Abstract
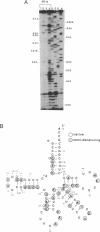
Cotranslational insertion of selenocysteine into selenoenzymes is mediated by a specialized transfer RNA, the tRNA(Sec). We have carried out the determination of the solution structure of the eucaryotic tRNA(Sec). Based on the enzymatic and chemical probing approach, we show that the secondary structure bears a few unprecedented features like a 9 bp aminoacid-, a 4 bp thymine- and a 6 bp dihydrouridine-stems. Surprisingly, the eighth nucleotide, although being a uridine, is base-paired and cannot therefore correspond to the single-stranded invariant U8 found in all tRNAs. Rather, experimental evidence led us to propose that the role of the invariant U8 is actually played by the tenth nucleotide which is an A, numbered A8 to indicate this fact. The experimental data therefore demonstrate that the cloverleaf structure we derived experimentally resembles the hand-folded model proposed by Böck et al (ref. 3). Using the solution data and computer modelling, we derived a three-dimensional structure model which shows some unique aspects. Basically, A8, A14, U21 form a novel type of tertiary interaction in which A8 interacts with the Hoogsteen sites of A14 which itself forms a Watson-Crick pair with U21. No coherent model containing the canonical 15-48 interaction could be derived. Thus, the number of tertiary interactions appear to be limited, leading to an uncoupling of the variable stem from the rest of the molecule.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron C., Böck A. The length of the aminoacyl-acceptor stem of the selenocysteine-specific tRNA(Sec) of Escherichia coli is the determinant for binding to elongation factors SELB or Tu. J Biol Chem. 1991 Oct 25;266(30):20375–20379. [PubMed] [Google Scholar]
- Baron C., Heider J., Böck A. Mutagenesis of selC, the gene for the selenocysteine-inserting tRNA-species in E. coli: effects on in vivo function. Nucleic Acids Res. 1990 Dec 11;18(23):6761–6766. doi: 10.1093/nar/18.23.6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. J., Banu L., Chen Y. Y., Mandel S. J., Kieffer J. D., Harney J. W., Larsen P. R. Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3' untranslated region. Nature. 1991 Sep 19;353(6341):273–276. doi: 10.1038/353273a0. [DOI] [PubMed] [Google Scholar]
- Böck A., Forchhammer K., Heider J., Baron C. Selenoprotein synthesis: an expansion of the genetic code. Trends Biochem Sci. 1991 Dec;16(12):463–467. doi: 10.1016/0968-0004(91)90180-4. [DOI] [PubMed] [Google Scholar]
- Carbon P., Krol A. Transcription of the Xenopus laevis selenocysteine tRNA(Ser)Sec gene: a system that combines an internal B box and upstream elements also found in U6 snRNA genes. EMBO J. 1991 Mar;10(3):599–606. doi: 10.1002/j.1460-2075.1991.tb07987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Dudock B., Hatfield D. Structure and properties of a bovine liver UGA suppressor serine tRNA with a tryptophan anticodon. Cell. 1981 Aug;25(2):497–506. doi: 10.1016/0092-8674(81)90068-4. [DOI] [PubMed] [Google Scholar]
- Dock-Bregeon A. C., Westhof E., Giegé R., Moras D. Solution structure of a tRNA with a large variable region: yeast tRNASer. J Mol Biol. 1989 Apr 20;206(4):707–722. doi: 10.1016/0022-2836(89)90578-0. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Baudin F., Mougel M., Romby P., Ebel J. P., Ehresmann B. Probing the structure of RNAs in solution. Nucleic Acids Res. 1987 Nov 25;15(22):9109–9128. doi: 10.1093/nar/15.22.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Cedergren R. J., McKay W. Structure in tRNA data. Biochimie. 1982 Jun;64(6):387–397. doi: 10.1016/s0300-9084(82)80576-2. [DOI] [PubMed] [Google Scholar]
- Hatfield D., Portugal F. H. Seryl-tRNA in mammalian tissues: chromatographic differences in brain and liver and a specific response to the codon, UGA. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1200–1206. doi: 10.1073/pnas.67.3.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider J., Baron C., Böck A. Coding from a distance: dissection of the mRNA determinants required for the incorporation of selenocysteine into protein. EMBO J. 1992 Oct;11(10):3759–3766. doi: 10.1002/j.1460-2075.1992.tb05461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider J., Leinfelder W., Böck A. Occurrence and functional compatibility within Enterobacteriaceae of a tRNA species which inserts selenocysteine into protein. Nucleic Acids Res. 1989 Apr 11;17(7):2529–2540. doi: 10.1093/nar/17.7.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A., Carbon P. A guide for probing native small nuclear RNA and ribonucleoprotein structures. Methods Enzymol. 1989;180:212–227. doi: 10.1016/0076-6879(89)80103-x. [DOI] [PubMed] [Google Scholar]
- Krol A., Westhof E., Bach M., Lührmann R., Ebel J. P., Carbon P. Solution structure of human U1 snRNA. Derivation of a possible three-dimensional model. Nucleic Acids Res. 1990 Jul 11;18(13):3803–3811. doi: 10.1093/nar/18.13.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. J., Rajagopalan M., Kim Y. S., You K. H., Jacobson K. B., Hatfield D. Selenocysteine tRNA[Ser]Sec gene is ubiquitous within the animal kingdom. Mol Cell Biol. 1990 May;10(5):1940–1949. doi: 10.1128/mcb.10.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. J., Worland P. J., Davis J. N., Stadtman T. C., Hatfield D. L. Identification of a selenocysteyl-tRNA(Ser) in mammalian cells that recognizes the nonsense codon, UGA. J Biol Chem. 1989 Jun 15;264(17):9724–9727. [PubMed] [Google Scholar]
- Mizutani T., Kurata H., Yamada K., Totsuka T. Some properties of murine selenocysteine synthase. Biochem J. 1992 Jun 15;284(Pt 3):827–834. doi: 10.1042/bj2840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T. Some evidence of the enzymatic conversion of bovine suppressor phosphoseryl-tRNA to selenocysteyl-tRNA. FEBS Lett. 1989 Jul 3;250(2):142–146. doi: 10.1016/0014-5793(89)80707-0. [DOI] [PubMed] [Google Scholar]
- Peattie D. A., Gilbert W. Chemical probes for higher-order structure in RNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4679–4682. doi: 10.1073/pnas.77.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond T. J. Solvent accessible surface area and excluded volume in proteins. Analytical equations for overlapping spheres and implications for the hydrophobic effect. J Mol Biol. 1984 Sep 5;178(1):63–89. doi: 10.1016/0022-2836(84)90231-6. [DOI] [PubMed] [Google Scholar]
- Schön A., Böck A., Ott G., Sprinzl M., Söll D. The selenocysteine-inserting opal suppressor serine tRNA from E. coli is highly unusual in structure and modification. Nucleic Acids Res. 1989 Sep 25;17(18):7159–7165. doi: 10.1093/nar/17.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturchler C., Carbon P., Krol A. An additional long-range interaction in human U1 snRNA. Nucleic Acids Res. 1992 Mar 25;20(6):1215–1221. doi: 10.1093/nar/20.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhof E., Dumas P., Moras D. Crystallographic refinement of yeast aspartic acid transfer RNA. J Mol Biol. 1985 Jul 5;184(1):119–145. doi: 10.1016/0022-2836(85)90048-8. [DOI] [PubMed] [Google Scholar]
- Westhof E., Romby P., Romaniuk P. J., Ebel J. P., Ehresmann C., Ehresmann B. Computer modeling from solution data of spinach chloroplast and of Xenopus laevis somatic and oocyte 5 S rRNAs. J Mol Biol. 1989 May 20;207(2):417–431. doi: 10.1016/0022-2836(89)90264-7. [DOI] [PubMed] [Google Scholar]
- Zinoni F., Birkmann A., Stadtman T. C., Böck A. Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4650–4654. doi: 10.1073/pnas.83.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]