Abstract
The major cellular antioxidant glutathione is depleted during HIV infection and in obesity. While the consequence of glutathione depletion on immune function is starting to emerge, it is currently not known whether glutathione dysregulation influences the differentiation and maturation of dendritic cells (DCs). Moreover, the effect of glutathione depletion on DC effector functions such as antigen presentation is poorly understood. Glutathione synthesis depends on the cystine/glutamate antiporter, which transports the rate-limiting precursor cystine into the cell in exchange for glutamate. Here we present a detailed study of antiporter function in DCs and demonstrate a role for the antiporter in DC differentiation and cross-presentation. We show that the antiporter is the major mechanism for transport of cystine and glutamate and modulates the intracellular glutathione content and glutathione efflux from DCs. Blocking antiporter-dependent cystine transport decreases intracellular glutathione levels and these effects correlate with reduced transcription of the functional subunit of the antiporter. We further demonstrate that blocking antiporter activity interferes with DC differentiation from monocyte precursors, but antiporter activity is not required for LPS-induced phenotypic maturation. Finally we show that inhibiting antiporter uptake of cystine interferes with presentation of exogenous antigen to class II MHC-restricted T cells and blocks cross-presentation on MHC class I. We conclude that aberrant antiporter function disrupts glutathione homeostasis in DCs and may contribute to impaired immunity in the diseased host.
Introduction
Glutathione is the most prevalent low molecular weight thiol in mammalian cells and the major determinant of cellular redox state (1–4). Glutathione plays a critical role in maintaining dendritic cell (DC) redox homeostasis and protecting DCs from oxidative stress, a state in which oxidants outnumber antioxidant defenses (5–11). The de novo synthesis of glutathione is regulated via the cystine/glutamate antiporter which transports cysteine into the cell in its oxidized form in exchange for glutamate (12). Inside the cell cystine is readily reduced to cysteine and enters the glutathione biosynthetic pathway (12–17). The cystine/glutamate antiporter, also termed system xc-, is a heterodimer composed of xCT and CD98. The xCT light chain confers the specificity of amino acid transport while the ubiquitously expressed CD98 heavy chain is common to other amino acid transport systems and is required for membrane expression of xCT (18, 19). In vitro, the antiporter also functions as a glutamate/glutamate exchanger to transport glutamate into the cell in exchange for the efflux of cellular glutamate. Glutamate can also be transported via the excitatory amino acid transporters (EAATs) and the two systems may be distinguished on the basis of their ionic requirements for transport. The cystine/glutamate antiporter is a chloride-dependent, sodium-independent transporter (20) while the EAATs are sodium-dependent transporters (21). The function of the cystine/glutamate antiporter has been well described in neutrophils, monocytes and macrophages, however its activity has not been rigorously characterized in DCs (22–24).
Currently, little is known about how glutathione homeostasis is maintained in DCs and the effect of glutathione dysregulation on DC phenotype and function remains to be completely defined. DCs are highly specialized in their ability to process and present exogenous antigen to CD4+ T helper cells and endogenous antigen to CD8+ T cytotoxic cells. DCs also present exogenous antigen in the context of MHC class I to CD8+ T cells, a process termed cross-presentation (25–27). Cross-presentation plays a pivotal role in generating CD8+ T cell responses against soluble, cell-associated or pathogen-derived antigens that are not endogenously expressed by DCs (28). Among antigen-presenting cells DCs are the only cells proficient at cross-priming (29). To present antigen, DCs must undergo functional maturation whereby the cell surface levels of MHC class II and co-stimulatory molecules required for T cell activation are increased and chemokine receptors that promote DC migration to lymph nodes are expressed (30, 31). The transition of an immature DC to the mature form is vital as only mature DCs can activate T cells; DCs arrested in an immature or semi-mature state induce T cell anergy resulting in the development of tolerance (32–34). Whether the cystine/glutamate antiporter, by modulating the intracellular glutathione concentration, regulates DC maturation and antigen presentation has not been explored. In addition, nothing is known about whether glutathione regulates DC differentiation from monocyte precursors.
The goal of this study was to analyze antiporter function in DCs in order to provide critical insight into how the disruption of glutathione homeostasis may impact DC function. Our results highlight an important role for the cystine/glutamate antiporter in DC differentiation and antigen presentation. Disturbances in glutathione homeostasis are implicated in the etiology and/or progression of several human diseases including cancer and inflammatory, immune, metabolic and neurodegenerative diseases (35). Thus, this study has relevance for understanding how glutathione depletion affects DC function in disease.
Materials and Methods
Materials
Escherichia coli 026:B6 lipopolysaccharide (LPS; γ-irradiated; total impurities <5% protein), FITC-dextran (40,000 Da), L-homocysteic acid (LHC), DL-threo-β-hydroxyaspartic acid (THA), dimethyl amiloride (DMA) and FITC-dextran were from Sigma (St. Louis, MI, USA). Recombinant human IL-4 was from R&D Systems (Minneapolis, MN, USA). Recombinant human GM-CSF (Leukine) was from Berlex Laboratories, Inc. (Montville, NJ, USA). RPMI 1640, FBS, penicillin, streptomycin sulfate and amphotericin B were from Invitrogen (Carlsbad, CA, USA). Cystine/cysteine-free medium was from MP Biomedicals (Solon, OH, USA). DL-threo-β-benzyloxyaspartate (TBOA) was from Tocris Bioscience (Ellisville, MO, USA). The fluorophore-conjugated isotype control antibody and mouse monoclonal antibodies to detect CD80, CD83, CD86, HLA-DR, HLA-ABC, CD16, CD1a, CD14, CD62L, CD11c and DC-SIGN were from BD Pharmingen (San Jose, CA, USA). Fluorophore-conjugated antibodies to detect murine CD11c, CD4, CD8, Vα2 and Vβ5 were from BD Pharmingen.
Human monocyte-derived dendritic cells
Human peripheral blood mononuclear cells (PBMCs) were isolated from normal human buffy coats (purchased from the Blood Donation Center of Louisiana) by centrifugation on Ficoll-Paque (GE Healthcare, Uppsala, Sweden). Monocytes were purified from PBMCs by positive selection using immunomagnetic cell separation (Human CD14 Microbeads, Miltenyi Biotec, Auburn, CA, USA). To derive DCs, monocytes (106 cells per ml) were cultured in complete medium (RPMI 1640 supplemented with 10% heat-inactivated FBS, 10U/ml penicillin, 10μg/ml streptomycin sulfate and 25ng/ml amphotericin B) containing IL-4 (10ng/ml; 290 U/ml) and GM-CSF (100ng/ml; 560 U/ml) for 4 days in a humidified atmosphere at 37°C with 5% CO2. Medium containing fresh cytokines was replenished every other day during culture. DC preparations routinely contained negligible quantities of CD3+ T cells (0.54±0.51%) (Ave±StDev) and CD19+ B cells (1.44±1.71%) (Ave±StDev). The average percentage of granulocytes (eosinophils and neutrophils) in DC preparations from three separate buffy coats was 10±4% (Ave±StDev) and the average percentage of DCs was 92±3% (Ave±StDev) as determined by Wright stain of CytoSpins (Diff-Quik, Baxter Scientific, Deerfield, IL, USA). To induce maturation, DCs were incubated for 24 h with LPS (1μg/ml). In all experiments, the viability of DCs following treatment with compounds (LPS, LHC, THA, TBOA, DMA, choline, acetate and cystine/cysteine-free medium) was verified by trypan blue exclusion. DC viability was not compromised in any of our experimental settings. The Institutional Review Boards of LSU Health Sciences Center and the Children’s Hospital of New Orleans have approved these studies.
Animals
Mice were used in accordance with NIH guidelines in experiments approved by Children’s Hospital, Boston and The Research Institute for Children’s Institutional Animal Care and Use Committees. C57BL/6, OT-I and OT-II mice were obtained from Charles River Laboratories (Wilmington, MA, USA). Mice were housed in a specific pathogen-free barrier facility and kept on sterile bedding with unrestricted access to autoclaved water and standard lab chow.
Isolation of splenic DCs
Spleens from C57BL/6 mice were minced and digested with collagenase (Sigma) and DNase I (Invitrogen) at 37°C in RPMI supplemented with 10% FBS, 100U/ml penicillin and 100μg/ml streptomycin. Cells were washed and resuspended in PBS containing 2% FBS and 2mM EDTA and undigested material was removed by filtration through a 70μm cell strainer. CD11c+ DCs were purified using anti-CD11c microbeads (Miltenyi Biotec) according to manufacturer's instructions. DCs were stained for CD11c and purity was assessed by flow cytometry. DCs were cultured in RPMI supplemented with 10% FBS, 100U/ml penicillin and 100μg/ml streptomycin.
Preparation of OT-I and OT-II cells
The spleen, inguinal lymph nodes and mesenteric lymph nodes from OT-I and OT-II mice were removed, resuspended in PBS supplemented with 2% FBS and 2mM EDTA and pressed through a wire mesh to obtain a single cell suspension. The cells were washed and red blood cells were lysed in Red Blood Cell Lysis Solution (Sigma). To determine the percentage of OT-I and OT-II cells in the preparations, cells were stained for CD4, CD8, Vα2 and Vβ5 and analyzed on a flow cytometer as detailed below. The percentage of OT-I (Vβ5+Vα2+CD8+) and OT-II (Vβ5+Vα2+CD4+) positive T cells was typically ~27% and ~7.5%, respectively.
Flow cytometry
Human DCs were resuspended at 106 cells per ml of flow buffer (PBS containing 0.5% BSA) and incubated with FITC-conjugated primary antibodies for 30 min at 4°C. Cells were then fixed with 4% paraformaldehyde for 15 min at 4°C, washed twice with flow buffer, resuspended in 2% paraformaldehyde and analyzed using a Becton Dickinson LSRII flow cytometer (Franklin Lakes, NJ, USA). Data analysis was performed using FlowJO software (TreeStar Inc., Ashlan, OR, USA). Murine DCs and cells purified from the spleen, inguinal lymph nodes and mesenteric lymph nodes of OT-I and OT-II mice were incubated for 5 min on ice with Fc block (BD Biosciences) in FACS buffer (Invitrogen) supplemented with 5% FCS. DCs were incubated with antibodies for 15 min on ice, washed and examined directly by flow cytometry on a FACS Canto II flow cytometer (BD Biosciences). Data were collected using FACS Diva software (BD Biosciences) and analyzed with FlowJo software. Gates were selected based on the staining pattern of DCs incubated with isotype control antibodies. 10,000 events were collected in each experiment.
Radiolabeled amino acid transport assays
The activity of the cystine/glutamate antiporter was measured as previously described with minor modifications (23). Briefly, DCs were equilibrated in transport medium (137mM NaCl, 0.7mM K2HPO4, 1mM CaCl2, 1mM MgCl2, 5mM glucose, 10mM HEPES, pH 7.4) at 37°C or 4°C in 96-well plates. Then, L-[3H] glutamate (18.5μCi/ml; PerkinElmer, Waltham, MA) or L-[14C] cystine (1.25μCi/ml; PerkinElmer) was added to cells at a final concentration of 50, 100 or 200μM and the cells were incubated at 37°C for 5 min. Amino acid transport was stopped by transferring 96-well plates to an ice water bath. DCs were then washed two times with ice-cold transport medium and lysed in 100mM NaOH. Radioactivity in lysates was measured by liquid scintillation counting and normalized to the quantity of protein in lysates as determined using the Pierce BCA (bicinchoninic acid) Protein Assay kit (Pierce, Rockford, IL, USA). To assess ionic dependence, assays were performed in transport medium containing choline in lieu of sodium, or acetate in lieu of chloride. To block the activity of the cystine/glutamate antiporter, DCs were incubated with LHC, THA or TBOA for 30 min at 37°C before the addition of the radiolabeled amino acid. The DC culture media, wash buffers and amino acid transport media were entirely free of reducing agents.
Measurement of GSH and GSSG by high performance liquid chromatography
GSH and GSSG were quantified in DC lysates and DC-conditioned medium by HPLC using an already established technique (36–38). DCs were incubated in complete medium in the presence or absence of LHC (2.5mM) or incubated in cystine/cysteine-free medium for 24 h. Cell viability was confirmed using Invitrogen’s LIVE/DEAD Cell Assay. None of the treatments resulted in significant cell death (non-treated immature DCs compared with LHC-treated immature DCs p= 0.24; non-treated immature DCs compared with immature DCs incubated in cystine/cysteine-free medium p= 0.88; non-treated mature DCs compared with LHC-treated mature DCs p= 0.76; non-treated mature DCs compared with mature DCs incubated in cystine/cysteine-free medium p= 0.43). At no time were reducing agents present in the medium. DCs were lysed in 5% trichloroacetic acid and centrifuged. Thiols in the acid supernatant were treated with iodoacetic acid and derivatized with 1-fluoro-2, 4-dinitrobenzene. Oxidized (GSSG) and reduced (GSH) glutathione were detected by reverse phase ion exchange HPLC on a 250 × 4.6-mm Waters Spherisorb 10-μM NH2 column (Waters Corp., Milford, MA, USA). Proteins captured in the acid pellet were solubilized in 0.1M NaOH and protein was determined using the Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA). The GSH and GSSG concentrations were determined by comparison with purified GSH and GSSG standards derivatized in the same manner.
mRNA measurements with nCounter
The NanoString nCounter gene expression system (NanoString Technologies Inc., Seattle, WA, USA) was used to quantify individual mRNA transcripts using an approach similar to that described by Geiss et al. (39). Immature DCs were treated with or without LPS (1μg/ml) for 4 h and then incubated incomplete medium or in cystine/cysteine-free medium in the continued presence of LPS for 6 or 16 h. These treatments did not result in significant cell death (LPS-treated DCs incubated in complete medium compared with LPS-treated DCs incubate in cystine/cysteine-free medium p= 0.58). Then, 50,000 DCs per condition were lysed in RLT buffer (Qiagen, Valencia, CA, USA) supplemented with β-mercaptoethanol (Sigma). 10% of the lysates was hybridized for 6 or 16 h with the CodeSet and loaded into the nCounter prep station followed by quantification using the nCounter Digital Analyzer. The nCounter data was normalized in two steps. First, we used the positive spiked-in controls provided by the nCounter instrument as per the manufacturer’s instructions. Second, we normalized copy numbers to the housekeeping genes GAPDH and HPRT1.
RT-PCR
RNA was extracted from DCs using RNAqueous-4PCR kit (Ambion, Austin, TX) according to the manufacturer’s instruction. RNA was treated with RNase-free DNase (Ambion) to remove DNA and the RNA (1 μg) was reverse transcribed to cDNA using SuperScript III First-Strand Synthesis SuperMix (Invitrogen) according to the manufacturer’s instruction. Then, xCT, EAAT1, EAAT2 and G3PDH cDNA were amplified by PCR using the Platinum PCR SuperMix (Invitrogen). PCR conditions were as follows: cDNA was denatured at 95°C for 1 min, followed by 35 cycles of amplification at 95°C for 15 seconds, 55°C for 30 seconds and 68°C for 1 min. Final products were then incubated at 72°C for 7 min. The EAAT1, EAAT2 and xCT primers used were described previously (40, 41). The G3PDH primers used were as follows: forward primer 5’-GGA AAT CCC ATC ACC ATC TTC CAG-3’ and reverse primer 5’-GTC ATA CCA GGA AAT GAG CTT GAC-3’. Amplification products were resolved by electrophoresis on a 1.5% agarose gel.
T cell proliferation assays
Splenic DCs (4 × 105) were cultured for 2 h or overnight (16 h) with LHC (10mM) or in cystine/cysteine-free medium. Following the incubation, DCs were seeded into round-bottom 96-well plates with 0–1,000μg chicken egg ovalbumin (OVA) (Sigma) for 3 h in the presence or absence of LHC (10mM). Then, DCs were irradiated with 3,000 rad, washed and incubated with OT-I or OT-II T cells (4 × 105) in RPMI supplemented with 10% FBS, 100U/ml penicillin, 100μg/ml streptomycin, 1% glutamine, 10mM HEPES and 55μM β-mercaptoethanol. After 3 days in culture cells were spiked with 1μCi [3H] thymidine (PerkinElmer) and incubated for 16 h after which T cell proliferation was measured by liquid scintillation counting. To examine T cell proliferation by CFSE dilution, splenic DCs were cultured in medium or in medium containing LHC (10mM) for 2 or 16 h. Then, DCs were pulsed with OVA (500μg) for 3 h. OT-I T cells were labeled with CFSE (Invitrogen) and washed with RPMI. DCs were then washed in PBS and cultured with CFSE-labeled OT-I cells at DC-to-T cell ratio of 1:1 and 1:3 in triplicate. Following 3 days of culture, CFSE dilution of CD8α+ T cells was examined by flow cytometry.
Granzyme B and IFN-γ ELISA
Granzyme B and IFN-γ in culture supernatants were analyzed using the Ready SET Go ELISA kit (eBioscience Inc., San Diego, CA, USA) according to the manufacturer's instructions.
Endocytosis assay
Endocytosis was measured as the cellular uptake of FITC–dextran and quantified by flow cytometry. DCs (2×105 cells per sample) were treated with or without LHC (10mM) for 2 or 24 h, washed and incubated with FITC-dextran (1mg/ml) in the presence or absence of LHC for 30, 60 and 90 mins at 37°C or 4°C. After incubation, cells were washed three times with complete medium to remove excess dextran and the uptake of FITC–dextran was determined by flow cytometry. 10,000 cells per sample were analyzed.
Statistical analysis
For the amino acid transport assays, cell viability assays, T cell proliferation, endocytosis assay, ELISA and the quantification of GSH and GSSG, differences between the means of experimental groups were analyzed by single factor ANOVA. NS, not statistically significant (p>0.05); a statistical difference between groups is denoted by * (*, p<0.05; **, p<0.01); a statistical significance between 37°C and 4°C controls, or between groups as they relate to the non-treated control is denoted by # (#, p<0.05; ##, p<0.01). The two-tailed t-test was used to analyze statistical significances between treatment groups in the digital RNA profiling studies and in studies comparing cell surface expressed molecules by flow cytometry. Evaluation of donor data was performed with the Mann-Whitney test using GraphPad Prism software (La Jolla, CA, USA).
Results
DC maturation is associated with increased transport of cystine and glutamate
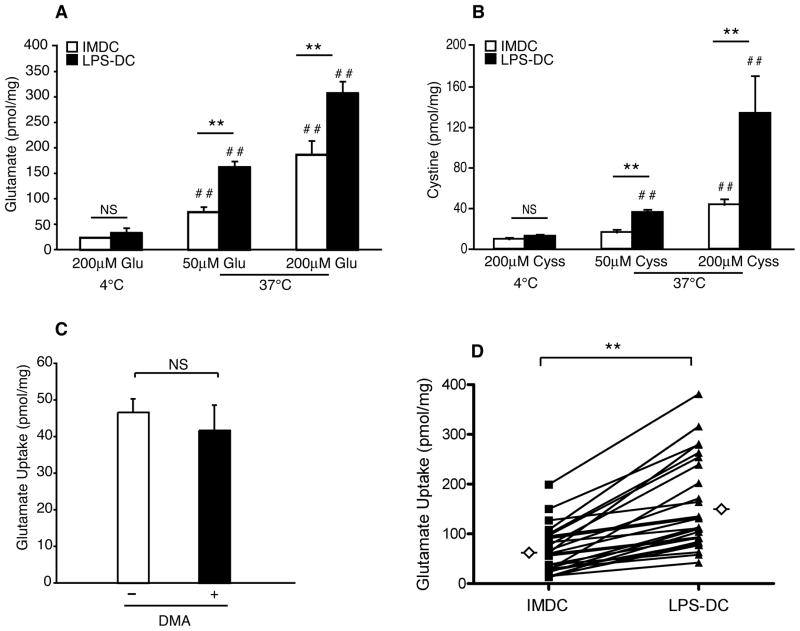
To elucidate a role for the cystine/glutamate antiporter in DC maturation and function, we used radiolabeled amino acid transport assays to compare the transport of glutamate and cystine in immature and mature human monocyte-derived DCs. Both immature DCs and DCs matured with LPS (hereafter referred to as mature DCs) internalized glutamate and cystine in a dose-dependent manner (Figure 1, A and B). Glutamate and cystine transport was significantly greater in mature DCs as compared with immature DCs, and transport did not occur via pinocytosis as transport was blocked at 4°C (Figure 1, A and B). Glutamate uptake also did not depend on the activity of the Na+/H+ exchanger, as dimethylamiloride (DMA), an inhibitor of pinocytosis, did not block the uptake of glutamate by immature DCs (Figure 1C). We next analyzed glutamate transport in DCs from 23 different donors. The data show that glutamate transport by mature DCs was significantly greater than by immature DCs (Figure 1D). Statistical evaluation of DCs from 10 male (aged 33±11; Ave±StDev) and 6 female (aged 36±19; Ave±StDev) donors revealed that in this set of donors, age and biological sex had no significant effect on glutamate transport (data not shown). However, there was a trend for greater glutamate transport in immature and mature DCs from male donors than in DCs from female donors, and slightly higher levels of transport were observed in older donors than in younger donors.
FIGURE 1.
Human DC maturation is associated with increased transport of cystine and glutamate. A and B, Immature (IMDC) and mature (LPS-DC) DCs were incubated for 5 min at 37°C or 4°C in medium containing [3H] glutamate or [14C] cystine. The quantity of glutamate or cystine transported into the cell is expressed in picomoles per milligram of cellular protein. Data are mean values ± SEM of triplicate samples from three independent experiments (i.e. three different donors). C, Immature DCs were treated for 30 min with 1mM dimethyl amiloride (DMA), washed and then incubated for 5 min at 37°C in uptake medium containing [3H] glutamate. Data are mean values ± SEM of triplicate samples from two independent experiments. D, IMDC and LPS-DC were incubated for 5 min at 37°C in medium containing [3H] glutamate. The quantity of glutamate transported into the cell is expressed in picomoles per milligram of cellular protein. Data are mean values ± SEM of triplicate samples using DCs prepared from 23 different donors. The means are indicated by open diamonds. Data are displayed as paired data points.
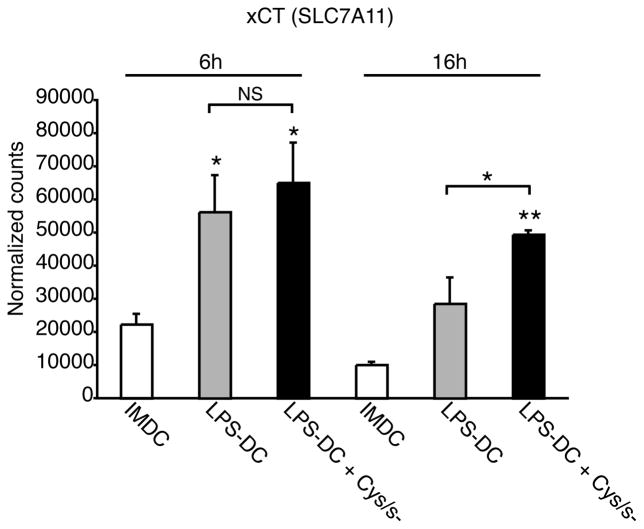
Our results show that the activity of the cystine/glutamate antiporter is greater in mature DCs than in immature DCs. To explain this result, we examined whether DC maturation correlated with an increase in transcription of the functional subunit of the antiporter, xCT. Immature DCs were incubated with or without LPS for 4 h and then cultured for 6 or 16 h in complete medium. RNA transcripts encoding xCT were then measured by digital mRNA profiling. LPS induced an increase in xCT mRNA transcripts at both time points. We then examined whether blocking antiporter activity could affect xCT mRNA expression levels. When DCs were incubated with LPS for 4 h and then cultured in cystine/cysteine-free medium for 6 or 16 h, we found that there was an increase in the level of xCT mRNA when compared with LPS-treated DCs incubated in complete medium (Figure 2). These data show that transcription of xCT is increased both during DC maturation and when the activity of the antiporter is inhibited.
FIGURE 2.
The functional subunit of the cystine/glutamate antiporter is transcriptionally regulated during human DC maturation. Immature DCs were incubated with or without LPS for 4 h and then cultured in complete medium or in cystine/cysteine-free medium for 6 or 16 h. RNA transcripts encoding xCT were quantified by digital mRNA profiling. Data are mean values ± SEM of duplicate samples from three independent experiments.
The cystine/glutamate antiporter is the major mechanism for glutamate and cystine transport in human DCs
To confirm that glutamate and cystine transport was dependent on cystine/glutamate antiporter activity, we compared glutamate and cystine transport in DCs treated with and without L-homocysteic acid (LHC). LHC is a potent competitive inhibitor of the antiporter that does not serve as a substrate for glutathione synthesis (42). Consistent with our previous results, glutamate and cystine transport was significantly greater in mature DCs than in immature DCs (Figure 3, A and B). LHC efficiently blocked both glutamate and cystine transport in both immature and mature DCs (Figure 3, A and B). Because the antiporter has similar affinities for cystine and glutamate (43), we next tested whether transport of cystine could be blocked in the presence of excess glutamate and vice versa. We found that excess cystine inhibited glutamate transport in immature and mature DCs (Figure 3A). The reciprocal experiment showed that cystine transport was inhibited in the presence of excess glutamate (Figure 3B).
FIGURE 3.
Functional characterization of the cystine/glutamate antiporter in human DCs. Immature (IMDC) and mature (LPS-DC) DCs were incubated in medium for 30 min at 37°C with or without LHC (10mM) or in medium containing excess glutamate (2.5mM) or cystine (1mM) and then examined for transport of [3H] glutamate (200μM) (A) or [14C] cystine (200μM) (B). Data are mean values ± SEM of triplicate samples from two independent experiments.
Glutamate transport is also a function of the excitatory amino acid transporters (EAATs). To determine whether the cystine/glutamate antiporter or EAATs mediate the transport of glutamate in DCs, we examined DCs for the presence of EAAT1 and EAAT2 mRNA by RT-PCR. Both immature and mature DCs expressed xCT mRNA as well as transcripts for EAAT1 and EAAT2 (data not shown). To determine whether EAATs participated in glutamate transport in DCs, we measured transport in the presence or absence of sodium or chloride ions. We found that transport of glutamate in immature and mature DCs was chloride-dependent and sodium-independent (Supplemental figure 1A and B). This pattern of ionic dependence is a hallmark of the cystine/glutamate antiporter (42) and argues against a major role for the sodium-dependent EAATs in the transport of glutamate in DCs. To confirm this, we treated DCs with DL-threo-β-hydroxyaspartic acid (THA), a competitive antagonist transported in the place of glutamate (40), or DL-threo-β-benzyloxyaspartate (TBOA), a non-transportable inhibitor specific for EAATs (44). In contrast to the block in glutamate transport when DCs were treated with LHC, THA and TBOA had no effect on glutamate transport in immature and mature DCs (Supplemental Figure 2A and B). When THA or TBOA was used in combination with LHC, there was no additional inhibition in glutamate transport when compared with the effect of LHC alone (Supplemental Figure 2A and B). These data show that the cystine/glutamate antiporter is the predominant mechanism for glutamate transport in DCs.
The cystine/glutamate antiporter regulates DC glutathione homeostasis
Next, we tested whether the cystine/glutamate antiporter could regulate intracellular glutathione levels in DCs. We first quantified reduced (GSH) and oxidized (GSSG) glutathione in DC lysates by HPLC. Immature and mature DCs contained similar levels of GSH and GSSG (Figure 4A and B) and thus the ratio of GSH-to-GSSG was also comparable between immature and mature DCs (Figure 4C). Next, we treated immature and mature DCs with LHC (2.5mM) for 24 h and examined the cellular GSH and GSSG content. Treatment with LHC did not affect DC viability when compared with non-treated DCs. LHC decreased the cellular GSH content by 24% in immature DCs and 36% in mature DCs (Figure 4A). In contrast, LHC had no effect on immature or mature DC GSSG levels (Figure 4B). The decline in cellular GSH with unchanged GSSG resulted in a decrease in the GSH-to-GSSG ratio in both immature and mature DCs treated with LHC (Figure 4C). Similarly, when DCs were incubated in cystine/cysteine-free medium, GSH levels were reduced by 47% in immature DCs and by 49% in mature DCs (Figure 4A). As with LHC, cystine/cysteine-free medium had no effect on DC GSSG levels (Figure 4B) and thus decreased the GSH-to-GSSG ratio (Figure 4C). This set of results suggests that a major function of the cystine/glutamate antiporter in DCs is to provide the cell with cystine to support the de novo biosynthesis of GSH. These data also show that blocking antiporter activity in DCs decreased the ratio of cellular GSH-to-GSSG, a hallmark of oxidative stress.
FIGURE 4.
The cystine/glutamate antiporter regulates human DC glutathione homeostasis. A-C, Immature (IMDC) and mature (LPS-DC) DCs were cultured for 24 h in complete medium, LHC-containing medium (2.5mM), or cystine/cysteine-free medium. Cells were then lysed and GSH and GSSG were measured in lysates by HPLC. Data were normalized to total cellular protein. Reduced glutathione (A), oxidized glutathione (B) and the GSH-to-GSSG ratio (C) were plotted (note differences in the y-axis scales between A and B). Data represent single measurements from four independent experiments. D and E, Glutathione efflux was measured by culturing immature (IMDC) and mature (LPS-DC) DCs for 24 h in complete medium, medium containing LHC (2.5mM) or cystine/cysteine-free medium. Cells were then removed by centrifugation and GSH and GSSG in the medium was measured by HPLC. Data were normalized to total cellular protein. Data are plotted as GSH or GSSG in nmol per mg of protein (D) and as GSH content relative to the non-treated immature and mature DCs (=100%) (E). Data represent single measurements from four independent experiments.
The cystine/glutamate antiporter controls the availability of glutathione for efflux from DCs
We next examined whether the cystine/glutamate antiporter regulates GSH efflux from DCs. In the extracellular compartment, GSH scavenges extracellular reactive oxygen species (ROS), facilitates cysteine transport by neighboring cells and reduces oxidized protein sulfhydryls (45, 46). We first measured GSH release from immature and mature DCs cultured in medium for 24 h. We also measured GSSG in the medium to determine whether GSH was oxidized following export from the cell. Immature and mature DCs exported similar quantities of GSH (Figure 4D). The GSH detected in the medium was exported from DCs and not already present in the culture medium because only low levels of GSH were detected in fresh, complete medium (~12pM GSH) and in cystine/cysteine-free medium (~6pM GSH). In contrast to GSH, only small quantities of GSSG were present in the medium of immature and mature DCs (Figure 4D).
To examine a role for the antiporter in the regulation of GSH efflux, DCs were incubated with LHC or in cystine/cysteine-free medium for 24 h after which GSH and GSSG were measured in the medium. LHC reduced GSH efflux from both immature and mature DCs by ~35% (Figure 4C and D). In contrast, LHC had no effect on GSSG concentrations in the medium of immature and mature DCs (Figure 4D). Incubation of DCs in cystine/cysteine-free medium also reduced GSH efflux from immature DCs by ~80% and from mature DC by ~82% (Figure 4D and E). Further, the incubation of DCs in cystine/cysteine-free medium had no effect on the GSSG levels in the medium of immature and mature DCs (Figure 4D). These data demonstrate that the cystine/glutamate antiporter controls the intracellular concentration of GSH and thus indirectly regulates GSH efflux from the cell by controlling the availability of GSH for export.
The cystine/glutamate antiporter regulates DC differentiation from monocyte precursors
We next tested whether the cystine/glutamate antiporter could regulate DC differentation from peripheral blood monocytes that, like DCs, express a functional cystine/glutamate antiporter (47). Monocytes were cultured with or without LHC for 5 days in the presence of IL-4 and GM-CSF to induce differentiation into immature DCs (LHC treatment did not induce significant cell death when compared with non-treated cells, p= 0.64). Then, DC expression of MHC class I and II, co-stimulatory molecules (CD80 and CD86), the activation marker CD83 and lineage markers were quantified by flow cytometry. LHC treatment significantly reduced the up-regulation of MHC class I and II, CD80 and DC-SIGN (Figure 5 and Supplemental figure 3). In contrast, the expression of the other markers remained the same as that observed for immature DCs. In control experiments, LHC had no effect on the staining pattern of the isotype control antibodies (data not shown). Taken together, these data suggest that the antiporter plays an important role in the development of DCs from monocytes.
FIGURE 5.
The cystine/glutamate antiporter regulates human DC development from monocyte precursors. Monocytes were stained for markers following culture for five days with IL-4 and GM-CSF in the presence or absence of LHC (10mM). The MFI (log scale) is plotted on the y-axis. Data are mean values ± SEM of triplicate samples from four independent experiments.
The cystine/glutamate antiporter does not regulate the phenotypic maturation of DCs
To determine whether the antiporter could regulate LPS-induced DC maturation, immature DCs were treated with or without LHC for 24 h and then incubated with or without LPS in the presence or absence of LHC for an additional 24 h after which the cell surface expression of MHC class I and II, CD80, CD83 and CD86 was measured by flow cytometry. Consistent with the ability of LPS to induce DC maturation, LPS increased the surface expression of MHC class I and II, CD80, CD83 and CD86 relative to that observed in immature DCs (Figure 6, compare white bars with black bars). When immature DCs were cultured for 24 h with LHC and then treated with LPS for an additional 24 h, we found that there was no change in the cell surface expression of MHC class II, CD80, CD83 or CD86 when compared with DCs treated with LPS alone (Figure 6, compare dark grey bars with black bars). However, we did observe a change in MHC class I; LHC treatment decreased the level of MHC class I on the DC cell surface. LHC also had no effect on the phenotype of immature DCs (Figure 6, compare white bars with light grey bars). Taken together, this set of data shows that the cystine/glutamate antiporter does not significantly regulate the phenotypic maturation of DCs.
FIGURE 6.
The activity of the cystine/glutamate antiporter does not regulate the phenotypic maturation of human DCs. Immature DCs were incubated with or without LPS for 24 h and then cultured with or without LHC (10mM) an additional 24 h. The cell surface expression of MHC class I, MHC class II, CD80, CD83 and CD86 was monitored by flow cytometry. The MFI (log scale) is plotted on the y-axis. Data are mean values ± SEM of triplicate samples from three independent experiments.
The cystine/glutamate antiporter does not interfere with DC uptake of antigen
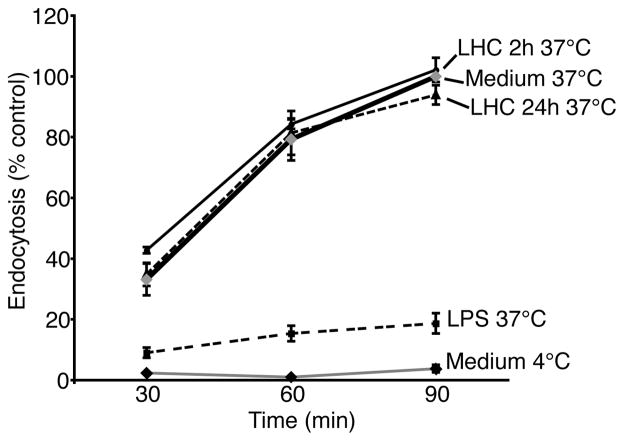
We next tested whether the cystine/glutamate antiporter could influence DC endocytosis as a parameter of functional maturation. DCs were treated for 2 or 24 h with LHC (2.5mM) and then incubated with FITC-dextran for 30, 60 and 90 min at 37°C after which FITC-dextran uptake was quantified by flow cytometry. Non-treated immature DCs actively took up FITC-dextran at each time point and uptake was significantly reduced in the fully mature LPS-treated DCs (Figure 7). When normalized to the maximum uptake of dextran by non-treated immature DCs at the 90 min interval (= 100%), DCs treated with LHC for 2 or 24 h exhibited the same rate of dextran endocytosis as the non-treated immature DCs. These data indicate that the activity of the antiporter is not required for DC uptake of antigen.
FIGURE 7.
Blocking the activity of the cystine/glutamate antiporter in human DCs does not interfere with antigen uptake. Immature DCs were treated with or without LHC (10mM) for 2 or 24 h, washed and incubated with FITC-dextran in the presence or absence of LHC for 30, 60 and 90 mins. The data were normalized to the MFI of non-treated DC incubated with FITC-dextran for 90 mins (= 100%). Means ± SEM of triplicate measurements for each time point from two independent experiments are shown.
The cystine/glutamate antiporter regulates the presentation of exogenous antigen
To explore a role for the antiporter in antigen presentation, we examined murine DC presentation of OVA to T cells using the OT-I and OT-II system. T cells isolated from OT-I mice express a transgenic T cell receptor that recognizes chicken ovalbumin (OVA) presented in the context of MHC class I. OT-II T cell receptors recognize OVA presented via MHC class II. Murine splenic DCs were treated with LHC for 2 or 16 h and the presentation of exogenous OVA to OT-I and OT-II T cells was measured by 3H-thymidine incorporation. LHC potently reduced the ability of DCs to stimulate OT-I proliferation at both time points (Figure 8A). When DCs were treated with LHC for 2 h, OT-I proliferation was reduced by 67%, 58% and 91% relative to non-treated control DCs incubated with decreasing concentrations of OVA (1,000, 100 and 10μg) (Figure 8A). At the 16 h time point, LHC reduced OT-I proliferation by 81%, 77% and 48% relative to control DCs incubated with decreasing concentrations of OVA (Figure 8A). Blocking antiporter transport of cystine also interfered with DC presentation of OVA to OT-II cells although the effect was not as pronounced as that observed for the OT-I cells. When DCs were treated with LHC for 2 or 16 h and incubated with OVA (1,000μg), OT-II proliferation was reduced by 14% and 61%, respectively (Figure 8C). When DCs were pulsed with lower concentrations of OVA, LHC more potently inhibited OT-II proliferation (Figure 8C).
FIGURE 8.
The cystine/glutamate antiporter regulates murine splenic DC antigen presentation to T cells. Murine splenic DCs were incubated for 2 or 16 h in complete medium or in complete medium containing LHC (10mM) and then pulsed with OVA (0–1,000μg). DCs were cultured with OT-I or OT-II cells for 3 days after which co-cultures were spiked with 3H-thymidine and incubated overnight. T cell proliferation was measured by liquid scintillation counting (A and C) and granzyme B and IFN-γ were quantified in culture supernatants by ELISA (B and D). Data are from one experiment in which samples were analyzed in triplicate (nd= not detectable).
In addition to measuring T cell proliferation, we quantified the secretion of the CD8-specific effector molecule granzyme B from OT-I cells and IFN-γ from CD4+ OT-II cells by ELISA. Although granzyme B is a marker for CD8+ T cells, we cannot exclude the posibility that it or IFN-γ was derived from other cells in the cultures. Consistent with the inhibitory effect of LHC on the ability of DCs to stimulate OT-I and OT-II proliferation, blocking antiporter activity reduced the levels of granzyme B and IFN-γ in the co-cultures (Figure 8B and D). Granzyme B was reduced 55% and 89% when DCs were treated with LHC for 2 h and pulsed with 1,000μg or 100μg OVA, respectively. When DCs were treated with LHC for 16 h and pulsed with 1,000μg OVA, granzyme B production was reduced by 97% (Figure 8B). Granzyme B was not detectable when DCs were treated with LHC for 2 h and pulsed with the lowest concentration of OVA, or when DCs were treated for 16 h with LHC pulsed with 100 or 10μg OVA (Figure 8B). LHC also blocked IFN-γ production from OT-II cells although the effect was less pronounced than that observed for granzyme B. IFN-γ secretion was not affected when DCs were treated with LHC for 2 h and pulsed with the highest concentration of OVA, and IFN-γ production was reduced 75% when DCs were treated with LHC for 16 h and pulsed with 1,000μg OVA (Figure 8D). IFN-γ was not detectable when DCs were treated with LHC for 2 or 16 h and pulsed with 100μg or 10μg OVA (Figure 8D). These data show that the antiporter plays a fundamental role in regulating DC presentation of exogenous antigen to both class I and class II MHC-restricted T cells.
Finally, we confirmed these data in two separate experiments. In the first, we examined the effect of cystine/cysteine-free medium on murine splenic DC presentation of OVA to OT-I and OT-II T cells (Supplemental figure 4). As with LHC treatment, incubation of DCs in cystine/cysteine-free medium for 16 h reduced their ability to stimulate OT-I and OT-II T cell proliferation (Supplemental figure 4A and B, respectively). This block in T cell proliferation correlated with a decrease in T cell secretion of granzyme B and IFN-γ (Supplemental figure 4C and D, respectively). In the second experiment, we showed that LHC inhibited splenic DC presentation of OVA to CFSE-labeled OT-I T cells (Figure 9 and Supplemental figure 5). These data confirm that the antiporter plays a fundamental role in cross-presentation.
FIGURE 9.
The cystine/glutamate antiporter regulates DC cross-presentation. Splenic DCs were incubated in complete medium or in complete medium containing LHC (10mM) for 2 or 16 h and then pulsed with OVA (500μg). DCs were then cultured with CFSE-labeled OT-I cells at a ratio of 1:1 (A) or 1:3 (B) for 3 days after which T cell proliferation was measured by CFSE dilution by flow cytometry. Data are from one experiment in which samples were analyzed in triplicate.
Discussion
The goal of this study was to examine the function of the cystine/glutamate antiporter in DCs to discern a role for the antiporter in the regulation of DC differentiation, maturation and antigen presentation. We show that blocking antiporter transport of cystine had several important effects on DCs. Inhibiting antiporter activity reduced the quantity of intracellular glutathione available for export from DCs. The reduction in GSH efflux from DCs is an indirect effect of inhibiting the antiporter. To date, there is no evidence to support a role for the antiporter in GSH efflux from cells. Rather, it is likely that transporters such as CFTR may be involved. Glutathione export is important as subtle changes in the extracellular redox status are known to regulate signaling in the immune synapse and shape the outcome of T cell activation (48). For example, extracellular cysteine accumulation results in a lower redox potential, which promotes T cell proliferation and modifies the redox status of proteins on the T cell surface.
Our data also show that antiporter activity is increased during LPS-induced maturation. Thus, the antiporter may play a role in regulating DC redox homeostasis during the maturation process. Microbial antigens such as LPS increase ROS in DCs by stimulating the activity of the NADPH oxidase (49). This raises the possibility that increased antiporter activity may be critical for maintaining the intracellular glutathione balance necessary to adjust to the physiological changes that occur during DC maturation. Similarly, the antiporter may also function to maintain the intracellular redox status during DC antigen presentation in the lymph nodes, a microenvironment characterized by high rates of T and B cell proliferation, apoptosis, inflammation and oxidative stress (50–52).
We also show that the antiporter plays a role in regulating DC differentiation from monocyte precursors. Blocking antiporter activity in monocytes prevented their differentiation into inflammatory DCs in the presence of IL-4 and GM-CSF. The most pronounced effect of LHC was on the expression levels of the antigen-presenting molecules MHC class I and II and DC-SIGN, a C-type lectin receptor which participates in the activation of CD4+ T cells by mediating the transient adhesion of DCs to T cells (53). Whether this change in phenotype translates to an effect on DC function remains to be examined. In vivo, inflammatory DCs are generated from monocytes at inflammatory foci and function to induce robust Th1 immunity (54–56). Because inflammatory DCs have robust Th1-polarizing activity (54–56), the generation of fewer inflammatory DCs during infection may also significantly impair Th1 immunity in the setting of global glutathione depletion that characterizes the HIV infected host.
In contrast to the role the antiporter plays in DC differentiation, antiporter activity was not required for DC maturation. Although oxidative stress is an important feature of DC maturation, we found that blocking antiporter activity did not impair LPS-induced maturation. LHC and cystine/cysteine-free medium decreased intracellular glutathione levels in immature DCs by 24% and 47%, respectively, but this did not affect LPS-induced up-regulation of MHC class II, CD80, CD83 or CD86. Our results also show that the antiporter was the main mechanism for cystine and glutamate transport in human DCs and that the activity of the antiporter was increased during LPS-induced DC maturation. Although DC maturation correlated with an increase in antiporter activity, we did not observe a change in the GSH-to-GSSG ratio when mature DCs were incubated with LPS for 24 h. This result is in line with a previous report showing that GSH levels were the same in immature DCs and DCs matured in the presence of LPS and INF-γ for 48 h (57). It is possible that the accumulation of reactive oxygen species following LPS exposure induced a rapid and early depletion of intracellular GSH and that at later time points GSH homeostasis was restored by the action of the antiporter. Consistent with this, others have shown that a decrease in the GSH-to-GSSG ratio in DCs occurs as early as 4 h of incubation with LPS (58).
Finally, our data show that the antiporter played a central role in exogenous antigen presentation to both class I and class II MHC-restricted T cells. While previous studies have examined the effect of glutathione depleting agents on murine DC antigen presentation, this study is the first to directly examine the effect of glutathione depletion on cross-presentation. In a previous report, for example, splenic antigen-presenting cells (APCs) were treated with the thiol-alkylating agent diethyl maleate (DEM) which forms irreversible adducts with glutathione. APCs were treated with DEM, washed and incubated with T cells in the presence of OVA for 72 h (59). While glutathione depletion had no effect on the ability of the APCs to stimulate T cell proliferation, it interfered with T cell production of IFN-γ following antigen presentation. In that study, incubation of APCs with T cells and antigen in the absence of DEM would likely allow the intracellular glutathione levels in the APCs to normalize during the 72 h in co-culture. This is in contrast to our study in which we observed a defect in both T cell proliferation and IFN-γ production. In our assay, DCs were pre-treated with LHC and incubated with OVA for 2 or 16 h in the continued presence of LHC before they were irradiated, washed and incubated with T cells. This eliminated the possibility that glutathione levels could be replenished in the DCs before they were incubated with antigen and T cells. Thus, we were able to demonstrate that glutathione depletion interfered with both exogenous antigen presentation to CD4+ T cells and cross-presentation to CD8+ T cells. Finally, in contrast to the clear defect in cross-presentation, we observed that OVA presentation on MHC class II molecules was relatively intact in DCs treated with LHC and pulsed with high concentrations of OVA. This suggests that blocking antiporter activity had little effect on antigen processing. The contribution of cystinosin, a lysosomal transmembrane protein involved in cystine export from the lysosomal compartment, was not examined in these studies. However, the studies presented here as well as studies in human macrophages, which express both the cystine/glutamate antiporter and EAATs, make clear that the intracellular GSH level is controlled by the activity of transporters for cystine, glutamate, and glutamine (60). Elucidating the precise molecular mechanisms by which the antiporter controls antigen presentation in DCs will be an important area of future investigation.
In summary, the results of this study have significance for understanding how impaired DC function may contribute to the pathogenesis of type 2 diabetes and obesity, as well as other diseases in which a perturbation of glutathione homeostasis plays a known role (8, 61–64).
Supplementary Material
Acknowledgments
We thank Ms. Alexandra Baker for excellent technical assistance.
Footnotes
This work was supported with funds from The Research Institute for Children and NIH grants DK44510 (T.Y.A.) and R01AI075037 (E.F.).
References
- 1.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 2.Reed DJ. Regulation of reductive processes by glutathione. Biochem Pharmacol. 1986;35:7–13. doi: 10.1016/0006-2952(86)90545-9. [DOI] [PubMed] [Google Scholar]
- 3.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 4.Das KC, White CW. Redox systems of the cell: possible links and implications. Proc Natl Acad Sci U S A. 2002;99:9617–9618. doi: 10.1073/pnas.162369199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klebanoff SJ. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980;93:480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- 6.Cook JA, Gius D, Wink DA, Krishna MC, Russo A, Mitchell JB. Oxidative stress, redox, and the tumor microenvironment. Semin Radiat Oncol. 2004;14:259–266. doi: 10.1016/j.semradonc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 7.De la Fuente M, Hernanz A, Vallejo MC. The immune system in the oxidative stress conditions of aging and hypertension: favorable effects of antioxidants and physical exercise. Antioxid Redox Signal. 2005;7:1356–1366. doi: 10.1089/ars.2005.7.1356. [DOI] [PubMed] [Google Scholar]
- 8.Richie JP., Jr The role of glutathione in aging and cancer. Exp Gerontol. 1992;27:615–626. doi: 10.1016/0531-5565(92)90015-r. [DOI] [PubMed] [Google Scholar]
- 9.Hernanz A, Fernandez-Vivancos E, Montiel C, Vazquez JJ, Arnalich F. Changes in the intracellular homocysteine and glutathione content associated with aging. Life Sci. 2000;67:1317–1324. doi: 10.1016/s0024-3205(00)00722-0. [DOI] [PubMed] [Google Scholar]
- 10.Hernanz A, Fernandez-Vivancos E, Salazar RM, Arnalich F. Homocysteine and other thiol compounds in ageing. Biofactors. 2000;11:47–49. doi: 10.1002/biof.5520110113. [DOI] [PubMed] [Google Scholar]
- 11.Droge W. Aging-related changes in the thiol/disulfide redox state: implications for the use of thiol antioxidants. Exp Gerontol. 2002;37:1333–1345. doi: 10.1016/s0531-5565(02)00175-4. [DOI] [PubMed] [Google Scholar]
- 12.Bannai S, Kitamura E. Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. J Biol Chem. 1980;255:2372–2376. [PubMed] [Google Scholar]
- 13.Bannai S, Tateishi N. Role of membrane transport in metabolism and function of glutathione in mammals. J Membr Biol. 1986;89:1–8. doi: 10.1007/BF01870891. [DOI] [PubMed] [Google Scholar]
- 14.Ishii T, Sugita Y, Bannai S. Regulation of glutathione levels in mouse spleen lymphocytes by transport of cysteine. J Cell Physiol. 1987;133:330–336. doi: 10.1002/jcp.1041330217. [DOI] [PubMed] [Google Scholar]
- 15.Bannai S, Kitamura E. Role of proton dissociation in the transport of cystine and glutamate in human diploid fibroblasts in culture. J Biol Chem. 1981;256:5770–5772. [PubMed] [Google Scholar]
- 16.Bannai S, Ishii T. Transport of cystine and cysteine and cell growth in cultured human diploid fibroblasts: effect of glutamate and homocysteate. J Cell Physiol. 1982;112:265–272. doi: 10.1002/jcp.1041120216. [DOI] [PubMed] [Google Scholar]
- 17.Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70:43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- 18.Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 19.Verrey F, Meier C, Rossier G, Kuhn LC. Glycoprotein-associated amino acid exchangers: broadening the range of transport specificity. Pflugers Arch. 2000;440:503–512. doi: 10.1007/s004240000274. [DOI] [PubMed] [Google Scholar]
- 20.Bridges CC, Kekuda R, Wang H, Prasad PD, Mehta P, Huang W, Smith SB, Ganapathy V. Structure, function, and regulation of human cystine/glutamate transporter in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:47–54. [PubMed] [Google Scholar]
- 21.Bridges RJ, Esslinger CS. The excitatory amino acid transporters: pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacol Ther. 2005;107:271–285. doi: 10.1016/j.pharmthera.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S. Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol. 2007;81:974–982. doi: 10.1189/jlb.0606385. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe H, Bannai S. Induction of cystine transport activity in mouse peritoneal macrophages. J Exp Med. 1987;165:628–640. doi: 10.1084/jem.165.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eck HP, Droge W. Influence of the extracellular glutamate concentration on the intracellular cyst(e)ine concentration in macrophages and on the capacity to release cysteine. Biol Chem Hoppe Seyler. 1989;370:109–113. doi: 10.1515/bchm3.1989.370.1.109. [DOI] [PubMed] [Google Scholar]
- 25.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brossart P, Bevan MJ. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–1599. [PMC free article] [PubMed] [Google Scholar]
- 27.Brossart P, Goldrath AW, Butz EA, Martin S, Bevan MJ. Virus-mediated delivery of antigenic epitopes into dendritic cells as a means to induce CTL. J Immunol. 1997;158:3270–3276. [PubMed] [Google Scholar]
- 28.Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–368. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- 29.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 30.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 32.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, Nussenzweig M. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 34.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009 doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980;106:55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- 37.Circu ML, Rodriguez C, Maloney R, Moyer MP, Aw TY. Contribution of mitochondrial GSH transport to matrix GSH status and colonic epithelial cell apoptosis. Free Radic Biol Med. 2008;44:768–778. doi: 10.1016/j.freeradbiomed.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Circu ML, Stringer S, Rhoads CA, Moyer MP, Aw TY. The role of GSH efflux in staurosporine-induced apoptosis in colonic epithelial cells. Biochem Pharmacol. 2009;77:76–85. doi: 10.1016/j.bcp.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 40.Rimaniol AC, Haik S, Martin M, Le Grand R, Boussin FD, Dereuddre-Bosquet N, Gras G, Dormont D. Na+-dependent high-affinity glutamate transport in macrophages. J Immunol. 2000;164:5430–5438. doi: 10.4049/jimmunol.164.10.5430. [DOI] [PubMed] [Google Scholar]
- 41.Pacheco R, Oliva H, Martinez-Navio JM, Climent N, Ciruela F, Gatell JM, Gallart T, Mallol J, Lluis C, Franco R. Glutamate released by dendritic cells as a novel modulator of T cell activation. J Immunol. 2006;177:6695–6704. doi: 10.4049/jimmunol.177.10.6695. [DOI] [PubMed] [Google Scholar]
- 42.Patel SA, Warren BA, Rhoderick JF, Bridges RJ. Differentiation of substrate and non-substrate inhibitors of transport system xc(−): an obligate exchanger of L-glutamate and L-cystine. Neuropharmacology. 2004;46:273–284. doi: 10.1016/j.neuropharm.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261:2256–2263. [PubMed] [Google Scholar]
- 44.Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- 45.Gmunder H, Eck HP, Benninghoff B, Roth S, Droge W. Macrophages regulate intracellular glutathione levels of lymphocytes. Evidence for an immunoregulatory role of cysteine. Cell Immunol. 1990;129:32–46. doi: 10.1016/0008-8749(90)90184-s. [DOI] [PubMed] [Google Scholar]
- 46.Kanner SB, Kavanagh TJ, Grossmann A, Hu SL, Bolen JB, Rabinovitch PS, Ledbetter JA. Sulfhydryl oxidation down-regulates T-cell signaling and inhibits tyrosine phosphorylation of phospholipase C gamma 1. Proc Natl Acad Sci U S A. 1992;89:300–304. doi: 10.1073/pnas.89.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sido B, Braunstein J, Breitkreutz R, Herfarth C, Meuer SC. Thiol-mediated redox regulation of intestinal lamina propria T lymphocytes. J Exp Med. 2000;192:907–912. doi: 10.1084/jem.192.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan Z, Garg SK, Kipnis J, Banerjee R. Extracellular redox modulation by regulatory T cells. Nat Chem Biol. 2009;5:721–723. doi: 10.1038/nchembio.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanlioglu S, Williams CM, Samavati L, Butler NS, Wang G, McCray PB, Jr, Ritchie TC, Hunninghake GW, Zandi E, Engelhardt JF. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J Biol Chem. 2001;276:30188–30198. doi: 10.1074/jbc.M102061200. [DOI] [PubMed] [Google Scholar]
- 50.Carta S, Castellani P, Delfino L, Tassi S, Vene R, Rubartelli A. DAMPs and inflammatory processes: the role of redox in the different outcomes. J Leukoc Biol. 2009;86:549–555. doi: 10.1189/jlb.1008598. [DOI] [PubMed] [Google Scholar]
- 51.Castellani P, Angelini G, Delfino L, Matucci A, Rubartelli A. The thiol redox state of lymphoid organs is modified by immunization: role of different immune cell populations. Eur J Immunol. 2008;38:2419–2425. doi: 10.1002/eji.200838439. [DOI] [PubMed] [Google Scholar]
- 52.Masciarelli S, Sitia R. Building and operating an antibody factory: redox control during B to plasma cell terminal differentiation. Biochim Biophys Acta. 2008;1783:578–588. doi: 10.1016/j.bbamcr.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 54.Leon B, Ardavin C. Monocyte-derived dendritic cells in innate and adaptive immunity. Immunol Cell Biol. 2008;86:320–324. doi: 10.1038/icb.2008.14. [DOI] [PubMed] [Google Scholar]
- 55.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 56.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuppner MC, Scharner A, Milani V, Von Hesler C, Tschop KE, Heinz O, Issels RD. Ifosfamide impairs the allostimulatory capacity of human dendritic cells by intracellular glutathione depletion. Blood. 2003;102:3668–3674. doi: 10.1182/blood-2003-05-1408. [DOI] [PubMed] [Google Scholar]
- 58.Yamada H, Arai T, Endo N, Yamashita K, Fukuda K, Sasada M, Uchiyama T. LPS-induced ROS generation and changes in glutathione level and their relation to the maturation of human monocyte-derived dendritic cells. Life Sci. 2006;78:926–933. doi: 10.1016/j.lfs.2005.05.106. [DOI] [PubMed] [Google Scholar]
- 59.Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A. 1998;95:3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rimaniol AC, Mialocq P, Clayette P, Dormont D, Gras G. Role of glutamate transporters in the regulation of glutathione levels in human macrophages. Am J Physiol Cell Physiol. 2001;281:C1964–1970. doi: 10.1152/ajpcell.2001.281.6.C1964. [DOI] [PubMed] [Google Scholar]
- 61.James SJ, Rose S, Melnyk S, Jernigan S, Blossom S, Pavliv O, Gaylor DW. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 2009;23:2374–2383. doi: 10.1096/fj.08-128926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- 63.Arnalich F, Hernanz A, Lopez-Maderuelo D, De la Fuente M, Arnalich FM, Andres-Mateos E, Fernandez-Capitan C, Montiel C. Intracellular glutathione deficiency is associated with enhanced nuclear factor-kappaB activation in older non-insulin dependent diabetic patients. Free Radic Res. 2001;35:873–884. doi: 10.1080/10715760100301371. [DOI] [PubMed] [Google Scholar]
- 64.Eck HP, Gmunder H, Hartmann M, Petzoldt D, Daniel V, Droge W. Low concentrations of acid-soluble thiol (cysteine) in the blood plasma of HIV-1-infected patients. Biol Chem Hoppe Seyler. 1989;370:101–108. doi: 10.1515/bchm3.1989.370.1.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.