Abstract
Members of the peroxisome proliferator-activated receptor γ coactivator-1 family (i.e. PGC-1α, PGC-1β, and the PGC-1-related coactivator (PRC)) are key regulators of mitochondrial biogenesis and function. These regulators serve as mediators between environmental or endogenous signals and the transcriptional machinery governing mitochondrial biogenesis. The FTC-133 and RO82 W-1 follicular thyroid carcinoma cell lines, which present significantly different numbers of mitochondria, metabolic mechanisms, and expression levels of PRC and PGC-1α, may employ retrograde signaling in response to respiratory dysfunction. Nitric oxide (NO) and calcium have been hypothesized to participate in this activity. We investigated the effects of the S-nitroso-N-acetyl-dl-penicillamine-NO donor, on the expression of genes involved in mitochondrial biogenesis and cellular metabolic functions in FTC-133 and RO82 W-1 cells by measuring lactate dehydrogenase and cytochrome c oxidase (COX) activities. We studied the action of ionomycin and 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester (BAPTA/AM) (i.e. a calcium ionophore and a cytosolic calcium chelator) on whole genome expression and mitochondrial biogenesis in RO82 W-1 cells. COX activity and the dynamics of endoplasmic reticulum and mitochondrial networks were analyzed in regard to calcium-modulating treatments. In the FTC-133 and RO82 W-1 cells, the mitochondrial biogenesis induced by NO was mainly related to PRC expression as a retrograde mitochondrial signaling. Ionomycin diminished COX activity and negatively regulated PRC-mediated mitochondrial biogenesis in RO82 W-1 cells, whereas BAPTA/AM produced the opposite effects with a reorganization of the mitochondrial network. This is the first demonstration that NO and calcium regulate mitochondrial biogenesis through the PRC pathway in thyroid cell lines.
Keywords: Calcium, Gene Regulation, Mitochondrial Metabolism, Nitric Oxide, Thyroid, Mitochondrial Biogenesis
Introduction
Mitochondrial proteins of the oxidative phosphorylation system are encoded by the nuclear as well as the mitochondrial genomes. The mitochondrial energy function requires coordination of the transcription of nuclear genes coding for the majority of oxidative phosphorylation subunits and the mitochondrial genes coding for 13 essential oxidative phosphorylation subunits. Several nucleus-encoded transcription factors, such as the nuclear respiratory factors (NRFs),2 estrogen-related receptors (ERRs), and the cAMP-response element-binding protein (CREB), are known to ensure this coordination. The transcriptional efficiency of these factors is controlled by coactivators from the peroxisome proliferator-activated receptor γ coactivator-1 (PGC-1) family (i.e. PGC-1α, PGC-1β, and the PGC-1-related coactivator (PRC)), which regulate mitochondrial biogenesis and function, depending on various environmental signals (1). The founding member of the coactivator family, PGC-1α, has been identified through its role in adaptative thermogenesis (2). PGC-1α and PGC-1β are highly expressed in oxidative tissues, such as the heart, kidney, muscle, brown adipose tissue, and brain. In contrast, PRC, which is ubiquitously and rapidly expressed by serum induction in proliferating cells, is considered to be a regulator of cell growth (3). PGC-1α and PRC interact with NRF-1, ERRα, CREB, and transactivating promoters of target genes involved in mitochondrial respiration, such as cytochrome c (4–6). In the cross-talk between nucleus and mitochondria, retrograde signaling has been shown to be induced between mitochondria and nucleus in response to respiratory dysfunction. The events initiating this process are not clearly understood, but nitric oxide (NO) and calcium are suspected of mediating this retrograde cross-talk in mammals (7).
NO has different effects on mitochondria, depending on the level and duration of its production. In various cell types, long term treatment with low levels of NO induces biogenesis of functional mitochondria through a cyclic guanosine monophosphate (cGMP)/PGC-1α pathway (8, 9). Moreover, compared with the case of wild-type animals, cold-induced mitochondrial biogenesis and oxygen consumption decreased in the brown adipose tissue of mice with a null mutation of endothelial nitric-oxide synthase. We have demonstrated that NO also mediates mitochondrial biogenesis through the cGMP/PRC pathway in a model of mitochondrion-rich thyroid tumors (10). Thus, it appears that PRC and PGC-1α induce mitochondrial biogenesis when endothelial nitric-oxide synthase produces a chronic supply of NO at a low level. However, if this activity is acute, NO may bind to cytochrome c oxidase (COX) and reversibly inhibit mitochondrial chain respiration by competing with oxygen (11, 12).
Calcium may also modulate respiratory chain activity and mitochondrial biogenesis. However, the reported effects of calcium on mitochondrial biogenesis and function are contradictory and may depend on the cellular model used. Thus, in the bovine heart, the increase in Ca2+ concentration enhanced complex I and ATP synthase activities while reversing the allosteric cAMP inhibition of COX by modulating its phosphorylation status (13–16). In contrast, in L6E9 myotubes and isolated rat brain mitochondria, the increase in Ca2+ concentration was found to inhibit COX activity (17, 18). Interestingly, COX inhibition may depend on NO production by the Ca2+-activated mitochondrial NOS. Increased cytosolic Ca2+ concentration by means of Ca2+ ionophores A23187 or ionomycin enhanced cytochrome c expression in muscle cells (18–20). This was associated with an increase in NRF-dependent transcription activity and the overexpression of COX I, PGC-1α, and the transcription factor A, mitochondrial (TFAM) (19–21). However, another study found no variation in the expression of nucleus-encoded COX subunits (COX IV, Vb, and VIc) and reported decreased expression in TFAM and mitochondrion-encoded COX subunits (COX II and III) (18). Finally, in mitochondrial DNA-depleted cells, mitochondrial biogenesis was stimulated by the increased concentration of cytosolic Ca2+ and CREB activation (22, 23).
We have shown that the XTC.UC1, FTC-133, and RO82 W-1 follicular thyroid carcinoma cell lines differ in their metabolic status. In effect, the metabolism of XTC.UC1 and FTC-133 cells, particularly rich in mitochondria, is essentially oxidative; in contrast, the metabolism of RO82 W-1 cells, which are rather poor in mitochondria, is mainly glycolytic (24). Moreover, in XTC.UC1 cells, only the PRC coactivator is overexpressed, whereas in FTC-133 and RO82 W-1 cells, the ratio between the mRNA expressions of PRC and PGC-1α varies. Thus, in our investigation of the endogenous cellular pathways that might be involved in the fine regulation of mitochondrial function and biogenesis, we examined the effects of NO and calcium in FTC-133 and RO82 W-1 cells. We measured the expression levels of the PRC and PGC-1α coactivators as well as those of the main factors required for mitochondrial biogenesis and studied their effects on the respiratory chain function and cell metabolism by measuring COX and lactate dehydrogenase (LDH) activities. In particular, we investigated the role of calcium in mitochondrial network remodeling in the RO82 W-1 glycolytic cell line.
MATERIALS AND METHODS
Human Follicular Thyroid Carcinoma Cell Lines and Conditions of Cell Growth
Two human follicular thyroid carcinoma cell lines cultured at 37 °C, 5% CO2 were used: FTC-133 and RO82 W-1 (Interlab Cell Line Collection, National Institute for Cancer Research, Genoa, Italy). FTC-133 cells were grown in Dulbecco's modified Eagle's medium (DMEM)/F-12 (Jacques Boy, Reims, France), and RO82 W-1 cells were grown in a mixed 60:30 DMEM/F-12 plus endothelial basal medium (PAA, Pasching, Austria). Both media were supplemented with 10% fetal bovine serum (Jacques Boy), 1% l-glutamine (Dominique Dutscher, Brumath, France), and an antibiotic mixture (Thermo Fisher Scientific, Waltham, MA).
To study the effects of NO, the two cell lines were treated once a day for 4 days with the nitric oxide donor S-nitroso-N-acetyl-dl-penicillamine (SNAP) (Merck Calbiochem, Darmstadt, Germany) made up to a final concentration of 50 or 100 μm (corresponding to 50 and 100 nm equivalent NO during several h) in the selected medium or with equivalent volumes of DMSO (Sigma-Aldrich) for the control cells. We verified that the 4.6-h half-life of SNAP was sufficient to increase nitrite production by 2.3-fold (50 μm) and 4.1-fold (100 μm) after 24 h of treatment. To control the mediating effect of NO/cGMP on PRC-related mitochondrial biogenesis, cells were pretreated with a 1 μm concentration of the protein kinase G inhibitor KT5823 (EMD, San Diego, CA) 30 min before 50 μm SNAP treatment during 96 h.
To study the effects of calcium on mitochondrial biogenesis and function, RO82 W-1 cells were treated 5 h/day for 4 days with the calcium ionophore ionomycin (Merck Calbiochem) made up to a final concentration of 2 μm in the culture medium. We further used the cytosolic calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester (BAPTA/AM) (Merck Calbiochem) made up to a final concentration of 2–8 μm, with a combination of ionomycin and BAPTA/AM or with equivalent volumes of DMSO for control cells. To avoid cell death due to the toxic effects of intracellular Ca2+ ion chelation, the BAPTA/AM concentration was adjusted to cell density in the flask for each day of treatment as follows: 2 μm the first day, 4 μm the second day, 6 μm the third day, and 8 μm the fourth day. Duplicate cell counts were made with a Z1 Coulter particle counter (Beckman Coulter, Fullerton, CA). To control the mediating effect of calcium on PRC-related mitochondrial biogenesis, cells were pretreated with a 10 μm concentration of the calcineurin inhibitor CN585 (EMD) during ionomycin and BAPTA/AM treatments.
Quantitative RT-PCR Analysis
Total RNA was isolated from cultured cells using the RNeasy kit (Qiagen, Hilden, Germany). The integrity of RNA was determined using a Bio-Analyzer 2100 (Agilent Technologies, Waldbronn, Germany). Reverse transcription was performed on 2 μg of RNA with the Advantage RT-for-PCR kit (Clontech), following the manufacturer's recommendations. cDNA real-time quantifications were performed in a 96-well plate on a Chromo4 apparatus (Bio-Rad) using SYBR Green I dye as a fluorescent signal (iQ SYBR Green Supermix, Bio-Rad) according to the manufacturer's instructions. The expression of eight selected genes (CREB1, cytochrome c (CYT C), ERRα, NRF-1, PGC-1α, PRC, SOD2, and UCP2) was measured to determine the impact of calcium and NO modulation on key factors controlling mitochondrial biogenesis and reactive oxygen species production. For RO82W-1 cells treated with ionomycin and BAPTA/AM, some of the most differentially expressed genes on microarray, such as DRP1, TEP1, CRYAB, FIS1, PDE1A, GREB1, TFAM, RINT1, and MFN2, were controlled by quantitative RT-PCR. The amount of cDNA for selected genes was normalized by the quantification of β2-microglobulin (B2M) or β-globin (for microarray confirmation) cDNA. Each sample was assayed in duplicate using the primers described in Table 1.
TABLE 1.
Primer sequences used for real-time quantitative RT-PCR
| Primer | Sequence |
|---|---|
| β-Globin | |
| Forward | 5′-ACACAACTGTGTTCACTAGC-3′ |
| Reverse | 5′-CAACTTCATCCACGTTCACC-3′ |
| β2-microglobulin | |
| Forward | 5′-TTGTCTTTCAGCAAGGACTG-3′ |
| Reverse | 5′-ATCTTGGGCTGTGACAAAGT-3′ |
| CREB1 | |
| Forward | 5′-GTGTTACGTGGGGAGAGAA-3′ |
| Reverse | 5′-GGGCTAATGTGGCAATCTGT-3′ |
| CRYAB | |
| Forward | 5′-GTTGGAGTCTGATCTTTTCCC-3′ |
| Reverse | 5′-GATAGCACTACCTGGACTATT-3′ |
| CYT C | |
| Forward | 5′-CCAGTGCCACACCGTTGAA-3′ |
| Reverse | 5′-TCCCCAGATGATGCCTTTGTT-3′ |
| DRP1 | |
| Forward | 5′-GGAGCCAGCTAGATATTAACA-3′ |
| Reverse | 5′-GCTCTTCTAGACGTTTGATTTG-3′ |
| ERRα | |
| Forward | 5′-AAGACAGCAGCCCCAGTGAA-3′ |
| Reverse | 5′-ACACCCAGCACCAGCACCT-3′ |
| FIS1 | |
| Forward | 5′-GGAGGAACAGCGGGATTACGT-3′ |
| Reverse | 5′-CTTCATGGCCTTGTCAATGAGC-3′ |
| GREB1 | |
| Forward | 5′-CCCGGGCAAGTCAGGGG-3′ |
| Reverse | 5′-GACTGCTCGGTGGATGTCAT-3′ |
| MFN2 | |
| Forward | 5′-GAAGAACAGGTTCTGGACGTC-3′ |
| Reverse | 5′-CCTCATGGCCATCTGTGCCC-3′ |
| NRF-1 | |
| Forward | 5′-TCAAAGGCATACAAAAGGTC-3′ |
| Reverse | 5′-TACTCTACAGGTCGGGGAAA-3′ |
| PDE1A | |
| Forward | 5′-CTCAAAAGCCGAAACTTCTTC-3′ |
| Reverse | 5′-CGTCTTAGTGCATCAGCAATG-3′ |
| PGC-1α | |
| Forward | 5′-GTTTCATTACCTACCGTTATAC-3′ |
| Reverse | 5′-GCCATCCCTCTGTCATCCTC-3′ |
| PRC | |
| Forward | 5′-CACTGGTTGACCCTGTTCCT-3′ |
| Reverse | 5′-GTGTTTCAGGGCTTCTCTGC-3′ |
| RINT1 | |
| Forward | 5′-CCACAGGCCACGCTGGCT-3′ |
| Reverse | 5′-GCATTGAGTCCATTTTTGAAG-3′ |
| SOD2 | |
| Forward | 5′-GCTGCACCACAGCAAGCAGTCC-3′ |
| Reverse | 5′-CCAGCAACTCCCCTTTGGGT-3′ |
| TEP1 | |
| Forward | 5′-GTTCCAGAGTCTACAGATATC-3′ |
| Reverse | 5′-GCCATCTTCTTTTCCTGAAG-3′ |
| TFAM | |
| Forward | 5′-GTTGGAGGGAACTTCCTGA-3′ |
| Reverse | 5′-CCCTTAGCTTCTTGGAATCT-3′ |
| UCP2 | |
| Forward | 5′-CCAGTGCGCGCTACAGTCA-3′ |
| Reverse | 5′-GTGGTGCTGCCTGCTAGGAG-3′ |
Western Blot Analysis
Dry cell pellets were mixed with a buffer containing 10 mmol/liter HEPES (pH 7.9), 1.5 mmol/liter MgCl2, 10 mmol/liter KCl, 0.5% Nonidet P-40, and a protease inhibitor mixture and then centrifuged at 13,000 × g for 5 min. Supernatants were collected, and the protein concentration was determined by the bicinchoninic assay kit (Uptima, Interchim, Montluçon, France) with bovine serum albumin as standard. Samples containing 20 μg of total protein were applied to 12% SDS-polyacrylamide gel electrophoresis and hybridized with either cytochrome c (Ab13575) or α-tubulin (Ab7291) antibodies at a dilution of 1:5000 (both from Abcam, Paris, France). Cytochrome c was chosen as validated target gene for direct regulation by PRC and PGC-1α coactivators (5). After overnight incubation with primary antibodies, membranes were washed before incubation with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibody, which was detected with a chemiluminescent detection system (ECL Plus, Amersham Biosciences). All of the assays were performed in triplicate.
Enzymatic Activities
The activities of COX, citrate synthase (CS), and LDH were measured on cell lysates, at 37 °C, in a cell buffer (250 mm saccharose, 20 mm Tris, 2 mm EGTA, 1 mg/ml bovine serum albumin, pH 7.2) (50 μl/106 cells) using a Beckman U 640B spectrophotometer (Beckman Coulter). COX activity was measured in a 100 mm KH2PO4 buffer containing 1 mg/ml bovine serum albumin using 15 μm reduced cytochrome c and 2.5 mm β-D dodecylmaltoside (25). The CS activity was measured in a reaction medium consisting of 0.15 mm 5,5′-dithiobis(2-nitrobenzoic acid), 0.5 mm oxaloacetic acid, 0.3 mm acetyl-CoA, and Triton X-100 (0.1%). The LDH activity was measured in a reaction medium consisting of 95 mm Tris, 10 mm pyruvate, and Triton X-100 (0.25%) using 300 μm NADH. Specific enzymatic activities were expressed in mIU (i.e. nmol of cytochrome c, 5,5′-dithiobis(2-nitrobenzoic acid), NADH/min/mg protein, respectively). The cellular protein content was determined using the bicinchoninic assay kit with bovine serum albumin as a standard. All of the reagents were obtained from Sigma-Aldrich except for Tris and saccharose (Eurobio, Les Ulis, France).
Measurement of Free Intracellular [Ca2+]
FTC-133 and RO82 W-1 cells were seeded in a 96-well plate to a density of 50,000 cells/well 15 h before the experiment and were grown in the selected medium. Cells were then loaded with 5 μm FURA-2/AM (Merck Calbiochem) and 0.1% pluronic acid F-127 (Invitrogen) at 37 °C for 40 min in a PSS buffer (140 mm NaCl, 4 mm KCl, 1 mm MgCl2, 330 μm NaH2PO4, 10 mm Hepes, 11.1 mm glucose, pH 7.4) with or without 2 mm CaCl2 (Redundant), supplemented with 0.1% bovine serum albumin (PSS/BSA). Cells were washed once with PSS/BSA and incubated for 30 min at 37 °C in 200 μl of PSS/BSA to allow the complete de-esterification of the dye. The buffer was then removed, and cells were loaded with 2 to 8 μm of BAPTA/AM at 37 °C for 40 min in PSS/BSA. Cells were washed once with PSS/BSA and incubated for 30 min at 37 °C in 200 μl of PSS/BSA. The buffer was then removed, and 90 μl of PSS/BSA were added to each well before kinetic measurement of fluorescence intensity at 37 °C using a Mithras LB940 spectrophotometer (Berthold Technologies, Bad Wildbad, Germany). For each well, the cell preparation was alternatively excited for 30 s at two wavelengths, 340 and 380 nm, and the fluorescence emission was detected at 510 nm with a counting time of 150 ms. The ionomycin solution was then injected to attain a final concentration of 2 μm/well, and the plate was shaken before measurement of fluorescence intensity at 510 nm for 160 s. The background was determined by measuring the fluorescence of a well loaded without FURA-2/AM and BAPTA/AM. The fluorescence intensity ratio, F340/F380, was used to evaluate variations in cytosolic calcium concentration ([Ca2+]c).
Microarray Analysis
cDNA preparations obtained from RO82 W-1 cells after treatments with ionomycin and BAPTA/AM were hybridized in duplicate on human 4 × 44,000 expression chips (Agilent Technologies, Santa Clara, CA) after RNA amplification and cDNA labeling using Cy3/Cy5 dyes according to the manufacturer's recommendations. Slides were analyzed with the feature extraction software after scanning on the DNA microarray scanner (Agilent Technologies). Data are available in the GEO data base (GSE 26237). The hierarchical gene clustering was computed on median gene-centered and log-transformed data using average linkage and uncentered correlation distances. Computations and visualization were performed using Cluster and TreeView software (26). The Expression Analysis Systematic Explorer (EASE) and Gene Set analysis were used to determine the statistical over represented and differentially expressed genes (27). Gene ontology enrichments in gene lists were searched for by means of the GOMiner (28). The most abundant gene ontology terms, representing at least 5% of the genes in the lists, with p values lower than 0.05, were considered for interpretation.
Confocal Microscopy
FTC-133 and RO82 W-1 cells were grown on coverslips and treated with either ionomycin (4 days at 2 μm) or BAPTA/AM (days 1–2, 2 μm; day 3, 4 μm; day 4, 6 μm). Mitochondrial staining was performed by incubating cells with MitoTracker Red CMXRos (100 nm) for 15 min at 37 °C, and endoplasmic reticulum staining was performed by incubating cells with ER-Tracker Green FM (1 μm) for 20 min at 37 °C. Cells were subsequently washed twice in PBS and fixed for 15 min in 3.7% paraformaldehyde, and nuclei were stained with 300 nm DAPI (Invitrogen). Cells were mounted with Vectorshield (Vector Laboratories, Burlingame, CA) and analyzed on a Zeiss LSM700 scanning microscope (Carl Zeiss, Göttingen, Germany) equipped with a ×63 oil immersion objective. The 555-nm laser was used for excitation of MitoTracker Red, the 488-nm laser was used for ER-Tracker Green, and the 405-nm laser was used for DAPI. Image analysis was performed using Zeiss Zen-light 4.8 software.
Statistical Analysis
Data are represented as mean values ± S.D., with n representing the number of experiments. The statistical significance of the variations observed was assessed using the Mann-Whitney test. Differences were considered significant at p < 0.05. Significances of differential expression between microarray and quantitative RT-PCR results were assessed by the t test. All analyses were performed using StatView 5.0 (SAS Institute, Gary, NC).
RESULTS
Effects of NO on Cell Proliferation and Mitochondrial Function in FTC-133 and RO82 W-1 Cells
At 96 h of SNAP treatment, cell proliferation decreased by 41.5% (50 μm SNAP) or 74.0% (100 μm SNAP) in FTC-133 cells and by 56.0% (50 μm SNAP) or 80.9% (100 μm SNAP) in RO82 W-1 cells, compared with time 0 (Fig. 1A). We controlled that the decrease in cell proliferation was due to quiescence rather than to cell death. Thus, the inhibitory action of SNAP was similarly effective on cell proliferation in the oxidative and the glycolytic cell lines.
FIGURE 1.
Cell proliferation and enzymatic activities of COX and LDH after SNAP treatment of FTC-133 and RO82 W-1 cells. FTC-133 and RO82 W-1 cells were treated once a day with 50 μm SNAP, 100 μm SNAP, or the equivalent volumes of DMSO (control) for 24–96 h. A, cell proliferation was analyzed by direct cell counts. Values are the averages ± S.E. for at least four separate determinations. *, p < 0.05 versus time 0. B, enzymatic activities of COX and LDH were measured, and values were normalized to enzymatic activity of CS and expressed relative to the controls, assigned the value of 1. Values are the averages ± S.E. (error bars) for at least five separate determinations. *, p < 0.05 versus controls.
To evaluate the effects of SNAP on mitochondrial function and glycolytic metabolism, we measured the enzymatic activities of mitochondrial complex IV (COX) and LDH (Fig. 1B). Whereas the inhibition of COX activity was proportional to SNAP concentration at 24 h in both cell lines, differences were observed at 96 h; with 100 μm SNAP, a toxic effect was observed in RO82 W-1 cells with complete inhibition of COX activity and enhancement of LDH activity. For FTC-133 cells, the inhibition of COX activity was proportional to the duration of treatment and the SNAP concentration, but the remaining COX activity was sufficient to maintain an oxidative metabolism with no increase of LDH activity. Conversely, the basal COX activity was very low in RO82 W-1 cells compared with FTC-133 cells (24).
Thus, 50 μm SNAP induced a greater decrease of COX activity at 96 h in FTC-133 cells, which mainly depend on oxidative phosphorylation to produce energy, compared with the glycolytic RO82 W-1 cells. The decrease in FTC-133 cell proliferation was proportional to that of COX activity, whereas the decrease in RO82 W-1 cell proliferation could not be exclusively attributed to changes in mitochondrial metabolism.
Effects of NO on Mitochondrial Biogenesis in FTC-133 and RO82 W-1 Cells
To determine the role of the NO pathway in the induction of mitochondrial biogenesis, we investigated the effects of SNAP on the transcriptional efficiency of several genes involved in mitochondrial function, transcriptional regulation, and coactivation (Fig. 2A). Because the 100 μm SNAP treatment led to a toxic effect at 96 h in RO82 W-1 cells, we explored the NO effect only for the 50 μm SNAP treatment.
FIGURE 2.
Gene expression after 50 μm SNAP treatment of FTC-133 and RO82 W-1 cells for 24 or 96 h. A, nuclear gene expression was monitored by quantitative RT-PCR. The genes examined represent coactivators (PRC and PGC-1α), transcription factors (CREB, NRF-1, and ERRα), CYT C, and UCP2. Data are expressed in relative units (mRNA copy number of a specific gene normalized to the β2-microglobulin mRNA copy number) and are represented relative to the controls, assigned the value of 1. Values are the averages ± S.E. (error bars) for six separate determinations. *, p < 0.05 versus controls (i.e. cells treated with equivalent volumes of DMSO). B, cytochrome c (CytC) protein expression relative to α-tubulin expression at 96 h of SNAP treatment using Western blot analysis. Control cells were treated with equivalent volumes of DMSO (n = 3). *, p ≤ 0.05 versus controls.
At 96 h of SNAP treatment, PRC expression was significantly higher in both cell lines compared with controls. However, this increase was greater in RO82 W-1 cells (108.5%) than in FTC-133 cells (48.0%). PGC-1α mRNA expression was also enhanced at 96 h of SNAP treatment but at a lower level than that of PRC: 72.8% in RO82 W-1 cells and 27.6% in FTC-133 cells. Among the transcription factors, CREB, NRF-1, and ERRα, known to be involved in either PGC-1α- or PRC-dependent mitochondrial biogenesis, NRF-1 and ERRα were overexpressed in RO82 W-1 cells at 96 h, whereas CREB and NRF-1 were overexpressed in FTC-133 cells. Interestingly, SNAP enhanced the mRNA expression of ERRα by 58.7% at 96 h only in RO82 W-1 cells in which the PRC/PGC-1α ratio as well as the induction of PRC were higher.
The expression of cytochrome c, a marker of mitochondrial biogenesis, was exclusively increased in RO82 W-1 cells, by 89.2% for mRNA and by 1.38-fold for protein at 96 h of SNAP treatment (Fig. 2B). No change in cytochrome c expression was found in FTC-133 cells at the same time. Thus, in these thyroid cell lines, NO induced mitochondrial biogenesis more efficiently through the PRC than the PGC-1α coactivator pathway. The overexpression of UCP2 in RO82 W-1 cells reflects the reduction in the efficiency of ATP production while mitochondrial biogenesis is being induced.
Effects of Ionomycin and BAPTA/AM on [Ca2+]c in FTC-133 and RO82 W-1 Cells
We measured the intracellular calcium response to the calcium ionophore ionomycin, in FTC-133 and RO82 W-1 cells (Fig. 3). Ionomycin injection (2 μm) led to a rapid increase in cytosolic calcium concentration. This increase could be due either to the generation of Ca2+-permeable pores in the plasma membrane, allowing the influx of Ca2+ from the extracellular medium, or to the induction of an efflux of Ca2+ from intracellular stores. When cells were incubated in PSS buffer containing 2 mm Ca2+, the calcium peak was 1.7-fold higher in FTC-133 cells than in RO82 W-1 cells, whereas in PSS containing 0 mm Ca2+, which revealed the part of ionomycin-induced [Ca2+]c increase due to the efflux from intracellular stores, the calcium peak in FTC-133 cells was similar to that in RO82 W-1 cells (Fig. 3A). Thus, in FTC-133 cells, the increase in [Ca2+]c was due in equal parts to the Ca2+ efflux from intracellular stores and to the extracellular Ca2+ entry. In contrast, in RO82 W-1 cells, ionomycin mainly induced the Ca2+ efflux from intracellular stores.
FIGURE 3.
Free intracellular Ca2+ measurement in FTC-133 and RO82 W-1 cells. Cells were loaded with 5 μm FURA-2/AM and 0.1% pluronic acid F-127 in PSS/BSA buffer for 40 min at 37 °C before measurement of cell fluorescence. Data are expressed as ratios of the fluorescence intensity, F340/F380, and are representative of six independent experiments. A, cell fluorescence was measured before and after ionomycin injection (arrows represent ionomycin injection) in cells incubated in PSS containing 2 mm Ca2+ (a) or 0 mm Ca2+ (b). B, cell fluorescence was measured before and after ionomycin injection (arrows represent ionomycin injection) in cells incubated in PSS containing 2 mm Ca2+ without BAPTA/AM (a) or with BAPTA/AM (b–e) treatment for 40 min.
When RO82 W-1 cells were preincubated with the cytosolic calcium chelator BAPTA/AM (Fig. 3B), the response to 2 μm ionomycin was partly reduced with 2 μm BAPTA/AM but strongly diminished with 4, 6, or 8 μm BAPTA/AM. Conversely, in FTC-133 cells, preincubation with BAPTA/AM (2–8 μm) failed to reduce the response to 2 μm ionomycin. This may be explained by the low cytosolic calcium store in RO82 W-1 cells.
Effects of Calcium on Cell Proliferation and Mitochondrial Biogenesis and Function in RO82 W-1 Cells
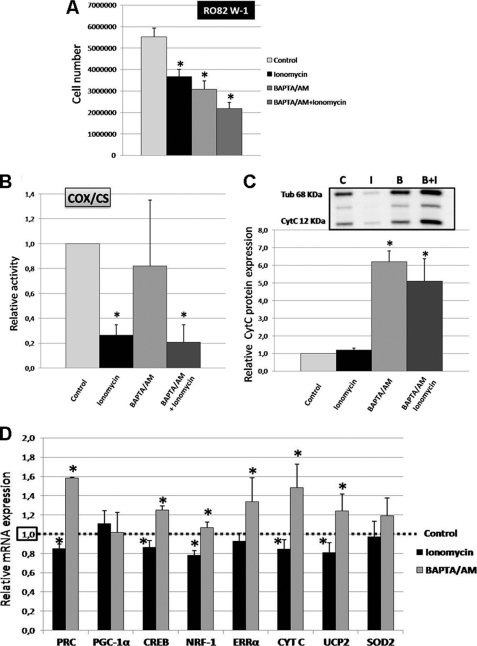
In RO82 W-1 cells, after 4 days of intermittent treatment, 2 μm ionomycin or 2–8 μm BAPTA/AM decreased cell proliferation by 33.5 and 44.5%, respectively, whereas combined treatment decreased it by 60.6% (Fig. 4A).
FIGURE 4.
Gene expression and COX activity in RO82 W-1 cells after ionomycin or BAPTA/AM treatments. RO82 W-1 cells were incubated in culture medium (containing 1.8 mm Ca2+) with either 2 μm ionomycin, 2–8 μm BAPTA/AM (to avoid cell death due to the toxic effects of intracellular Ca2+ ion chelation, the BAPTA/AM concentration was adjusted to cell density in the flask for each day of treatment as follows: 2 μm the first day, 4 μm the second day, 6 μm the third day, and 8 μm the fourth day), or both treatments compared with equivalent volumes of DMSO (control) 5 h/day for 4 days. A, cell proliferation was analyzed by direct cell counts. Values are the averages ± S.E. (error bars) for six separate determinations. *, p < 0.05 versus control. B, nuclear gene expression was monitored by quantitative RT-PCR. The genes explored represent coactivators (PRC and PGC-1α), transcription factors (CREB, NRF-1, and ERRα), cytochrome c (CYT C), UCP2, and SOD2. Data are expressed in relative units (mRNA copy numbers of specific genes normalized to the β2-microglobulin mRNA copy number) and expressed relative to the controls, assigned the value of 1. Values are the averages ± S.E. for at least three separate determinations. *, p < 0.05 versus controls. C, cytochrome c protein expression relative to α-tubulin using Western blot analysis (n = 3). *, p ≤ 0.05 relative to control cells (C). D, measurements of the enzymatic activity of COX were normalized to the enzymatic activity of CS and expressed relative to the controls, assigned the value of 1. Values are the averages ± S.E. for at least five separate determinations. *, p < 0.05 versus untreated cells as controls.
Concerning the effects of intracellular calcium levels on mitochondrial function, there was a drastic (73.5%) inhibition of COX activity in ionomycin-treated cells, whereas the chelation of cytosolic Ca2+ by BAPTA/AM had no significant effect (Fig. 4B). After combined treatment with ionomycin and BAPTA/AM, COX activity was also strongly decreased, as observed after ionomycin treatment alone. Thus, our results suggest that COX activity depends closely on the mobilization of calcium stores in RO82 W-1 cells.
The effects of the variation of cytosolic Ca2+ levels on the transcription of genes involved in mitochondrial biogenesis and function are shown in Fig. 4, C and D. After 4 days of intermittent treatment, ionomycin and BAPTA/AM induced opposite effects on gene transcription in RO82 W-1 cells. Ionomycin reduced the expression of PRC, CREB, NRF-1, CYT C, and UCP2 by 15.0, 13.9, 22.1, 15.7, and 19.0%, respectively, whereas the expression of ERRα remained unchanged. In contrast, BAPTA/AM increased the expression of PRC, CREB, ERRα, CYT C, and UCP2 by 58.5, 25.0, 33.6, 48.3, and 24.2%, respectively, whereas neither ionomycin nor BAPTA/AM modified the expression of PGC-1α mRNA. These results showed that the increase in [Ca2+]c led to the negative regulation of mitochondrial biogenesis at the transcriptional level, independent of a PGC-1α effect. Similar results were obtained after treatment with BAPTA/AM alone or combined treatment with ionomycin and BAPTA/AM (data not shown). In accordance with mRNA results, BAPTA/AM treatment induced a 6.2-fold increase in the cytochrome c protein level compared with controls (Fig. 4C). These results suggest that cytosolic calcium levels play an important role in the regulation of mitochondrial biogenesis.
Effects of Calcium on Mitochondrial and Endoplasmic Reticulum Networks in RO82 W-1 Cells
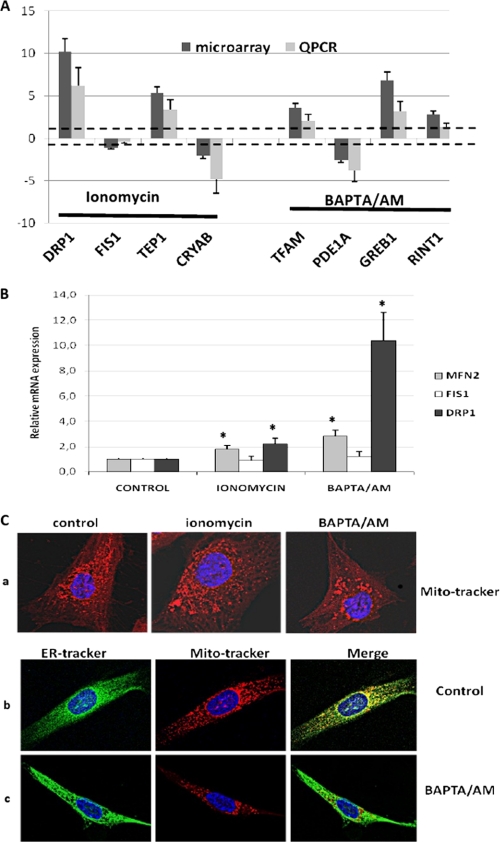
Regarding pangenomic microarray results and main gene ontologies (supplemental Fig. 1), we observed that ionomycin treatment induced the differential expression of 55 genes, whereas BAPTA/AM treatment induced the differential expression of 169 genes. Using quantitative RT-PCR analysis, we verified that seven (DRP1, TEP1, CRYAB, TFAM, PDE1A, GREB, and RINT1) of the eight most differential genes identified on microarray were confirmed in their expression (Fig. 5A). Among significant ontologies, no pathways involving NO were identified with ionomycin and BAPTA/AM treatments, suggesting that the NO and calcium pathways were not interrelated in the RO82 W-1 cells. Microarray analysis revealed that mitochondrial dynamics was regulated by calcium in RO82 W-1 cells. The expression of three genes involved in mitochondrial fusion (MFN2) and fission (DRP1 and FIS1) was specifically analyzed by quantitative RT-PCR after ionomycin and BAPTA/AM treatments (Fig. 5B). The fission/fusion gene expression ratio (DRP1/MFN2) was in favor of fission activation in the case of BAPTA/AM treatment (ratio 3.6) compared with ionomycin (ratio 1.2) and controls (ratio 1).
FIGURE 5.
Dynamics of mitochondrial and endoplasmic reticulum networks in RO82 W-1 cells after ionomycin or BAPTA/AM treatments. A, expression level of eight of the most differential genes observed after ionomycin or BAPTA/AM treatments on microarray analysis compared with real-time quantitative RT-PCR (QPCR). The expression of DRP1, FIS1, TEP1, CRYAB, TFAM, PDE1A, GREB1, and RINT1 genes was referred to the β-globin expression level and log-transformed to be compared with microarray data. Significances of differential expression between microarray and real-time quantitative RT-PCR results were assessed by t tests. Heavy dotted lines indicate the significant level for differential expression between treated (ionomycin or BAPTA/AM) and untreated cells. B, expression levels of three nuclear genes involved in the mitochondrial fusion/fission process (MFN2, FIS1, and DRP1) measured by quantitative RT-PCR in ionomycin- or BAPTA/AM-treated RO82 W-1 cells. Data are expressed in relative units (mRNA copy number of specific genes normalized to the β2-microglobulin mRNA copy number) and expressed relative to the controls, assigned the value of 1. Values are the averages ± S.E. (error bars) for at least three separate determinations. *, p < 0.05 versus controls. C, mitochondrial and endoplasmic reticulum (ER) networks after either ionomycin (4 days at 2 μm) or BAPTA/AM (from 2 to 8 μm during 4 days) treatments. a, MitoTracker Red fluorescence after ionomycin and BAPTA/AM treatments relative to control cells. b and c, ER-Tracker Green and MitoTracker Red relative to nuclear DAPI fluorescence and overlaid images in control (b) and BAPTA/AM-treated cells (c).
To better understand the role of calcium distribution in cellular compartments in mitochondrial biogenesis and function in RO82 W-1 cells, we examined the mitochondrial and endoplasmic reticulum networks after ionomycin or BAPTA/AM treatments (Fig. 5C). The BAPTA/AM treatment led to fragmentation of the mitochondrial network (Fig. 5C, a) independent of the modification of the endoplasmic reticulum (Fig. 5C, b and c). This fragmentation affecting specifically the mitochondrial network could be due to an imbalance between the fission and fusion processes. Thus, the remodeling of the mitochondrial network and the availability of mitochondrial calcium following BAPTA/AM treatment may lead to the induction of mitochondrial biogenesis.
Molecular Pathways Involved in NO- and Ca2+-mediated Mitochondrial Biogenesis in RO82 W-1 Cells
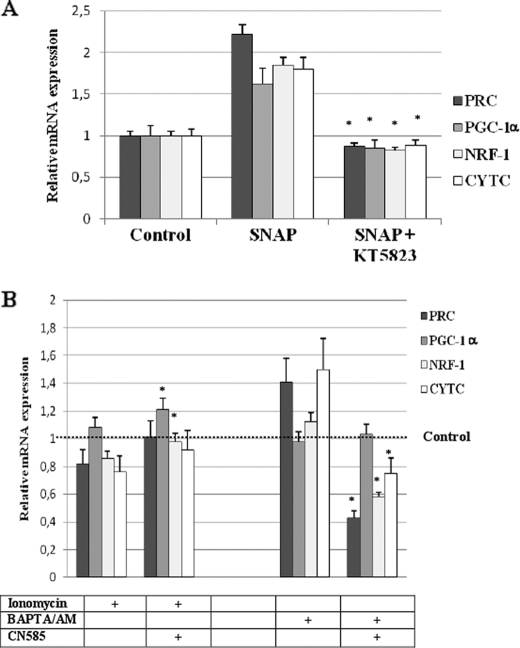
To explore whether variations of intracellular NO and calcium concentrations were causative or correlative of mitochondrial biogenesis regulation, we examined the effects of kinase or phosphatase inhibitors on the expression of key genes for mitochondrial biogenesis. When the NO/cGMP pathway was inhibited by the protein kinase G inhibitor KT5823 at 1 μm, the expression of PRC, PGC-1α, NRF-1, and CYTC was significantly down-regulated (Fig. 6A). This showed the involvement of the NO/cGMP pathway in the regulation of PRC and PGC-1α expression impacting mitochondrial biogenesis.
FIGURE 6.
Gene expression after modulation of molecular pathways involved in NO or Ca2+ signaling in RO82 W-1 cells. A, PRC, PGC-1α, NRF-1, and cytochrome c mRNA expression at 96 h of 50 μm SNAP and 1 μm KT5823 (protein kinase G inhibitor) treatments were compared with those of controls (i.e. cells treated with an equivalent volume of DMSO). *, p ≤ 0.05 versus SNAP-treated cells (n = 3). B, PRC, PGC-1α, NRF-1, and cytochrome c mRNA expression after 4 days of intermittent treatment with 2 μm ionomycin or 2–8 μm BAPTA/AM and 10 μm CN585 (calcineurin inhibitor) were compared with those of controls (i.e. cells treated with equivalent volume of DMSO). *, p ≤ 0.05 versus ionomycin or BAPTA/AM-treated cells (n = 3). Error bars, S.D.
Because activation of the calcineurin pathway was recently shown to be involved in the translocation of DRP1 to mitochondria and in nuclear translocation of a number of transcription factors in response to mitochondrial respiratory stress (29, 30), we have chosen to explore the effects of the calcineurin inhibitor CN585 on the regulation of mitochondrial biogenesis by calcium. The expression of PGC-1α and NRF-1 was significantly increased when CN585 at 10 μm was added during ionomycin treatment (Fig. 6B). However, this was not associated with significant variation of CYT C expression. On the contrary, the addition of CN585 calcineurin inhibitor during BAPTA/AM treatment led to significant down-regulation of PRC, NRF-1, and CYT C expression without variation of PGC-1α expression. This showed the involvement of calcineurin in the regulation of PRC-related mitochondrial biogenesis induced by BAPTA/AM treatment.
DISCUSSION
The two follicular thyroid carcinoma cell lines, FTC-133 and RO82 W-1, which we investigated for their differential mitochondrial content, metabolic status, and PRC/PGC-1α expression ratios, were expected to serve as suitable models for examining the endogenous modulation of mitochondrial biogenesis through the PRC and PGC-1α pathways.
Our study showed that the expression of PRC, PGC-1α, and NRF-1 mRNA increased after 96 h of 50 μm SNAP treatment in FTC-133 as well as in RO82 W-1 cells. This increase was greater in the glycolytic RO82 W-1 cells, which have a higher basal PRC/PGC-1α ratio than the mainly oxidative FTC-133 cells. In addition, we found that the expression of PRC was more sensitive to NO induction than that of PGC-1α. Interestingly, SNAP treatment increased the mRNA expression of ERRα and cytochrome c as well as the protein level of cytochrome c in RO82 W-1 cells, which present higher basal and NO-inductive levels of PRC expression than FTC-133 cells. We therefore postulate that NO-dependent mitochondrial biogenesis is more effectively induced by PRC, especially when interacting with ERRα. This is in accordance with our earlier finding that both ERRα and PRC are necessary for the activation of gene transcription and mitochondrial biogenesis (24). In addition, ERRα has also been shown to modulate the expression of endothelial nitric-oxide synthase (31), underscoring the critical role of the NO/PRC/ERRα pathway in the fine regulation of mitochondrial biogenesis.
The greater induction of genes involved in mitochondrial biogenesis could be related to the activation of the retrograde pathway by the modification of respiratory chain activity and COX inhibition (7). Thus, cells presenting respiratory defects should be more sensitive to signaling agents previously identified for their role in the initialization of the retrograde pathway like NO and calcium, leading to compensatory processes for energy production. We showed that the induction of mitochondrial biogenesis was higher in the glycolytic RO82 W-1 cells than in the oxidative FTC-133 cells. Unfortunately, this was not associated with an increase in mitochondrial respiratory function because LDH activity persisted at a high level after SNAP treatment. Our findings indicate that although NO preferentially induces mitochondrial biogenesis through a PRC/ERRα pathway in the glycolytic RO82 W-1 cells, it does not reverse the metabolic pathway of these cells. We postulate that the glucose-enriched medium used to grow these cells is capable of preventing the metabolic shift as it has been described in the case of colon cancer cells (32).
Our investigation into the effects of intracellular Ca2+ variations in the glycolytic RO82 W-1 cells showed that ionomycin mainly increased [Ca2+]c by inducing the efflux from the intracellular stores. COX activity was strongly inhibited after ionomycin treatment, whereas it remained unchanged after treatment with BAPTA/AM, the cytoplasmic Ca2+ chelator. Interestingly, the inhibition of COX activity was similar whether ionomycin or a combination of ionomycin and BAPTA/AM was added, suggesting that ionomycin mainly exerted its effect on the calcium store in the mitochondrial matrix. It has been shown that mitochondrial Ca2+-dependent kinases and phosphatases modulate the respiratory complex activity by acting on the phosphorylation status of COX or other respiratory complexes (13, 33). Thus, the inhibition of COX activity by ionomycin in RO82 W-1 cells may result from the deregulation of the phosphorylation status.
In a recent study, we demonstrated the involvement of PRC in the regulation of phosphorylation levels of several mitogen-activated protein kinases (10). The present study shows that mitochondrial biogenesis in RO82 W-1 cells is modulated through the PRC pathway by variations in the [Ca2+]c level. In these cells, calcium may inhibit mitochondrial biogenesis by modulating the activity of cytosolic targets involved in the regulation of nuclear genes. Interestingly, our results show that calcineurin is involved in the regulation of PRC-related mitochondrial biogenesis induced by [Ca2+]c modulation. Our work with microarray analysis and confocal microscopy also indicates that calcium affects the dynamics of the mitochondrial network in RO82 W-1 cells. Mitochondrial biogenesis induced by treatment with the cytosolic calcium chelator BAPTA/AM is associated with a disruption of the mitochondrial network and a great increase of DRP1 mRNA expression. These findings agree with observations on skeletal muscle showing that training-induced mitochondrial biogenesis involves remodeling of the mitochondrial network corresponding to an increased expression of DRP1 (34).
Among the cytosolic kinases affected by calcium, the Ca2+/calmodulin-dependent protein kinase type IV has been reported to regulate PGC-1α-mediated mitochondrial biogenesis in muscle (35). Moreover, the authors showed that Ca2+/calmodulin-dependent protein kinase also contributed to the activation of constitutive endothelial nitric-oxide synthase, leading to increased NO production. Moreover, calcium release from the sarcoplasm has been found to partially increase mitochondrial biogenesis via the NOS/PGC-1α pathway in the L6E9 myotube model (36). No such effects have been described for PRC. Here, we show that treatment of RO82 W-1 thyroid cells with the SNAP-NO donor increased mitochondrial biogenesis, whereas the rise in [Ca2+]c induced by ionomycin decreased it. This suggests that Ca2+/calmodulin-dependent protein kinase is not involved in the modulation of mitochondrial biogenesis by calcium. Moreover, SNAP treatment increased the expression of the PRC as well as the PGC-1α coactivators, whereas variations in [Ca2+]c modulated only the expression of PRC. The opposite effects of calcium on mitochondrial biogenesis in thyroid cell and myotube models may thus be due to a preferential use of calcium in the PRC or PGC-1α coactivator pathways.
Although further investigation will be required to elucidate each pathway, our results suggest that independent intracellular signaling cascades may be involved in the NO- and calcium-dependent modulation of mitochondrial biogenesis in thyroid cells. In our study, calcium chelation stimulated mitochondrial biogenesis through the PRC pathway, inducing the expression of ERRα exclusively, whereas NO acted through the PRC and PGC-1α pathways, inducing the expression of NRF-1 as well as that of ERRα. Thus, our results highlight the critical role of the PRC/ERRα pathway to control the maintenance of mitochondrial network as described previously for PGC1α (37).
Moreover, in thyroid cell models expressing PRC as well as PGC-1α, the expression of UCP2 mRNA was related to that of PRC mRNA after the modulation of NO and intracellular calcium levels. Indeed, unlike the oxidative FTC-133 cells, the glycolytic RO82 W-1 cells overexpressed UCP2 mRNA after SNAP and BAPTA/AM treatments, with little or no change in PGC-1α expression. These results indicate that in follicular thyroid carcinoma cells, the efficiency of the oxidative phosphorylation process is regulated by PRC rather than by PGC-1α when endogenous signals, such as NO and calcium, are activated (10, 38). In conclusion, our work highlights the role of NO and calcium as regulators of PRC-dependent mitochondrial biogenesis and function, suggesting that these PRC-regulating pathways could be potential therapeutic targets for solid tumors presenting metabolic alterations.
Supplementary Material
Acknowledgments
We are grateful to Kanaya Malkani for critical reading and comments on the manuscript and to Dominique Couturier for technical help.
This work was supported by grants from the Région Pays de la Loire (Ciblage Moléculare et Applications Thérapeutiques).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- NRF
- nuclear respiratory factor
- ERR
- estrogen-related receptor
- CREB
- cAMP-response element-binding protein
- PRC
- PGC-1-related coactivator
- COX
- cytochrome c oxidase
- CYT C
- cytochrome c
- TFAM
- transcription factor A, mitochondrial
- LDH
- lactate dehydrogenase
- BAPTA/AM
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester
- CS
- citrate synthase
- [Ca2+]c
- cytosolic calcium concentration
- SNAP
- S-nitroso-N-acetyl-dl-penicillamine.
REFERENCES
- 1. Scarpulla R. C. (2008) Physiol. Rev. 88, 611–638 [DOI] [PubMed] [Google Scholar]
- 2. Puigserver P., Wu Z., Park C. W., Graves R., Wright M., Spiegelman B. M. (1998) Cell 92, 829–839 [DOI] [PubMed] [Google Scholar]
- 3. Andersson U., Scarpulla R. C. (2001) Mol. Cell. Biol. 21, 3738–3749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gleyzer N., Vercauteren K., Scarpulla R. C. (2005) Mol. Cell. Biol. 25, 1354–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vercauteren K., Pasko R. A., Gleyzer N., Marino V. M., Scarpulla R. C. (2006) Mol. Cell. Biol. 26, 7409–7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vercauteren K., Gleyzer N., Scarpulla R. C. (2008) J. Biol. Chem. 283, 12102–12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butow R. A., Avadhani N. G. (2004) Mol. Cell 14, 1–15 [DOI] [PubMed] [Google Scholar]
- 8. Nisoli E., Clementi E., Paolucci C., Cozzi V., Tonello C., Sciorati C., Bracale R., Valerio A., Francolini M., Moncada S., Carruba M. O. (2003) Science 299, 896–899 [DOI] [PubMed] [Google Scholar]
- 9. Nisoli E., Falcone S., Tonello C., Cozzi V., Palomba L., Fiorani M., Pisconti A., Brunelli S., Cardile A., Francolini M., Cantoni O., Carruba M. O., Moncada S., Clementi E. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 16507–16512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raharijaona M., Le Pennec S., Poirier J., Mirebeau-Prunier D., Rouxel C., Jacques C., Fontaine J. F., Malthiery Y., Houlgatte R., Savagner F. (2009) PLoS One 4, e7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cleeter M. W., Cooper J. M., Darley-Usmar V. M., Moncada S., Schapira A. H. (1994) FEBS Lett. 345, 50–54 [DOI] [PubMed] [Google Scholar]
- 12. Moncada S., Bolaños J. P. (2006) J. Neurochem. 97, 1676–1689 [DOI] [PubMed] [Google Scholar]
- 13. Bender E., Kadenbach B. (2000) FEBS Lett. 466, 130–134 [DOI] [PubMed] [Google Scholar]
- 14. Harris D. A., Das A. M. (1991) Biochem. J. 280, 561–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moreno-Sánchez R. (1985) J. Biol. Chem. 260, 12554–12560 [PubMed] [Google Scholar]
- 16. Papa S., Sardanelli A. M., Cocco T., Speranza F., Scacco S. C., Technikova-Dobrova Z. (1996) FEBS Lett. 379, 299–301 [DOI] [PubMed] [Google Scholar]
- 17. Calderón-Cortés E., Cortés-Rojo C., Clemente-Guerrero M., Manzo-Avalos S., Villalobos-Molina R., Boldogh I., Saavedra-Molina A. (2008) Mitochondrion 8, 262–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freyssenet D., Irrcher I., Connor M. K., Di Carlo M., Hood D. A. (2004) Am. J. Physiol. Cell Physiol. 286, C1053–C1061 [DOI] [PubMed] [Google Scholar]
- 19. Ojuka E. O., Jones T. E., Han D. H., Chen M., Wamhoff B. R., Sturek M., Holloszy J. O. (2002) Am. J. Physiol. Endocrinol. Metab. 283, E1040–E1045 [DOI] [PubMed] [Google Scholar]
- 20. Ojuka E. O., Jones T. E., Han D. H., Chen M., Holloszy J. O. (2003) FASEB J. 17, 675–681 [DOI] [PubMed] [Google Scholar]
- 21. Irrcher I., Adhihetty P. J., Sheehan T., Joseph A. M., Hood D. A. (2003) Am. J. Physiol. Cell Physiol. 284, C1669–C1677 [DOI] [PubMed] [Google Scholar]
- 22. Arnould T., Vankoningsloo S., Renard P., Houbion A., Ninane N., Demazy C., Remacle J., Raes M. (2002) EMBO J. 21, 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mercy L., Pauw A., Payen L., Tejerina S., Houbion A., Demazy C., Raes M., Renard P., Arnould T. (2005) FEBS J. 272, 5031–5055 [DOI] [PubMed] [Google Scholar]
- 24. Mirebeau-Prunier D., Le Pennec S., Jacques C., Gueguen N., Poirier J., Malthiery Y., Savagner F. (2010) FEBS J. 277, 713–725 [DOI] [PubMed] [Google Scholar]
- 25. Rustin P., Munnich A., Rötig A. (2004) Methods Enzymol. 382, 81–88 [DOI] [PubMed] [Google Scholar]
- 26. Eisen M. B., Spellman P. T., Brown P. O., Botstein D. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tusher V. G., Tibshirani R., Chu G. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeeberg B. R., Feng W., Wang G., Wang M. D., Fojo A. T., Sunshine M., Narasimhan S., Kane D. W., Reinhold W. C., Lababidi S., Bussey K. J., Riss J., Barrett J. C., Weinstein J. N. (2003) Genome Biol. 4, R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cereghetti G. M., Stangherlin A., Martins de Brito O., Chang C. R., Blackstone C., Bernardi P., Scorrano L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 15803–15808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guha M., Tang W., Sondheimer N., Avadhani N. G. (2010) Biochim. Biophys. Acta 1797, 1055–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sumi D., Ignarro L. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14451–14456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. López-Ríos F., Sánchez-Aragó M., García-García E., Ortega A. D., Berrendero J. R., Pozo-Rodríguez F., López-Encuentra A., Ballestín C., Cuezva J. M. (2007) Cancer Res. 67, 9013–9017 [DOI] [PubMed] [Google Scholar]
- 33. Miyazaki T., Neff L., Tanaka S., Horne W. C., Baron R. (2003) J. Cell Biol. 160, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perry C. G., Lally J., Holloway G. P., Heigenhauser G. J., Bonen A., Spriet L. L. (2010) J. Physiol. 588, 4795–4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu H., Kanatous S. B., Thurmond F. A., Gallardo T., Isotani E., Bassel-Duby R., Williams R. S. (2002) Science 296, 349–352 [DOI] [PubMed] [Google Scholar]
- 36. McConell G. K., Ng G. P., Phillips M., Ruan Z., Macaulay S. L., Wadley G. D. (2010) J. Appl. Physiol. 108, 589–595 [DOI] [PubMed] [Google Scholar]
- 37. Soriano F. X., Liesa M., Bach D., Chan D. C., Palacín M., Zorzano A. (2006) Diabetes 55, 1783–1791 [DOI] [PubMed] [Google Scholar]
- 38. Wu Z., Puigserver P., Andersson U., Zhang C., Adelmant G., Mootha V., Troy A., Cinti S., Lowell B., Scarpulla R. C., Spiegelman B. M. (1999) Cell 98, 115–124 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.