genome-wide linkage analyses using a set of 3,797 markers spanning the genome and the mapping module of the GeneNetwork.org (43). Genome-wide linkage statistics (likelihood ratio scores) were computed using a fast regression-based method (44). Significance thresholds were computed based on 1000 permutations of the trait values (45).
Election of Positional Candidates
We used the informatics resources at GeneNetwork.org to elect positional candidates within the linkage interval (46, 47). The relevant QTL was defined as the 2-LOD support interval centered on the peak LRS (48). Array-based transcriptional phenotypes for multiple brain regions and microarray platforms were available for a subset of the strains measured in the current study. We relied most heavily on expression data for hippocampus (49), neocortex (50), and dorsal striatum (51). We also examined normative data on the mesocorticolibmic system (ventral tegmental area, nucleus accumbers, and prefrontal cortex) generated by Michael Miles and colleagues that are publically available in GeneNetwork.
We computed genetic correlations between the two primary behavioral phenotypes and the levels of mRNA expression, with particular attention to how expression of genes within the chromosome 10 QTL related to reversal learning. We reasoned that some genes and transcripts within this region would have the following attributes: 1) significant sequence differences between the parental strains, and 2) significant correlation between quantitative measures of expression and reversal learning performance, and 3) demonstrate cis-regulation (quantitative expression regulated from the same locus on chromosome 10), determined by submitting quantitative mRNA expression across the panel to QTL (eQTL) analysis. Transcripts that were correlated with reversal learning TTC at an uncorrected p < .05 level in three brain regions were mapped to determine if they were cis-regulated (52) and to assess the degree to which the regulatory region overlapped the reversal learning QTL (50). When a microarray probe targets a transcript with sequence variation between individual animals, anomalous hybridization may occur (one transcript will hybridize more efficiently than the other by virtue of being a better match for the probe, rather than because there are higher levels of it), resulting in spurious eQTLs (53). We therefore filtered probes that overlapped B-vs-D SNPs.
Results
Descriptive Statistics
Figure 1 shows that the initial discrimination acquisition (panel A) and reversal learning (panel B) phenotypes are distributed continuously, though differently, across the BXD strains: an outcome indicative of complexly-determined traits (also see Table S2 in the Supplement). Both phenotypes were moderately heritable (R2 = 0.29 for acquisition and R2 = 0.31 for reversal), adequate for mapping traits with the current sample sizes (40). A paired-samples two-tailed t-test (TTCacquisition – TTCreversal) revealed that reversal (mean = 173.2 trials, SD = 56.4; range 77 to 310.5) was significantly more difficult than acquisition (mean = 106.6 trials, SD = 45.3; range 23.6 to 200, t(174) = − 9.12, p < .001). The strain means were not significantly correlated across experimental phases (N = 51, r = 0.220, p = .12), and several patterns of strain variation between phases are evident. For example, the BXD16 strain performed poorly on both phases, suggesting poor learning or discrimination generally, while BXD2 performed very well on acquisition but very poorly on reversal, suggesting a reversal-specific effect in that strain. More commonly, strain ranks shifted by moderate amounts in either direction across phases (i.e. BXD13 improved by 20 rank positions between phases, while BXD12 dropped back by 22 rank positions, etc.). The diversity of strain variation between experimental phases suggests that the genetic influences on the cognitive and behavioral demands of each phase are at least somewhat independent.
Prior to conducting the genome scan, we considered whether the behavioral measure used in mice (reversal learning) bears mechanistic relationships to related human phenotypes, as hypothesized. If there were common molecular correlates of reversal learning in mice and relevant phenotypes in humans, this would support the idea that the relationship between these measures is more than simply descriptive. In human subjects, trait impulsivity, which is linked to reversal learning and inhibitory control (54), is related to low ventral mesencephalic D2-like receptor availability, measured in vivo (6), and the Genenetwork.org phenotype database contains data on dopamine transporter density, D1 protein density, and D2-like receptor density in prefrontal cortex (PFC), nucleus accumbens, dorsal striatum and ventral mesencephalon in a subset of BXD strains (55). We found that D2-like receptor expression in the ventral mesencephalon and PFC are inversely related to TTC in reversal (poor performance) in our sample (ventral mesencephalon: r = −0.75, p < 0.01, n = 11; prefrontal cortex: r= −0.67, p < 0.01, n=11; dopamine transporter: r = −0.70, p < 0.001; see Figure S1 in the Supplement). Other measures did not significantly correlate. These findings indicate that the current reversal learning phenotype in mice relates to a set of molecular correlates of human traits.
Genome Scan
No significant or suggestive QTL were observed for performance during the acquisition of the discrimination (data not shown), but a genome-wide significant QTL was mapped to a locus on chromosome 10 (77–82.4Mb) for the reversal learning phenotype (see figure 2A). Peak LRS-values were noted around marker CEL-10_86482491 (86.197002Mb; maximum LRS = 19.29, genome-wide p< 0.05). The locus-specific heritability was estimated at 31.5% based on a single QTL model. While variability within strain was generally low throughout the sample, a few strains in the reversal condition had notably high standard errors. If the two strains with the highest errors BXD2 and BXD29 (exceeding 60% of the strain mean) are removed, the QTL scan generates statistically identical results (LRS = 18.567 around marker CEL-10_86482491, genome-wide p < 0.05), demonstrating the robustness of the effect.
Figure 2.

Genome scan of the reversal learning trait. A. LRS-values are plotted across each chromosome. The red line near the top represents the genome-wide significance threshold at the p< 0.05 level. B. The reversal learning data regressed on acquisition shows a slightly reduced but statistically identical result at the same genomic locus.
Despite the lack of correlation between the acquisition and reversal phenotypes, it remained possible that some small amount of the variance driving the reversal QTL is due to general learning abilities rather than the specific ability to learn a reversal. To rule out this possibility, TTC for the reversal phase was regressed on TTC for the acquisition phase at the individual subject level, and the mean residual scores per strain (the portion of variance in the reversal data that could not be accounted for by variance in the acquisition data) were submitted to a second genome scan, yielding statistically identical results on chromosome 10, around marker CEL-10_86482491 (LRS = 17.487, p < 0.05; see figure 2B). It is therefore reasonable to assume that the chromosome 10 QTL is specific to those cognitive functions required for reversal, but not acquisition, namely inhibitory control over a pre-potent response.
The Region of Interest and Specification of Positional Candidates
The confidence interval around the peak LRS defining the genomic ROI for reversal learning ranged from 77Mb to 92.4Mb on mouse chromosome 10, which is syntenic with portions of human chromosomes 12, 19 and 22. The ROI contains 288 previously-named transcripts according to the GeneNetwork.org interval analysis tool (http://genenetwork.org/webqtl/main.py?FormID=intervalAnalyst), including both known and predicted genes. Of these 288 transcripts, 141 contained sequence variation in the BXD panel (see Table S3 in the Supplement); array-based expression for each of these transcripts was subjected to correlation analyses with reversal learning performance (see Tables S4-6 in the Supplement for correlated probes by brain region). Expression phenotypes for 7 unique transcripts (29 transcript/probe combinations) correlated with the reversal learning phenotype consistently in 3 different brain regions and across two microarray platforms (uncorrected p < 0.05), indicating that the expression data are reliable and the relationships with the reversal learning phenotype are robust (see table 1). Finally, three of these transcripts resulted in significant eQTL in (Hcfc2, Syn3 and Nt5dc3; uncorrected p < 0.05) that substantially overlapped the reversal learning ROI, also in all three brain regions (see figure 3). According to the Allen Mouse Brain Atlas (http://mousebrain-map.org/welcome.do), all three candidate genes are expressed in brain tissue.
Table 1.
Transcripts within the ROI whose multi-regional expression correlates with reversal learning performance across a subset of BXD strains. The N’s reported are the number of strains common to the microarray and the current studies. The p-values are uncorrected for multiple comparisons. The probe identifiers are provided by the array manufacturers. The asterisks denote that increased mRNA expression is related to better reversal learning performance.
| Symbol | Region | N | r2 | p | Probe ID |
|---|---|---|---|---|---|
| Hcfc2@82.16Mb | Hippocampus | 41 | 0.1971 | 0.0032 | 1428617_at |
| Neocortex | 38 | 0.4624 | 9.00E-07 | ILM105550446 | |
| Neocortex | 38 | 0.2981 | 0.0003 | ILM3780142 | |
| Striatum | 39 | 0.1858 | 0.006 | ILM105550446 | |
| Nfyb@82.21Mb | Hippocampus | 41 | 0.0955 | 0.0491 | 1419267_at |
| Neocortex | 38 | 0.3564 | 5.00E-05 | ILM103390059 | |
| Neocortex | 38 | 0.1806 | 0.0072 | ILM1850053 | |
| Striatum | 39 | 0.1459 | 0.0156 | ILM103390059 | |
| Polr3b@84.09Mb | Hippocampus | 41 | 0.1037 | 0.0397 | 1452949_at |
| Hippocampus | 41 | 0.1089 | 0.0347 | 1443466_s_at | |
| Hippocampus | 41 | 0.1253 | 0.0226 | 1457345_at | |
| Neocortex | 38 | 0.2125 | 0.0032 | ILM5900551 | |
| Fbxo7@85.49Mb | Striatum | 39 | 0.2275 | 0.0018 | ILM5900551 |
| Hippocampus | 41 | 0.1998 | 0.003 | 1435311_s_at | |
| Neocortex | 38 | 0.2043 | 0.003 | ILM2030397 | |
| Striatum | 39 | 0.1781 | 0.0069 | ILM2030397 | |
| Syn3*@85.52Mb | Hippocampus | 41 | 0.1875 | 0.0043 | 1443006_at |
| Neocortex | 38 | 0.3147 | 0.0002 | ILM100610369 | |
| Striatum | 39 | 0.1918 | 0.0048 | ILM100610369 | |
| Nt5dc3*@86.24Mb | Hippocampus | 41 | 0.3844 | 8.00E-06 | 1427057_at |
| Hippocampus | 41 | 0.4970 | 6.00E-08 | 1443558_s_at | |
| Neocortex | 38 | 0.3136 | 0.0002 | ILM105050575 | |
| Striatum | 39 | 0.2228 | 0.0021 | ILM105050575 | |
| Gnptab*@87.84Mb | Hippocampus | 41 | 0.3434 | 3.00E-05 | 1456620_at |
| Neocortex | 38 | 0.3352 | 9.00E-05 | ILM5290767 | |
| striatum | 39 | 0.3014 | 0.0002 | ILM5290767 | |
Figure 3.
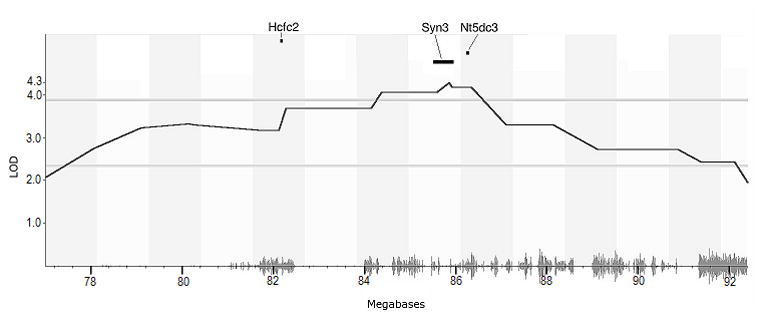
The reversal-learning 2-LOD interval on mouse chromosome 10. The red line shows the cut off for genome-wide significance (p < 0.05). The candidate genes and their genomic length are shown in their respective genomic positions. The seismograph along the bottom of the figure depicts SNP density through the region.
Discussion
Genome-wide, discovery-type studies for neuropsychiatric-related phenotypes have the potential to identify novel molecular-genetic influences on behavior, as well as to confirm existing candidate genes, both highly desirable outcomes. With that in mind, we used mice from the BXD recombinant inbred strains and the informatics resources at GeneNetwork.org to explore the biological basis of a trait conceptually related to various neuropsychiatric disorders, including ADHD and addictions.
Reversal learning in mice is genetically correlated with midbrain dopamine D2 receptor density, suggesting important biological determinants that are shared between reversal learning and trait impulsivity in species from rodents (10) to humans (6, 8). This represents a relatively novel association for the reversal learning task that is supported by a longitudinal study of preadolescents demonstrating a relationship between reversal learning and risk taking behaviors that is mediated by the largest predictor of risk taking: trait impulsivity (54). We then mapped a significant and novel QTL to a region on mouse chromosome 10 for reversal learning performance, the first genome-wide significant result for a complex behavioral phenotype related to impulse control and response inhibition. The finding has far-reaching implications for understanding behavior and pathological impulsivity, because measures of inhibitory control may index genetic risk for ADHD and addictions. While only association analysis of genetically independent subjects would definitively rule in, or out, genes residing in the locus, stringent informatics analysis identified three high-priority positional candidates deserving of intensive future study.
Positional Candidates
Variation in the expression of genes within a QTL may be useful for delineating positional candidates for further analysis (56). This argument stems from the concept that continuous variation in a trait is likely linked to genetically-determined continuous variation in expression of genes within the QTL (47, 57, 58). Transcriptome data were available for three brain regions in a large sample of BXD mice, and we were able to use these data to identify seven genes whose expression consistently correlated with the reversal learning phenotype in datasets generated from three separate regions, despite the fact that the genomic data was obtained by different investigators, sampling from different mouse cohorts and using different array platforms. Of these robustly correlated transcripts, three were advanced as high priority candidates because their quantitative expression is controlled from the same locus that influences reversal learning performance.
Of the positional candidates elected, Syn3 has been previously studied for its regulation of synaptic function. The gene codes for synapsin III, a member of a family of neuron-specific phosphoproteins known to affiliate with the cytoplasmic surface of synaptic vesicles (59), regulate vesicular reserve pools, and influence calcium-dependent synaptic dynamics (60–62). While all of the synapsin proteins influence vesicular trafficking, synapsin III demonstrates unique functions under a variety of circumstances. For example, evoked inhibitory post-synaptic currents are reduced in amplitude in hippocampal neurons of Syn3 knockout mice, while evoked excitatory post-synaptic responses are unaffected (60). Synapsin III knockout mice also demonstrate larger and longer striatal dopamine transients, measured by sub-second voltammetry, in response to electrical stimulation (63), while serotonin function is not affected; this change is consistent with reduced dopamine uptake, and it is noteworthy that reversal learning is correlated with dopamine transporter density in some regions of forebrain. The current findings demonstrate that increased Syn3 mRNA levels relate to better reversal learning performance, which together with Kile et al. invites a DA release dysregulation hypothesis of impulse control, mediated by Syn3 function.
The relationship between Syn3 and reversal learning may, therefore, involve intermediate adaptations in dopamine system dynamics. Separately, we showed that D2 receptor expression in the ventral midbrain (autoreceptor expression) negatively correlates with reversal learning; furthermore, Syn3 expression tends to positively relate to ventral midbrain D2 levels (n = 17, r = 0.451, p = 0.069), providing some evidence of a genetic relationship between Syn3 expression and D2 function. Trait impulsivity is related to diminished D2 autoreceptor activity (6), and other animal studies show that reducing D2 activity genetically or pharmacologically interferes with reversal learning (64–66), which is interesting in light of these findings, as together they suggest a common pathway to poor impulse control – namely, Syn3 modulation of dopamine dynamics, leading to behavioral inflexibility and impulsivity.
Human Syn3 resides on chromosome 22 (22q12), near a genomic locus that has been repeatedly implicated in psychosis (67); and deficits in reversal learning are often observed among schizophrenia patients (68, 69). Indeed, human studies of polymorphisms in Syn3 and post-mortem Syn3 expression have sometimes implied a relationship to schizophrenia, but these results are neither consistent nor conclusive (70–75). However, the Syn3 locus spans 545,839 bases and at least 3 haplotype blocks. We estimate that more than 125 SNP markers would be required to fully impute variation in the transcript. The majority of Syn3-schizophrenia studies, interrogating but a few SNPs at most, are therefore inconclusive. While questions about Syn3 and schizophrenia remain, the current finding substantially advances understanding of the genomic locus and its relation to impulse control, which is where the future direction of Syn3 research is best aimed. If there is a relationship between Syn3 and schizophrenia, it is most likely a relationship with a symptom-type that plays a small role in the overall syndrome, but is more characteristic of other neuropsychiatric disorders, like ADHD and behavior addictions.
Nt5dc3 (5′-nucleotidase domain containing 3), a member of a 50-nucleotidase domain containing family, codes for a largely uncharacterized transmembrane protein. Polymorphisms in Nt5dc3 and surrounding loci on human chromosome 12 have been associated with ADHD in two genome-wide mapping studies. Fisher et al. (2002) reported weak evidence for linkage with ADHD diagnosis at markers near the 12q23 locus (D12S78–D12S79, p = 0.0039), and in a more recent study, Lesch et al. (2008) identified a SNP within Nt5dc3 (rs4964805) as one of the top 30 out of ~500,000 SNPs (p = 4.74X10-6). A marker at a locus (rs1862032) near human Nt5dc3 (12q23.3) was also suggestively associated with a performance on a stop signal reaction time task (LOD = 2.627, uncorrected p = 0.0003), another measure of inhibitory control function(76). While none of these findings were significant at genome-wide levels on their own, their convergence was worthy of note even before the current study. The current finding alone, however, does provide convincing evidence for genome-wide significant linkage of a clinically relevant translational phenotype with the homologous locus on the mouse genome. Further investigation of Nt5dc3 as a risk gene for disorders of impulse control, including ADHD, is well warranted, as is basic neuroscience research to specify the functions of the encoded protein.
Hcfc2 (host cell factor C2) is also near the human 12q23 locus and is known to interact with a specific herpes simplex protein, VP16, during the infection process. (77). To date, no regulatory functions or interactions with endogenous proteins have been characterized for this transcript. Therefore, Hcfc2 represents the potential for identifying a completely novel genetic mechanism with implications for cognitive control.
Conclusions
We have implemented, in a murine genetic reference population, a task that measures a clinically relevant cognitive-behavioral phenotype, and have shown that performance on the task shares mechanistic features of the same cognitive-behavioral function in humans. Using this approach, we identified important new genomic mechanisms that explain individual variation in the inhibitory control phenotype and that likely have substantial implications for homologous human phenotypes and related neuropsychiatric disorders. While the methods applied to the election of positional candidates for the current QTL cannot convincingly eliminate other transcripts from consideration as potential contributors to the observed phenotypes, they do provide strong evidence for inclusion of the candidates that were advanced, and vigorous follow up investigation of these candidates and their functions is warranted. In addition to adding converging evidence supporting human findings implicating Syn3 and Nt5dc and their surrounding genomic loci to neuropsychiatric disorders, the current study, definitively and for the first time, demonstrates the power of murine genetic reference populations for uncovering the genomic bases for phenotypes of interest to neuropsychiatry. This demonstration of the translational value of the reversal learning phenotype and the elucidation of its genetic underpinnings strongly suggest that the mouse is a powerful system for advancing the understanding of the etiology of impulse control disorders, and their potential treatments.
Supplementary Material
Acknowledgments
This work was supported by PHS Grants UL1-DE019580, PL1-NS062410, RL1-MH083270, RL1-MH083269 (JDJ), T32-NS048004 (REL), the NIAAA Integrative Neuroscience Initiative on Alcoholism (U01AA13499, U24AA13513, U01AA014425) and NIDA, NIMH, and NIAAA P20-DA 21131 (RWW). In addition, we thank Nelson Freimer and Glen D. Rosen for reviewing and commenting on an earlier draft of this report, and Alex S. James for intellectual and technical support. Preliminary data from the current study were previously presented at the BXD World Conference (Braunsweig, Germany, 2009) and the Society for Neuroscience Meeting (San Diego, CA., 2010).
Footnotes
Financial Disclosures
All authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Malloy-Diniz L, Fuentes D, Leite WB, Correa H, Bechara A. Impulsive behavior in adults with attention deficit/hyperactivity disorder: characterization of attentional, motor and cognitive impulsiveness. J Int Neuropsychol Soc. 2007;13:693–698. doi: 10.1017/S1355617707070889. [DOI] [PubMed] [Google Scholar]
- 2.Wodushek TR, Neumann CS. Inhibitory capacity in adults with symptoms of Attention Deficit/Hyperactivity Disorder (ADHD) Arch Clin Neuropsychol. 2003;18:317–330. [PubMed] [Google Scholar]
- 3.Dong G, Lu Q, Zhou H, Zhao X. Impulse inhibition in people with internet addiction disorder: electrophysiological evidence from a Go/NoGo study. Neurosci Lett. 2010;485:138–142. doi: 10.1016/j.neulet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James AS, Groman SM, Seu E, Jorgensen M, Fairbanks LA, Jentsch JD. Dimensions of impulsivity are associated with poor spatial working memory performance in monkeys. J Neurosci. 2007;27:14358–14364. doi: 10.1523/JNEUROSCI.4508-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cools R, Sheridan M, Jacobs E, D’Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27:5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson OJ, Standing HR, DeVito EE, Cools R, Sahakian BJ. Dopamine precursor depletion improves punishment prediction during reversal learning in healthy females but not males. Psychopharmacology (Berl) 2010;211:187–195. doi: 10.1007/s00213-010-1880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fillmore MT, Rush CR. Polydrug abusers display impaired discrimination-reversal learning in a model of behavioural control. J Psychopharmacol. 2006;20:24–32. doi: 10.1177/0269881105057000. [DOI] [PubMed] [Google Scholar]
- 19.Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- 20.Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- 23.Itami S, Uno H. Orbitofrontal cortex dysfunction in attention-deficit hyperactivity disorder revealed by reversal and extinction tasks. Neuroreport. 2002;13:2453–2457. doi: 10.1097/00001756-200212200-00016. [DOI] [PubMed] [Google Scholar]
- 24.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on-line” processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butters N, Butter C, Rosen J, Stein D. Behavioral effects of sequential and one-stage ablations of orbital prefrontal cortex in the monkey. Exp Neurol. 1973;39:204–214. doi: 10.1016/0014-4886(73)90223-9. [DOI] [PubMed] [Google Scholar]
- 28.Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, et al. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:1225–1236. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- 29.Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groman SM, James AS, Jentsch JD. Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2009;33:690–698. doi: 10.1016/j.neubiorev.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug Addiction Endophenotypes: Impulsive Versus Sensation-Seeking Personality Traits. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol. 2008;75:63–75. doi: 10.1016/j.bcp.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Congdon E, Lesch KP, Canli T. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: implications for impulsivity. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:27–32. doi: 10.1002/ajmg.b.30557. [DOI] [PubMed] [Google Scholar]
- 36.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 37.Gubner NR, Wilhelm CJ, Phillips TJ, Mitchell SH. Strain differences in behavioral inhibition in a Go/No-go task demonstrated using 15 inbred mouse strains. Alcohol Clin Exp Res. 2010;34:1353–1362. doi: 10.1111/j.1530-0277.2010.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams RW, Gu J, Qi S, Lu L. The genetic structure of recombinant inbred mice: high-resolution consensus maps for complex trait analysis. Genome Biol. 2001;2:RESEARCH0046. doi: 10.1186/gb-2001-2-11-research0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peirce JL, Lu L, Gu J, Silver LM, Williams RW. A new set of BXD recombinant inbred lines from advanced intercross populations in mice. BMC Genet. 2004;5:7. doi: 10.1186/1471-2156-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belknap JK. Effect of within-strain sample size on QTL detection and mapping using recombinant inbred mouse strains. Behav Genet. 1998;28:29–38. doi: 10.1023/a:1021404714631. [DOI] [PubMed] [Google Scholar]
- 41.Rosen GD, Azoulay N, Griffen EG, Newbury AJ, Li Z, Wang X, et al. Program# 1309/A9 2010 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience Online; 2010. Midline neocortical nodular heterotopias and partial callosal agenesis in a spontaneous mutation of a BXD recombinant inbred strain. [Google Scholar]
- 42.Broman KW, Wu H, Sen S, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19:889–890. doi: 10.1093/bioinformatics/btg112. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Williams RW, Manly KF. WebQTL: web-based complex trait analysis. Neuroinformatics. 2003;1:299–308. doi: 10.1385/NI:1:4:299. [DOI] [PubMed] [Google Scholar]
- 44.Haley CS, Knott SA. A simple regression method for mapping quantitative trait loci in line crosses using flanking markers. Heredity. 1992;69:315–324. doi: 10.1038/hdy.1992.131. [DOI] [PubMed] [Google Scholar]
- 45.Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chesler EJ, Lu L, Wang J, Williams RW, Manly KF. WebQTL: rapid exploratory analysis of gene expression and genetic networks for brain and behavior. Nat Neurosci. 2004;7:485–486. doi: 10.1038/nn0504-485. [DOI] [PubMed] [Google Scholar]
- 47.Chesler EJ, Wang J, Lu L, Qu Y, Manly KF, Williams RW. Genetic correlates of gene expression in recombinant inbred strains: a relational model system to explore neurobehavioral phenotypes. Neuroinformatics. 2003;1:343–357. doi: 10.1385/NI:1:4:343. [DOI] [PubMed] [Google Scholar]
- 48.Lander ES, Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989;121:185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Overall RW, Kempermann G, Peirce J, Lu L, Goldowitz D, Gage FH, et al. Genetics of the hippocampal transcriptome in mouse: a systematic survey and online neurogenomics resource. Front Neurosci. 2009;3:55. doi: 10.3389/neuro.15.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaglani SM, Lu L, Williams RW, Rosen GD. The genetic control of neocortex volume and covariation with neocortical gene expression in mice. BMC Neurosci. 2009;10:44. doi: 10.1186/1471-2202-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosen GD, Pung CJ, Owens CB, Caplow J, Kim H, Mozhui K, et al. Genetic modulation of striatal volume by loci on Chrs 6 and 17 in BXD recombinant inbred mice. Genes Brain Behav. 2009;8:296–308. doi: 10.1111/j.1601-183X.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Burmeister M. Genetical genomics: combining genetics with gene expression analysis. Hum Mol Genet. 2005;14(Spec No 2):R163–169. doi: 10.1093/hmg/ddi267. [DOI] [PubMed] [Google Scholar]
- 53.Ciobanu DC, Lu L, Mozhui K, Wang X, Jagalur M, Morris JA, et al. Detection, validation, and downstream analysis of allelic variation in gene expression. Genetics. 2010;184:119–128. doi: 10.1534/genetics.109.107474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romer D, Betancourt L, Giannetta JM, Brodsky NL, Farah M, Hurt H. Executive cognitive functions and impulsivity as correlates of risk taking and problem behavior in preadolescents. Neuropsychologia. 2009;47:2916–2926. doi: 10.1016/j.neuropsychologia.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones BC, Tarantino LM, Rodriguez LA, Reed CL, McClearn GE, Plomin R, et al. Quantitative-trait loci analysis of cocaine-related behaviours and neurochemistry. Pharmacogenetics. 1999;9:607–617. [PubMed] [Google Scholar]
- 56.Farber CR, Lusis AJ. Integrating global gene expression analysis and genetics. Adv Genet. 2008;60:571–601. doi: 10.1016/S0065-2660(07)00420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bystrykh L, Weersing E, Dontje B, Sutton S, Pletcher MT, Wiltshire T, et al. Uncovering regulatory pathways that affect hematopoietic stem cell function using ‘genetical genomics’. Nat Genet. 2005;37:225–232. doi: 10.1038/ng1497. [DOI] [PubMed] [Google Scholar]
- 58.de Koning DJ, Cabrera CP, Haley CS. Genetical genomics: combining gene expression with marker genotypes in poultry. Poult Sci. 2007;86:1501–1509. doi: 10.1093/ps/86.7.1501. [DOI] [PubMed] [Google Scholar]
- 59.Kao HT, Porton B, Czernik AJ, Feng J, Yiu G, Haring M, et al. A third member of the synapsin gene family. Proc Natl Acad Sci U S A. 1998;95:4667–4672. doi: 10.1073/pnas.95.8.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng J, Chi P, Blanpied TA, Xu Y, Magarinos AM, Ferreira A, et al. Regulation of neurotransmitter release by synapsin III. J Neurosci. 2002;22:4372–4380. doi: 10.1523/JNEUROSCI.22-11-04372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hosaka M, Sudhof TC. Synapsin III, a novel synapsin with an unusual regulation by Ca2+ J Biol Chem. 1998;273:13371–13374. doi: 10.1074/jbc.273.22.13371. [DOI] [PubMed] [Google Scholar]
- 62.Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kile BM, Guillot TS, Venton BJ, Wetsel WC, Augustine GJ, Wightman RM. Synapsins differentially control dopamine and serotonin release. J Neurosci. 2010;30:9762–9770. doi: 10.1523/JNEUROSCI.2071-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Steno DA, Schmauss C. A role for dopamine D2 receptors in reversal learning. Neuroscience. 2009;162:118–127. doi: 10.1016/j.neuroscience.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology. 2007;32:2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- 66.Kruzich PJ, Mitchell SH, Younkin A, Grandy DK. Dopamine D2 receptors mediate reversal learning in male C57BL/6J mice. Cogn Affect Behav Neurosci. 2006;6:86–90. doi: 10.3758/cabn.6.1.86. [DOI] [PubMed] [Google Scholar]
- 67.Williams NM, Owen MJ. Genetic abnormalities of chromosome 22 and the development of psychosis. Curr Psychiatry Rep. 2004;6:176–182. doi: 10.1007/s11920-004-0062-4. [DOI] [PubMed] [Google Scholar]
- 68.Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murray GK, Cheng F, Clark L, Barnett JH, Blackwell AD, Fletcher PC, et al. Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull. 2008;34:848–855. doi: 10.1093/schbul/sbn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Porton B, Wetsel WC. Reduction of synapsin III in the prefrontal cortex of individuals with schizophrenia. Schizophr Res. 2007;94:366–370. doi: 10.1016/j.schres.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 71.Lachman HM, Stopkova P, Papolos DF, Pedrosa E, Margolis B, Aghalar MR, et al. Analysis of synapsin III-196 promoter mutation in schizophrenia and bipolar disorder. Neuropsychobiology. 2006;53:57–62. doi: 10.1159/000091720. [DOI] [PubMed] [Google Scholar]
- 72.Lachman HM, Stopkova P, Rafael MA, Saito T. Association of schizophrenia in African Americans to polymorphism in synapsin III gene. Psychiatr Genet. 2005;15:127–132. doi: 10.1097/00041444-200506000-00009. [DOI] [PubMed] [Google Scholar]
- 73.Porton B, Ferreira A, DeLisi LE, Kao HT. A rare polymorphism affects a mitogen-activated protein kinase site in synapsin III: possible relationship to schizophrenia. Biol Psychiatry. 2004;55:118–125. doi: 10.1016/j.biopsych.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 74.Tsai MT, Hung CC, Tsai CY, Liu MY, Su YC, Chen YH, et al. Mutation analysis of synapsin III gene in schizophrenia. Am J Med Genet. 2002;114:79–83. doi: 10.1002/ajmg.10116. [DOI] [PubMed] [Google Scholar]
- 75.Chen Q, Che R, Wang X, O’Neill FA, Walsh D, Tang W, et al. Association and expression study of synapsin III and schizophrenia. Neurosci Lett. 2009;465:248–251. doi: 10.1016/j.neulet.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rommelse NN, Arias-Vasquez A, Altink ME, Buschgens CJ, Fliers E, Asherson P, et al. Neuropsychological endophenotype approach to genome-wide linkage analysis identifies susceptibility loci for ADHD on 2q21.1 and 13q12.11. Am J Hum Genet. 2008;83:99–105. doi: 10.1016/j.ajhg.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson KM, Mahajan SS, Wilson AC. Herpes simplex virus transactivator VP16 discriminates between HCF-1 and a novel family member, HCF-2. J Virol. 1999;73:3930–3940. doi: 10.1128/jvi.73.5.3930-3940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


