Abstract
Ligands of the EGF family regulate autocrine keratinocyte proliferation and IL-1 family cytokines orchestrate epithelial defense responses. While members of both families are overexpressed in wound healing and psoriasis, their roles in regulating the innate immune functions of keratinocytes remain incompletely explored. Using sensitive assays, we found significant increases of HB-EGF, TGF-α and amphiregulin mRNA and protein in lesional psoriasis compared with uninvolved or control skin. In normal human keratinocyte (NHK) monolayers, EGFR ligands were ineffective in inducing DEFB4, S100A7, and CCL20 mRNAs and hBD-2 peptide. Combined with IL-1α, however, EGFR ligands provoked 250× more DEFB4 and CCL20 and a 9-fold rise in S100A7 mRNA relative to the EGFR ligand alone. This synergy was also reflected in secreted hBD-2 protein, both from NHK and reconstituted human epidermis. Keratinocyte differentiation was critical for these responses, as postconfluent NHK yielded mRNA and protein levels an order of magnitude greater than subconfluent cells. Differentiation also influenced signal transduction, with subconfluent cells utilizing NF-κB and postconfluent cells utilizing EGFR, MEK1/2, and p38. We propose that EGFR ligands are important modifiers of IL-1 activity, synergizing with IL-1 to stimulate epidermal production of hBD-2, S100A7 and CCL20, three of the most upregulated transcripts in psoriatic plaques.
Introduction
Many members of both the interleukin (IL)-1 and the epidermal growth factor (EGF) families are overexpressed in wounded skin (Barrientos et al., 2008; Marikovsky et al., 1993) and inflammatory dermatoses such as psoriasis (Camp et al., 1986; Cook et al., 1992; Elder et al., 1989; Piepkorn, 1996; Piepkorn et al., 2003; Stoll and Elder, 1998; Yoshida et al., 2008). Epidermal keratinocytes (KCs) respond to injury by becoming hyperproliferative, migratory and pro-inflammatory in processes regulated by cytokines and growth factors. IL-1 was the first cytokine detected in skin (Luger et al., 1982) and plays an important role in directing these responses. Both pro-IL-1α and pro-IL-1β are present in normal epidermis (Feldmeyer et al., 2007; Kupper et al., 1986). Pro-IL-1α is active and pre-formed pro-IL-1α is secreted by KCs under conditions of cell stress (Kondo et al., 1994), whereas pro-IL-1β requires inflammasome-mediated processing for activity (Franchi et al., 2009). Once secreted, these isoforms have similar functions, acting in an autocrine fashion and also on local fibroblasts, vascular endothelium and lymphocytes. Interestingly, IL-1α-treated normal human KCs (NHK) have a transcriptional signature which closely resembles differences seen in lesional versus non-lesional psoriatic skin (Mee et al., 2007).
The family of human growth factors capable of binding to the epidermal growth factor receptor (EGFR) is comprised of 7 members: epidermal growth factor (EGF), transforming growth factor (TGF)-α, heparin-binding-EGF-like growth factor (HB-EGF), amphiregulin (AREG), epiregulin (EREG), betacellulin (BTC) and epigen (EPGN). Many of these have been reported to be overexpressed in wounded skin (Barrientos et al., 2008; Marikovsky et al., 1993) and in psoriasis (Cook et al., 1992; Elder et al., 1989; Piepkorn, 1996; Piepkorn et al., 2003; Stoll and Elder, 1998; Yoshida et al., 2008) with the exception of BTC which is less abundant in lesional psoriasis skin (Piepkorn et al., 2003) and EGF which is undetectable in skin (Rittie et al., 2007). These growth factors bind to homo- and heterodimers formed by the four members of the ErbB family of receptor tyrosine kinases in a hierarchical fashion (Olayioye et al., 2000). EGFR (ErbB1) is expressed on the surface of human skin KCs, whereas ErbB2 is located intracelluarly until KCs reach the upper suprabasal layers (Stoll et al., 2001). Although there is likely some redundancy within this system, each of the EGFR ligands differ in their ability to stimulate EGFR tyrosine phosphorylation and exhibit context-dependent functional specificity (Stoll et al., 2010). However, their specific roles in the pathophysiology of epidermal hyperplasia remain unclear.
Recently there has been an increased awareness of the role of the innate immune system in skin diseases (Yamasaki and Gallo, 2008). Several antimicrobial peptides (AMPs) are highly overexpressed in psoriasis, including S100A7(Madsen et al., 1991), S100A8/S100A9 (Kelly et al., 1989) and human β-defensin-2 (hBD-2)(Harder et al., 1997). Moreover, peptides such as CCL20 (MIP-3α),discovered on the basis of their chemotactic properties also have antimicrobial activity (Chan et al., 2008). IL-1α and IL-1β markedly increase the expression of AMPs by human KCs (Liu et al., 2002) and positive interactions between the EGF and IL-1 signaling systems have been reported in human KCs (Chen et al., 1995; Lee et al., 1991; Wan et al., 2001). However, the interaction of the IL-1 and EGF signaling systems in regulating the expression of AMPs has not been explored.
To address these issues, we assessed EGFR ligand expression in healthy and psoriasis skin and then investigated the potential synergism between these growth factors and IL-1α for the induction of AMPs. We show that IL-1α synergizes with all EGF-like growth factors for the induction of hBD-2, CCL20 and S100A7. The response of KCs was highly dependent on their state of differentiation and corresponded to the sequence of stratified differentiation in skin. These data strongly suggest that multiple EGFR and IL-1R ligands are active in the epidermis and synergize at multiple levels for the induction of AMP and chemokines in the context of epidermal stress responses.
Results
Multiple EGFR ligands are overexpressed in lesional psoriasis skin
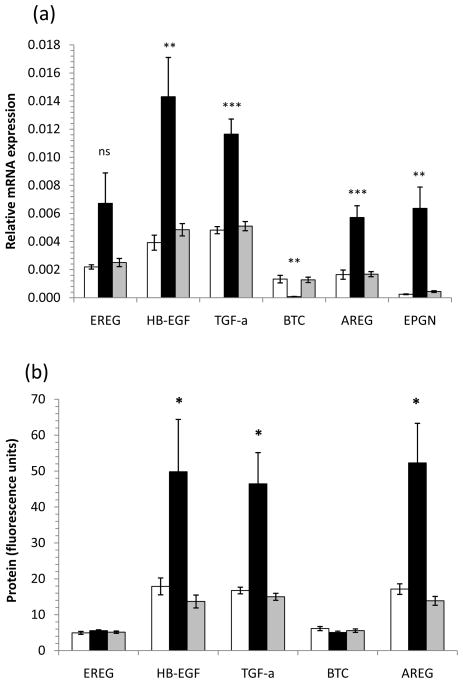
To globally analyze the overexpression of EGF-like growth factors in lesional psoriasis skin, we prepared RNA and protein fractions from lysates of 6mm skin biopsies and analyzed EGFR ligand mRNA expression by qRT-PCR and protein expression by multiplex bead array assay. Transcripts of HB-EGF were increased 3-fold (p<0.01), TGF-α 2.3-fold (p<0.001), AREG 3.4-fold (p<0.001) and EPGN 14-fold (p<0.01) in plaque psoriasis (PP) compared with uninvolved psoriasis (PN) (Figure 1a). In contrast, BTC mRNA expression was decreased 10-fold (p<0.001) in PP skin compared with PN skin (Figure 1a). EGFR ligand mRNA was not significantly different in PN or healthy control (NN) skin. In support of this we found the tissue level of HB-EGF protein was increased 3.6-fold (p<0.05), TGF-α 3.0-fold (p<0.05) and AREG 3.8-fold (p<0.05) in tissue lysates from PP versus PN skin (Figure 1b). Consistent with transcript levels, PN and NN skin did not have significantly different protein expression levels of EGF-like growth factors (Figure 1b).
Figure 1. EGFR ligand expression in skin.
Epiregulin (EREG), HB-EGF, TGF-α, amphiregulin (AREG) and epigen (EPGN) RNA transcripts were all more abundant in plaque psoriasis (PP, black) than symptomless psoriasis (PN, grey) or healthy control (NN, open bars) skin, whereas betacellulin (BTC) expression was decreased in PP skin (a). In support of this we found that HB-EGF, TGF-α and AREG proteins were all substantially elevated in PP versus PN or NN skin (b). Bars represent mean ± S.D. n=10 donors for mRNA n=4 for protein measurements. Statistical significance determined by 2-tailed t-test and indicated * p<0.05, ** p<0.01, *** p<0.001.
IL-1α and key EGFR ligands synergize for the production of antimicrobial peptides
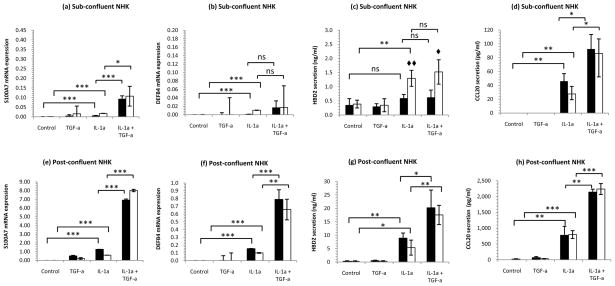
To understand the combined effect of elevated levels of IL-1 and EGFR ligands in the epidermis, we treated monolayer NHK cultures with EGF, HB-EGF, TGF-α, AREG, BTC, and EREG in combination with IL-1α. When NHK were treated with EGFR ligands alone, we did not detect appreciable fold-increases in AMP mRNA (Figure 2a, b). However, in combination with 10ng/ml IL-1α, all of the ErbB ligands induced robust increases in CCL20 (on average 900-fold), DEFB4 (400-fold) and S100A7 (26-fold) mRNA levels (Figure 2a, b). Neither S100A8 nor S100A9 mRNAs were synergistically increased (Figure 2b). The induction of CCL20 occurred with faster kinetics compared with DEFB4 (Figure 2c). The synergy between IL-1α and the EGFR ligands was also evident from the secretion of hBD-2 which was increased 3.4-fold (p<0.01, Figure 2d).
Figure 2. IL-1α and EGFR ligands synergize for the induction of CCL20, S100A7 but not S100A8 or S100A9 expression by KCs.
Postconfluent NHK were treated with 10ng/ml IL-1α in combination with 2nM EGF, 2nM HB-EGF, 4nM TGF-α, 2 or 20nM AREG, 2nM BTC or 2nM EREG. RNA was quantified by qRT-PCR normalized to the housekeeping gene RPLP0. (a) DEFB4, CCL20 and (b) S100 family mRNA expression at 4h of stimulation demonstrates synergism between IL-1α and EGFR ligands for DEFB4, CCL20, S100A7 but not S100A8 or S100A9 induction. (c) CCL20 (circles) and DEFB4 (squares) have markedly different kinetics with CCL20 mRNA occurring as an early response to IL-1α (closed symbols) or IL-1α+TGF-α (open symbols). (d) The synergism is evident at the protein level as shown by secreted hBD-2 at 24h of culture measured by ELISA. Open bars, EGFR ligand alone, filled bars IL-1α+EGFR ligand. Mean ± S.D. n=3. Statistical significance determined by 2-tailed t-test versus control or IL-1α treated cultures, indicated * p<0.05, ** p<0.01, *** p<0.001.
Cell density and calcium concentration are critical factors governing the responses of NHK in vitro
In human epidermis, AMP expression is typically a feature of the suprabasal, more differentiated, KCs and not the proliferative basal layers. This behavior is mimicked by monolayer NHK cultures, which, at a subconfluent cell density showed relatively little inducible DEFB4 mRNA and hBD-2 protein expression (Figure 3a–d). However, as the cell density increased through confluence to 4 days post-confluence we observed a dramatic increase in AMP transcription and protein secretion: S100A7 and DEFB4 mRNA were increased 80 and 40-fold respectively, and hBD-2 and CCL20 secretion increased by 30 and 66-fold for cells stimulated with IL-1α+TGF-α (Figure 3e-h). The effect of increased extracellular calcium was only evident in subconfluent cultures, where hBD-2 secretion could be doubled (p<0.01 for IL-1α stimulation, Figure 3c).
Figure 3. Cell density rather than extracellular calcium concentration is crucial to the responses of KCs in vitro.
The effect of increased extracellular Ca2+ is only obvious under proliferative conditions (a–d). Subconfluent, proliferating NHK or 4-day postconfluent NHK were starved of growth factors then treated with 10ng/ml IL-1α and/or 4nM TGF-α for 24h. Secreted hBD-2 and CCL20 were assayed by ELISA, S100A7, DEFB4 and CCL20 mRNA quantified by qRT-PCR relative to the housekeeping gene RPLP0. Filled bars 0.1mM, open bars 1.4mM Ca2+ indicate mean ± SD, n=3. Statistical significance (2-tailed t-test) indicated ◆ p<0.05, ◆◆ p<0.01 for low versus high calcium and * p<0.05, ** p<0.01, *** p<0.001 for untreated versus IL-1α and IL-1α+TGF-α. Note differences in y-axis scaling.
Postconfluent KCs mainly express suprabasal keratins and have altered IL-1 and ErbB receptor expression
To begin to examine the differences between sub- and postconfluent NHK cultures, we surveyed cytokeratin expression. Postconfluent NHK had keratin expression profiles similar to epidermal suprabasal KCs, with elevated levels of the suprabasal-associated cytokeratin (K)1 (p<0.01) and K10 (p<0.05) and significantly decreased levels of basal-layer associated K5 (p<0.001) and K14 (p<0.001) compared with their subconfluent counterparts (Figure 4a). Next, we examined whether these cells differed in their expression of EGF or IL-1 receptors. There was no significant difference in EGFR (ErbB1) transcript expression by sub- or postconfluent cells, however, both ErbB2 and ErbB3 were expressed at significantly higher levels by postconfluent cells (p<0.001, Figure 4c). There were no differences in receptor transcript expression from NHK maintained in low (0.1mM) or high (1.4mM) calcium environments (not shown). In terms of IL-1 receptors, subconfluent NHK expressed 6 times more mRNA for the IL-1 decoy receptor, IL-1RII (p<0.001, Figure 4b) compared with postconfluent cells. In contrast, there were no significant differences in IL-1RI or IL-1RAcP expression.
Figure 4. Subconfluent and 4-day postconfluent KCs show differential keratin, IL-1R and ErbB receptor expression.
Postconfluent NHK express less K5 and K14 mRNA and more K1 and K10 mRNA, characteristic of more differentiated KCs of the skin (a). Subconfluent NHK express higher levels of mRNA for the IL-1 decoy receptor IL-1RII (b). Sub- and postconfluent cells exhibited similar levels of ErbB1 transcript expression, no detectable ErbB4 expression, but significantly different levels of ErbB2 and ErbB3 (c). Open bars, subconfluent, filled bars postconfluent NHK. Bars indicate mean ± SD, n=8. Statistical significance denoted: * p< 0.05, ** p<0.01, *** p<0.001, 2-tailed t-test.
Subconfluent and postconfluent KCs utilize different signal transduction pathways
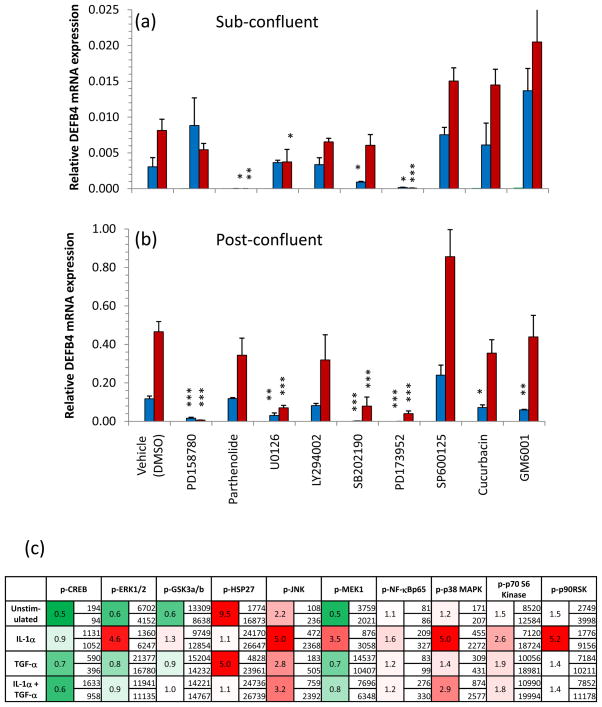
In an effort to identify the signal transduction pathways involved in the synergism between IL-1R and EGFR, we utilized a number of pharmacological signaling inhibitors. We found that the IL-1α and IL-1α+TGF-α-induced DEFB4 mRNA expression could be blocked by pre-treatment with the NF-κB inhibitor parthenolide in subconfluent (Figure 5a, p<0.05 and p<0.001) but not in postconfluent NHK cultures (Figure 5b), suggesting a switch in signaling pathways. Due to the low levels of secreted hBD-2 by subconfluent NHK, the effect of parthenolide pre-treatment was not apparent at the protein level (not shown). Further, inhibition of Src family kinase activity with PD173952 blocked IL-1α and IL-1α+TGF-α-induced DEFB4 mRNA expression in both subconfluent and postconfluent NHK (p<0.001, Figure 5a,b). In contrast, DEFB4 expression in postconfluent cells was much more sensitive to inhibition of ErbB tyrosine kinases (PD158780, p<0.001), MEK1/2 (U0126, p<0.001) and p38MAPK(SB202190, p<0.001) than in subconfluent NHK (Figure 5a,b). We also analyzed the expression of 10 key phosphoproteins involved in KC signal transduction. Figure 5c shows these data expressed both as absolute values and as the fold change of phosphoprotein expression of postconfluent over subconfluent cells. It is evident that IL-1α-stimulated postconfluent cells increase their usage of ERK1/2(4.6-fold, p=0.002), MEK1(3.5-fold, p<0.001), JNK(5-fold, p=0.01) and p38 MAPK(5-fold, p<0.05) compared with subconfluent cultures.
Figure 5. Differential utilization of signal transduction pathways by (a) sub- and (b) postconfluent KCs in culture.
NHK were grown to the specified confluency, starved of growth factors overnight then treated with one of each of the following inhibitors for 1h before stimulation with 10ng/ml IL-1α (blue bars) or 10ng/ml IL-1α + 4nM TGF-α (red bars) for 24h. Green bars, where present, represent unstimulated, inhibitor-treated NHK. Subconfluent NHK were particularly sensitive to inhibition of NF-κB (parthenolide) and Src family kinases (PD173952) but were largely unaffected by inhibition of EGFR (PD158780) or p38 (SB202190). Bars indicate mean±SD, n=3 representative of 3 separate experiments. Statistical significance indicated * p<0.05, ** p<0.01, *** p<0.001, 2-tailed t-test versus vehicle control. (c) Sub- and postconfluent NHK were stimulated with 10ng/ml IL-1α and/or 4nM TGF-α for 30 min and assayed for 10 phosphoproteins. Values indicate the concentration of each phosphoprotein in subconfluent (upper value) and postconfluent cells (lower value) and the fold increase (red) or fold decrease (green). Note differences in y-axis scaling. Values are representative of 2 independent experiments.
To further explore the mechanism by which IL-1α and EGFR ligands synergize, we examined IL-1α, IL-1β, IL-1ra and IL-1R (Figure S1) mRNA expression by EGFR-treated keratinocytes. EGF, HB-EGF and TGF-α significantly increased IL-1α, IL-1β and IL-1RA and also increased the expression of IL-1R2. Conversely, IL-1α significantly increased AREG(1.8-fold), EGF(2.8-fold), Ereg(1.9-fold), HB-EGF(2.5-fold), and TGF-α(3-fold) mRNA at 24h (Figure S2a-h) but failed to alter ErbB receptor expression (Figure S2g). Further, sequential treatment experiments revealed that both IL-1α and TGF-α must be present simultaneously to synergistically induce hBD-2 secretion (Figure S3).
IL-1α and EGFR synergistically induce AMP expression in reconstituted epidermal cultures
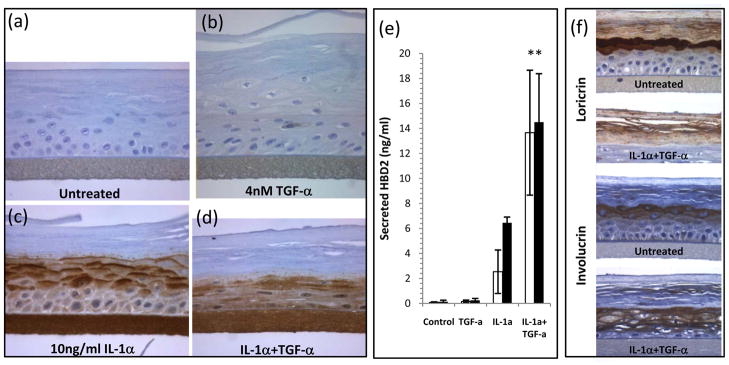
Synergism between IL-1α and EGFR ligands was also apparent in RHE cultures (Figure 6). TGF-α alone induced a hyperplastic response, but no hBD-2 (Figure 6b). hBD-2 was clearly induced in the suprabasal layers of the RHE by IL-1α, in a strikingly differentiation-related expression pattern (Figure 6c). Indicative of secretion, large amounts of hBD-2 were found in the underlying culture medium of IL-1α-treated cultures (Figure 6e). In combination with TGF-α, IL-1α induced a more diffuse pattern of hBD-2 expression throughout the basal and spinous layers (Figure 6d) which was accompanied by a 5-fold increase in hBD-2 secretion (24h, p=0.003) when IL-1α was combined with TGF-α (Figure 6e). The IL-1α+TGF-α-treated cultures exhibited a more compressed morphology of their viable cell layers and loss of the granular layer (Figure 6d) which was accompanied by loss of loricrin expression and a more widespread expression of involucrin in the cultures (Figure 6f).
Figure 6. The synergistic effects of IL-1α and TGF-α are evident on reconstituted human epidermal cultures.
Cultures were treated for 48 hours with 10ng/ml IL-1a, 4nM TGF-α or both, then processed and immunohistochemically stained for hBD-2 (a-d), loricrin or involucrin (f). Untreated cultures show no hBD-2 expression (a), this is true also of TGF-α-treatment which resulted in a thickened epidermal layer (b), IL-1α treatment induced a strong vectorial hBD-2 expression (c) while treatment with both IL-1α+TGF-α resulted in altered tissue morphology with enhanced hBD-2 expression (d). The altered epidermal structure was accompanied by decreased loricrin and more extensive involucrin expression in IL-1α+TGF-α treated cultures (f). To confirm the secretion of hBD-2, culture medium was sampled at 24h (open bars) and 48h (filled bars) and hBD-2 assayed by ELISA (e). Bars indicate mean ± SD, n=5. Statistical significance determined by 2-tailed t-test IL-1α versus IL-1α+TGF-α, ** p<0.01. Scale bar = 100μm.
Discussion
In addition to increased proliferation, keratinocyte differentiation is markedly altered in psoriasis (Ghadially et al., 1996), and the processes occurring in wound healing and psoriatic epidermis have been likened to a distinctive pattern of epidermal differentiation termed regenerative hyperplasia (Mansbridge et al., 1984). EGFR ligand expression is increased in wounded skin, and signaling through EGFR has been strongly implicated in the re-epithelialization phase of skin (Repertinger et al., 2004) and corneal wound healing (Nakamura et al., 2001). Despite considerable evidence for increased expression of EGFR ligands in psoriasis lesions, reversion of psoriasis-associated phenotypes in response to inhibition of EGFR in skin organ culture (Varani et al., 1998), and in response to anti-amphiregulin antibodies in xenografted lesional psoriatic skin (Bhagavathula et al., 2005) the precise role of EGFR signaling in psoriasis remains to be fully elucidated.
We demonstrate significant overexpression of TGF-α, HB-EGF, AREG, EREG, and EPGN mRNAs, and TGF-α, HB-EGF, and AREG proteins in psoriasis, in the most globally comparative assessment of these parameters to date. We then show that multiple EGFR ligands synergize with IL-1α to induce AMP expression, both in NHK and in RHE, resulting in robustly increased CCL20, DEFB4 and S100A7 mRNA, and secreted hBD-2 peptide from monolayer NHK and stratified RHE cultures. Such synergy would be beneficial for the control of AMP expression at a site of wounding, and likely contributes to the hyperplasia, AMP expression and immunocyte infiltrate in psoriasis. However, using purified IL-1 and TGF-α to stimulate three-dimensional RHE cultures, we were unable to recapitulate the pronounced and distinctive epidermal hyperplasia characteristic of psoriasis (Figure 6d), suggesting that other mechanisms are likely to be at play in vivo.
A mixed immunocytic infiltrate is characteristic of both wound healing and psoriasis. Recently, we have found that stimulated blood monocytes express IL-1, EREG and HB-EGF (A. Johnston, unpublished observations), and could therefore be a source of both IL-1 and EGFR ligands. As EGFR and IL-1R family members also synergize to induce expression of chemokines such as CCL20 by KC this interaction may promote and/or sustain the inflammatory response by driving further leukocyte infiltration.
AMP expression is restricted to the spinous and granular layers of the skin (de Jongh et al., 2005; Ong et al., 2002), and cytokine-induced expression of hBD-2 by NHK is markedly increased after in vitro differentiation (Harder et al., 2004). Consistent with this, we found that postconfluent NHK, which share the keratin expression profile of suprabasal KC (Figure 4a) (De Potter et al., 2001), were much more responsive to IL-1α stimulation than their subconfluent counterparts, expressing on average 10 times more S100A7 and DEFB4 mRNA and hBD-2 and CCL20 protein than subconfluent cells (Figure 3). To begin to elucidate the source of this difference we measured EGFR and IL-1R transcript expression. We found that although IL-1RI and IL-1RAcP are not differentially expressed, the decoy receptor, IL-1RII is 6-fold more abundant in subconfluent cultures (p<0.001, Figure 4b). This receptor, like IL-1RI, binds IL-1α and β and IL-1RAcP but cannot initiate signal transduction due to the lack of a cytoplasmic TIR domain. Thus increased expression of this receptor leads to decreased responsiveness to IL-1α/β which is supported by the increased utilization of MEK1, p38, p65 and JNK in postconfluent NHK (Figure 5c). The loss of IL-1RII expression as KC mature in normal epidermis would confer a greater degree of sensitivity to IL-1 signals and appropriate expression of AMPs in the higher strata of the epidermis. EGFR (ErbB1) was not significantly different between sub- and postconfluent cells, and as anticipated, ErbB4 was undetectable (De Potter et al., 2001; Stoll et al., 2001). However, ErbB2 and ErbB3 transcripts were both significantly elevated in postconfluent cultures, in accordance with the increased expression of ErbB2 by the more differentiated KC in the suprabasal layers of the skin (De Potter et al., 2001; Stoll et al., 2001). If these results were indicative of protein expression in the cell membrane, then ErbB heterodimer formation could contribute to the increased responses to IL-1α and EGFR ligand stimulation seen here, as ErbB hetereodimers have been reported to show increased ligand affinity and higher signal potency (Pinkas-Kramarski et al., 1996).
The increased sensitivity of postconfluent NHK to inhibition of MEK1/2 and p38MAPK (Figure 5a,b) was also reflected in their enhanced usage (Figure 5c). We observed a 10-fold increase in HSP27 phosphorylation in unstimulated postconfluent compared with subconfluent NHK which is consistent with increased HSP27 expression during epidermal cell differentiation (Gandour-Edwards et al., 1994; Jonak et al., 2002) and that oligomers of phospho-HSP27 are required for the folding of transglutaminase, loricrin and filaggrin for the formation of the cornified cell envelope (Jonak et al., 2002). The most consistently and dramatically down-regulated phosphoprotein was the transcription factor CREB, which was expressed in postconfluent cells at only half the levels observed for subconfluent NHK (Figure 5c), consistent with its role in repressing loricrin, the major protein of the cornified envelope (Jang and Steinert, 2002). While the signaling profiles of sub-and postconfluent cells presented in Figure 5 do not fully elucidate the mechanism of synergy between EGFR and IL-1 signaling, they do mirror the functional differences the basal and suprabasal keratinocytes.
In an effort to better understand the mechanism of IL-1-EGFR ligand synergy, we assessed the effect of one family of ligands on expression of ligands and receptors for the other. In accord with earlier findings for TGF-α (Lee et al., 1991), EGF, HB-EGF and TGF-α all induced IL-1α and IL-1β mRNA in NHK (Figure S1). Conversely, IL-1α induced EGFR ligands (Figure S2), including secreted AREG and TGF-α proteins. We also observed induction of IL-1RA and IL-1RII (Figure S1c,e) transcripts by EGF, HB-EGF and TGF-α, which may to some extent balance the induced IL-1α and IL-1β.
Finally, with RHE cultures when IL-1α and TGF-α were combined we measured a 5-fold increase in secreted hBD-2 and observed the loss of the granular layer (Figure 6d) and loricrin expression (Figure 6f), which occurs in psoriasis lesions, suggesting that the combined effects of IL-1 and EGFR ligands can contribute to the altered maturation of KC evident in psoriasis skin.
Based on these studies, we propose that EGFR ligands are important modifiers of the activity of IL-1α, and these data are consistent with the co-operation of the IL-1 and EGFR systems in promoting efficient wound healing with EGFR ligands enhancing KC motility and the synergistic induction of AMPs to suppress opportunistic microbial growth both by direct antimicrobial activity and by recruitment of immunocytes to the skin. However, the experimental systems utilized here do not fully recapitulate the complex, multicellular environment of human skin. Future studies should explore the responses studied in this report in more complex experimental models of the skin.
Materials and Methods
Study Population
Ten individuals with chronic plaque psoriasis and ten normal controls were enrolled (mean age 26.9 years, range 18–75 years). Entry criteria were the manifestation of one or more well-demarcated, scaly, erythematous psoriatic plaques that were not limited to the scalp and no systemic anti-psoriatic treatments for 2 weeks before biopsy. Biopsies sites varied between patients depending on site of active plaques whereas biopsies of uninvolved and control skin were from the buttocks. Informed written consent was obtained from all subjects, under protocols approved by the Institutional Review Board of the University of Michigan. This study was conducted in compliance with good clinical practice and according to the Declaration of Helsinki Principles.
Cell Cultures
Monolayer NHK cultures were established as described (Elder et al., 1991) and used in the second or third passage. Cultures were grown to 40 or 80% confluence, or maintained to 4 days post-confluency. Cultures were starved of growth factors in unsupplemented M154 medium (Invitrogen/Cascade Biologics, Portland, OR) for 24 hours before use. Experiments were carried out under low calcium(0.1mM) and high calcium(1.4mM) conditions. Cultures were stimulated with recombinant human cytokines and growth factors from R&D Systems (Minneapolis, MN): 0.56nM (10ng/ml) IL-1α, 2nM EGF, 2nM HB-EGF, 4nM TGF-α, 2 or 20nM AREG, 2nM BTC, 2nM EREG or combinations thereof. These EGFR ligand concentrations were selected on the basis of their ability to equivalently stimulate EGFR tyrosine phosphorylation (Stoll et al., 2010).
Where used, cultures were incubated with inhibitors 1 hour before cytokine addition. Reagents included inhibitors of EGFR tyrosine kinase (PD158780, 1μM, EMD Chemicals, San Diego, CA), NF-κB(parthenolide, 10μM, Sigma), MEK1/2(U0126, 10μM, EMD), phosphoinositol (PI)-3-kinase(LY294002, 1μM, EMD), p38MAPK(SB202190, 5μM), Src family kinases (PD173952, 1μM, kindly provided by Drs. Alan Kraker and Wilbur Leopold, Pfizer Global Research and Development, Ann Arbor, MI), JNK (SP600125, 5μM, A.G. Scientific, San Diego, CA) and STAT3(JSI-124/cucurbatacin, 10μM, EMD) and matrix metalloproteinases (GM6001, 40μM, EMD).
Reconstituted human epidermal cultures (RHE) were obtained from MatTek Inc (EPI-200, Ashland, MA). After overnight equilibration, medium was refreshed and supplemented with recombinant human IL-1α (0.56nM, 10ng/ml) or TGF-α(4nM) or both. Medium was sampled at 24 and 48hours. 4mm discs were punched from RHE cultures and formalin fixed, paraffin embedded, sectioned at 5μm and stained for hBD-2 expression (goat polyclonal, Peprotech, Rocky Hill, NJ), loricrin (rabbit polyclonal, Covance, Berkeley, CA), or involucrin (Clone SY5, Novocastra/Leica, Bannockburn, IL) and visualized with 3,3′-diaminobenzidine (BD Pharmingen, San Diego, CA).
Tissue processing
6mm skin biopsies were snap frozen, pulverized and either dissolved in complete RLT buffer (Qiagen, Valencia, CA) for RNA extraction or complete RIPA buffer for protein quantitation. Tissue lysates for protein quantitation were normalized to 1mg/ml total protein before analysis. Similarly, NHK were dissolved into 300μl complete RLT buffer for RNA extraction.
Real Time Quantitative Reverse Transcription PCR (qRT-PCR)
RNA was isolated (RNeasy Mini kit, Qiagen), reverse transcribed (High Capacity cDNA Transcription kit, Applied Biosystems Inc., Foster City, CA) and transcripts quantified using a 7900HT Fast Real-time PCR system (Applied Biosystems). Taqman primer sets were purchased from Applied Biosystems (Supplemental Table 1). All values were normalized to the expression of the housekeeping gene ribosomal protein, large, P0 (RPLP0) (Minner and Poumay, 2009).
ELISAs and Multiplexed Bead Assays
Conditioned culture medium was analyzed by ELISA for CCL20(R&D Systems) and hBD-2 (Supplemental Materials and Methods). Phosphoproteins in NHK lysates and EGFR ligands in medium were quantified by multiplexed assays (Supplemental Materials and Methods).
Statistics
Data sets were tested for normality using the Kolmogorov–Smirnov test, and statistical significance determined by Student’s t-test or Mann–Whitney rank sum tests where appropriate using SigmaPlot v10(Systat, Erkrath, Germany).
Supplementary Material
Acknowledgments
This work was in part supported by the National Institute for Arthritis, Musculoskeletal and Skin Disease(NIAMS), National Institutes of Health (K01 AR050462 and R03 AR049420 to SWS and R01 AR 052889 to JTE), American Skin Association and Dermatology Foundation (JEG). JTE is supported by the Ann Arbor Veterans Affairs Hospital.
Abbreviations
- AMP
anti-microbial peptide
- AREG
amphiregulin
- BTC
betacellulin
- EGF
epidermal growth factor
- EPGN
epigen
- EREG
epiregulin
- HBD
human beta-defensin
- HB-EGF
heparin-binding-EGF-like growth factor
- KC
keratinocyte
- NHK
normal human keratinocytes
- NN
normal control skin
- PN
uninvolved psoriasis
- PP
Plaque psoriasis
- QRT-PCR
quantitative real-time reverse transcriptase
- PCR RHE
reconstituted human epidermis
- TGF
transforming growth factor
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Bhagavathula N, Nerusu KC, Fisher GJ, Liu G, Thakur AB, Gemmell L, et al. Amphiregulin and epidermal hyperplasia: amphiregulin is required to maintain the psoriatic phenotype of human skin grafts on severe combined immunodeficient mice. Am J Pathol. 2005;166:1009–16. doi: 10.1016/S0002-9440(10)62322-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp RD, Fincham NJ, Cunningham FM, Greaves MW, Morris J, Chu A. Psoriatic skin lesions contain biologically active amounts of an interleukin 1-like compound. J Immunol. 1986;137:3469–74. [PubMed] [Google Scholar]
- Chan DI, Hunter HN, Tack BF, Vogel HJ. Human macrophage inflammatory protein 3alpha: protein and peptide nuclear magnetic resonance solution structures, dimerization, dynamics, and anti-infective properties. Antimicrob Agents Chemother. 2008;52:883–94. doi: 10.1128/AAC.00805-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JD, Lapiere JC, Sauder DN, Peavey C, Woodley DT. Interleukin-1 alpha stimulates keratinocyte migration through an epidermal growth factor/transforming growth factor-alpha-independent pathway. J Invest Dermatol. 1995;104:729–33. doi: 10.1111/1523-1747.ep12606970. [DOI] [PubMed] [Google Scholar]
- Cook PW, Pittelkow MR, Keeble WW, Graves-Deal R, Coffey RJ, Jr, Shipley GD. Amphiregulin messenger RNA is elevated in psoriatic epidermis and gastrointestinal carcinomas. Cancer Res. 1992;52:3224–7. [PubMed] [Google Scholar]
- de Jongh GJ, Zeeuwen PL, Kucharekova M, Pfundt R, van der Valk PG, Blokx W, et al. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J Invest Dermatol. 2005;125:1163–73. doi: 10.1111/j.0022-202X.2005.23935.x. [DOI] [PubMed] [Google Scholar]
- De Potter IY, Poumay Y, Squillace KA, Pittelkow MR. Human EGF receptor (HER) family and heregulin members are differentially expressed in epidermal keratinocytes and modulate differentiation. Exp Cell Res. 2001;271:315–28. doi: 10.1006/excr.2001.5390. [DOI] [PubMed] [Google Scholar]
- Elder JT, Fisher GJ, Lindquist PB, Bennett GL, Pittelkow MR, Coffey RJ, Jr, et al. Overexpression of transforming growth factor alpha in psoriatic epidermis. Science. 1989;243:811–4. doi: 10.1126/science.2916128. [DOI] [PubMed] [Google Scholar]
- Elder JT, Fisher GJ, Zhang QY, Eisen D, Krust A, Kastner P, et al. Retinoic acid receptor gene expression in human skin. J Invest Dermatol. 1991;96:425–33. doi: 10.1111/1523-1747.ep12469889. [DOI] [PubMed] [Google Scholar]
- Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer HD. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17:1140–5. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–7. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandour-Edwards R, McClaren M, Isseroff RR. Immunolocalization of low-molecular-weight stress protein HSP 27 in normal skin and common cutaneous lesions. Am J Dermatopathol. 1994;16:504–9. doi: 10.1097/00000372-199410000-00005. [DOI] [PubMed] [Google Scholar]
- Ghadially R, Reed JT, Elias PM. Stratum corneum structure and function correlates with phenotype in psoriasis. J Invest Dermatol. 1996;107:558–64. doi: 10.1111/1523-1747.ep12582813. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- Harder J, Meyer-Hoffert U, Wehkamp K, Schwichtenberg L, Schroder JM. Differential gene induction of human beta-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J Invest Dermatol. 2004;123:522–9. doi: 10.1111/j.0022-202X.2004.23234.x. [DOI] [PubMed] [Google Scholar]
- Jang SI, Steinert PM. Loricrin expression in cultured human keratinocytes is controlled by a complex interplay between transcription factors of the Sp1, CREB, AP1, and AP2 families. J Biol Chem. 2002;277:42268–79. doi: 10.1074/jbc.M205593200. [DOI] [PubMed] [Google Scholar]
- Jonak C, Klosner G, Kokesch C, Odinger D, Onigsmann H, Trautinger F. Subcorneal colocalization of the small heat shock protein, hsp27, with keratins and proteins of the cornified cell envelope. Br J Dermatol. 2002;147:13–9. doi: 10.1046/j.1365-2133.2002.04667.x. [DOI] [PubMed] [Google Scholar]
- Kelly SE, Jones DB, Fleming S. Calgranulin expression in inflammatory dermatoses. J Pathol. 1989;159:17–21. doi: 10.1002/path.1711590107. [DOI] [PubMed] [Google Scholar]
- Kondo S, Sauder DN, Kono T, Galley KA, McKenzie RC. Differential modulation of interleukin-1 alpha (IL-1 alpha) and interleukin-1 beta (IL-1 beta) in human epidermal keratinocytes by UVB. Exp Dermatol. 1994;3:29–39. doi: 10.1111/j.1600-0625.1994.tb00263.x. [DOI] [PubMed] [Google Scholar]
- Kupper TS, Ballard DW, Chua AO, McGuire JS, Flood PM, Horowitz MC, et al. Human keratinocytes contain mRNA indistinguishable from monocyte interleukin 1 alpha and beta mRNA. Keratinocyte epidermal cell-derived thymocyte-activating factor is identical to interleukin 1. J Exp Med. 1986;164:2095–100. doi: 10.1084/jem.164.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Morhenn VB, Ilnicka M, Eugui EM, Allison AC. Autocrine stimulation of interleukin-1 alpha and transforming growth factor alpha production in human keratinocytes and its antagonism by glucocorticoids. J Invest Dermatol. 1991;97:106–10. doi: 10.1111/1523-1747.ep12478503. [DOI] [PubMed] [Google Scholar]
- Liu AY, Destoumieux D, Wong AV, Park CH, Valore EV, Liu L, et al. Human beta-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Invest Dermatol. 2002;118:275–81. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- Luger TA, Stadler BM, Luger BM, Mathieson BJ, Mage M, Schmidt JA, et al. Murine epidermal cell-derived thymocyte-activating factor resembles murine interleukin 1. J Immunol. 1982;128:2147–52. [PubMed] [Google Scholar]
- Madsen P, Rasmussen HH, Leffers H, Honore B, Dejgaard K, Olsen E, et al. Molecular cloning, occurrence, and expression of a novel partially secreted protein “psoriasin” that is highly up-regulated in psoriatic skin. J Invest Dermatol. 1991;97:701–12. doi: 10.1111/1523-1747.ep12484041. [DOI] [PubMed] [Google Scholar]
- Mansbridge JN, Knapp AM, Strefling AM. Evidence for an alternative pathway of keratinocyte maturation in psoriasis from an antigen found in psoriatic but not normal epidermis. J Invest Dermatol. 1984;83:296–301. doi: 10.1111/1523-1747.ep12340429. [DOI] [PubMed] [Google Scholar]
- Marikovsky M, Breuing K, Liu PY, Eriksson E, Higashiyama S, Farber P, et al. Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc Natl Acad Sci U S A. 1993;90:3889–93. doi: 10.1073/pnas.90.9.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee JB, Johnson CM, Morar N, Burslem F, Groves RW. The psoriatic transcriptome closely resembles that induced by interleukin-1 in cultured keratinocytes: dominance of innate immune responses in psoriasis. Am J Pathol. 2007;171:32–42. doi: 10.2353/ajpath.2007.061067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minner F, Poumay Y. Candidate housekeeping genes require evaluation before their selection for studies of human epidermal keratinocytes. J Invest Dermatol. 2009;129:770–3. doi: 10.1038/jid.2008.247. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Sotozono C, Kinoshita S. The Epidermal Growth Factor Receptor (EGFR): Role in Corneal Wound Healing and Homeostasis. Experimental Eye Research. 2001;72:511–7. doi: 10.1006/exer.2000.0979. [DOI] [PubMed] [Google Scholar]
- Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–67. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Piepkorn M. Overexpression of amphiregulin, a major autocrine growth factor for cultured human keratinocytes, in hyperproliferative skin diseases. Am J Dermatopathol. 1996;18:165–71. doi: 10.1097/00000372-199604000-00010. [DOI] [PubMed] [Google Scholar]
- Piepkorn M, Predd H, Underwood R, Cook P. Proliferation-differentiation relationships in the expression of heparin-binding epidermal growth factor-related factors and erbB receptors by normal and psoriatic human keratinocytes. Arch Dermatol Res. 2003;295:93–101. doi: 10.1007/s00403-003-0391-x. [DOI] [PubMed] [Google Scholar]
- Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–67. [PMC free article] [PubMed] [Google Scholar]
- Repertinger SK, Campagnaro E, Fuhrman J, El-Abaseri T, Yuspa SH, Hansen LA. EGFR Enhances Early Healing After Cutaneous Incisional Wounding. J Investig Dermatol. 2004;123:982–9. doi: 10.1111/j.0022-202X.2004.23478.x. [DOI] [PubMed] [Google Scholar]
- Rittie L, Kansra S, Stoll SW, Li Y, Gudjonsson JE, Shao Y, et al. Differential ErbB1 signaling in squamous cell versus basal cell carcinoma of the skin. Am J Pathol. 2007;170:2089–99. doi: 10.2353/ajpath.2007.060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll SW, Elder JT. Retinoid regulation of heparin-binding EGF-like growth factor gene expression in human keratinocytes and skin. Exp Dermatol. 1998;7:391–7. doi: 10.1111/j.1600-0625.1998.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Stoll SW, Johnson JL, Bhasin A, Johnston A, Gudjonsson JE, Rittie L, et al. Metalloproteinase-Mediated, Context-Dependent Function of Amphiregulin and HB-EGF in Human Keratinocytes and Skin. J Invest Dermatol. 2010;130:295–304. doi: 10.1038/jid.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll SW, Kansra S, Peshick S, Fry DW, Leopold WR, Wiesen JF, et al. Differential utilization and localization of ErbB receptor tyrosine kinases in skin compared to normal and malignant keratinocytes. Neoplasia. 2001;3:339–50. doi: 10.1038/sj.neo.7900170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Kang S, Stoll S, Elder JT. Human psoriatic skin in organ culture: comparison with normal skin exposed to exogenous growth factors and effects of an antibody to the EGF receptor. Pathobiology. 1998;66:253–9. doi: 10.1159/000028031. [DOI] [PubMed] [Google Scholar]
- Wan YS, Wang ZQ, Voorhees J, Fisher G. EGF receptor crosstalks with cytokine receptors leading to the activation of c-Jun kinase in response to UV irradiation in human keratinocytes. Cell Signal. 2001;13:139–44. doi: 10.1016/s0898-6568(00)00146-7. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Gallo RL. Antimicrobial peptides in human skin disease. Eur J Dermatol. 2008;18:11–21. doi: 10.1684/ejd.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Kanno H, Watabe D, Akasaka T, Sawai T. The role of heparin-binding EGF-like growth factor and amphiregulin in the epidermal proliferation of psoriasis in cooperation with TNFalpha. Arch Dermatol Res. 2008;300:37–45. doi: 10.1007/s00403-007-0809-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.