Abstract
Proper developmental, neural cell type-specific and activity-dependent regulation of GABAergic transmission is essential for virtually all aspects of CNS function. The number of GABAA receptors in the postsynaptic membrane directly controls the efficacy of GABAergic synaptic transmission. Thus, regulated trafficking of GABAA receptors is essential for understanding of brain function in both health and disease. Here we summarize recent progress in understanding of mechanisms that allow dynamic adaptation of cell surface expression and postsynaptic accumulation and function of GABAA receptors. This includes activity-dependent and cell type-specific changes in subunit gene expression, assembly of subunits into receptors, as well as exocytosis, endocytic recycling, diffusion dynamics and degradation of GABAA receptors. In particular, we focus on the roles of receptor-interacting proteins, scaffold proteins, synaptic adhesion proteins, and enzymes that regulate the trafficking and function of receptors and associated proteins. In addition, we review neuropeptide signaling pathways that affect neural excitability through changes in GABAAR trafficking.
Introduction
Inhibitory neurotransmission in the brain is largely mediated by γ-aminobutyric acid (GABA) acting through GABA type A receptors (GABAARs). These receptors are heteropentameric GABA-gated chloride channels that belong to the Cys-loop ligand-gated ion channel superfamily (Figure 1A) (Barnard et al., 1998). In addition to fast actions of GABA via GABAARs, GABA also modulates neural activity on a slower time scale through activation of GABABRs belonging to the G protein-coupled receptor superfamily. GABAARs are expressed ubiquitously in neurons along the entire neuraxis. Dynamic changes in their expression and function accordingly are implicated in the regulation of virtually all aspects of brain function. In addition, GABAAR activity controls important aspects of brain development, including proliferation and differentiation of neural progenitors, neural migration, and dendritic maturation of neurons. Deficits in GABAAR-mediated GABAergic transmission are implicated in the etiology of epilepsy (Fritschy, 2008), anxiety disorders (Lydiard, 2003), mood disorders (Craddock et al., 2010; Luscher et al., 2011), and schizophrenia (Charych et al., 2009). A detailed understanding of the mechanisms that regulate functional expression of GABAARs at synapses therefore is a prerequisite for an understanding of the causes of these disorders.
Figure 1. GABAAR subunit structure and intracellular loop sequences.
A. Schematic representation of GABAAR heteropentamers consisting of two α, two β and a single γ2 subunit assembled in a counterclockwise γ–β-α-β-α arrangement. B. Every subunit includes an extracellular N-terminal domain, four transmembrane domains (TM1–4) separated by an extended cytoplasmic loop region between TM3 and TM4, and a short extracellular C-terminus. The cytoplasmic loop and TM4 regions of the γ2 subunit are essential for postsynaptic clustering of GABAARs (Alldred et al., 2005). C. Sequences of cytoplasmic loop regions of representative subunits (γ2L, β3, α2) with amino acid numbers referring to mature polypeptides from the mouse. Interaction sites for binding partners are marked by brackets beneath the sequence, along with amino acid numbers of known Ser/Thr and Tyr phosphorylation sites. Phosphorylation sites are shown in blue, Lys residues representing putative ubiquitination sites are in orange. Note that the minimal interaction site for CAML includes the C-terminal region of the cytoplasmic loop as well as the TM4 domain of the γ2 subunit. For CaMKII phosphorylation sites see Houston et al (2009). For other references see text.
Experimental evidence indicates that synaptically released neurotransmitters saturate their receptors (Clements, 1996) and hence, that the functional strength of GABAergic synapses changes in proportion with the number of postsynaptic GABAARs (Otis et al., 1994; Nusser et al., 1997). Consistent with this idea, even modest reductions in postsynaptic GABAARs (5–35%) in GABAAR mutant mice have significant behavioral consequences (Crestani et al., 1999; Shen et al., 2010b). The focus of this review is on mechanisms that underlie dynamic changes in the posttranslational biogenesis, surface accumulation, turnover and trafficking of GABAARs, which arguably represent the most important and diverse biological means to adjust GABAergic transmission. First, we will provide brief overviews of the structure-function relationships of different GABAAR subtypes and the different modes of regulation of postsynaptic GABAergic function. We will then summarize current understanding of the processes that regulate the assembly of subunits into transport-competent GABAARs, the exocytosis of receptors to the plasma membrane, and the endocytic recycling and degradation of GABAARs. Next we will focus on mechanisms that regulate the differential distribution of GABAARs at the cell surface between synaptic and extrasynaptic membrane sites, the interaction with postsynaptic protein scaffold and adhesion molecules, and dynamic changes in surface mobility of GABAARs. Finally, we discuss neuropeptide signaling systems that act upstream of GABAARs and exert their neural effects in part through altered GABAAR trafficking.
Structure of GABAARs in relation to cellular distribution and function
GABAARs are members of the superfamily of heteropentameric ligand-gated ion channels that also include the nicotinic acetylcholine receptors, glycine receptors, and 5-HT3 receptors (Figure 1A) (reviewed in Unwin, 1989; Barnard et al., 1998). The subunits of all these receptors share a common ancestral structure that includes an extracellular N-terminal domain, four transmembrane domains (TM1–4), and an extended cytoplasmic loop region between TM3 and TM4 that mediates interactions with trafficking and signaling factors (Figure 1B, C). GABAAR subunits are encoded by 19 different genes that have been grouped into eight subclasses based on sequence homology (α1–6, β1–3, γ1–3, δ, ε, θ, π, ρ1–3). Alternative splicing contributes to additional receptor diversity. In particular, the γ2 (Whiting et al., 1990) and β2 subunits (McKinley et al., 1995) exist as short and long splice variants distinguished by the presence or absence of eight and 38 amino acids, respectively.
Different subunit combinations give rise to a large number of structurally and functionally distinct GABAAR subtypes. Based on a recent conservative count, 11 structurally and functionally distinct receptor subtypes have been conclusively identified and are reasonably abundant in at least parts of the brain. They represent combinations of 2α and 2β subunits together with a single γ2 or δ subunit. An additional 15 receptor subtypes exist with high probability and a more limited distribution (Olsen and Sieghart, 2008). These numbers do not account for additional heterogeneity based on two different types of α or β subunits in one receptor complex (Khan et al., 1996; Benke et al., 2004), or due to alternative splicing of subunits. GABAARs with different subunit compositions exhibit different pharmacology and channel gating properties, are differentially expressed during development and in the adult brain, accumulate at different neuronal cell surfaces, and are subject to differential regulation by extracellular cues.
The subsets of GABAARs at synapses are composed of two α1, α2, or α3 subunits together with two β2 or β3 subunits and a single γ2 subunit. Compared to other GABAAR subtypes discussed below these receptors exhibit low affinity for GABA and thus are optimized to respond selectively to relatively high concentrations of GABA released into the synaptic cleft (300 μM, Perrais and Ropert, 1999). The γ2 subunit is essential for postsynaptic clustering of GABAARs (Essrich et al., 1998). However, the γ3 subunit can substitute for the γ2 subunit and contribute to postsynaptic GABAARs in the developing postnatal brain (Baer et al., 1999). Freeze-fracture replica immunogold labeling indicates that α2, α3 and β3 subunit-containing receptors are 50–130 times more concentrated at synapses than in the extrasynaptic membrane (Kasugai et al., 2010). However, in absolute numbers the majority of these receptors are localized in the extrasynaptic space, which greatly exceeds the synaptic membrane area. Moreover, not all γ2-containing receptors are concentrated at synapses. In particular, α5βγ2 receptors are found almost exclusively at extrasynaptic sites (Brunig et al., 2002a; Crestani et al., 2002; Serwanski et al., 2006) and contribute to tonic GABAergic currents (Caraiscos et al., 2004; Glykys et al., 2008), although synaptic α5βγ2 receptors have been reported also (Serwanski et al., 2006; Zarnowska et al., 2009).
The most prominent population of non-synaptic GABAARs mediating tonic inhibition consists of α4βδ receptors in the forebrain and α6βδ receptors in the cerebellum. In addition, α1βδ receptors underlie tonic inhibition of hippocampal interneurons (Glykys et al., 2007). The δ-containing receptor subtypes exhibit high agonist affinity and therefore are tailored to function at ambient sub-micromolar concentrations of GABA outside of synapses (Saxena and Macdonald, 1996; Haas and Macdonald, 1999; Ke et al., 2000; Bianchi et al., 2001; Brown et al., 2002; Terpstra et al., 2002). Lastly, GABAARs also are present on axons, including the axon initial segment of pyramidal cells (Nusser et al., 1996; Brunig et al., 2002a; Szabadics et al., 2006), mossy fiber terminals of hippocampal granule cells (Ruiz et al., 2003; Jang et al., 2006; Alle and Geiger, 2007), axon terminals of retinal bipolar neurons (Shields et al., 2000) and cerebellar parallel fibers (Stell et al., 2007). Axonal GABAARs are thought to modulate action potential conductance and neurotransmitter release (Kullmann et al., 2005).
GABAergic inhibition: multiple modes of regulation
Changes in GABAAR subunit gene expression
Regulated expression of GABAAR subunit genes determines cell type-specific and developmental changes in the subunit composition and function of GABAARs. In addition, significant changes in subunit mRNA levels are observed in adulthood. For example, the subunit gene expression of α4βδ receptors in granule cells of the dentate gyrus is dynamically altered during epileptogenesis in a rat model of epilepsy (Brooks-Kayal et al., 1998; Peng et al., 2004) and during the estrus cycle of the mouse (Maguire et al., 2005). The levels of mRNAs encoding subunits of these receptors in CA1 pyramidal cells of rats is changed during puberty (Shen et al., 2007; Shen et al., 2010a), at the end of pregnancy (Sanna et al., 2009) and in a progesterone withdrawal model of premenstrual syndrome (Sundstrom-Poromaa et al., 2002). These studies in rodents indicate that alterations in subunit mRNA levels are generally paralleled by corresponding changes in the surface accumulation and function of GABAARs that contribute to changes in neural excitability. Significant changes in subunit gene expression have also been described in postmortem brain of depressed patients and suicide victims (Klempan et al., 2009; Sequeira et al., 2009). Such changes are predicted to disrupt the subunit composition of GABAARs and are consistent with the GABAergic deficit hypothesis of major depression (Luscher et al., 2011).
Dynamic changes in the Cl− equilibrium potential
The neural response to GABAAR activation depends on the Cl− equilibrium (ECl) potential, which determines the electrochemical driving force for Cl−. ECl is determined chiefly by the relative expression of the Cl−-transporters KCC2 and NKCC1, which increase and decrease, respectively, during animal development and neural differentiation (for reviews see Ben-Ari, 2002; Fiumelli and Woodin, 2007; Andang and Lendahl, 2008). The ensuing hyperpolarizing shift in ECl leads to a gradual conversion of GABAergic depolarization in immature neurons to mainly hyperpolarizing function in mature neurons. This switch in the function of GABAARs is essential for structural and functional maturation of neurons (Tozuka et al., 2005; Ge et al., 2006; Cancedda et al., 2007) and for termination of interneuron migration in the developing neocortex (Bortone and Polleux, 2009). Recent evidence further suggests that the ECl of mature neurons may be subject to synaptic input-specific modulation by the voltage- and Cl− sensitive Cl− channel ClC-2 (Foldy et al., 2010). The proposed function of ClC-2 is to prevent excessive accumulation of intracellular Cl− following strong GABAergic stimulation. While GABAergic inputs to mature neurons are mostly inhibitory, depolarizing GABAergic effects are also common (reviewed by Marty and Llano, 2005; Kahle et al., 2008). In particular, the aforementioned axo-axonic synapses at the axon initial segment of cortical pyramidal cells (Szabadics et al., 2006), at hippocampal mossy fiber terminals (Jang et al., 2006), and on parallel fibers of the cerebellum (Stell et al., 2007; Pugh and Jahr, 2011) are depolarizing and excitatory due to the local absence of KCC2 (Szabadics et al., 2006). Moreover, dynamic changes in the functional expression of KCC2 can lead to pathophysiological adaptations of neural excitability. For example, chronic stress-induced downregulation of KCC2 results in a depolarizing shift of the chloride reversal potential of neurons in the paraventricular nucleus of the hypothalamus, which renders GABA inputs ineffective (Hewitt et al., 2009). This posttranslational mechanism is thought to contribute to hypothalamus-pituitary-adrenal (HPA) axis hyperactivity and to the neuropathology of stress-associated neuropsychiatric disorders. Moreover, KCC2 mRNA and/or protein expression is down-regulated following focal ischemia (Jaenisch et al., 2010) and status epilepticus (Pathak et al., 2007). The sustained loss of GABAergic inhibition observed following status epilepticus has been proposed to underlie injury-induced long-term increases in seizure susceptibility. Mechanistically, status-epilepticus-induced downregulation of KCC2 involves BDNF/TrkB-mediated activation of cAMP response element-binding protein (CREB) (Rivera et al., 2004) and phosphorylation of tyrosine residues in the KCC2 C-terminal domain, which triggers its lysosomal degradation (Lee et al., 2010).
Regulation of GABAAR assembly
The functional expression of GABAARs at the cell surface is first controlled at the level of assembly of subunits into heteropentameric complexes. A detailed understanding of this step is limited by the overabundance of different subunits coexpressed in individual neurons. Nevertheless, the use of concatenated subunit constructs representative of the most abundant GABAAR subtype (α1β2γ2) established that assembly of heteropentamers follows strict rules, which ensure that the subunits assume a counterclockwise γ–β-α-β-α arrangement when viewed from the synaptic cleft (Baumann et al., 2001, 2002; Baur et al., 2006). Interestingly, corresponding analyses of αβδ receptors indicate that the δ subunit does not simply take the place of the γ2 subunit. Instead the optimal subunit arrangement of δ-containing receptors depends on the type of α subunit present (Sigel et al., 2009). Forced expression of subunits in heterologous cells can lead to homomeric assemblies and complexes between α and γ or β and γ subunits that are, however, in most cases retained in the endoplasmic reticulum (ER) (Connolly et al., 1996). Formation of such nonproductive dimers or oligomers renders assembly of functional receptors rather inefficient, at least in heterologous cells (Gorrie et al., 1997). Unlike the α/γ or β/γ subunit combinations, coexpression of α and β subunits in heterologous cells results in formation of functional receptors that can reach the surface. Moreover, some evidence suggests that αβ receptors may exist naturally in small numbers and contribute to tonic inhibition of neurons (Brickley et al., 1999; Mortensen and Smart, 2006). However, when α, β and γ2 subunits are coexpressed the formation of receptors containing all three types of subunits is strongly favored over receptors composed of α and β subunits alone (Angelotti and Macdonald, 1993). Moreover, single channel analyses of γ2 subunit knock-out neurons indicate that receptors composed of α and β subunits alone are gated inefficiently by GABA and have much lower single channel conductances than naturally occurring receptors (Lorez et al., 2000).
The assembly of complexes that are translocated to the cell surface involves the initial formation of αβ subunit heterodimers and is principally controlled by the N-terminal/luminal domain of subunits (Taylor et al., 1999; Klausberger et al., 2000; Taylor et al., 2000; Klausberger et al., 2001; Sarto et al., 2002; Bollan et al., 2003a; Ehya et al., 2003; Sarto-Jackson et al., 2006). This process involves interaction with ER-associated chaperones such as calnexin and binding immunoglobulin protein (BiP) (Connolly et al., 1996; Bradley et al., 2008). The fundamental role of α and β subunits in the assembly of GABAARs is further evidenced by data from knock-out mice, which indicate that deletion of α or β subunits results in loss of corresponding receptors (Homanics et al., 1997; Jones et al., 1997; Kralic et al., 2002a). By contrast, deletion of the γ2 subunit results in only a modest reduction of GABA binding sites (−22%) and is therefore largely dispensable for assembly of α and β subunits (Gunther et al., 1995). Intriguingly, a recent study analyzing the expression of GABAARs in transfected human embryo kidney (HEK) cells suggests that GABA might act as an intracellular chaperone important for GABAAR biogenesis in the early secretory pathway (Eshaq et al., 2010). Consistent with such a function, the above mentioned N-terminal assembly signals are located proximal to the GABA- and benzodiazepine-binding sites of GABAARs (Boileau et al., 1999; Teissere and Czajkowski, 2001). The importance of subunit N-terminal domains for receptor assembly in vivo is exemplified by a naturally occurring point mutation (R43Q) in the γ2 subunit that is associated with childhood absence epilepsy and febrile seizures (Wallace et al., 2001; Kang and Macdonald, 2004; Hales et al., 2005; Frugier et al., 2006; Tan et al., 2007). Moreover, a small naturally-occurring N-terminal deletion mutant of the rat α6 subunit abolishes assembly of corresponding receptors (Korpi et al., 1994).
The rules that govern differential assembly in cells that co-express multiple GABAAR subtypes remain little explored, although some evidence indicates that assembly may be mass-driven by the rate of co-translation of compatible subunits. Transgenic mice that express ectopic α6 subunits in hippocampal pyramidal cells exhibit a gain of extrasynaptic α6βγ2 receptors at a cost of postsynaptic receptors (Wisden et al., 2002). Deletion of the α1 subunit in mice leads to compensatory upregulation of receptors containing other α subunits (Sur et al., 2001; Kralic et al., 2002a; Kralic et al., 2002b; Kralic et al., 2006). Furthermore, a residue (R66) in the N-terminal domain of the α1 subunit is essential for assembly of α1β2 receptors but dispensable for formation of α1β1 and α1β3 complexes (Bollan et al., 2003b). Recent evidence further suggests that entry of transport competent GABAAR assemblies into the secretory pathway depends on subunit glycosylation (Tanaka et al., 2008; Lo et al., 2010).
Regulation of GABAAR exocytosis
The exit of GABAARs from the ER is limited by constitutive ER-associated degradation (ERAD) of α and β subunits (Gallagher et al., 2007; Saliba et al., 2007; Bradley et al., 2008), suggesting that receptor assembly is relatively inefficient (Figure 2). ERAD of GABAARs is further enhanced by chronic blockade of neural activity (Saliba et al., 2009). Neural activity blockade-induced ubiquitination and degradation of GABAAR subunits involves reduced Ca2+ entry through voltage-gated Ca2+ channels (VGCCs). The entry of GABAARs into the secretory pathway is facilitated by interaction of α and β subunits with the protein that links integrin-associated protein with the cytoskeleton-1 (PLIC-1, also known as ubiquilin) (Bedford et al., 2001) (interaction sites of GABAAR trafficking factors in GABAAR subunit intracellular loop regions are indicated in Figure 1C). PLIC-1 is concentrated in the perinuclear ER in association with aggresomes (Heir et al., 2006) but also present in the nucleus (Mah et al., 2000) and in association with intracellular membranes in dendrites and near synapses (Bedford et al., 2001). PLIC-1 and its paralog PLIC-2 contain ubiquitin-like (ubl) proteasome binding domains and ubiquitin-associated (uba) domains, and the two proteins are known to interfere with ubiquitin-mediated proteolysis of diverse substrates (Wu et al., 1999; Kleijnen et al., 2000; Walters et al., 2002; Kleijnen et al., 2003). Accordingly, overexpression of PLIC-1 in neurons promotes the surface expression of GABAARs (Bedford et al., 2001), presumably by inhibiting ubiquitination and ERAD of α and β subunits (Figure 2).
Figure 2. Regulated trafficking of GABAARs in the secretory pathway.
GABAAR heteropentamers assemble in the ER and interact with chaperones including calnexin and BiP. Unassembled or improperly folded receptor subunits are subject to ubiquitination and proteasomal degradation. This process is inhibited by interaction of α and β subunits with the ubiquitin-like protein PLIC, which in turn promotes the exit of receptors from the ER to the Golgi. The Golgi resident palmitoyltransferase GODZ palmitoylates the receptor γ2 subunit at cytoplasmic cysteine residues, which promotes translocation of receptors through the Golgi apparatus to the plasma membrane and to synapses. Exit of GABAARs from the Golgi may be facilitated by interaction of the GTP exchange factor BIG2 with GABAAR β subunits. The surface delivery of GABAARs is further promoted by a number of other proteins that currently cannot be assigned to a specific trafficking compartment (shaded gray).
ER to Golgi translocation of GABAARs
The γ2 subunit of GABAARs is subject to palmitoylation at cytoplasmic cysteine residues, and this modification regulates the accumulation of GABAARs at inhibitory synapses (Keller et al., 2004; Rathenberg et al., 2004). The Golgi-specific DHHC zinc finger protein (GODZ, zDHHC3) interacts with and palmitoylates the γ2 subunit in vitro (Figure 1C) (Keller et al., 2004; Fang et al., 2006). In brain, GODZ is selectively expressed in neurons and highly restricted to Golgi membranes, including Golgi outposts in primary dendrites (Keller et al., 2004). The protein is a member of a family of at least 23 structurally related palmitoyltransferases characterized by the presence of a DHHC motif-containing cysteine-rich domain (DHHC-CRD). Among these, only GODZ and its paralog SERZ-β (zDHHC7) are able to palmitoylate the γ2 subunit in heterologous cells (Fang et al., 2006). Reducing the expression of GODZ by shRNA or dominant negative constructs leads to selective loss of GABAARs at synapses, along with reduced GABAergic innervation and corresponding reductions in amplitude and frequency of miniature inhibitory synaptic currents (mIPSCs), as well as whole cell currents (Fang et al., 2006). Palmitoylation is a reversible posttranslational modification and therefore may dynamically regulate the association of cytoplasmic substrates with membranous structures. In the case of integral membrane proteins, however, palmitoylation may extend the effective length of an adjacent transmembrane domain, as suggested by analysis of the palmitoylation-dependent trafficking of the Wnt coreceptor LRP6 (lipoprotein receptor-related protein 6) (Abrami et al., 2008). The restricted localization of GODZ to Golgi membranes, together with the notion that ER membranes are thinner than Golgi and plasma membranes (Bretscher and Munro, 1993; Mitra et al., 2004), suggests that GODZ serves to facilitate ER to Golgi translocation of γ2-containing GABAARs (Figure 2).
Translocation of GABAARs from Golgi to the plasma membrane
The brefeldin A-inhibited GDP/GTP exchange factor 2 (BIG2) interacts with a sequence motif in the intracellular loop of GABAAR β subunits that overlaps with the PLIC binding site (Figure 1C) (Charych et al., 2004b). BIG2 is a Sec7 domain-containing guanine exchange factor (GEF) that catalyzes GDP/GTP exchange of class I ADP-ribosylation factors (ARF) 1 and 3 (Morinaga et al., 1997; Togawa et al., 1999). GEF activation of these G-proteins is required for membrane budding of vesicles from the Golgi apparatus, thereby enabling proteins to proceed through the trans-Golgi network (TGN) towards the plasma membrane (Shin et al., 2004) (Figure 2). Coexpression of BIG2 with the β3 subunit in heterologous cells promotes the translocation of this subunit to the cell surface. Consistent with a role in exocytosis of GABAARs, BIG2 immunoreactivity is concentrated in the TGN and has been detected in somatic and dendritic vesicle-like structures, as well as in the postsynaptic density of both inhibitory and excitatory synapses (Charych et al., 2004b). Interestingly, independent studies have identified BIG2 as a component of recycling endosomes and provided evidence that BIG2-mediated activation of ARFs contributes to the structural integrity of this trafficking compartment (Shin et al., 2004; Shin et al., 2005; Boal and Stephens, 2010). Thus, BIG2 is implicated in facilitating the exit of GABAARs from the Golgi towards the plasma membrane as well as in endocytic recycling of GABAARs.
The GABAAR associated protein (GABARAP) represents the first GABAAR interacting protein isolated and accordingly has received considerable attention (Wang et al., 1999; reviewed in Chen and Olsen, 2007). It belongs to a family of ubiquitin-like proteins that in mammals includes the paralogs GEC-1 (guinea-pig endometrial cells-1, also known as GABARAP-like 1, GABARAPL1), GATE-16 (Golgi-associated ATPase enhancer of 16 kDa, also known as ganglioside expression factor-2 or GABARAPL2), GABARAPL3, GABARAPL4 and the more distantly related MAP-LC3 (microtubule-associated protein light chain 3). GABARAP interacts with all γ subunits and with microtubules in vitro and in vivo (Figure 1C) (Wang et al., 1999; Nymann-Andersen et al., 2002b). The protein is enriched in Golgi and other somatodendritic membrane compartments but absent at synapses (Kneussel et al., 2000; Kittler et al., 2001). Upon overexpression in hippocampal neurons, GABARAP facilitates the translocation of GABAARs to the cell surface (Leil et al., 2004). Interestingly, GABARAP-mediated trafficking of GABAARs involves an evolutionarily conserved lipid conjugation and delipidation cycle first described in yeast (Tanida et al., 2004). The attachment of phosphatidyl ethanolamine (PE) to the C-terminus of GABARAP family proteins involves activating, conjugating, and deconjugating enzymes analogous to the ubiquitin conjugation system (Hemelaar et al., 2003; Tanida et al., 2003; Kabeya et al., 2004). Experiments in transfected cultured neurons indicate that conjugation to PE is required for dendritic accumulation of GABARAP and for GABARAP-induced cell surface expression of GABAARs (Chen and Olsen, 2007).
Activity-induced translocation of GABAARs to the plasma membrane
Acute knockdown of GABARAP by siRNA in cultured neurons has revealed a role of GABARAP in rapid NMDA-induced functional plasticity of inhibitory synapses (Marsden et al., 2007). NMDA receptor-mediated Ca2+ influx following moderate stimulation of neurons with NMDA leads to a rapid increase in the number of postsynaptic GABAAR clusters and mIPSC amplitudes (see also further below and Figure 5C). In addition to GABARAP this mechanism involves Ca2+ calmodulin-dependent kinase II (CaMKII), the vesicular trafficking factor N-ethylmaleimide-sensitive factor (NSF), and glutamate receptor interacting protein (GRIP). The rate of GABAAR endocytosis following treatment with NMDA was unaltered, suggesting that GABARAP-dependent potentiation of inhibitory synapses involves increased exocytosis rather than reduced endocytosis of GABAARs (Marsden et al., 2007). These findings represent the so far only loss-of-function experiments showing an essential role for endogenous GABARAP in GABAAR trafficking. The relevant protein-protein interactions and CaMKII phosphorylation targets have so far not been identified. However, experiments in heterologous cells allow speculation that this mechanism might involve CaMKII-induced phosphorylation of the β3 subunit at S383 (Houston et al., 2007).
Figure 5. Regulation of GABAAR clustering and lateral mobility at synaptic and extrasynaptic sites.
A. The biosynthesis of gephyrin is regulated by the peptidyl-prolyl cis/trans isomerase Pin1. Cytosolic soluble gephyrin exists as a trimer. The deposition of gephyrin trimers at the plasma membrane is facilitated by cooperative interactions of gephyrin with CBSH3+ (tethered to the plasma membrane by phosphoinositide binding of its PH domain) and NL2, which unlock the CBSH3+-dependent clustering function, presumably by releasing an intramolecular inhibition of CBSH3+ by its SH3 domain. The gephyrin/NL2/collybistin complex enables the postsynaptic clustering of gephyrin and, through interaction with presynaptic neurexins, helps to align the postsynaptic complex with GABAergic terminals. The GABAAR α2 subunit may substitute for NL2 and enable collybistin-dependent clustering of gephyrin. The clustering of GABAARs in the postsynaptic specialization is facilitated by interaction of specific subunits (α2, α3) with gephyrin. Postsynaptic gephyrin further interacts with Mena/VASP and profilin I/II. Competition of gephyrin and G-actin for interaction with profilin I/II is implicated in regulation of the microfilament-dependent receptor packing density. The density of postsynaptic gephyrin clusters is regulated by GSK3β-mediated phosphorylation of gephyrin, which enhances the susceptibility of gephyrin to cleavage by the Ca2+-dependent protease calpain-1. Constitutive proteolytic cleavage of gephyrin limits the confinement and accumulation of postsynaptic GABAARs, by facilitating their lateral diffusion. Conversely, inhibition of GSK3β by Li+ or of calpain-1 by its natural antagonist calpastatin stabilizes gephyrin and thereby promotes the density of gephyrin clusters as well as GABAergic synaptic function. B. Postsynaptic GABAARs (α1,2,3β2/3γ2) are confined (red arrows) by interactions with gephyrin and presumably other postsynaptic scaffold proteins. However, on leaving this area they become highly mobile within the plane of the phospholipid bilayer (yellow arrows). The interaction of GABAARs with the postsynaptic cytoskeleton is regulated by the activity-dependent and calcineurin-regulated phosphorylation state of the γ2 subunit. Robust excitation of glutamate receptors leads to NMDAR/Ca2+- and Ca2+/calmodulin-mediated activation of calcineurin and dephosphorylation of γ2(S270), which reduces the postsynaptic confinement of GABAARs allowing their diffusion away from synapses. CaMKII is activated in parallel but its translocation to synapses is prevented by calcineurin by an unknown mechanism. In contrast to α1,2,3β2/3γ2 receptors, α5βγ2 receptors are clustered extrasynaptically by interaction with phospho-activated radixin, which links these receptors to submembrane microfilaments. C. Modest stimulation of neurons as mimicked by treatment of neurons with NMDA leads to more limited influx of Ca2+ and preferential activation of CaMKII. Activated CaMKII is translocated to synapses and stimulates the insertion of GABAARs into the plasma membrane where they are trapped at synapses by interaction with the postsynaptic cytoskeleton. Insertion of GABAARs into the plasma membrane involves GABARAP, NSF and GRIP. The relevant target proteins interacting with and phosphorylated by CaMKII are not yet known.
GABARAP - a multitasker?
The data summarized thus far suggest that GABARAP promotes the regulated, activity-dependent and CaMKII-mediated translocation of GABAARs from intracellular compartments to the somatodendritic plasma membrane. However, a more general role of GABARAP in exocytosis of GABAARs is difficult to reconcile with other findings. First, GABARAP has been proposed to contribute to rebound potentiation, a neural activity-induced postsynaptic form of long-term potentiation (LTP) of inhibitory synapses on Purkinje cell neurons (Kawaguchi and Hirano, 2007). Using electrical stimulation of cultured Purkinje cells to mimic rebound potentiation, the authors found evidence that this form of plasticity is critically dependent on a CaMKII-dependent conformational alteration of GABARAP. However, LTP of GABAergic synapses occurred without measurable changes in the cellular distribution and cell surface expression of GABAARs. Given that GABARAP is absent at synapses (Kneussel et al., 2000; Kittler et al., 2001) the exact role of GABARAP in this form of plasticity requires further clarification. Second, the aforementioned PE conjugation of GABARAP is critically involved in autophagy, an evolutionarily conserved form of bulk transport of membranes and cytoplasm to lysosomes for protein degradation (Tanida et al., 2004). Consistent with a role of GABARAP in autophagy, there is evidence that GABAARs are subject to autophagy in worms. Body wall muscle cells of C. elegans lacking proper GABAergic and cholinergic innervation show selective accumulation of GABAARs, but not nicotinic acetylcholine receptors in autophagosomes. This indicates that in worms autophagy represents a pathway for endocytic lysosomal degradation of GABAARs (Rowland et al., 2006). Third, GABARAP knock-out mice show normal expression and punctate distribution of γ2-containing GABAARs (O’Sullivan et al., 2005), possibly due to functional redundancy of GABARAP with GEC1 (Mansuy-Schlick et al., 2006) and other GABARAP family members. Third, the function of GABARAP is complicated by its interactions with a very large number of other proteins. Among these the ER luminal Ca2+-dependent chaperone calreticulin stands out in that it binds GABARAP with exceptionally high affinity (Mohrluder et al., 2007). Compared to calreticulin the interaction of GABARAP with γ2-derived peptides shows low affinity, suggesting that GABARAP might promote protein trafficking unspecifically along the secretory pathway (Knight et al., 2002).
Several GABARAP-interacting proteins contribute to GABAAR trafficking independently of GABARAP. The aforementioned NMDAR-induced and GABARAP-dependent increase in GABAAR clustering also depends on the synaptic PDZ domain-containing protein GRIP (Marsden et al., 2007), which interacts with GABARAP in vitro and in vivo (Kittler et al., 2004a). GRIP was first described as a trafficking factor of AMPARs (Dong et al., 1997). It is present at both glutamatergic and GABAergic synapses, consistent with functions at both types of synapses (Dong et al., 1999; Charych et al., 2004a; Kittler et al., 2004a; Li et al., 2005). GABARAP further interacts with the phospholipase C-related catalytically inactive proteins 1 and 2 [PRIP1/2, PRIP1 was previously named p130 (Kanematsu et al., 2002)], a pair of GABAAR-associated adaptor proteins for phosphatases and kinases (Figure 3A) (Kanematsu et al., 2002; Uji et al., 2002). Likewise, GABARAP and its paralog GATE-16 (Sagiv et al., 2000) interact with NSF, an ATPase and chaperone of SNARE complexes that is critically important for regulated neurotransmitter release and also involved in trafficking of neurotransmitter receptors (Morgan and Burgoyne, 2004; Zhao et al., 2007).
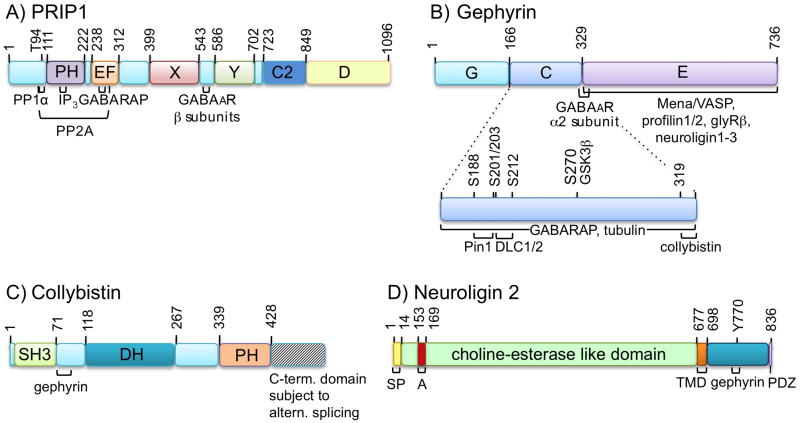
Figure 3. Schematic representation of proteins pivotal for intracellular trafficking of postsynaptic GABAARs.
A. PRIP consists of an N-terminal domain that incudes a binding site for the catalytic domain of PP1α, a PH domain that includes a binding site for IP3 (D-myo-inositol 1,4,5 triphosphate), an EF-hand domain that includes a binding site for GABARAP, and homologies to the catalytic (X, Y) and C2 domains of phospholipase Cδ. The GABAAR β subunit interaction domain is located between the X and Y domains of PRIP. Amino acid numbers refer to murine PRIP-1 (Kanematsu et al., 2005). B. Gephyrin consists of an N-terminal G-domain involved in the formation of gephyrin trimers, a central C domain with interaction sites for Pin1, DLC1/2, collybistin, and a GSK3β phosphorylation site that regulates susceptibility to cleavage by calpain-1, and a C-terminal E domain that dimerizes in vitro and regulates clustering in vivo. In vitro assays suggest that the C domain also interacts with GABARAP and tubulin, although these proteins are not colocalized with postsynaptic gephyrin. The E domain binds Mena/VASP, profilin, the glycine receptor β subunit and neuroligins. The gephyrin motif at the C-E domain interface that interacts with collybistin (PFPLTSMDKA) (Harvey et al., 2004) overlaps with the α2 subunit binding site (SMDKAFITVLEMPTVLGTE) (Saiepour et al., 2010).
Amino acid numbers refer to rat gephyrin (Prior et al., 1992). C. Collybistin exists in three alternatively spliced versions that differ in sequence and length of their C-terminal domain (striped area). In addition, its clustering function is regulated by the presence or absence of an SH3 domain. Also shown are the dbl homology (DH) domain that regulates nucleotide exchange and the pleckstrin homology (PH) domain required for interaction with membrane phosphoinositides. The gephyrin binding site has been mapped to the linker domain between SH3 and DH domains. Amino acid numbers refer to rat collybistin (Kins et al., 2000). D. NL2 is composed of an N-terminal signal peptide (SP), followed by a large choline-esterase-like domain with the alternatively spliced exon A, a transmembrane domain (TM), and an intracellular cytoplasmic domain that includes a 15-amino-acid tyrosine-containing binding site for gephyrin and a C-terminal binding site for PDZ domain proteins such as S-SCAM. Amino acid numbers refer to mouse NL2 (Ichtchenko et al., 1996).
Both PRIP1/2 and NSF interact with GABAARs indirectly through GABARAP and directly via GABAAR β subunits (Figure 1C) (Kanematsu et al., 2002; Kittler et al., 2004a; Terunuma et al., 2004; Goto et al., 2005). PRIP1/2 double knock-out mice exhibit reduced expression and altered behavioral pharmacology of GABAARs, suggesting deficits in mainly γ2-containing GABAARs (Kanematsu et al., 2002; Kanematsu et al., 2006; Mizokami et al., 2007). Brain extracts of these mice further show reduced association of GABAARs with GABARAP, indicating that PRIP facilitates indirect association of GABARAP with GABAARs (Mizokami et al., 2007). Moreover, PRIP and the γ2 subunit compete for binding to the same binding site on GABARAP (Kanematsu et al., 2002; Uji et al., 2002). The minimal interaction site for GABARAP in the γ2 subunit further overlaps with a γ2 domain that interacts with itself and with γ1, γ3 and β subunits in vitro (Figure 1C)(Nymann-Andersen et al., 2002a). Circumstantial evidence suggests that GABAARs are endocytosed as dimers (Kittler et al., 2008). It is therefore conceivable that overexpressed GABARAP and γ2 subunit peptides designed to compete for interaction with GABARAP affect the surface expression of GABAARs in part by competing for other protein interactions (receptor dimerization, interaction of receptors with PRIP and NSF) that facilitate the endocytic trafficking of GABAARs.
Regulated endocytosis of GABAARs
Clathrin-mediated endocytosis
Regulated endocytosis of neurotransmitter receptors is known to underlie physiological and pathological adaptations of neural excitability. Endocytosis of GABAARs occurs primarily via clathrin- and dynamin-dependent mechanisms that are facilitated by interactions of the GABAAR β and γ subunits with the clathrin adaptor protein AP2 (Kittler et al., 2000; Kittler et al., 2005; Kittler et al., 2008)(Figure 4). Accordingly, blocking the function of dynamin results in increased accumulation of postsynaptic GABAARs along with increased mIPSC amplitudes (Kittler et al., 2000). When measured in about one-week-old cultures, approximately 25% of cell surface GABAARs are endocytosed within 30 minutes, and 70% of these receptors are recycled back to the cell surface within one hour. On a slower time scale (6 h), about 30% of neuronal GABAARs are targeted to late endosomes where they become subject to lysosomal degradation (Kittler et al., 2004b). However, constitutive endocytosis of GABAARs is significantly reduced in mature neurons as indicated by studies that analyzed the diffusion dynamics of GABAARs within the plasma membrane (discussed later in this review).
Figure 4. Regulated endocytosis and recycling of GABAARs.
GABAAR endocytosis through clathrin-coated vesicles is regulated by phospho-sensitive interactions of β and γ2 subunits with the clathrin adaptor AP2. Phosphorylation of β subunits (S408/409 in β3) and the γ2 subunit (Y365/367) by PKA/PKC and Fyn/Src, respectively interferes with these interactions and thereby stabilizes GABAARs at the cell surface. Phosphorylation of β subunits by PKA and PKC is facilitated by the kinase adaptors AKAP and RACK, respectively. Dephosphorylation is modulated by PRIP-associated PP1α and PP2A. GABAARs in the plasma membrane are subject to lateral diffusion. Interaction of GABAARs with gephyrin (i.e. through α2/3 subunits) and collybistin (α2 subunit) leads to their accumulation at synapses. Interaction of gephyrin/collybistin/GABAAR complexes with the NL2-neurexin synaptic adhesion complex contributes to proper alignment of pre- and postsynaptic complexes at inhibitory synapses. Conversely, dephosphorylation of β subunits by PP1α and PP2A (β3 subunit S408/409 site) and unidentified tyrosine phosphatases (γ2 Y365/367) facilitates interaction of extrasynaptic GABAARs with AP2, which then triggers clathrin-mediated internalization. Dephosphorylation of β subunits by PP1α is inhibited or facilitated by the phosphatase adaptor PRIP, depending on its own phosphorylation state. Endocytosed receptors in early endosomes are ubiquitinated at lysine and possibly other residues of the γ2 subunit, which then leads to lysosomal degradation. Alternatively, interactions of CAML with the γ2 subunit cytoplasmic and transmembrane domains and of HAP1 with the β subunit cytoplasmic domain facilitate KIF5-dependent vesicular transport and recycling of GABAARs to the plasma membrane.
The search for sequence motifs important for AP2/clathrin/dynamin-mediated endocytosis of GABAARs first lead to the identification of a di-leucine motif in β subunits that is critical for receptor internalization in heterologous cells (Herring et al., 2003; Herring et al., 2005). However, whether this particular mechanism operates in neurons has not been established. A second motif that is important for AP2/clathrin/dynamin-mediated GABAAR internalization in neurons has been mapped to a highly basic 10-amino acid sequence motif that includes a major phosphorylation site conserved in the cytoplasmic loop region of β1–3 subunits (S408, S409 in β3, Figure 1C) (Kittler et al., 2005; Kittler et al., 2008). Importantly, interaction of the AP2 μ2 subunit with this site is negatively regulated by phosphorylation of GABAAR β subunits, indicating that AP2 binds GABAARs with high affinity and triggers their internalization preferentially when this site is dephosphorylated. Accordingly, perfusion of neurons with an unphosphorylated β3-derived peptide that competes with this interaction results in enhanced mIPSCs and whole cell currents (Kittler et al., 2005).
Regulation of endocytosis by phospho-sensitive interactions with GABAAR β subunits
The AP2 interaction site on β1 and β3 subunits (Figure 1C) can be phosphorylated by both protein kinase A (PKA) (McDonald et al., 1998) and protein kinase C (PKC) (Brandon et al., 2000; Brandon et al., 2002), while the same site in the β2 subunit is phosphorylated by PKC only (McDonald et al., 1998; Brandon et al., 2003), allowing for receptor subtype-specific modulation of GABAAR endocytosis. However, the same site can also be phosphorylated by CaMKII (McDonald and Moss, 1994) and Akt (also known as PKB) (Wang et al., 2003b; Xu et al., 2006). The latter is discussed further below in the context of insulin-induced exocytosis of GABAARs. PKC-mediated phosphorylation is facilitated by stable interaction of this kinase with β subunits, either directly as shown for the PKC-βII isozyme or indirectly through the receptor for activated C-Kinase (RACK-1), which recognizes a binding site in the β1 subunit adjacent to the PKC binding site (Brandon et al., 1999; Brandon et al., 2002). Reductions in the PKC-mediated phosphorylation of GABAAR β subunits are implicated in the dramatic loss of GABAergic inhibition in animal models of status epilepticus, which is thought to underlie pharmaco-resistance to benzodiazepines following prolonged seizures in epileptic patients (Terunuma et al., 2008).
The β subunit phosphostate-dependent endocytosis of GABAARs is further regulated by interaction of β subunits with PRIP1/2 and their function as adaptors for the serine/threonine-specific phosphatases PP1α and PP2A (Yoshimura et al., 2001; Uji et al., 2002; Terunuma et al., 2004; Kanematsu et al., 2006; Kanematsu et al., 2007). Phosphorylation of PRIP at a threonine residue (T94 in PRIP1) leads to dissociation of the catalytically inactive PRIP/PP1α complex and activation of PP1α and hence dephosphorylation of the β3 subunit at the AP2 interaction site (Terunuma et al., 2004). Unlike PP1α, PP2A is constitutively active when bound to PRIP (Kanematsu et al., 2006). Consistent with a role of PRIP-associated phosphatases in endocytosis of GABAARs, the PRIP/PP1α/PP2A complex can be coimmunoprecipitated with AP2 and clathrin from brain extracts (Kanematsu et al., 2007). Moreover, PRIP facilitates GABAAR endocytosis in transfected heterologous cells. The association of PRIP with PP2A (Kanematsu et al., 2006) is implicated in brain-derived neurotrophic factor (BDNF)-induced downregulation of GABAARs (Jovanovic et al., 2004), as discussed in further detail below. The end effect of PRIP on GABAAR cell surface expression appears to depend on the cellular state of several other signal transduction pathways. The aforementioned phenotype of PRIP1/2 double knock-out mice, which includes functional deficits of GABAARs, suggests that PRIP primarily facilitates the exocytosis or cell surface stability of GABAARs (Kanematsu et al., 2002; Kanematsu et al., 2006; Mizokami et al., 2007). Recent evidence summarized further below indicates that PRIP1/2 also serve as an adaptor for the serine/threonine kinase Akt, which promotes the de novo insertion of GABAARs into the plasma membrane (Fujii et al., 2010).
Intriguingly, the AP2 interaction site in the β1–3 subunits overlaps with the binding site for the vesicular ATPase and trafficking factor NSF (Figure 1C) (Goto et al., 2005). NSF interacts with phorbol ester-activated PKCε. Moreover, PKCε phosphorylates and activates the ATPase function of NSF. PKCε-mediated phosphorylation of NSF induces its translocation to the plasma membrane and to synapses and concurrently reduces the cell surface expression of GABAARs (Chou et al., 2010). PKCε knock-out mice are less anxious and produce lower levels of stress hormone than WT mice (Hodge et al., 2002), which is the opposite of the anxious-depressive-like phenotype of GABAAR γ2 subunit heterozygous mice and therefore consistent with increased functional expression of GABAARs (Crestani et al., 1999; Luscher et al., 2011). Therefore, pharmacological inhibitors of PKCε activity may have therapeutic potential for the treatment of neuropathological conditions that involve deficits in GABAergic transmission. This NSF-dependent trafficking mechanism is reminiscent of aforementioned earlier experiments conducted in heterologous cells, showing phorbol ester-induced and PKC and clathrin-mediated endocytosis of GABAARs from the plasma membrane by a mechanism that is independent of GABAAR phosphorylation (Chapell et al., 1998; Connolly et al., 1999). PKCε is one of seven PKC isozymes activated by phorbol esters. It therefore seems likely that PKCε contributes to phorbol ester-induced endocytosis of GABAARs. However, one might predict that PKCε and NSF-dependent endocytosis of GABAARs is counteracted by the aforementioned PKC-βII-mediated phosphorylation of β subunits, which limits endocytosis of GABAARs. Consistent with multiple PKC and PKA-regulated modes of GABAAR trafficking, these kinases can have cell type specific and functionally opposite effects on mIPSC amplitudes in vivo (Poisbeau et al., 1999).
Regulation of endocytosis by phospho-sensitive interactions with the GABAAR γ2 subunit
A third interaction of GABAARs with AP2 involves a bipartite motif in the intracellular loop region of the γ2 subunit (Figure 1C). It consists of a 12-amino-acid basic domain that is homologous to the AP2 binding site in β subunits and a more C-terminal γ2-specific YGYECL motif (Smith et al., 2008). These two domains interact cooperatively with separate domains in the μ2 subunit of AP2. The γ2-specific YGYECL motif is of particular interest as it exhibits high affinity for AP2 that is sensitive to phosphorylation at γ2 Tyr365/367 (Kittler et al., 2008). These residues are phosphorylated by Fyn and other Src kinase family members in vivo (Lu et al., 1999; Jurd et al., 2010). A non-phosphorylated YGYECL peptide effectively competes with the AP2-γ2 subunit interaction, thereby increasing the GABAARs surface expression and mIPSCs amplitude and showing that this site is constitutively phosphorylated in cultured neurons (Kittler et al., 2008). This mechanism is also important in vivo as evidenced by reduced expression and altered function of GABAARs in Fyn knock-out mice (Boehm et al., 2004), and by an embryonic lethal phenotype of knock-in mice in which the γ2 Tyr365/367 residues were mutated to phenylalanine, which interferes with AP2 binding (Tretter et al., 2009). Heterozygous γ2Y365/7F mice, however, are viable. In the stratum pyramidale of the hippocampus they show a CA3-region-specific increase in the postsynaptic accumulation of GABAARs, suggesting different basal levels of γ2 Tyr365/367 phosphorylation in the CA3 versus CA1 region. The lethal phenotype of homozygous γ2Y365/7F mutants indicates that excessive GABAergic transmission is detrimental during early development, probably due to excessive GABAergic excitation, which may interfere with normal neurogenesis and neural migration (Wang and Kriegstein, 2009).
Collectively, there is now conclusive evidence that GABAARs are subject to at least two major mechanisms of regulated endocytosis. These mechanisms involve different phospho-sensitive interactions of the clathrin adaptor AP2 with β and γ2 subunits, respectively. The phospho-states of the relevant β and γ2 subunit motifs are subject to regulation by multiple Ser/Thr and Tyr kinases, as well as phosphatases and their respective adaptor proteins. Dynamic changes in the phosphorylation state of NSF and PRIP and their interaction with the AP2 binding site of β subunits provide additional levels of regulation. Future experiments will need to address whether NSF and PRIP compete with AP2 for GABAAR interaction and whether their interaction with GABAARs is regulated by phosphorylation of GABAARs.
Regulation of recycling and degradation of GABAARs
The decision of whether endocytosed GABAARs are recycled or degraded is regulated by interaction of GABAAR β subunits with huntingtin-associated protein 1 (HAP1) (Figure 4) (Kittler et al., 2004b). HAP1 interacts with the Huntington disease protein huntingtin (Li et al., 1995; Li et al., 2002) and is involved in motor protein-dependent transport of neuronal cargo (Engelender et al., 1997; Gauthier et al., 2004; McGuire et al., 2006). In addition, HAP1 interacts with the cytoplasmic loop region of β1–3 subunits. When overexpressed in cultured neurons, HAP1 interferes with the degradation of endocytosed GABAARs and thereby increases the recycling and surface expression of GABAARs (Kittler et al., 2004b). More recent experiments have identified HAP1 as an adaptor for the kinesin superfamily motor protein 5 (KIF5), interacting directly with all three isoforms (A-C) of KIF5 heavy chains (Twelvetrees et al., 2010). HAP1, KIF5 heavy chains and γ2-containing GABAARs are partly colocalized in dendrites and can be isolated as a complex from brain lysates. Moreover, live imaging and electrophysiological recordings revealed that HAP1-KIF5-dependent vesicular trafficking controls the delivery of GABAARs to the plasma membrane and thereby promotes the function of GABAergic inhibitory synapses. Interestingly, mutant huntingtin with an expanded polyGln repeat that causes Huntington’s disease interferes with normal HAP1-KIF-dependent vesicular transport and thereby reduces the amplitude of GABAergic mIPSCs. Thus, reduced expression and function of GABAARs may contribute to neurodegeneration associated with Huntington’s disease (Twelvetrees et al., 2010).
Of note, the γ-aminobutyric acid(A) receptor-interacting factor, GRIF-1 (also known as OIP98, ALS2CR3, huMilt2, or TRAK2), which has been shown to interact selectively with the β2 subunit in vitro (Beck et al., 2002), also interacts with KIF5 motor proteins (Brickley et al., 2005). The precise function of GRIF-1 in trafficking of GABAARs is unknown but the protein provides a second potential link between GABAARs and the KIF5 vesicular trafficking machinery. Furthermore, the GRIF-1 paralog TRAK-1, which also interacts with KIF5 (Brickley et al., 2005), has been isolated as the gene that causes a spontaneous hypertonic mutant phenotype of mice associated with elevated basal activity of motor neurons (Gilbert et al., 2006). TRAK-1 can be immunoprecipitated with GABAARs from brain extracts and results in reduced GABAAR immunostaining when mutated, probably due to a dominant negative effect of mutant TRAK-1. Consistent with an underlying GABAAR deficit, the hypertonic phenotype of TRAK-1 mutants can be ameliorated by potentiation of GABAAR function with benzodiazepines (Gilbert et al., 2006).
An independent line of experiments identified calcium-modulating cyclophilin ligand (CAML) as a regulator of post-endocytic trafficking of GABAARs (Figure 4) (Yuan et al., 2008). CAML is an integral membrane protein that is essential for normal embryonic development and for differentiation of neurons in culture. However, conditional deletion of CAML in differentiated neurons results in reduced accumulation of GABAARs at the plasma membrane and at synapses, along with selective GABAergic but not glutamatergic functional deficits. Interestingly, CAML interacts with the C-terminal cytoplasmic and transmembrane domains of γ subunits (Yuan et al., 2008). These domains are essential for clustering and function of GABAARs at synapses, as was shown for the γ2 subunit (Alldred et al., 2005; Christie et al., 2006). Reduced plasma membrane accumulation and function of GABAARs in CAML-deficient neurons is associated with normal endocytosis from the plasma membrane but reduced recycling of GABAARs from endocytic pools (Yuan et al., 2008). This function of CAML in endocytic recycling of GABAARs is consistent with a similar role of CAML in recycling of endocytosed epidermal growth factor (EGF) receptor (Tran et al., 2003).
A recent report has identified Maf1 and a Maf1-interacting coiled-coil protein named Macoco as additional GABAAR β3 subunit interacting proteins (Smith et al., 2010). Maf1 was originally identified in yeast as a nuclear regulator of t-RNA transcription {Pluta, 2001 #7125}. However, in neurons Maf1 is also present in the somatodendritic cytoplasm. Macoco was isolated as a Maf1-interacting protein and then found to also interact with GABAAR γ2 and β3 subunits independently of Maf1. Both proteins are highly expressed in hippocampus, and they are partially colocalized with postsynaptic GABAARs in cultured neurons. Overexpression of Macoco facilitates the surface expression of GABAARs suggesting a function in the secretory pathway (Smith et al., 2010). However, the precise mechanism for this effect remains to be determined.
Endocytosed GABAARs that fail to be recycled are targeted for lysosomal degradation as demonstrated by reduced degradation in the presence of the lysosomal protease inhibitor leupeptin (Figure 4) (Kittler et al., 2004b). This route of trafficking is facilitated by ubiquitination of a series of lysine residues within the intracellular domain of the γ2 subunit (Figure 1C). Blockade of lysosomal activity or disruption of the trafficking of ubiquitinated cargo to lysosomes specifically increases the accumulation of GABAARs at synapses as well as the efficacy of GABAergic synaptic inhibition (Arancibia-Carcamo et al., 2009). Moreover, mutation of the cytoplasmic γ2 Lys residues retards the lysosomal targeting of GABAARs and is sufficient to block the loss of synaptic GABAARs induced by anoxic insult. Thus, in addition to ubiquitin-mediated proteasomal degradation of α and β subunits at the ER, the number of GABAARs at synapses is also regulated by ubiquitin-mediated degradation of the γ2 subunit in the endocytic lysosomal pathway (Arancibia-Carcamo et al., 2009). The ubiquitin ligases involved in degradation of GABAARs are not yet known. However, a recent preliminary report has identified brain-expressed ring finger protein (BERP, also known as TRIM3, RNF22) as a putative ubiquitin ligase that, counter intuitively, facilitates the cell surface expression and synaptic function of GABAARs (Cheung et al., 2010). Whether BERP acts directly on GABAARs or other protein(s) as a substrate has not yet been determined.
The mechanisms of endocytic recycling summarized above have been explored with a focus on γ2-containing GABAARs that are confined to synapses. Emerging evidence indicates that similar mechanism may apply to nonsynaptic, δ-containing receptors. In particular, phosphorylation of Ser443 in the α4 subunit promotes the cell surface stability of α4βδ receptors (Abramian et al., 2010).
Regulation of GABAAR interactions with the postsynaptic protein scaffold
Molecular imaging of bungarotoxin-labeled recombinant GABAARs suggests that the delivery to the cell surface and endocytosis occur at nonsynaptic plasma membrane sites (Bogdanov et al., 2006). Consistent with these observations, the insertion of GABAARs into the plasma membrane can proceed normally in the absence of subsynaptic scaffold proteins (Levi et al., 2002; Levi et al., 2004). However, the distribution of GABAARs between synaptic and extrasynaptic sites in the plasma membrane is dynamically regulated by direct and indirect interactions of GABAARs with the postsynaptic scaffold, as detailed in the following.
Gephyrin
Arguably the most important protein for stabilization of GABAARs at synapses is gephyrin, the principal subsynaptic scaffold protein of both GABAergic and glycinergic synapses (Figures 3B, 5A) (Fritschy et al., 2008). Gephyrin was first identified as a 93-KDa polypeptide that copurified with affinity-purified glycine receptors (Pfeiffer et al., 1982), the principal inhibitory neurotransmitter receptors in the spinal cord. Molecular cloning and targeted deletion in mice revealed that gephyrin is a multifunctional protein that is broadly expressed and essential for postsynaptic clustering of glycine receptors and also for molybdenum cofactor (Moco) biosynthesis in nonneural tissues (Prior et al., 1992; Kirsch et al., 1993; Feng et al., 1998; Sola et al., 2004; Dumoulin et al., 2009). Gephyrin interacts with microtubules (Kirsch et al., 1995) as well as several regulators of microfilament dynamics including profilin I and II (Mammoto et al., 1998) and members of the mammalian enabled (Mena)/vasodilator stimulated phosphoprotein (VASP) family (Figure 3B, 5A) (Giesemann et al., 2003). The N-terminal gephyrin domain known as G-gephyrin assumes a trimeric structure (Schwarz et al., 2001; Sola et al., 2001), whereas the C-terminal E domain forms a dimer (Schwarz et al., 2001; Xiang et al., 2001; Sola et al., 2004). These domain interactions are essential for oligomerization and clustering of gephyrin at postsynaptic sites (Saiyed et al., 2007). The clustering function of gephyrin is regulated by select residues within the E-domain that are dispensable for E domain dimerization (Lardi-Studler et al., 2007). Moreover, the linker region between E and G domains of gephyrin is thought to interact with microtubules (Ramming et al., 2000). Thus, gephyrin has the structural prerequisites to form a microtubule and microfilament-associated hexagonal protein lattice that may organize the spatial distribution of receptors and other proteins in the postsynaptic membrane.
Gephyrin has long been established as a phosphoprotein (Langosch et al., 1992), although to date few studies have addressed the relevance of this modification. Zita et al. (2007) showed preliminary evidence that gephyrin is phosphorylated by proline-directed kinase(s) and that this is essential for interaction of gephyrin with the peptidyl-prolyl cis/trans isomerase Pin1 (Figure 5A). Pin1-induced conformational changes of gephyrin were found to be essential for maximal clustering of glycine receptors, suggesting a similar function for Pin1 in regulating gephyrin destined for GABAergic synapses. Recently, an unbiased proteomic screen using mass spectrometry mapped the first specific phosphorylation sites to S188, S194 and S200 of gephyrin (Huttlin et al., 2010). Treatment of cultured neurons with inhibitors of the phosphatases PP1α and PP2A caused a significant loss of gephyrin from inhibitory synapses (Bausen et al., 2010). However, mutation of S188/194 to alanine or glutamate resulted in only a modest change in gephyrin or GABAAR cluster size, indicating that the effect of phosphatases was due to dephosphorylation of other PP1α/PP2A substrates.
An elegant study has identified glycogen synthase kinase 3β (GSK3β) as a proline-directed kinase that controls phosphorylation- and proteolytic cleavage-induced turnover of gephyrin (Figure 5A) (Tyagarajan et al., 2011). Using tandem mass spectrometry of gephyrin, the authors identified S270 as a residue that is basally phosphorylated in brain tissue. Transfection of cultured neurons with phosphorylation-deficient gephyrinS270A increased the density of gephyrin clusters and the amplitude and frequency of GABAergic mIPSCs, indicating that gephyrin clustering is limited by phosphorylation at S270. However, mutations of S270 had no effect on cluster size. Using kinase-specific inhibitors in in vitro phosphorylation assays the authors identified GSK3β as an important kinase for S270. To address the mechanism by which phosphorylation might increase gephyrin turnover they focused on calpain-1. This Ca2+-dependent cysteine protease was previously shown to cleave gephyrin and to produce a stable C-terminal gephyrin fragment of 48–50 kDa (Kawasaki et al., 1997). Transfection of neurons with the natural calpain-1 inhibitor calpastatin increased the gephyrin cluster density (Tyagarajan and Fritschy, 2010). Moreover, this effect was enhanced in the presence of the phosphomimetic mutant gephyrinS270E as a substrate, indicating that calpain-1-mediated degradation of gephyrin is triggered by phosphorylation of S270. Lastly, the authors showed that S270 phosphostate-dependent clustering of gephyrin is enhanced by chronic treatment of cultured neurons or mice with Li+, a potent inhibitor of GSK3β used as mood-stabilizing agent for the treatment of bipolar disorder. The findings strongly suggest that Li+-induced enhancement of GABAergic synaptic transmission contributes to the mood stabilizing effects of Li+ in patients (Tyagarajan and Fritschy, 2010). GSK3β is inhibited as a downstream target of both the canonical Wnt signaling pathway (Inestrosa and Arenas, 2010) and the insulin receptor signaling pathway. Both pathways promote the postsynaptic clustering of GABAARs by additional, gephyrin-independent mechanisms, as detailed further below. Gephyrin forms a stable complex with affinity-purified glycine receptors (Pfeiffer et al., 1982). By contrast, GABAARs in detergent-solubilized membrane extracts do not stably associate with gephyrin (Meyer et al., 1995). Moreover, a major subset of GABAARs comprised of α1βγ2 receptors can accumulate and cluster at synapses independently of gephyrin (Kneussel et al., 2001; Levi et al., 2004). Nevertheless, in brain gephyrin serves as a reliable postsynaptic marker for all GABAergic synapses (Sassoe-Pognetto et al., 1995; Essrich et al., 1998; Sassoè-Pognetto and Fritschy, 2000). Moreover, reducing the expression of gephyrin in cultured neurons or mice results in the selective loss of synaptic localization of GABAARs composed of α2βγ2 or α3βγ2 subunits (Essrich et al., 1998; Kneussel et al., 1999). These data indicate that the exact role of gephyrin at synapses is receptor subtype-specific. Conversely, however, GABAARs are essential for postsynaptic clustering of gephyrin at all synapses regardless of the GABAAR subtype normally present (Essrich et al., 1998; Schweizer et al., 2003; Kralic et al., 2006; Studer et al., 2006; Patrizi et al., 2008).
Receptor subtype-specific functions of gephyrin may be explained at least in part by different modes of interaction of gephyrin with GABAARs. Tretter et al (2008) described a detergent-sensitive interaction of gephyrin with a hydrophobic motif in the cytoplasmic loop region of the receptor α2 subunit (Figure 1C). Yeast two-hybrid assays further suggest a similar interaction between gephyrin and the α3 subunit (Saiepour et al., 2010). Curiously, however, the gephyrin binding motif of the α2 subunit but not the homologous sequence of the α1 subunit is sufficient to target a heterologous membrane protein to synapses (Tretter et al., 2008). A lower affinity interaction between GABAARs and gephyrin than between glycine receptors and gephyrin is consistent with weaker synaptic confinement of GABAA than glycine receptors (Levi et al., 2008).
The neuroligin-neurexin complex
The structural and functional maturation of synapses is critically dependent on synaptic adhesion complexes. One such complex involves a transsynaptic interaction of presynaptic neurexins and postsynaptic neuroligins (Figures 3D, 4, 5A) (Ushkaryov et al., 1992; Ushkaryov et al., 1994; Ichtchenko et al., 1995; Ullrich et al., 1995; Ichtchenko et al., 1996; Jamain et al., 2008). Overexpression of different neuroligins in neurons or heterologous cells cocultured with neurons can induce presynaptic development of glutamatergic and GABAergic synapses (Scheiffele et al., 2000; Chih et al., 2005; Chubykin et al., 2007; Dong et al., 2007; Fu and Vicini, 2009). Conversely, β-neurexins presented on beads or overexpressed in heterologous cells can induce the formation of separate postsynaptic GABAergic or glutamatergic hemisynapses in co-cultured neurons (Graf et al., 2004). Of special interest is NL2 as it is localized selectively at inhibitory synapses (Graf et al., 2004; Varoqueaux et al., 2004) and required for structural and functional maturation of subsets of GABAergic but not glutamatergic or glycinergic synapses in vivo (Varoqueaux et al., 2006; Gibson et al., 2009; Hoon et al., 2009; Poulopoulos et al., 2009). By contrast, NL3 is found at both glutamatergic and GABAergic synapses (Budreck and Scheiffele, 2007), while NL1 and NL4 are found primarily at glutamatergic (Song et al., 1999) and glycinergic (Hoon et al., 2011) synapses, respectively. A recent report has identified gephyrin as a direct interaction partner of NLs (Poulopoulos et al., 2009). Although this interaction lacks selectivity for NL2 (Poulopoulos et al., 2009), this finding provides important clues, detailed further below, for the mechanisms underlying the selective deposition of gephyrin at inhibitory synapses.
Interactions between postsynaptic NL2 and presynaptic neurexins are thought to contribute to proper alignment of pre- and postsynaptic molecules at inhibitory synapses. Nevertheless, NL2 is dispensable for clustering and synaptic localization of gephyrin in most brain areas (Varoqueaux et al., 2006; Hoon et al., 2009) (except dentate gyrus Jedlicka et al., 2010), suggesting that other so far unknown synaptogenic complexes might exist. A transsynaptic interaction between the postsynaptic dystrophin-associated glycoprotein (DG) complex and presynaptic neurexins might contribute to the structural integrity of a subset of inhibitory synapses (Sugita et al., 2001). The DG complex consists of the peripheral membrane protein α-dystroglycan, the integral membrane spanning protein β-dystroglycan, and the subsynaptic cytoskeletal component dystrophin. However, this complex appears late during synaptogenesis and is present at a subset of GABAergic synapses only (Knuesel et al., 1999). Moreover, the DG complex is dispensable for postsynaptic clustering of GABAARs and unable to promote the accumulation of GABAARs and gephyrin at synapses (Brunig et al., 2002b; Levi et al., 2002). Recently the synaptic scaffolding and PDZ domain-containing protein S-SCAM (also known as membrane-associated guanylate kinase inverted-2, MAGI-2) was isolated as a β-dystroglycan interacting protein that might physically link the DG complex to NL2 (Sumita et al., 2007). However, S-SCAM also interacts with NL1 and is found at both excitatory and a subset of inhibitory synapses, suggesting an unspecific role in maturation of synapses.
Collybistin
The gephyrin interacting protein collybistin (CB) is a member of the Dbl family of guanine nucleotide exchange factors (RhoGEFs) that selectively activates the small GTPase Cdc42 (Figure 3C, 4, 5A) (Reid et al., 1999; Kins et al., 2000; Grosskreutz et al., 2001). However, analyses of Cdc42 knock-out mice indicate that Cdc42 is dispensable for gephyrin and GABAAR clustering (Reddy-Alla et al., 2010). In neurons, CB is colocalized with gephyrin at inhibitory synapses (Saiepour et al., 2010). When coexpressed with gephyrin in heterologous cells CB has the unique ability to transform cytoplasmic aggregates of gephyrin into submembrane micro-clusters that resemble postsynaptic gephyrin clusters of neurons (Kins et al., 2000). Moreover, CB is required for postsynaptic clustering of gephyrin and GABAARs, as shown by analyses of naturally occurring mutations of the CB gene (ARHGEF9) associated with hyperekplexia, epilepsy and mental retardation in patients (Harvey et al., 2004; Kalscheuer et al., 2009) as well as by CB gene knock-out in mice (Papadopoulos et al., 2007). Loss of gephyrin and GABAAR clusters in CB knock-out mice is most pronounced in the hippocampus and amygdala. However, in brainstem, neocortex and several other brain areas the clustering of gephyrin and GABAA or glycine receptors is unaffected, indicating that other GEFs can compensate for the loss of CB function in these brain areas.
Multiple CB splice variants exist that differ in their C-terminal structures and by the presence or absence of an N-terminal SH3 domain (Kins et al., 2000; Harvey et al., 2004). Intriguingly, the predominant CB isoforms detected in vivo contain an SH3 domain, which inhibits the aforementioned CB-dependent formation of submembrane gephyrin clusters, indicating that CB is negatively regulated by its SH3 domain (Kins et al., 2000; Harvey et al., 2004). However, cotransfection of CBSH3+ and gephyrin with NL2 negates the inhibitory effect of the SH3 domain (Poulopoulos et al., 2009). CB splice variants invariably contain a pleckstrin homology (PH) domain that is required for its interaction with plasma membrane-restricted phosphoinositides and for clustering of gephyrin at inhibitory synapses (Harvey et al., 2004; Reddy-Alla et al., 2010). The data are consistent with a heterotrimeric membrane-associated complex that consists of NL2, CBSH3+ and gephyrin and that enables the selective deposition of gephyrin at NL2-containing inhibitory synapses. Experiments in heterologous cells indicate that NL1 can potentially substitute for NL2 and similarly induce submembrane gephyrin clusters but only with constitutively active CB isoform that lack an SH3 domain. In addition, preliminary evidence suggests that the α2 subunit can substitute for NL2 and activate the gephyrin-clustering function of CBSH3+ (Saiepour et al., 2010). This GABAAR-dependent function of CB is specific for α2-containing receptors and abolished by a naturally occurring missense mutation (CBG55A) that disrupts the clustering of α2-containing GABAARs and gephyrin in cultured neurons and associated with mental retardation, epilepsy and hyperekplexia in a patient (Harvey et al., 2004; Saiepour et al., 2010). NL1- and α2 subunit-mediated activation of CB might contribute to residual clustering of gephyrin seen in NL2 KO mice (Jedlicka et al., 2010).
Other gephyrin binding proteins that are concentrated at synapses include the Ser/Thr kinase mTor (mammalian target of rapamycin, also known as RAFT1 and FRAP1) (Sabatini et al., 1999) and the dynein light chains (DLC) 1 and 2 (Fuhrmann et al., 2002). mTor functions as an important regulator of mRNA translation, allowing for speculation that gephyrin might contribute to translational control of postsynaptic protein synthesis. This idea is supported by recent evidence that both gephyrin and collybistin are part of the eukaryotic translation initiation factor 3 complex (Sertie et al., 2010). However, whether gephyrin and collybistin play a role in translational control in dendrites remains to be elucidated. The interaction between gephyrin and the DLC is implicated in retrograde vesicular transport of gephyrin-glycine receptor complexes from glycinergic synapses (Maas et al., 2009). The significance of this interaction for GABAARs is unclear as intracellular GABAAR-gephyrin-DLC complexes have not been described and the DLC-gephyrin interaction is dispensable for normal localization of gephyrin at GABAergic synapses (Fuhrmann et al., 2002).
Radixin-mediated extrasynaptic clustering of α5βγ2 receptors
Among different γ2-containing GABAARs the α5βγ2 receptors are unique in that they are localized mostly extrasynaptically, as mentioned earlier. Interestingly, even extrasynaptic α5βγ2 receptors are clustered at the plasma membrane (Christie and de Blas, 2002) (Figure 5B). Loebrich et al (2006) have identified radixin as a α5 subunit-interacting protein that is essential for extrasynaptic clustering of α5βγ2 receptors. Radixin is a member of the ERM (ezrin, radixin, moesin) family of proteins, which are known to link transmembrane proteins to the actin cytoskeleton. Transfection of neurons with a dominant negative radixin construct abolishes the clustering of α5-containing receptors but does not affect GABAAR surface expression nor GABAergic tonic and phasic currents (Loebrich et al., 2006). The data suggest that radixin-independent mechanism prevent α5-containing receptors from accumulation at synapses. The functional relevance of α5βγ2 receptor clustering in the extrasynaptic membrane is not known.
Regulation of GABAergic transmission by changes in lateral diffusion dynamics of GABAARs
Postsynaptic GABAAR clusters represent diffusional confinement areas containing laterally mobile GABAARs stabilized by gephyrin. Fluorescence recovery after photobleaching (FRAP) was used to compare the mobility of fluorescently-tagged GABAARs at postsynaptic and extrasynaptic plasma membrane sites (Jacob et al., 2005). These experiments revealed significantly greater fluorescence recovery rates at extrasynaptic than postsynaptic membrane domains, thereby indicating greater mobility of extrasynaptic than postsynaptic GABAARs (Figure 5B). Moreover, the fluorescence recovery rate at the periphery of the photobleached area was greater than that at the center, consistent with replenishment of GABAARs from within the plane of the plasma membrane, rather than by insertion into the plasma membrane from intracellular receptor pools. To assess the role of gephyrin in modulating lateral diffusion, FRAP experiments were combined with RNAi knockdown of gephyrin, a treatment that effectively reduced the expression of gephyrin but did not affect the accumulation of GABAARs at the plasma membrane. Interestingly, postsynaptic GABAARs of gephyrin-RNAi-treated neurons showed significantly greater FRAP recovery rates than control neurons, indicating that the mobility of GABAARs at postsynaptic sites is restrained by direct or indirect interactions with gephyrin (Jacob et al., 2005). An independent study relied on an ingenious method to mutate and functionally tag GABAARs such that they are permanently inactivated by an inhibitor after receptor activation by GABA (Thomas et al., 2005). This study showed that postsynaptic GABAARs are subject to rapid constitutive exchange with non-synaptic receptor pools without measurable contribution by exocytosis of GABAARs from intracellular pools.
NMDAR- and calcineurin-mediated long-term depression of GABAergic synapses
Experiments that tracked the lateral movement of quantum dot-labeled, single GABAAR molecules showed that the diffusion coefficient of postsynaptic receptors is about half of that of nonsynaptic receptors (Bannai et al., 2009). Increasing neural activity with a K+-channel blocker increased the diffusion coefficient of both synaptic and extrasynaptic GABAARs and decreased the postsynaptic cluster size of gephyrin and GABAARs, concomitant with a reduction in the amplitude of mIPSCs (Figure 5B). This effect of increased neural activity was dependent on Ca2+ influx and activation of calcineurin, did not involve receptor internalization, and was reversed when normal neural activity was restored. These results are consistent with EPSC-induced long-term depression (LTD) of unitary IPSCs observed in association with high frequency stimulation-induced LTP of the Schaffer collateral-CA1 pathway (Lu et al., 2000; Wang et al., 2003a). LTD of IPSCs required NMDA receptor-dependent recruitment of calcineurin to the GABAAR complex and calcineurin-mediated dephosphorylation of S327 of the γ2 subunit. The findings by Wang et al. and Bannai et al. were confirmed by a recent study that combined live imaging of fluorescently tagged GABAAR clusters with single molecule tracking of quantum dot-labeled single GABAAR molecules (Muir et al., 2010). As expected, glutamate-induced dispersal of GABAAR clusters and enhancement of GABAAR mobility was critically dependent on NMDA receptor and calcineurin activation and independent of dynamin and therefore did not involve endocytosis of GABAARs. Moreover, Glu-induced and calcineurin-mediated dephosphorylation of γ2 S327 increased the lateral mobility and reduced the synaptic residency time of quantum dot-labeled single GABAAR molecules (Muir et al., 2010). Future experiments will need to address how γ2 S327 regulates interaction of GABAARs with the synaptic protein scaffold.
The NMDAR- and calcineurin-mediated form of LTD of inhibitory synapses (Wang et al., 2003a; Bannai et al., 2009; Muir et al., 2010) at first seems in conflict with the aforementioned NMDAR-mediated potentiation of mIPSCs (Marsden et al., 2007). However, more recent evidence suggests that opposite functional effects observed in these two sets of experiments reflect different neuronal stimulation intensities (Marsden et al., 2010). NMDAR-dependent LTD of hippocampal pyramidal cells associated with calcineurin-dependent diffusional dispersal of GABAARs reflects robust stimulation of both NMDA and AMPA receptors achieved either by high frequency stimulation of glutamatergic afferents, or treatment of neurons with K+-channel blockers or glutamate (Figure 5B). These conditions result in activation of both CaMKII and calcineurin. However, calcineurin inhibits the targeting of CaMKII to inhibitory synapses (Marsden et al., 2010). By contrast, NMDAR-mediated membrane insertion of GABAARs and potentiation of mIPSCs is observed following more moderate chemical stimulation of NMDARs only (Figure 5C). Moderate stimulation of neurons with NMDA seemingly fails to activate calcineurin and thereby allows the activation and translocation of CaMKII to inhibitory synapses (Marsden et al., 2010), As mentioned earlier, NMDAR-induced de novo insertion of GABAARs into the plasma membrane is further dependent on GABARAP, NSF and GRIP (Marsden et al., 2007). Thus, the directionality of neural activity-induced trafficking of GABAARs is strictly stimulus intensity-dependent.
Regulation of GABAAR trafficking as a downstream effector of extracellular neuropeptide cues
Insulin-regulated cell surface expression of GABAARs
Signaling by pancreatic insulin is pivotal for the regulation of peripheral glucose and lipid metabolism. However, insulin is also produced in brain (Havrankova et al., 1981; Stevenson, 1983) and released from neurons in an activity-dependent manner (Clarke et al., 1986). Signaling by insulin receptors contributes to structural maturation of neuronal dendrites, as well as functional synaptic plasticity (reviewed in Chiu and Cline, 2010). In addition, insulin signaling leads to a rapid increase in the cell surface accumulation and function of postsynaptic GABAARs (Wan et al., 1997; Wang et al., 2003b).
A first line of investigation indicates that insulin-induced translocation of GABAARs to the cell surface requires activation of the serine-threonine kinase Akt, a primary target of insulin signaling downstream of phosphoinositide 3 kinase (PI3K, Figure 6A) (Wang et al., 2003b). PI3K-mediated phosphorylation of membrane lipids is established as a mechanism that leads to recruitment of Akt to the plasma membrane where it is phosphorylated and activated by the serine-threonine kinase, phosphoinositide-dependent kinase 1 (PDK1) (Cantley, 2002). In vitro assays showed that activated Akt phosphorylates a conserved phosphorylation site present in all three β subunits of GABAARs (S409 in β1, S410 in β2, S408/409 in β3) (Wang et al., 2003b; Xu et al., 2006). Cotransfection of Akt with α2β2γ2 receptors increased the cell surface expression of these receptors in HEK293 cells. Lastly, phosphorylation of β2 S410 was shown to be essential for Akt-induced surface expression of corresponding receptors in transfected neurons (Wang et al., 2003b). Curiously, the Akt phosphorylation site of β1–3 subunits is identical with the aforementioned motif in β subunits that regulates clathrin-mediated endocytosis of GABAARs. One might therefore conclude that insulin-induced surface expression and function of GABAARs reflects reduced clathrin-mediated endocytosis of GABAARs. However, insulin-induced potentiation of GABA-evoked currents was completely abolished by pretreatment of neurons with brefeldin A (BFA), an inhibitor of anterograde transport from ER to Golgi (Fujii et al., 2010). In the presence of BFA, insulin induced a modest run-down of GABA-evoked currents, thereby facilitating rather than inhibiting GABAAR endocytosis. These results are consistent with Akt-mediated insertion of newly synthesized GABAARs (and to a lesser extent increased endocytosis of GABAARs). Together with the aforementioned studies by Kittler et al. (2005), these findings indicate that phosphorylation of a single site in GABAAR β subunits can have different effects on trafficking of GABAARs depending on the kinase involved, most likely reflecting different subcellular compartments where phosphorylation of GABAARs occurs. Interestingly, Fujii et al (2010) found that the effects of insulin on GABA-evoked currents are absent in neurons from PRIP1/2 double knock-out mice. PRIP1 interacts with Akt directly and is required for insulin-induced association of Akt with GABAARs. Thus, PRIP serves as a multifunctional adaptor for both kinases and phosphatases and thereby contributes to both regulated membrane translocation and endocytosis of GABAARs (Fujii et al., 2010). Interestingly, insulin-mediated activation of Akt further results in inhibitory serine-phosphorylation of GSK3β (Cross et al., 1995). GSK3β in turn promotes postsynaptic GABAAR clustering and mIPSCs by reducing calpain-1-mediated cleavage of gephyrin (Tyagarajan et al., 2011) (Figures 5A, 6A).
Figure 6. Insulin receptor-mediated surface expression of GABAARs.
Two distinct mechanisms have been proposed for insulin-induced surface expression of GABAARs that involve either Ser or Tyr phosphorylation of the GABAAR β subunit, respectively and may function independently or cooperatively. A. In a first mechanism, insulin receptor-mediated phosphorylation of the insulin receptor substrate-1 (IRS-1) leads to activation of PI3K and accumulation of phosphoinositide (PIP3) at the plasma membrane. PIP3-mediated recruitment of Akt to the plasma membrane facilitates PDK1-mediated phosphorylation and activation of Akt. Phosphorylated Akt forms a ternary complex with PRIP and vesicular GABAARs, which causes phosphorylation (β3S408/9) and translocation of GABAARs to the plasma membrane. As an additional primary downstream target of insulin, Akt is known to inhibit GSK3β. This kinase in turn phosphorylates gephyrin, which triggers calpain-1-mediated degradation of gephyrin. Thus, insulin signaling may promote GABAergic synaptic function by increasing the surface expression and gephyrin-dependent synaptic confinement of GABAARs. B. In an alternate mechanism, the PI3K P85 subunit interacts directly with GABAARs. This complex is abundant already under basal condition and dependent on phosphorylated Tyr residues (Y372/9) of the GABAAR β3 subunit. On stimulation with insulin, the abundance of this complex and its association with phosphorylated lipids (PIP3) is rapidly increased, concurrent with translocation of the complex to the plasma membrane.
A second proposed mechanism involves direct interaction of the insulin receptor target PI3K with GABAARs (Figure 6B) (Vetiska et al., 2007). The PI3K-GABAAR complex was found to be present constitutively in brain tissue, presumably in an intracellular inactive state. When brain slices were treated with insulin the abundance of this complex rapidly increased as well as its association with phosphorylated membrane lipids. This suggests that the complex is translocated to the plasma membrane in concert with PI3K-mediated phosphorylation of lipids. In vitro analyses revealed that the PI3K-GABAAR complex involves binding of the PI3K p85 subunit SH2 domain to phospho-tyrosines (Tyr 372/379) in the intracellular loop region of β subunits. These Tyr residues were essential for insulin-induced surface expression of β2-containing receptors in transfected neurons. However, several aspects of this mechanism remain to be resolved. First, it is unclear whether this mechanism applies to GABAARs independently of the type of β subunit. Second, it is not known whether Tyr phosphorylation of β subunits is itself modulated by insulin, which Tyr kinase is involved in β subunit phosphorylation, and whether interaction of PI3K with β subunit phospho-tyrosines contributes to activation of PI3K enzyme function. Lastly, it is not known whether and how insulin-induced interaction between PI3K and GABAARs corroborates with the aforementioned Akt- and PRIP-dependent downstream effects of PI3K.
BDNF-regulated trafficking of GABAARs
Signaling by BDNF and its cognate receptor (receptor tropomyosin-related kinase B, TrkB) is critically important for neurogenesis (Bergami et al., 2008; Li et al., 2008) and inhibitory synapse formation (Seil, 2003). BDNF is further involved in structural and functional neuronal plasticity in the adult nervous system (Xu et al., 2000). At GABAergic synapses, BDNF leads to a rapid and transient increase followed by a lasting reduction in the amplitude of mIPSCs (Brunig et al., 2001; Jovanovic et al., 2004). The lasting reduction in mIPSC amplitude is correlated with reduced surface expression of GABAARs (Brunig et al., 2001). Mechanistically, BDNF-induced up- and downregulation of mIPSCs involves a biphasic modulation of the Ser408/409 phosphorylation state of β3 subunits (Jovanovic et al., 2004). Initial rapid phosphorylation is correlated with a transient association of GABAARs with PKC and the receptor for activated C-kinase (RACK-1). Subsequent dephosphorylation of the β3 subunit is predominantly mediated by PP2A. As discussed earlier, dephosphorylation of β3 Ser408/409 by PP2A promotes the association of GABAARs with AP2, which in turn facilitates clathrin-mediated endocytosis of GABAARs (Kittler et al., 2005) and explains the lasting effects of BDNF on GABAARs surface expression and mIPSCs. Interestingly, the recruitment of PP2A to GABAARs is critically dependent on the phosphatase adaptor PRIP (Kanematsu et al., 2006). Treatment of hippocampal PRIP1/2 double knock-out neurons with BDNF resulted in a steady rise in β3 phosphorylation accompanied by increased GABAergic whole cell currents, indicating that PKC-mediated phosphorylation remained intact while the subsequent PRIP-dependent and PP2A-mediated dephosphorylation step was disrupted (Kanematsu et al., 2006). Thus, PRIP plays essential roles both in BDNF-induced downregulation and insulin-induced potentiation of GABAergic postsynaptic function.
Wnt-regulated cell surface expression of GABAARs
Wnt signaling is critically involved in diverse aspects of embryonic development, neural differentiation and adult synaptic plasticity (reviewed by Inestrosa and Arenas, 2010; Budnik and Salinas, 2011). Wnt proteins encoded by 19 different genes act through several different frizzled family receptors to induce multiple signal transduction pathways. The canonical Wnt pathway involves inhibition of GSK3β in the axin/GSK3β/APC complex, which leads to accumulation and nuclear translocation of β-catenin and activation of β-catenin-dependent gene expression. By contrast, two non-canonical Wnt pathways activate either c-Jun N-terminal kinase (Wnt/JNK pathway) or CaMKII (Wnt/Ca2+ pathway) as downstream targets. All three pathways are implicated in the regulation of synaptic plasticity, primarily of excitatory synapses and both pre- and post synaptically (Inestrosa & Arenas, 2010). In addition, Wnt-5a was recently shown to result in rapid (5 min) and significant (+40%) upregulation of GABAAR clusters in cultured neurons (Cuitino et al., 2010). This effect was due to postsynaptic changes as it was paralleled by increased amplitudes but not frequency of mIPSCs recorded from cultured neurons. Consistent with this interpretation, the time course and paired-pulse relationship of evoked IPSCs recorded from hippocampal slices were unaffected by Wnt-5a. The effect of Wnt-5a on mIPSCs was further potentiated by blockade of endocytosis with a dynamin inhibitor peptide. In addition, Wnt-5a treatment reduced the pool of previously surface biotinylated and internalized GABAARs, suggesting that increased clustering of GABAARs reflected enhanced recycling of endocytosed receptors. In support of this mechanism, treatment of neurons with a Wnt-5a-mimicking peptide (Foxy-5) that specifically activates non-canonical Wnt pathways replicated the Wnt-5a effect on GABAAR clustering. Moreover, co-treatment with Foxy-5 and pathway-specific pharmacological inhibitors allowed the conclusion that Wnt-5A-induced clustering of GABAARs involved the non-canonical Wnt/Ca2+ pathway and CaMKII. The CaMKII targets that are phosphorylated in response to Wnt-5a have so far not been determined. In addition to the Wnt/Ca2+ pathway the canonical Wnt pathway is strongly implicated in the regulation of GABAergic inhibition by the aforementioned effects of Li+ and GSK3β on the stability and postsynaptic clustering of gephyrin (Tyagarajan et al., 2011). However, in apparent conflict with this study, the canonical Wnt ligand Wnt-7A and Li+ had no significant effect on GABAAR clustering in the study by Cuitino et al (2010).
Conclusions and outlook
There has been remarkable progress in understanding of mechanisms that regulate GABAergic transmission. Dynamic changes in GABAAR trafficking represent prevalent forms of GABAergic neural plasticity, although changes in subunit gene expression, Cl- reversal potential, and GABA release are also important, especially under pathological conditions. GABAAR-associated proteins and signaling factors involved in GABAAR trafficking are shared with other signal transduction pathways, thereby allowing for complex interactions among multiple neurotransmitter and signaling systems.
Developmental imbalances between neural excitation and inhibition are broadly implicated in the etiology of the most prevalent neuropsychiatric disorders. Such imbalances may be further amplified by trafficking deficits in GABAARs, as suggested by activity and anoxia-induced loss of postsynaptic GABAARs (Mielke and Wang, 2005; Terunuma et al., 2008; Arancibia-Carcamo et al., 2009). Indeed, deficits in GABAergic transmission may be central to the etiology of neuropsychiatric disorders such as major depressive disorder (Luscher et al., 2011), bipolar disorder (Craddock et al., 2010) and schizophrenia (Charych et al., 2009). Conversely, the cell surface trafficking and synaptic accumulation of GABAAR is modulated by Wnt pathway kinases (GSK3β, Akt) that are central to the therapeutic action of mood stabilizing and antidepressant drugs (Logan and Nusse, 2004; Okamoto et al., 2010; Tyagarajan et al., 2011). Further progress in understanding of GABAAR trafficking mechanisms should provide better mechanistic insights into these disorders and facilitate the development of more effective drug therapies.
Despite the recent progress, diverse aspects of GABAAR trafficking remain poorly understood. For example, current understanding of trafficking mechanisms largely fails to account for the structural heterogeneity of GABAARs. We can rationally explain the postsynaptic accumulation of α2βγ2 and α3βγ2 receptors. By contrast the postsynaptic clustering of α1βγ2 receptors can occur in the absence of gephyrin and therefore likely depends on alternative mechanisms. Disruption of Tyr phosphorylation in γ2Y365/7F knock-in mice results in selective upregulation of GABAARs in CA3 pyramidal cells but, so far unexplained, not in CA1 pyramidal cells (Tretter et al., 2009). Phosphorylation of γ2S327 is established to modulate the diffusional dynamics of postsynaptic GABAARs (Muir et al., 2010), yet the functionally relevant interaction partner(s) for this effect remain unknown. Lastly, gephyrin exists in multiple splice variants (Paarmann et al., 2006) and is phosphorylated at multiple sites (Huttlin et al., 2010; Tyagarajan et al., 2011), yet the functional relevance of different gephyrin isoforms and their posttranslational modifications remain largely unexplored.
One attractive mechanism underlying postsynaptic differentiation involves gephyrin-mediated interaction of GABAARs with NL2, which accumulates at synapses through interaction with presynaptic neurexin. However, the loss of GABAARs and gephyrin from inhibitory synapses of NL2 knock-out mice is incomplete (Hoon et al., 2009; Jedlicka et al., 2010), suggesting that additional so far unidentified synaptic adhesion complexes exist that substitute for NL2 and contribute to accumulation of GABAARs and gephyrin at many synapses. Interestingly, a recently described transsynaptic interaction between presynaptic neurexins and postsynaptic GABAARs appears to inhibit rather than promote the function of GABAergic synapses (Zhang et al., 2010). This negative effect of neurexin on GABAergic transmission was preserved in NL2 KO neurons and also observed in GABAAR expressing heterologous cells exposed to soluble neurexin constructs, indicating that it does not involve competition between GABAARs and NL2 for interaction with neurexin. The relevance of this interaction for native synapses and trafficking of GABAARs remains to be explored.
A complete understanding of the role of GABAAR trafficking in GABAergic synaptic plasticity will require that these knowledge gaps be filled. In addition, downstream consequences of altered GABAergic transmission on other signaling pathways will need to be explored. Ultimately, the function of these mechanisms will need to be explored at the level of neural network activity, behavior and cognition, including in appropriate disease models.
Acknowledgments
We thank Victoria Cavener and Pam Mitchell for critical comments on the manuscript. Research in the Luscher laboratory has been supported by Grants MH62391, MH60989 and RC1MH089111 from the National Institutes of Mental Health (NIMH) and grants from the Pennsylvania Department of Health using Tobacco Settlement Funds. The contents of this review are solely the responsibility of the authors and do not necessarily represent the views of the NIMH or the NIH. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions.
Footnotes
Conflicts of Interest. The authors declare no con ict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrami L, Kunz B, Iacovache I, van der Goot FG. Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2008;105:5384–5389. doi: 10.1073/pnas.0710389105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramian AM, Comenencia-Ortiz E, Vithlani M, Tretter EV, Sieghart W, Davies PA, Moss SJ. Protein kinase C phosphorylation regulates membrane insertion of GABA(A) receptor subtypes that mediate tonic inhibition. J Biol Chem. 2010;285:41795–41805. doi: 10.1074/jbc.M110.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B. Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alle H, Geiger JR. GABAergic spill-over transmission onto hippocampal mossy fiber boutons. J Neurosci. 2007;27:942–950. doi: 10.1523/JNEUROSCI.4996-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andang M, Lendahl U. Ion fluxes and neurotransmitters signaling in neural development. Current opinion in neurobiology. 2008;18:232–236. doi: 10.1016/j.conb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Angelotti TP, Macdonald RL. Assembly of GABAA receptor subunits: alpha 1 beta 1 and alpha 1 beta 1 gamma 2S subunits produce unique ion channels with dissimilar single-channel properties. J Neurosci. 1993;13:1429–1440. doi: 10.1523/JNEUROSCI.13-04-01429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancibia-Carcamo IL, Yuen EY, Muir J, Lumb MJ, Michels G, Saliba RS, Smart TG, Yan Z, Kittler JT, Moss SJ. Ubiquitin-dependent lysosomal targeting of GABA(A) receptors regulates neuronal inhibition. Proc Natl Acad Sci U S A. 2009;106:17552–17557. doi: 10.1073/pnas.0905502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer K, Essrich C, Benson JA, Benke D, Bluethmann H, Fritschy JM, Luscher B. Postsynaptic clustering of GABA(A) receptors by the gamma3 subunit in vivo. Proc Natl Acad Sci USA. 1999;96:12860–12865. doi: 10.1073/pnas.96.22.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai H, Levi S, Schweizer C, Inoue T, Launey T, Racine V, Sibarita JB, Mikoshiba K, Triller A. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. doi: 10.1016/j.neuron.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Subunit arrangement of gamma-aminobutyric acid type A receptors. J Biol Chem. 2001;276:36275–36280. doi: 10.1074/jbc.M105240200. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- Baur R, Minier F, Sigel E. A GABA(A) receptor of defined subunit composition and positioning: concatenation of five subunits. FEBS lett. 2006;580:1616–1620. doi: 10.1016/j.febslet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Bausen M, Weltzien F, Betz H, O’Sullivan GA. Regulation of postsynaptic gephyrin cluster size by protein phosphatase 1. Mol Cell Neurosci. 2010;44:201–209. doi: 10.1016/j.mcn.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Beck M, Brickley K, Wilkinson HL, Sharma S, Smith M, Chazot PL, Pollard S, Stephenson FA. Identification, molecular cloning, and characterization of a novel GABA(A) receptor-associated protein, GRIF-1. J Biol Chem. 2002;277:30079–30090. doi: 10.1074/jbc.M200438200. [DOI] [PubMed] [Google Scholar]
- Bedford FK, Kittler JT, Muller E, Thomas P, Uren JM, Merlo D, Wisden W, Triller A, Smart TG, Moss SJ. GABAA receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat Neurosci. 2001;4:908–916. doi: 10.1038/nn0901-908. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Benke D, Fakitsas P, Roggenmoser C, Michel C, Rudolph U, Mohler H. Analysis of the presence and abundance of GABAA receptors containing two different types of alpha subunits in murine brain using point-mutated alpha subunits. J Biol Chem. 2004;279:43654–43660. doi: 10.1074/jbc.M407154200. [DOI] [PubMed] [Google Scholar]
- Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci USA. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. Structural determinants of fast desensitization and desensitization-deactivation coupling in GABAa receptors. J Neurosci. 2001;21:1127–1136. doi: 10.1523/JNEUROSCI.21-04-01127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boal F, Stephens DJ. Specific functions of BIG1 and BIG2 in endomembrane organization. PLoS ONE. 2010;5:e9898. doi: 10.1371/journal.pone.0009898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm IS, Peden L, Harris RA, Blednov YA. Deletion of the fyn-kinase gene alters sensitivity to GABAergic drugs: dependence on beta2/beta3 GABAA receptor subunits. J Pharmacol Exp Ther. 2004;309:1154–1159. doi: 10.1124/jpet.103.064444. [DOI] [PubMed] [Google Scholar]
- Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ. Synaptic GABA(A) receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau AJ, Evers AR, Davis AF, Czajkowski C. Mapping the agonist binding site of the GABAA receptor: evidence for a beta-strand. J Neurosci. 1999;19:4847–4854. doi: 10.1523/JNEUROSCI.19-12-04847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollan K, King D, Robertson LA, Brown K, Taylor PM, Moss SJ, Connolly CN. GABA(A) receptor composition is determined by distinct assembly signals within alpha and beta subunits. J Biol Chem. 2003a;278:4747–4755. doi: 10.1074/jbc.M210229200. [DOI] [PubMed] [Google Scholar]
- Bollan K, Robertson LA, Tang H, Connolly CN. Multiple assembly signals in gamma-aminobutyric acid (type A) receptor subunits combine to drive receptor construction and composition. Biochem Soc Trans. 2003b;31:875–879. doi: 10.1042/bst0310875. [DOI] [PubMed] [Google Scholar]
- Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:63–71. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CA, Taghibiglou C, Collingridge GL, Wang YT. Mechanisms involved in the reduction of GABAA receptor alpha1-subunit expression caused by the epilepsy mutation A322D in the trafficking-competent receptor. J Biol Chem. 2008;283:22043–22050. doi: 10.1074/jbc.M801708200. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, Smart TG, Moss SJ. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem. 2000;275:38856–38862. doi: 10.1074/jbc.M004910200. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Jovanovic JN, Colledge M, Kittler JT, Brandon JM, Scott JD, Moss SJ. A-kinase anchoring protein 79/150 facilitates the phosphorylation of GABA(A) receptors by cAMP-dependent protein kinase via selective interaction with receptor beta subunits. Mol Cell Neurosci. 2003;22:87–97. doi: 10.1016/s1044-7431(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Jovanovic JN, Smart TG, Moss SJ. Receptor for activated C kinase-1 facilitates protein kinase C- dependent phosphorylation and functional modulation of GABA(A) receptors with the activation of G-protein-coupled receptors. J Neurosci. 2002;22:6353–6361. doi: 10.1523/JNEUROSCI.22-15-06353.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Uren JM, Kittler JT, Wang H, Olsen R, Parker PJ, Moss SJ. Subunit-specific association of protein kinase C and the receptor for activated C kinase with GABA type A receptors. J Neurosci. 1999;19:9228–9234. doi: 10.1523/JNEUROSCI.19-21-09228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher MS, Munro S. Cholesterol and the Golgi apparatus. Science. 1993;261:1280–1281. doi: 10.1126/science.8362242. [DOI] [PubMed] [Google Scholar]
- Brickley K, Smith MJ, Beck M, Stephenson FA. GRIF-1 and OIP106, members of a novel gene family of coiled-coil domain proteins: association in vivo and in vitro with kinesin. J Biol Chem. 2005;280:14723–14732. doi: 10.1074/jbc.M409095200. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur J Neurosci. 2001;13:1320–1328. doi: 10.1046/j.0953-816x.2001.01506.x. [DOI] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002a;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Brunig I, Suter A, Knuesel I, Luscher B, Fritschy J-M. GABAergic terminals are required for postsynaptic clustering of dystrophin but not GABA(A) receptors and gephyrin. J Neurosci. 2002b;22:4805–4813. doi: 10.1523/JNEUROSCI.22-12-04805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Salinas PC. Wnt signaling during synaptic development and plasticity. Current opinion in neurobiology. 2011 doi: 10.1016/j.conb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budreck EC, Scheiffele P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur J Neurosci. 2007;26:1738–1748. doi: 10.1111/j.1460-9568.2007.05842.x. [DOI] [PubMed] [Google Scholar]
- Cancedda L, Fiumelli H, Chen K, Poo MM. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J Neurosci. 2007;27:5224–5235. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapell R, Bueno OF, Alvarez-Hernandez X, Robinson LC, Leidenheimer NJ. Activation of protein kinase C induces gamma-aminobutyric acid type A receptor internalization in Xenopus oocytes. J Biol Chem. 1998;273:32595–32601. doi: 10.1074/jbc.273.49.32595. [DOI] [PubMed] [Google Scholar]
- Charych EI, Liu F, Moss SJ, Brandon NJ. GABA(A) receptors and their associated proteins: implications in the etiology and treatment of schizophrenia and related disorders. Neuropharmacology. 2009;57:481–495. doi: 10.1016/j.neuropharm.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych EI, Yu W, Li R, Serwanski DR, Miralles CP, Li X, Yang BY, Pinal N, Walikonis R, De Blas AL. A four PDZ domain-containing splice variant form of GRIP is localized in GABAergic and glutamatergic synapses in brain. J Biol Chem. 2004a;279:38978–38990. doi: 10.1074/jbc.M405786200. [DOI] [PubMed] [Google Scholar]
- Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, De Blas AL. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem. 2004b;90:173–189. doi: 10.1111/j.1471-4159.2004.02481.x. [DOI] [PubMed] [Google Scholar]
- Chen ZW, Olsen RW. GABA receptor associated proteins: a key factor regulating GABA receptor function. J Neurochem. 2007;100:279–294. doi: 10.1111/j.1471-4159.2006.04206.x. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Yang C, Berger T, Zaugg K, Reilly P, Elia AJ, Wakeham A, You-Ten A, Chang N, Li L, et al. Identification of BERP (brain-expressed RING finger protein) as a p53 target gene that modulates seizure susceptibility through interacting with GABA(A) receptors. Proc Natl Acad Sci USA. 2010;107:11883–11888. doi: 10.1073/pnas.1006529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chiu SL, Cline HT. Insulin receptor signaling in the development of neuronal structure and function. Neural Dev. 2010;5:7. doi: 10.1186/1749-8104-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou W-H, Wang D, McMahon T, Qi Z-H, Spong M, Zhang C, Shokat KM, Messing R. GABA(A) receptor trafficking is regulated by PKCε and the N- ethylmaleimide-sensitive factor. J Neurosci. 2010;30:13955–13965. doi: 10.1523/JNEUROSCI.0270-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, de Blas AL. alpha5 Subunit-containing GABA(A) receptors form clusters at GABAergic synapses in hippocampal cultures. Neuroreport. 2002;13:2355–2358. doi: 10.1097/00001756-200212030-00037. [DOI] [PubMed] [Google Scholar]
- Christie SB, Li RW, Miralles CP, Yang BY, De Blas AL. Clustered and non-clustered GABAA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2006;31:1–14. doi: 10.1016/j.mcn.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DW, Mudd L, Boyd FTJ, Fields M, Raizada MK. Brain insulin is released from neurons upon depolarization. J Neurochem. 1986;47:831–836. doi: 10.1111/j.1471-4159.1986.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Clements JD. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci. 1996;19:163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Kittler JT, Thomas P, Uren JM, Brandon NJ, Smart TG, Moss SJ. Cell surface stability of γ-aminobutyric acid type A receptors. Dependence on protein kinase C activity and subunit composition. J Biol Chem. 1999;274:36565–36572. doi: 10.1074/jbc.274.51.36565. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- Craddock N, Jones L, Jones IR, Kirov G, Green EK, Grozeva D, Moskvina V, Nikolov I, Hamshere ML, Vukcevic D, et al. Strong genetic evidence for a selective influence of GABA(A) receptors on a component of the bipolar disorder phenotype. Mol Psychiatry. 2010;15:146–153. doi: 10.1038/mp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy JM, Luscher B, Mohler H. Decreased GABA(A)-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Cuitino L, Godoy JA, Farias GG, Couve A, Bonansco C, Fuenzalida M, Inestrosa NC. Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. J Neurosci. 2010;30:8411–8420. doi: 10.1523/JNEUROSCI.5736-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhang P, Song I, Petralia RS, Liao D, Huganir RL. Characterization of the glutamate receptor-interacting proteins GRIP1 and GRIP2. J Neurosci. 1999;19:6930–6941. doi: 10.1523/JNEUROSCI.19-16-06930.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Qi J, Chen G. Molecular reconstitution of functional GABAergic synapses with expression of neuroligin-2 and GABA(A) receptors. Mol Cell Neurosci. 2007;35:14–23. doi: 10.1016/j.mcn.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, Triller A, Kneussel M. Cellular transport and membrane dynamics of the glycine receptor. Frontiers in Molecular Neuroscience. 2009 doi: 10.3389/neuro.02.028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehya N, Sarto I, Wabnegger L, Sieghart W. Identification of an amino acid sequence within GABA(A) receptor beta3 subunits that is important for receptor assembly. J Neurochem. 2003;84:127–135. doi: 10.1046/j.1471-4159.2003.01509.x. [DOI] [PubMed] [Google Scholar]
- Engelender S, Sharp AH, Colomer V, Tokito MK, Lanahan A, Worley P, Holzbaur EL, Ross CA. Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum Mol Genet. 1997;6:2205–2212. doi: 10.1093/hmg/6.13.2205. [DOI] [PubMed] [Google Scholar]
- Eshaq RS, Stahl LD, Stone R, 2nd, Smith SS, Robinson LC, Leidenheimer NJ. GABA acts as a ligand chaperone in the early secretory pathway to promote cell surface expression of GABAA receptors. Brain Res. 2010;1346:1–13. doi: 10.1016/j.brainres.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson J, Fritschy J-M, Luscher B. Postsynaptic clustering of major GABA(A) receptor subtypes requires the gamma2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B. GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci. 2006;26:12758–12768. doi: 10.1523/JNEUROSCI.4214-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282:1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- Fiumelli H, Woodin MA. Role of activity-dependent regulation of neuronal chloride homeostasis in development. Curr Opin Neurobiol. 2007;17:81–86. doi: 10.1016/j.conb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Foldy C, Lee SH, Morgan RJ, Soltesz I. Regulation of fast-spiking basket cell synapses by the chloride channel ClC-2. Nat Neurosci. 2010;13:1047–1049. doi: 10.1038/nn.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM. Epilepsy, E/I Balance and GABA(A) Receptor Plasticity. Front Mol Neurosci. 2008;1:5. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 2008;31:257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Frugier G, Coussen F, Giraud MF, Odessa MF, Emerit MB, Boue-Grabot E, Garret M. A gamma 2(R43Q) mutation, linked to epilepsy in humans, alters GABAa receptor assembly and modifies subunit composition on the cell surface. J Biol Chem. 2006;282:3819–3828. doi: 10.1074/jbc.M608910200. [DOI] [PubMed] [Google Scholar]
- Fu Z, Vicini S. Neuroligin-2 accelerates GABAergic synapse maturation in cerebellar granule cells. Mol Cell Neurosci. 2009;42:45–55. doi: 10.1016/j.mcn.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann JC, Kins S, Rostaing P, El Far O, Kirsch J, Sheng M, Triller A, Betz H, Kneussel M. Gephyrin interacts with dynein light chains 1 and 2, components of motor protein complexes. J Neurosci. 2002;22:5393–5402. doi: 10.1523/JNEUROSCI.22-13-05393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M, Kanematsu T, Ishibashi H, Fukami K, Takenawa T, Nakayama KI, Moss SJ, Nabekura J, Hirata M. Phospholipase C-related but catalytically inactive protein is required for insulin-induced cell surface expression of gamma-aminobutyric acid type A receptors. J Biol Chem. 2010;285:4837–4846. doi: 10.1074/jbc.M109.070045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher MJ, Ding L, Maheshwari A, Macdonald RL. The GABA(A) receptor alpha1 subunit epilepsy mutation A322D inhibits transmembrane helix formation and causes proteasomal degradation. Proc Natl Acad Sci U S A. 2007;104:12999–13004. doi: 10.1073/pnas.0700163104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, Saudou F. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Huber KM, Sudhof TC. Neuroligin-2 deletion selectively decreases inhibitory synaptic transmission originating from fast-spiking but not from somatostatin-positive interneurons. J Neurosci. 2009;29:13883–13897. doi: 10.1523/JNEUROSCI.2457-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesemann T, Schwarz G, Nawrotzki R, Berhorster K, Rothkegel M, Schluter K, Schrader N, Schindelin H, Mendel RR, Kirsch J, Jockusch BM. Complex formation between the postsynaptic scaffolding protein gephyrin, profilin, and Mena: a possible link to the microfilament system. J Neurosci. 2003;23:8330–8339. doi: 10.1523/JNEUROSCI.23-23-08330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SL, Zhang L, Forster ML, Anderson JR, Iwase T, Soliven B, Donahue LR, Sweet HO, Bronson RT, Davisson MT, et al. Trak1 mutation disrupts GABA(A) receptor homeostasis in hypertonic mice. Nat Genet. 2006;38:245–250. doi: 10.1038/ng1715. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Gorrie GH, Vallis Y, Stephenson A, Whitfield J, Browning B, Smart TG, Moss SJ. Assembly of GABA(A) receptors composed of alpha1 and beta2 subunits in both cultured neurons and fibroblasts. J Neurosci. 1997;17:6587–6596. doi: 10.1523/JNEUROSCI.17-17-06587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H, Terunuma M, Kanematsu T, Misumi Y, Moss SJ, Hirata M. Direct interaction of N-ethylmaleimide-sensitive factor with GABA(A) receptor beta subunits. Mol Cell Neurosci. 2005;30:197–206. doi: 10.1016/j.mcn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang XZ, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskreutz Y, Hermann A, Kins S, Fuhrmann JC, Betz H, Kneussel M. Identification of a gephyrin-binding motif in the GDP/GTP exchange factor collybistin. Biol Chem. 2001;382:1455–1462. doi: 10.1515/BC.2001.179. [DOI] [PubMed] [Google Scholar]
- Gunther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, Crestani F, Aguzzi A, Arigoni M, Lang Y, et al. Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KF, Macdonald RL. GABA(A) receptor subunit gamma2 and delta subtypes confer unique kinetic properties on recombinant GABA(A) receptor currents in mouse fibroblasts. J Physiol. 1999;514(Pt 1):27–45. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales TG, Tang H, Bollan KA, Johnson SJ, King DP, McDonald NA, Cheng A, Connolly CN. The epilepsy mutation, gamma2(R43Q) disrupts a highly conserved inter-subunit contact site, perturbing the biogenesis of GABAA receptors. Mol Cell Neurosci. 2005;29:120–127. doi: 10.1016/j.mcn.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Harvey K, Duguid IC, Alldred MJ, Beatty SE, Ward H, Keep NH, Lingenfelter SE, Pearce BR, Lundgren J, Owen MJ, et al. The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering. J Neurosci. 2004;24:5816–5826. doi: 10.1523/JNEUROSCI.1184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havrankova J, Roth J, Brownstein M. Insulin and insulin receptors in rodent brain. Diabetologia. 1981;20:268–273. [PubMed] [Google Scholar]
- Heir R, Ablasou C, Dumontier E, Elliott M, Fagotto-Kaufmann C, Bedford FK. The UBL domain of PLIC-1 regulates aggresome formation. EMBO Rep. 2006;7:1252–1258. doi: 10.1038/sj.embor.7400823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemelaar J, Lelyveld VS, Kessler BM, Ploegh HL. A single protease, Apg4B, is specific for the autophagy-related ubiquitin-like proteins GATE-16, MAP1-LC3, GABARAP, and Apg8L. J Biol Chem. 2003;278:51841–51850. doi: 10.1074/jbc.M308762200. [DOI] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Dillon GH, Leidenheimer NJ. PKC modulation of GABA(A) receptor endocytosis and function is inhibited by mutation of a dileucine motif within the receptor beta 2 subunit. Neuropharmacology. 2005;48:181–194. doi: 10.1016/j.neuropharm.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Robinson LC, Dillon GH, Leidenheimer NJ. Constitutive GABA(A) receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin binding motif within the beta 2 subunit of the receptor. J Biol Chem. 2003;278:24046–24052. doi: 10.1074/jbc.M301420200. [DOI] [PubMed] [Google Scholar]
- Hewitt SA, Wamsteeker JI, Kurz EU, Bains JS. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat Neurosci. 2009;12:438–443. doi: 10.1038/nn.2274. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Raber J, McMahon T, Walter H, Sanchez-Perez AM, Olive MF, Mehmert K, Morrow AL, Messing RO. Decreased anxiety-like behavior, reduced stress hormones, and neurosteroid supersensitivity in mice lacking protein kinase Cepsilon. J Clin Invest. 2002;110:1003–1010. doi: 10.1172/JCI15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homanics GE, DeLorey TM, Firestone LL, Quinlan JJ, Handforth A, Harrison NL, Krasowski MD, Rick CE, Korpi ER, Makela R, et al. Mice devoid of gamma-aminobutyrate type A receptor beta3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc Natl Acad Sci U S A. 1997;94:4143–4148. doi: 10.1073/pnas.94.8.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon M, Bauer G, Fritschy JM, Moser T, Falkenburger BH, Varoqueaux F. Neuroligin 2 controls the maturation of GABAergic synapses and information processing in the retina. J Neurosci. 2009;29:8039–8050. doi: 10.1523/JNEUROSCI.0534-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon M, Soykan T, Falkenburger B, Hammer M, Patrizi A, Schmidt KF, Sassoe-Pognetto M, Lowel S, Moser T, Taschenberger H, et al. Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. Proc Natl Acad Sci USA. 2011;108:3053–3058. doi: 10.1073/pnas.1006946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston CM, He Q, Smart TG. CaMKII phosphorylation of the GABA(A) receptor: receptor subtype- and synapse-specific modulation. J Physiol. 2009;587:2115–2125. doi: 10.1113/jphysiol.2009.171603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston CM, Lee HH, Hosie AM, Moss SJ, Smart TG. Identification of the sites for CaMK-II dependent phosphorylation of GABA(A) receptors. J Biol Chem. 2007 doi: 10.1074/jbc.M611533200. [DOI] [PubMed] [Google Scholar]
- Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villen J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Nguyen T, Sudhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nature reviews Neuroscience. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABA(A) receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch N, Witte OW, Frahm C. Downregulation of potassium chloride cotransporter KCC2 after transient focal cerebral ischemia. Stroke. 2010;41:e151–159. doi: 10.1161/STROKEAHA.109.570424. [DOI] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IS, Nakamura M, Ito Y, Akaike N. Presynaptic GABAA receptors facilitate spontaneous glutamate release from presynaptic terminals on mechanically dissociated rat CA3 pyramidal neurons. Neuroscience. 2006;138:25–35. doi: 10.1016/j.neuroscience.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Jedlicka P, Hoon M, Papadopoulos T, Vlachos A, Winkels R, Poulopoulos A, Betz H, Deller T, Brose N, Varoqueaux F, Schwarzacher SW. Increased dentate gyrus excitability in neuroligin-2-deficient mice in vivo. Cereb Cortex. 2010;21:357–367. doi: 10.1093/cercor/bhq100. [DOI] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Mäkelä R, Mellor R, Pollard S, Bahn S, Stephenson A, et al. Ligand-gated ion channel subunit partnership: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J Neurosci. 2004;24:522–530. doi: 10.1523/JNEUROSCI.3606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurd R, Tretter V, Walker J, Brandon NJ, Moss SJ. Fyn kinase contributes to tyrosine phosphorylation of the GABA(A) receptor gamma2 subunit. Mol Cell Neurosci. 2010;44:129–134. doi: 10.1016/j.mcn.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci. 2004;117:2805–2812. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, Mount DB. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4:490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- Kalscheuer VM, Musante L, Fang C, Hoffmann K, Fuchs C, Carta E, Deas E, Venkateswarlu K, Menzel C, Ullmann R, et al. A balanced chromosomal translocation disrupting ARHGEF9 is associated with epilepsy, anxiety, aggression, and mental retardation. Hum Mutat. 2009;30:61–68. doi: 10.1002/humu.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T, Fujii M, Mizokami A, Kittler JT, Nabekura J, Moss SJ, Hirata M. Phospholipase C-related inactive protein is implicated in the constitutive internalization of GABA(A) receptors mediated by clathrin and AP2 adaptor complex. J Neurochem. 2007;101:898–905. doi: 10.1111/j.1471-4159.2006.04399.x. [DOI] [PubMed] [Google Scholar]
- Kanematsu T, Jang IS, Yamaguchi T, Nagahama H, Yoshimura K, Hidaka K, Matsuda M, Takeuchi H, Misumi Y, Nakayama K, et al. Role of the PLC-related, catalytically inactive protein p130 in GABA(A) receptor function. EMBO J. 2002;21:1004–1011. doi: 10.1093/emboj/21.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T, Takeuchi H, Terunuma M, Hirata M. PRIP, a novel Ins(1,4,5)P3 binding protein, functional significance in Ca2+ signaling and extension to neuroscience and beyond. Mol Cells. 2005;20:305–314. [PubMed] [Google Scholar]
- Kanematsu T, Yasunaga A, Mizoguchi Y, Kuratani A, Kittler JT, Jovanovic JN, Takenaka K, Nakayama KI, Fukami K, Takenawa T, et al. Modulation of GABA(a) receptor phosphorylation and membrane trafficking by phospholipase C-related inactive protein/protein phosphatase 1 and 2A signaling complex underlying BDNF-dependent regulation of GABAergic inhibition. J Biol Chem. 2006;281:22180–22189. doi: 10.1074/jbc.M603118200. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Macdonald RL. The GABA(A) receptor gamma2 subunit R43Q mutation linked to childhood absence epilepsy and febrile seizures causes retention of alpha1beta2gamma2S receptors in the endoplasmic reticulum. J Neurosci. 2004;24:8672–8677. doi: 10.1523/JNEUROSCI.2717-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasugai Y, Swinny JD, Roberts JD, Dalezios Y, Fukazawa Y, Sieghart W, Shigemoto R, Somogyi P. Quantitative localisation of synaptic and extrasynaptic GABA(A) receptor subunits on hippocampal pyramidal cells by freeze-fracture replica immunolabelling. Eur J Neurosci. 2010;32:1868–1888. doi: 10.1111/j.1460-9568.2010.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi SY, Hirano T. Sustained structural change of GABA(A) receptor-associated protein underlies long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. J Neurosci. 2007;27:6788–6799. doi: 10.1523/JNEUROSCI.1981-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki BT, Hoffman KB, Yamamoto RS, Bahr BA. Variants of the receptor/channel clustering molecule gephyrin in brain: distinct distribution patterns, developmental profiles, and proteolytic cleavage by calpain. J Neurosci Res. 1997;49:381–388. doi: 10.1002/(sici)1097-4547(19970801)49:3<381::aid-jnr13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Ke Y, Cohen BM, Bang JY, Yang M, Renshaw PF. Assessment of GABA concentration in human brain using two-dimensional proton magnetic resonance spectroscopy. Psychiatry Res. 2000;100:169–178. doi: 10.1016/s0925-4927(00)00075-5. [DOI] [PubMed] [Google Scholar]
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred MJ, Sassoè-Pognetto M, Luscher B. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZU, Gutierrez A, De Blas AL. The alpha 1 and alpha 6 subunits can coexist in the same cerebellar GABA(A) receptor maintaining their individual benzodiazepine-binding specificities. J Neurochem. 1996;66:685–691. doi: 10.1046/j.1471-4159.1996.66020685.x. [DOI] [PubMed] [Google Scholar]
- Kins S, Heinrich Betz H, Kirsch J. Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nat Neurosci. 2000;3:22–29. doi: 10.1038/71096. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Kushe J, Betz H. Targeting of glycine receptor subunits to gephyrin-rich domains in transfected human embryonic kidney cells. Mol Cell Neurosci. 1995;6:450–461. doi: 10.1006/mcne.1995.1033. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Wolters I, Triller A, Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993;366:745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Arancibia-Carcamo IL, Moss SJ. Association of GRIP1 with a GABA(A) receptor associated protein suggests a role for GRIP1 at inhibitory synapses. Biochem Pharmacol. 2004a;68:1649–1654. doi: 10.1016/j.bcp.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, Arancibia-Carcamo IL, Jovanovic JN, Pangalos MN, Haucke V, Yan Z, Moss SJ. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABA(A) receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci U S A. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Kukhtina V, Vahedi-Faridi A, Gu Z, Tretter V, Smith KR, McAinsh K, Arancibia-Carcamo IL, Saenger W, et al. Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor gamma2 subunit. Proc Natl Acad Sci U S A. 2008;105:3616–3621. doi: 10.1073/pnas.0707920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Rostaing P, Schiavo G, Fritschy JM, Olsen R, Triller A, Moss SJ. The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABAA receptors. Mol Cell Neurosci. 2001;18:13–25. doi: 10.1006/mcne.2001.1005. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci U S A. 2004b;101:12736–12741. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Ehya N, Fuchs K, Fuchs T, Ebert V, Sarto I, Sieghart W. Detection and binding properties of GABA(A) receptor assembly intermediates. J Biol Chem. 2001;276:16024–16032. doi: 10.1074/jbc.M009508200. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Fuchs K, Mayer B, Ehya N, Sieghart W. GABA(A) receptor assembly. Identification and structure of gamma(2) sequences forming the intersubunit contacts with alpha(1) and beta(3) subunits. J Biol Chem. 2000;275:8921–8928. doi: 10.1074/jbc.275.12.8921. [DOI] [PubMed] [Google Scholar]
- Kleijnen MF, Alarcon RM, Howley PM. The ubiquitin-associated domain of hPLIC-2 interacts with the proteasome. Mol Biol Cell. 2003;14:3868–3875. doi: 10.1091/mbc.E02-11-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijnen MF, Shih AH, Zhou P, Kumar S, Soccio RE, Kedersha NL, Gill G, Howley PM. The hPLIC proteins may provide a link between the ubiquitination machinery and the proteasome. Mol Cell. 2000;6:409–419. doi: 10.1016/s1097-2765(00)00040-x. [DOI] [PubMed] [Google Scholar]
- Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, Ffrench-Mullen J, Turecki G. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry. 2009;14:175–189. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstätter JH, Gasnier B, Feng G, Sanes JR, Betz H. Gephyrin-independent clustering of postsynaptic GABAA receptor subtypes. Mol Cell Neurosci. 2001;17:973–982. doi: 10.1006/mcne.2001.0983. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstätter JH, Laube B, Stahl S, Müller U, Betz H. Loss of postsynaptic GABAA receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Haverkamp S, Fuhrmann JC, Wang H, Wassle H, Olsen RW, Betz H. The gamma-aminobutyric acid type A receptor (GABAAR)-associated protein GABARAP interacts with gephyrin but is not involved in receptor anchoring at the synapse. Proc Natl Acad Sci U S A. 2000;97:8594–8599. doi: 10.1073/pnas.97.15.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D, Harris R, McAlister MS, Phelan JP, Geddes S, Moss SJ, Driscoll PC, Keep NH. The X-ray Crystal Structure and Putative Ligand-derived Peptide Binding Properties of gamma -Aminobutyric Acid Receptor Type A Receptor- associated Protein. J Biol Chem. 2002;277:5556–5561. doi: 10.1074/jbc.M109753200. [DOI] [PubMed] [Google Scholar]
- Knuesel I, Mastrocola M, Zuellig RA, Bornhauser B, Schaub MC, Fritschy JM. Altered synaptic clustering of GABAA receptors in mice lacking dystrophin (mdx mice) Eur J Neurosci. 1999;11:4457–4462. doi: 10.1046/j.1460-9568.1999.00887.x. [DOI] [PubMed] [Google Scholar]
- Korpi E, Kuner T, Kristo P, Kohler M, Herb A, Luddens H, Seeburg P. Small N-terminal deletion by splicing in cerebellar alpha 6 subunit abolishes GABAA receptor function. J Neurochem. 1994;63:1167–1170. doi: 10.1046/j.1471-4159.1994.63031167.x. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Korpi ER, O’Buckley TK, Homanics GE, Morrow AL. Molecular and pharmacological characterization of GABA(A) receptor alpha1 subunit knockout mice. J Pharmacol Exp Ther. 2002a;302:1037–1045. doi: 10.1124/jpet.102.036665. [DOI] [PubMed] [Google Scholar]
- Kralic JE, O’Buckley TK, Khisti RT, Hodge CW, Homanics GE, Morrow AL. GABA(A) receptor alpha-1 subunit deletion alters receptor subtype assembly, pharmacological and behavioral responses to benzodiazepines and zolpidem. Neuropharmacology. 2002b;43:685–694. doi: 10.1016/s0028-3908(02)00174-0. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Sidler C, Parpan F, Homanics GE, Morrow AL, Fritschy JM. Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of gamma-aminobutyric acid type A receptor alpha1 subunit knockout mice. J Comp Neurol. 2006;495:408–421. doi: 10.1002/cne.20866. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langosch D, Hoch W, Betz H. The 93 kDa protein gephyrin and tubulin associated with the inhibitory glycine receptor are phosphorylated by an endogenous protein kinase. FEBS lett. 1992;298:113–117. doi: 10.1016/0014-5793(92)80034-e. [DOI] [PubMed] [Google Scholar]
- Lardi-Studler B, Smolinsky B, Petitjean CM, Koenig F, Sidler C, Meier JC, Fritschy JM, Schwarz G. Vertebrate-specific sequences in the gephyrin E-domain regulate cytosolic aggregation and postsynaptic clustering. J Cell Sci. 2007;120:1371–1382. doi: 10.1242/jcs.003905. [DOI] [PubMed] [Google Scholar]
- Lee HH, Jurd R, Moss SJ. Tyrosine phosphorylation regulates the membrane trafficking of the potassium chloride co-transporter KCC2. Mol Cell Neurosci. 2010;45:173–179. doi: 10.1016/j.mcn.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leil TA, Chen ZW, Chang CS, Olsen RW. GABA(A) receptor-associated protein traffics GABA(A) receptors to the plasma membrane in neurons. J Neurosci. 2004;24:11429–11438. doi: 10.1523/JNEUROSCI.3355-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Grady RM, Henry MD, Campbell KP, Sanes JR, Craig AM. Dystroglycan is selectively associated with inhibitory GABAergic synapses but is dispensable for their differentiation. J Neurosci. 2002;22:4274–4285. doi: 10.1523/JNEUROSCI.22-11-04274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Schweizer C, Bannai H, Pascual O, Charrier C, Triller A. Homeostatic regulation of synaptic GlyR numbers driven by lateral diffusion. Neuron. 2008;59:261–273. doi: 10.1016/j.neuron.2008.05.030. [DOI] [PubMed] [Google Scholar]
- Li RW, Serwanski DR, Miralles CP, Li X, Charych E, Riquelme R, Huganir RL, de Blas AL. GRIP1 in GABAergic synapses. J Comp Neurol. 2005;488:11–27. doi: 10.1002/cne.20566. [DOI] [PubMed] [Google Scholar]
- Li XJ, Li SH, Sharp AH, Nucifora FC, Jr, Schilling G, Lanahan A, Worley P, Snyder SH, Ross CA. A huntingtin-associated protein enriched in brain with implications for pathology. Nature. 1995;378:398–402. doi: 10.1038/378398a0. [DOI] [PubMed] [Google Scholar]
- Li Y, Chin LS, Levey AI, Li L. Huntingtin-associated protein 1 interacts with hepatocyte growth factor-regulated tyrosine kinase substrate and functions in endosomal trafficking. J Biol Chem. 2002;277:28212–28221. doi: 10.1074/jbc.M111612200. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WY, Lagrange AH, Hernandez CC, Harrison R, Dell A, Haslam SM, Sheehan JH, Macdonald RL. Glycosylation of beta2 subunits regulates GABAA receptor biogenesis and channel gating. J Biol Chem. 2010;285:31348–31361. doi: 10.1074/jbc.M110.151449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebrich S, Bahring R, Katsuno T, Tsukita S, Kneussel M. Activated radixin is essential for GABAA receptor alpha5 subunit anchoring at the actin cytoskeleton. EMBO J. 2006;25:987–999. doi: 10.1038/sj.emboj.7600995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annual review of cell and developmental biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lorez M, Benke D, Luscher B, Mohler H, Benson JA. Single-channel properties of neuronal GABAA-receptors lacking the g2 subunit. J Physiol. 2000;527:11–31. doi: 10.1111/j.1469-7793.2000.t01-1-00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YF, Kojima N, Tomizawa K, Moriwaki A, Matsushita M, Obata K, Matsui H. Enhanced synaptic transmission and reduced threshold for LTP induction in fyn-transgenic mice. Eur J Neurosci. 1999;11:75–82. doi: 10.1046/j.1460-9568.1999.00407.x. [DOI] [PubMed] [Google Scholar]
- Lu YM, Mansuy IM, Kandel ER, Roder J. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26:197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;15:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydiard RB. The role of GABA in anxiety disorders. J Clin Psychiatry. 2003;64(Suppl 3):21–27. [PubMed] [Google Scholar]
- Maas C, Belgardt D, Lee HK, Heisler FF, Lappe-Siefke C, Magiera MM, van Dijk J, Hausrat TJ, Janke C, Kneussel M. Synaptic activation modifies microtubules underlying transport of postsynaptic cargo. Proc Natl Acad Sci U S A. 2009;106:8731–8736. doi: 10.1073/pnas.0812391106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Mah AL, Perry G, Smith MA, Monteiro MJ. Identification of ubiquilin, a novel presenilin interactor that increases presenilin protein accumulation. J Cell Biol. 2000;151:847–862. doi: 10.1083/jcb.151.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Sasaki T, Asakura T, Hotta I, Imamura H, Takahashi K, Matsuura Y, Shirao T, Takai Y. Interactions of drebrin and gephyrin with profilin. Biochem Biophys Res Commun. 1998;243:86–89. doi: 10.1006/bbrc.1997.8068. [DOI] [PubMed] [Google Scholar]
- Mansuy-Schlick V, Tolle F, Delage-Mourroux R, Fraichard A, Risold PY, Jouvenot M. Specific distribution of gabarap, gec1/gabarap Like 1, gate16/gabarap Like 2, lc3 messenger RNAs in rat brain areas by quantitative real-time PCR. Brain Res. 2006;1073–1074:83–87. doi: 10.1016/j.brainres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Marsden KC, Beattie JB, Friedenthal J, Carroll RC. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABA(A) receptors. J Neurosci. 2007;27:14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden KC, Shemesh A, Bayer KU, Carroll RC. Selective translocation of Ca2+/calmodulin protein kinase IIalpha (CaMKIIalpha) to inhibitory synapses. Proc Natl Acad Sci USA. 2010;107:20559–20564. doi: 10.1073/pnas.1010346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A, Llano I. Excitatory effects of GABA in established brain networks. Trends Neurosci. 2005;28:284–289. doi: 10.1016/j.tins.2005.04.003. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG. Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci. 1998;1:23–28. doi: 10.1038/223. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Moss SJ. Differential phosphorylation of intracellular domains of gamma-aminobutyric acid type A receptor subunits by calcium/calmodulin type 2-dependent protein kinase and cGMP-dependent protein kinase. J Biol Chem. 1994;269:18111–18117. [PubMed] [Google Scholar]
- McGuire JR, Rong J, Li SH, Li XJ. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J Biol Chem. 2006;281:3552–3559. doi: 10.1074/jbc.M509806200. [DOI] [PubMed] [Google Scholar]
- McKinley DD, Lennon DJ, Carter DB. Cloning, sequence analysis and expression of two forms of mRNA coding for the human beta 2 subunit of the GABAA receptor. Brain Res Mol Brain Res. 1995;28:175–179. doi: 10.1016/0169-328x(94)00228-7. [DOI] [PubMed] [Google Scholar]
- Meyer G, Kirsch J, Betz H, Langosch D. Identification of a gephyrin binding motif on the glycine receptor beta subunit. Neuron. 1995;15:563–572. doi: 10.1016/0896-6273(95)90145-0. [DOI] [PubMed] [Google Scholar]
- Mielke JG, Wang YT. Insulin exerts neuroprotection by counteracting the decrease in cell-surface GABA receptors following oxygen-glucose deprivation in cultured cortical neurons. J Neurochem. 2005;92:103–113. doi: 10.1111/j.1471-4159.2004.02841.x. [DOI] [PubMed] [Google Scholar]
- Mitra K, Ubarretxena-Belandia I, Taguchi T, Warren G, Engelman DM. Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol. Proc Natl Acad Sci U S A. 2004;101:4083–4088. doi: 10.1073/pnas.0307332101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizokami A, Kanematsu T, Ishibashi H, Yamaguchi T, Tanida I, Takenaka K, Nakayama KI, Fukami K, Takenawa T, Kominami E, et al. Phospholipase C-related inactive protein is involved in trafficking of gamma2 subunit-containing GABA(A) receptors to the cell surface. J Neurosci. 2007;27:1692–1701. doi: 10.1523/JNEUROSCI.3155-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrluder J, Stangler T, Hoffmann Y, Wiesehan K, Mataruga A, Willbold D. Identification of calreticulin as a ligand of GABARAP by phage display screening of a peptide library. The FEBS journal. 2007;274:5543–5555. doi: 10.1111/j.1742-4658.2007.06073.x. [DOI] [PubMed] [Google Scholar]
- Morgan A, Burgoyne RD. Membrane traffic: controlling membrane fusion by modifying NSF. Curr Biol. 2004;14:R968–970. doi: 10.1016/j.cub.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Morinaga N, Moss J, Vaughan M. Cloning and expression of a cDNA encoding a bovine brain brefeldin A-sensitive guanine nucleotide-exchange protein for ADP-ribosylation factor. Proc Natl Acad Sci U S A. 1997;94:12926–12931. doi: 10.1073/pnas.94.24.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic alpha beta subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir J, Arancibia-Carcamo IL, MacAskill AF, Smith KR, Griffin LD, Kittler JT. NMDA receptors regulate GABAA receptor lateral mobility and clustering at inhibitory synapses through serine 327 on the 2 subunit. Proc Natl Acad Sci U S A. 2010;107 doi: 10.1073/pnas.1000589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABA(A) receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nymann-Andersen J, Sawyer GW, Olsen RW. Interaction between GABAA receptor subunit intracellular loops: implications for higher order complex formation. J Neurochem. 2002a;83:1164–1171. doi: 10.1046/j.1471-4159.2002.01222.x. [DOI] [PubMed] [Google Scholar]
- Nymann-Andersen J, Wang H, Chen L, Kittler JT, Moss SJ, Olsen RW. Subunit specificity and interaction domain between GABA(A) receptor- associated protein (GABARAP) and GABA(A) receptors. J Neurochem. 2002b;80:815–823. doi: 10.1046/j.0022-3042.2002.00762.x. [DOI] [PubMed] [Google Scholar]
- O’Sullivan GA, Kneussel M, Elazar Z, Betz H. GABARAP is not essential for GABA receptor targeting to the synapse. Eur J Neurosci. 2005;22:2644–2648. doi: 10.1111/j.1460-9568.2005.04448.x. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Voleti B, Banasr M, Sarhan M, Duric V, Girgenti MJ, Dileone RJ, Newton SS, Duman RS. Wnt2 expression and signaling is increased by different classes of antidepressant treatments. Biol Psychiatry. 2010;68:521–527. doi: 10.1016/j.biopsych.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis T, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of g-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic cuurents. Proc Natl Acad Sci USA. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paarmann I, Saiyed T, Schmitt B, Betz H. Gephyrin: does splicing affect its function? Biochem Soc Trans. 2006;34:45–47. doi: 10.1042/BST0340045. [DOI] [PubMed] [Google Scholar]
- Papadopoulos T, Korte M, Eulenburg V, Kubota H, Retiounskaia M, Harvey RJ, Harvey K, O’Sullivan GA, Laube B, Hulsmann S, et al. Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. EMBO J. 2007;26:3888–3899. doi: 10.1038/sj.emboj.7601819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak HR, Weissinger F, Terunuma M, Carlson GC, Hsu FC, Moss SJ, Coulter DA. Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci. 2007;27:14012–14022. doi: 10.1523/JNEUROSCI.4390-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizi A, Scelfo B, Viltono L, Briatore F, Fukaya M, Watanabe M, Strata P, Varoqueaux F, Brose N, Fritschy JM, Sassoe-Pognetto M. Synapse formation and clustering of neuroligin-2 in the absence of GABAA receptors. Proc Natl Acad Sci U S A. 2008;105:13151–13156. doi: 10.1073/pnas.0802390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the delta subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D, Ropert N. Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses. J Neurosci. 1999;19:578–588. doi: 10.1523/JNEUROSCI.19-02-00578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer F, Graham D, Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982;257:9389–9393. [PubMed] [Google Scholar]
- Poisbeau P, Cheney MC, Browning MD, Mody I. Modulation of synaptic GABAA receptor function by PKA and PKC in adult hippocampal neurons. J Neurosci. 1999;19:674–683. doi: 10.1523/JNEUROSCI.19-02-00674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, et al. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63:628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Prior P, Schmitt B, Grenningloh G, Pribilla I, Multhaup G, Beyreuther K, Maulet Y, Werner P, Langosh D, Kirsch J, Betz H. Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron. 1992;8:1161–1170. doi: 10.1016/0896-6273(92)90136-2. [DOI] [PubMed] [Google Scholar]
- Pugh JR, Jahr CE. Axonal GABAA receptors increase cerebellar granule cell excitability and synaptic activity. J Neurosci. 2011;31:565–574. doi: 10.1523/JNEUROSCI.4506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramming M, Kins S, Werner N, Hermann A, Betz H, Kirsch J. Diversity and phylogeny of gephyrin: tissue-specific splice variants, gene structure, and sequence similarities to molybdenum cofactor- synthesizing and cytoskeleton-associated proteins. Proc Natl Acad Sci U S A. 2000;97:10266–10271. doi: 10.1073/pnas.97.18.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathenberg J, Kittler JT, Moss SJ. Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Mol Cell Neurosci. 2004;26:251–257. doi: 10.1016/j.mcn.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Reddy-Alla S, Schmitt B, Birkenfeld J, Eulenburg V, Dutertre S, Bohringer C, Gotz M, Betz H, Papadopoulos T. PH-domain-driven targeting of collybistin but not Cdc42 activation is required for synaptic gephyrin clustering. Eur J Neurosci. 2010;31:1173–1184. doi: 10.1111/j.1460-9568.2010.07149.x. [DOI] [PubMed] [Google Scholar]
- Reid T, Bathoorn A, Ahmadian MR, Collard JG. Identification and characterization of hPEM-2, a guanine nucleotide exchange factor specific for Cdc42. J Biol Chem. 1999;274:33587–33593. doi: 10.1074/jbc.274.47.33587. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipila S, Payne JA, Minichiello L, Saarma M, Kaila K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland AM, Richmond JE, Olsen JG, Hall DH, Bamber BA. Presynaptic terminals independently regulate synaptic clustering and autophagy of GABAA receptors in Caenorhabditis elegans. J Neurosci. 2006;26:1711–1720. doi: 10.1523/JNEUROSCI.2279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Fabian-Fine R, Scott R, Walker MC, Rusakov DA, Kullmann DM. GABAA receptors at hippocampal mossy fibers. Neuron. 2003;39:961–973. doi: 10.1016/s0896-6273(03)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM, Barrow RK, Blackshaw S, Burnett PE, Lai MM, Field ME, Bahr BA, Kirsch J, Betz H, Snyder SH. Interaction of RAFT1 with gephyrin required for rapamycin-sensitive signaling. Science. 1999;284:1161–1164. doi: 10.1126/science.284.5417.1161. [DOI] [PubMed] [Google Scholar]
- Sagiv Y, Legesse-Miller A, Porat A, Elazar Z. GATE-16, a membrane transport modulator, interacts with NSF and the Golgi v-SNARE GOS-28. EMBO J. 2000;19:1494–1504. doi: 10.1093/emboj/19.7.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiepour L, Fuchs C, Patrizi A, Sassoe-Pognetto M, Harvey RJ, Harvey K. Complex role of collybistin and gephyrin in GABAA receptor clustering. J Biol Chem. 2010;285:29623–29631. doi: 10.1074/jbc.M110.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiyed T, Paarmann I, Schmitt B, Haeger S, Sola M, Schmalzing G, Weissenhorn W, Betz H. Molecular basis of gephyrin clustering at inhibitory synapses: role of G- and E-domain interactions. J Biol Chem. 2007;282:5625–5632. doi: 10.1074/jbc.M610290200. [DOI] [PubMed] [Google Scholar]
- Saliba RS, Gu Z, Yan Z, Moss SJ. Blocking L-type voltage-gated Ca2+ channels with dihydropyridines reduces gamma-aminobutyric acid type A receptor expression and synaptic inhibition. J Biol Chem. 2009;284:32544–32550. doi: 10.1074/jbc.M109.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba RS, Michels G, Jacob TC, Pangalos MN, Moss SJ. Activity-dependent ubiquitination of GABA(A) receptors regulates their accumulation at synaptic sites. J Neurosci. 2007;27:13341–13351. doi: 10.1523/JNEUROSCI.3277-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Murru L, Carta M, Talani G, Zucca S, Mura ML, Maciocco E, Biggio G. Changes in expression and function of extrasynaptic GABA(A) receptors in the rat hppocampus during pregnancy and after delivery. J Neurosci. 2009;29:1755–1765. doi: 10.1523/JNEUROSCI.3684-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarto I, Klausberger T, Ehya N, Mayer B, Fuchs K, Sieghart W. A novel site on gamma 3 subunits important for assembly of GABA(A) receptors. J Biol Chem. 2002;277:30656–30664. doi: 10.1074/jbc.M203597200. [DOI] [PubMed] [Google Scholar]
- Sarto-Jackson I, Ramerstorfer J, Ernst M, Sieghart W. Identification of amino acid residues important for assembly of GABA receptor alpha1 and gamma2 subunits. J Neurochem. 2006;96:983–995. doi: 10.1111/j.1471-4159.2005.03626.x. [DOI] [PubMed] [Google Scholar]
- Sassoè-Pognetto M, Fritschy J-M. Gephyrin, a major postsynaptic protein of GABAergic synapses. Eur J Neurosci. 2000;12:2205–2210. doi: 10.1046/j.1460-9568.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Kirsch J, Grunert U, Greferath U, Fritschy JM, Mohler H, Betz H, Wassle H. Colocalization of gephyrin and GABAA-receptor subunits in the rat retina. J Comp Neurol. 1995;357:1–14. doi: 10.1002/cne.903570102. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar gamma-aminobutyric acid A receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Schwarz G, Schrader N, Mendel RR, Hecht HJ, Schindelin H. Crystal structures of human gephyrin and plant Cnx1 G domains: comparative analysis and functional implications. J Mol Biol. 2001;312:405–418. doi: 10.1006/jmbi.2001.4952. [DOI] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy M, Fritschy JM, Mohler H, Luscher B. The gamma2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Seil FJ. TrkB receptor signaling and activity-dependent inhibitory synaptogenesis. Histol Histopathol. 2003;18:635–646. doi: 10.14670/HH-18.635. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, Rehal S, Klempan T, Gratton A, Benkelfat C, et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS ONE. 2009;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sertie AL, de Alencastro G, De Paula VJ, Passos-Bueno MR. Collybistin and gephyrin are novel components of the eukaryotic translation initiation factor 3 complex. BMC Res Notes. 2010;3:242. doi: 10.1186/1756-0500-3-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL. Synaptic and nonsynaptic localization of GABA(A) receptors containing the alpha5 subunit in the rat brain. J Comp Neurol. 2006;499:458–470. doi: 10.1002/cne.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at alpha4beta2delta GABA(A) receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for alpha4betadelta GABA(A) receptors in shaping learning deficits at puberty in mice. Science. 2010a;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, Luscher B. Gamma-aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol Psychiatry. 2010b;68:512–520. doi: 10.1016/j.biopsych.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CR, Tran MN, Wong RO, Lukasiewicz PD. Distinct ionotropic GABA receptors mediate presynaptic and postsynaptic inhibition in retinal bipolar cells. J Neurosci. 2000;20:2673–2682. doi: 10.1523/JNEUROSCI.20-07-02673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HW, Morinaga N, Noda M, Nakayama K. BIG2, a guanine nucleotide exchange factor for ADP-ribosylation factors: its localization to recycling endosomes and implication in the endosome integrity. Mol Biol Cell. 2004;15:5283–5294. doi: 10.1091/mbc.E04-05-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HW, Shinotsuka C, Nakayama K. Expression of BIG2 and analysis of its function in mammalian cells. Methods Enzymol. 2005;404:206–215. doi: 10.1016/S0076-6879(05)04020-6. [DOI] [PubMed] [Google Scholar]
- Sigel E, Kaur KH, Luscher BP, Baur R. Use of concatamers to study GABA(A) receptor architecture and function: application to delta-subunit-containing receptors and possible pitfalls. Biochem Soc Trans. 2009;37:1338–1342. doi: 10.1042/BST0371338. [DOI] [PubMed] [Google Scholar]
- Smith KR, McAinsh K, Chen G, Arancibia-Carcamo IL, Haucke V, Yan Z, Moss SJ, Kittler JT. Regulation of inhibitory synaptic transmission by a conserved atypical interaction of GABA(A) receptor beta- and gamma-subunits with the clathrin AP2 adaptor. Neuropharmacology. 2008;55:844–850. doi: 10.1016/j.neuropharm.2008.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Oliver PL, Lumb MJ, Arancibia-Carcamo IL, Revilla-Sanchez R, Brandon NJ, Moss SJ, Kittler JT. Identification and characterisation of a Maf1/Macoco protein complex that interacts with GABAA receptors in neurons. Mol Cell Neurosci. 2010;44:330–341. doi: 10.1016/j.mcn.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola M, Bavro VN, Timmins J, Franz T, Ricard-Blum S, Schoehn G, Ruigrok RW, Paarmann I, Saiyed T, O’Sullivan GA, et al. Structural basis of dynamic glycine receptor clustering by gephyrin. EMBO J. 2004;23:2510–2519. doi: 10.1038/sj.emboj.7600256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola M, Kneussel M, Heck IS, Betz H, Weissenhorn W. X-ray crystal structure of the trimeric N-terminal domain of gephyrin. J Biol Chem. 2001;26:26. doi: 10.1074/jbc.M101923200. [DOI] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Rostaing P, Triller A, Marty A. Activation of presynaptic GABA(A) receptors induces glutamate release from parallel fiber synapses. J Neurosci. 2007;27:9022–9031. doi: 10.1523/JNEUROSCI.1954-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RW. Further Evidence for Non-Pancreatic Insulin Immunoreactivity in Guinea-Pig Brain. Horm Metab Res. 1983;15:526–529. doi: 10.1055/s-2007-1018779. [DOI] [PubMed] [Google Scholar]
- Studer R, von Boehmer L, Haenggi T, Schweizer C, Benke D, Rudolph U, Fritschy JM. Alteration of GABAergic synapses and gephyrin clusters in the thalamic reticular nucleus of GABAA receptor alpha3 subunit-null mice. Eur J Neurosci. 2006;24:1307–1315. doi: 10.1111/j.1460-9568.2006.05006.x. [DOI] [PubMed] [Google Scholar]
- Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumita K, Sato Y, Iida J, Kawata A, Hamano M, Hirabayashi S, Ohno K, Peles E, Hata Y. Synaptic scaffolding molecule (S-SCAM) membrane-associated guanylate kinase with inverted organization (MAGI)-2 is associated with cell adhesion molecules at inhibitory synapses in rat hippocampal neurons. J Neurochem. 2007;100:154–166. doi: 10.1111/j.1471-4159.2006.04170.x. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Wafford KA, Reynolds DS, Hadingham KL, Bromidge F, Macaulay A, Collinson N, O’Meara G, Howell O, Newman R, et al. Loss of the major GABA(A) receptor subtype in the brain is not lethal in mice. J Neurosci. 2001;21:3409–3418. doi: 10.1523/JNEUROSCI.21-10-03409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Tan HO, Reid CA, Single FN, Davies PJ, Chiu C, Murphy S, Clarke AL, Dibbens L, Krestel H, Mulley JC, et al. Reduced cortical inhibition in a mouse model of familial childhood absence epilepsy. Proc Natl Acad Sci U S A. 2007;104:17536–17541. doi: 10.1073/pnas.0708440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Olsen RW, Medina MT, Schwartz E, Alonso ME, Duron RM, Castro-Ortega R, Martinez-Juarez IE, Pascual-Castroviejo I, Machado-Salas J, et al. Hyperglycosylation and reduced GABA currents of mutated GABRB3 polypeptide in remitting childhood absence epilepsy. Am J Hum Genet. 2008;82:1249–1261. doi: 10.1016/j.ajhg.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Komatsu M, Ueno T, Kominami E. GATE-16 and GABARAP are authentic modifiers mediated by Apg7 and Apg3. Biochem Biophys Res Commun. 2003;300:637–644. doi: 10.1016/s0006-291x(02)02907-8. [DOI] [PubMed] [Google Scholar]
- Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36:2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PM, Connolly CN, Kittler JT, Gorrie GH, Hosie A, Smart TG, Moss SJ. Identification of residues within GABA(A) receptor alpha subunits that mediate specific assembly with receptor beta subunits. J Neurosci. 2000;20:1297–1306. doi: 10.1523/JNEUROSCI.20-04-01297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PM, Thomas P, Gorrie GH, Connolly CN, Smart TG, Moss SJ. Identification of amino acid residues within GABA(A) receptor beta subunits that mediate both homomeric and heteromeric receptor expression. J Neurosci. 1999;19:6360–6371. doi: 10.1523/JNEUROSCI.19-15-06360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissere JA, Czajkowski C. A (beta)-strand in the (gamma)2 subunit lines the benzodiazepine binding site of the GABA A receptor: structural rearrangements detected during channel gating. J Neurosci. 2001;21:4977–4986. doi: 10.1523/JNEUROSCI.21-14-04977.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra M, Ugurbil K, Gruetter R. Direct in vivo measurement of human cerebral GABA concentration using MEGA-editing at 7 Tesla. Magn Reson Med. 2002;47:1009–1012. doi: 10.1002/mrm.10146. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Jang IS, Ha SH, Kittler JT, Kanematsu T, Jovanovic JN, Nakayama KI, Akaike N, Ryu SH, Moss SJ, Hirata M. GABAA receptor phospho-dependent modulation is regulated by phospholipase C-related inactive protein type 1, a novel protein phosphatase 1 anchoring protein. J Neurosci. 2004;24:7074–7084. doi: 10.1523/JNEUROSCI.1323-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ. Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28:376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Mortensen M, Hosie AM, Smart TG. Dynamic mobility of functional GABA(A) receptors at inhibitory synapses. Nat Neurosci. 2005;8:889–897. doi: 10.1038/nn1483. [DOI] [PubMed] [Google Scholar]
- Togawa A, Morinaga N, Ogasawara M, Moss J, Vaughan M. Purification and cloning of a brefeldin A-inhibited guanine nucleotide-exchange protein for ADP-ribosylation factors. J Biol Chem. 1999;274:12308–12315. doi: 10.1074/jbc.274.18.12308. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Tran DD, Russell HR, Sutor SL, van Deursen J, Bram RJ. CAML is required for efficient EGF receptor recycling. Dev Cell. 2003;5:245–256. doi: 10.1016/s1534-5807(03)00207-7. [DOI] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha2 subunits to gephyrin. J Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Revilla-Sanchez R, Houston C, Terunuma M, Havekes R, Florian C, Jurd R, Vithlani M, Michels G, Couve A, et al. Deficits in spatial memory correlate with modified gamma-aminobutyric acid type A receptor tyrosine phosphorylation in the hippocampus. Proc Natl Acad Sci U S A. 2009;106:20039–20044. doi: 10.1073/pnas.0908840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, Macaskill AF, Rostaing P, Lumb MJ, Humbert S, Triller A, Saudou F, Yan Z, Kittler JT. Delivery of GABA(A)Rs to Synapses Is Mediated by HAP1-KIF5 and Disrupted by Mutant Huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagarajan SK, Fritschy JM. GABA(A) receptors, gephyrin and homeostatic synaptic plasticity. J Physiol. 2010;588:101–106. doi: 10.1113/jphysiol.2009.178517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagarajan SK, Ghosh H, Yévenes GE, Nikonenko I, Ebeling C, Schwerdel C, Sidler C, Zeilhofer HU, Gerrits B, Muller D, Fritschy JM. Regulation of GABAergic synapse formation and plasticity by GSK3(beta)-dependent phosphorylation of gephyrin. Proc Natl Acad Sci U S A. 2011;108:379–384. doi: 10.1073/pnas.1011824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uji A, Matsuda M, Kukita T, Maeda K, Kanematsu T, Hirata M. Molecules interacting with PRIP-2, a novel Ins(1,4,5)P3 binding protein type 2: Comparison with PRIP-1. Life Sci. 2002;72:443–453. doi: 10.1016/s0024-3205(02)02275-0. [DOI] [PubMed] [Google Scholar]
- Ullrich B, Ushkaryov YA, Sudhof TC. Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron. 1995;14:497–507. doi: 10.1016/0896-6273(95)90306-2. [DOI] [PubMed] [Google Scholar]
- Unwin N. The structure of ion channels in membranes of excitable cells. Neuron. 1989;3:665–676. doi: 10.1016/0896-6273(89)90235-3. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Hata Y, Ichtchenko K, Moomaw C, Afendis S, Slaughter CA, Sudhof TC. Conserved domain structure of beta-neurexins. Unusual cleaved signal sequences in receptor-like neuronal cell-surface proteins. J Biol Chem. 1994;269:11987–11992. [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Sudhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Vetiska SM, Ahmadian G, Ju W, Liu L, Wymann MP, Wang YT. GABAA receptor-associated phosphoinositide 3-kinase is required for insulin-induced recruitment of postsynaptic GABAA receptors. Neuropharmacology. 2007;52:146–155. doi: 10.1016/j.neuropharm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, Berkovic SF. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- Walters KJ, Kleijnen MF, Goh AM, Wagner G, Howley PM. Structural studies of the interaction between ubiquitin family proteins and proteasome subunit S5a. Biochemistry. 2002;41:1767–1777. doi: 10.1021/bi011892y. [DOI] [PubMed] [Google Scholar]
- Wan G, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABAA receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- Wang DD, Kriegstein AR. Defining the role of GABA in cortical development. J Physiol. 2009;587:1873–1879. doi: 10.1113/jphysiol.2008.167635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABAA-receptor-associated protein links GABAA receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu S, Haditsch U, Tu W, Cochrane K, Ahmadian G, Tran L, Paw J, Wang Y, Mansuy I, et al. Interaction of calcineurin and type-A GABA receptor gamma2 subunits produces long-term depression at CA1 inhibitory synapses. J Neurosci. 2003a;23:826–836. doi: 10.1523/JNEUROSCI.23-03-00826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Control of synaptic strength, a novel function of Akt. Neuron. 2003b;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- Whiting P, McKernen RM, Iversen LL. Another mechanism for creating diversity in gamma-aminobutyrate type A receptors: RNA splicing directs expression of two forms of gamma 2 phosphorylation site. Proc Natl Acad Sci USA. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Cope D, Klausberger T, Hauer B, Sinkkonen ST, Tretter V, Lujan R, Jones A, Korpi ER, Mody I, et al. Ectopic expression of the GABA(A) receptor alpha6 subunit in hippocampal pyramidal neurons produces extrasynaptic receptors and an increased tonic inhibition. Neuropharmacology. 2002;43:530–549. doi: 10.1016/s0028-3908(02)00151-x. [DOI] [PubMed] [Google Scholar]
- Wu AL, Wang J, Zheleznyak A, Brown EJ. Ubiquitin-related proteins regulate interaction of vimentin intermediate filaments with the plasma membrane. Mol Cell. 1999;4:619–625. doi: 10.1016/s1097-2765(00)80212-9. [DOI] [PubMed] [Google Scholar]
- Xiang S, Nichols J, Rajagopalan KV, Schindelin H. The crystal structure of Escherichia coli MoeA and its relationship to the multifunctional protein gephyrin. Structure (Camb) 2001;9:299–310. doi: 10.1016/s0969-2126(01)00588-3. [DOI] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006;3:47–58. doi: 10.1016/j.cmet.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Takeuchi H, Sato O, Hidaka K, Doira N, Terunuma M, Harada K, Ogawa Y, Ito Y, Kanematsu T, Hirata M. Interaction of p130 with, and consequent inhibition of, the catalytic subunit of protein phosphatase 1alpha. J Biol Chem. 2001;276:17908–17913. doi: 10.1074/jbc.M009677200. [DOI] [PubMed] [Google Scholar]
- Yuan X, Yao J, Norris D, Tran DD, Bram RJ, Chen G, Luscher B. Calcium-modulating cyclophilin ligand regulates membrane trafficking of postsynaptic GABA(A) receptors. Mol Cell Neurosci. 2008;38:277–289. doi: 10.1016/j.mcn.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnowska ED, Keist R, Rudolph U, Pearce RA. GABAA receptor alpha5 subunits contribute to GABAA,slow synaptic inhibition in mouse hippocampus. Journal of neurophysiology. 2009;101:1179–1191. doi: 10.1152/jn.91203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Atasoy D, Arac D, Yang X, Fucillo MV, Robison AJ, Ko J, Brunger AT, Sudhof TC. Neurexins physically and functionally interact with GABA(A) receptors. Neuron. 2010;66:403–416. doi: 10.1016/j.neuron.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Slevin JT, Whiteheart SW. Cellular functions of NSF: not just SNAPs and SNAREs. FEBS lett. 2007;581:2140–2149. doi: 10.1016/j.febslet.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zita MM, Marchionni I, Bottos E, Righi M, Del Sal G, Cherubini E, Zacchi P. Post-phosphorylation prolyl isomerisation of gephyrin represents a mechanism to modulate glycine receptors function. EMBO J. 2007;26:1761–1771. doi: 10.1038/sj.emboj.7601625. [DOI] [PMC free article] [PubMed] [Google Scholar]