This paper describes a novel perspective on the neural mediation of prostaglandin's effect on fever and anorexia, emphasizing a neuroanatomical distribution of targeted sites rather than a single “center.”
Abstract
Fever and anorexia are induced by immune system challenges. Because these responses are adaptive when short lasting but deleterious when prolonged, an understanding of the mediating neural circuitry is important. Prostaglandins (PGE) are a critical signaling element for these immune responses. Despite the widespread distribution of PGE receptors throughout the brain, research focuses on the hypothalamic preoptic area as the mediating site of PGE action. Paraventricular nucleus of the hypothalamus (PVH), parabrachial nucleus (PBN), and nucleus tractus solitarius (NTS) neurons also express PGE receptors and are activated during systemic pathogen infection. A role for these neurons in PGE-induced fever, tachycardia, and anorexia is unexplored and is the subject of this report. A range of PGE2 doses was microinjected into third or fourth ventricles (v), or directly into the dorsal PVH, lateral PBN, and medial NTS, and core and brown adipose tissue temperature, heart rate, locomotor activity, and food intake were measured in awake, behaving rats. PGE2 delivery to multiple brain sites (third or fourth v, PVH, or PBN) induced a short- latency (<10 min) fever and tachycardia. By contrast, an anorexic effect was observed only in response to third v and PVH stimulation. NTS PGE2 stimulation was without effect; locomotor activity was not affected for any of the sites. The data are consistent with a view of PGE2-induced effects as mediated by anatomically distributed sites rather than a single center. The data also underscore a potential anatomical dissociation of the neural pathways mediating pyrogenic and anorexic effects of PGE2.
Pathogens detected by the immune system induce a variety of signals that trigger acute-phase responses via actions on the central nervous system. Among these responses are fever, tachycardia, anorexia, lethargy, hyperalgesia, hypothalamic-pituitary-adrenal axis activation, and circadian changes (1, 2). These responses are well conserved evolutionarily and are generally adaptive (3–5), but when excessive or chronic, they can become deleterious (6). For this reason, it is crucial to determine the central neural mechanism that mediates the production of specific responses. Data presented here indicate the potential role of several central nervous system sites, previously not evaluated, in the mediation of prostaglandin 2 (PGE2)-induced fever, tachycardia, and anorexia.
PGE are produced by endothelial cells, perivascular microglia, and meningeal macrophages and, to a lesser extent, by neurons (7–9). PGE2, produced at the blood-brain interface in response to immune cell-triggered cytokine release, is considered the final humoral mediator for fever and other observed responses (10, 11). This key role of PGE2 is supported by data indicating that blockade of PGE2 synthesis, by deletion of synthesizing enzyme cyclooxygenase 2 or microsomal PGE2 synthase or deletion of PGE2 receptors, alleviates many immune responses including fever (12, 13) and anorexia (14, 15). Consistent with these data, it is also known that infections elevate endogenous PGE2 levels in cerebrospinal fluid (16), and exogenous central PGE2 application elicits the fever, tachycardia, hyperalgesia, and anorexia that are also observed in response to pathogen introduction (17, 18).
Despite the broad access of PGE2 to the brain parenchyma and the broad anatomical distribution of its receptors (19–21), investigations of the central site of PGE2 action have focused almost exclusively on the neurons of the hypothalamic preoptic area (POA) (22–25). Direct POA application of PGE2 mimics the pyrogenic and tachycardic action of PGE2 applied to the third ventricle (v) (22–24), responses likely mediated by the PGE2 receptor 3 (EP3) that is densely expressed in POA (20).
As already noted, PGE receptor (EP-R)-expressing neurons are also located outside of the POA (19–21), but little attention has been given to them functionally. To date, four subtypes of PGE2 receptors are identified (EP1–4); each has a distinct expression pattern, but when viewed collectively, these EP-R are anatomically distributed (19–21). Several of these extra-POA, EP-R-expressing nuclei have neuroanatomical connections to thermoregulatory (26), cardiovascular, and feeding control circuitry and are activated (increased Fos-like immunoreactivity) during immune challenge (27). These studies, along with two other studies questioning the crucial role of POA in PGE fever generation (28, 29), raise the hypothesis that PGE-triggered fever is mediated by a distributed neural system engaged by multisite action of PGE2 rather than via a site-specific (POA) mechanism. Here, using awake, behaving rats, we pursue the idea that EP-R-bearing neurons outside of the POA contribute to pyrogenic, tachycardic, and anorexic actions of PGE2. A functional role for EP-R-expressing neurons in the caudal brainstem in these responses was probed via application of PGE2 into the hindbrain (fourth) v and with direct parenchymal injection of PGE2 into nucleus of the solitary tract (NTS; EP3-expressing neurons) and parabrachial nucleus (PBN; EP3- and -4-expressing neurons), two major visceroceptive and autonomic control areas in the caudal brainstem that are activated during an immune challenge (30–33). Neurons of the paraventricular nucleus of the hypothalamus (PVH) express EP-R (EP1 and EP4), and these neurons have been implicated in the control of thermoregulation and feeding (21, 34–36). In addition, a wealth of molecular and electrophysiological evidence suggests a role of PVH in immune response (35, 37–39), but no assessments of the effect of direct PGE2 application on pyrogenesis, tachycardia, and anorexia have been made in nonanesthetized rats. A role for PVH neurons in mediating PGE2 responses was therefore also examined here.
Materials and Methods
Subjects
Male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 300–400 g (10–12 wk old) at surgery and housed individually in plastic bins under a 12-h light, 12-h dark cycle (0800 h lights on), participated in experiments described below. Pelleted chow (Purina 5001; Purina, St. Louis, MO) and water were available ad libitum unless otherwise noted. All procedures conformed to the institutional standards of the animal care and use committee (University of Pennsylvania).
Surgery
For all surgeries, rats were anesthetized with a mixture of ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) delivered im.
Fourth and third v intracerebroventricular and intraparenchymal (PVH, PBN, and NTS) cannula implantation
Rats (n = 30) were implanted with both third and fourth v cannulae (22 gauge; Plastics One, Inc., Roanoke, VA). Third v guide cannulae were positioned on the midline, 2.0 mm posterior to bregma, and 5.5 mm ventral to dura mater, with the injector aimed 7.5 mm ventral to dura. Fourth v guide cannulae were stereotaxically positioned 2.0 mm above the fourth v (coordinates were on the midline, 2.5 mm anterior to the occipital suture, and 4.5 mm ventral to the dura, with the injector aimed 6.5 mm ventral from dura). Parenchymal guide cannulae were located 2.0 mm above intended target, coordinates chosen as reported previously (40). NTS cannulae were placed 0.5 mm from the midline, on the occipital suture, 5.9 mm ventral to skull (n = 12). PBN cannulae were positioned 2.0 mm lateral to midline, 9.5 mm posterior to bregma, and 4.5 mm ventral to the dura, with injector aimed 6.5 mm ventral to dura (n = 33). PVN cannulae were placed 0.5 mm lateral to midline, 1.8 mm posterior to bregma, and 5.7 mm ventral to the dura, with injector aimed 7.7 mm ventral to dura (n = 16). All cannulae were attached to the skull with dental acrylic and jeweler's screws and closed with an obturator as previously described (41).
Telemetric transponder surgery
For recording core temperature (TC), heart rate (HR), and spontaneous physical activity (SPA), rats were implanted with telemetric transponders (HRC 4000 VitalView; Mini Mitter/Respironics, Bend, OR). Transponders were inserted into the abdominal cavity, with the leads positioned sc and secured to the chest muscles on either side of the heart with sutures. In a separate group of rats, IPTT-300 (Bio Medic Data Systems, Seaford, DE) transponders were implanted just ventral to the interscapular brown adipose tissue (IBAT) for measurement of TIBAT.
Experimental procedures
Cannula position verification
At least 7 d after surgery, the placement of third, fourth v, and NTS cannulae was assessed by measurement of the sympathoadrenal-mediated glycemic response elicited by the cytoglucopenia induced by central injection of 5-thio-d-glucose [210 μg in 2 μl artificial cerebral spinal fluid (aCSF) (Harvard Apparatus, Holliston, MA) for third and fourth v and 21 μg in 0.1 μl for NTS] (42). A postinjection elevation of at least 100% of baseline plasma glucose level was required for subject inclusion. In addition for NTS, PVH, and PBN placements, at the conclusion of the parenchymal experiments, pontamine sky blue dye was injected at the same volume used for earlier drug infusion, and brains were examined using light microscopy to identify the injection sites. Only rats whose injection placements were deemed to reside within the boundaries of the targeted structures were included in the statistical analyses. Photomicrographs of representative injection sites from the study for each target area are included in panel E of Figs. 4–6.
Fig. 4.
A–D, Effect of PBN PGE2 administration (0.1 μl) on TC (A), HR (B), SPA (C), and 24-h food intake (D); E, photomicrograph with a representative injection in the PBN. Line graphs represent across-rat average parameter measurements throughout the recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms below. The histograms below provide 1.5-h postinjection averages + sem for each parameter at each dose. **, P < 0.005; ***, P < 0.0005. LPB, Lateral parabrachial nucleus; LPBC, lateral parabrachial nucleus, central part; LPBE, lateral parabrachial nucleus, external part; KF, Killiker-Fuse nucleus; MPB, medial parabrachial nucles.
Fig. 5.
A–D, Effect of NTS PGE2 administration (0.1 μl) on TC (A), HR (B), SPA (C), and 24-h food intake (D); E, photomicrograph with a representative injection in the NTS. Line graphs represent across-rat average parameter measurements throughout the recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms below. The histograms below provide 1.5-h postinjection averages + sem for each parameter at each dose.
Fig. 6.
A–D, Effect of PVH PGE2 administration (0.1 μl) on TC (A), HR (B), SPA (C), and 24-h food intake (D); E, photomicrograph with a representative injection in the PVH. Line graphs represent across-rat average parameter measurements throughout the recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms below. The histograms below provide 1.5 h postinjection averages + sem for each parameter at each dose. *, P < 0.05; **, P < 0.005. PaDC, Paraventricular hypothalamic nucleus, dorsal cap; PaLM, paraventricular hypothalamic nucleus, lateral magnocellular part; PaMP, paraventricular hypothalamic nucleus, medial parvicellular part; PaV, paraventricular hypothalamic nucleus, ventral part.
Habituation training
Before the start of experimental testing, rats were acclimated for 1 wk to the handling and injection procedures to be used in a given experiment.
Experiment 1: evaluation of hindbrain (fourth v) and forebrain (third v) PGE2 administration on TC, TIBAT, HR, SPA, and food intake
To compare the energetic/sympathetic effects of hindbrain (fourth v) PGE2 application with that of third v PGE2 administration, rats received counterbalanced fourth or third v vehicle injections (1 μl aCSF), counterbalanced with several doses of PGE2 (Cayman, Ann Arbor, MI): 0.01, 0.1, 1.0, and 5.0 μg. Doses were selected based on results from pilot experiments. In experiment 1A TC, HR, and SPA were recorded telemetrically for 1 h before injections and 4 h after injections every 5 min (TC and SPA) or 60 sec (HR). In experiment 1B, TIBAT was recorded telemetrically before injections and every 30 min after drug injections for 2 h. For both experiments, food was removed at the time of injections (early in the light cycle) and returned 4 h later, late in the light phase. Food was not available during the period of energetic/sympathetic response measurement to eliminate the influence of the thermic effect of food.
Shorter latency effects of PGE2 administration to forebrain and hindbrain ventricles on food intake were assessed in experiment 1C, where rats received third or fourth v vehicle injections (1 μl aCSF) counterbalanced with several doses of PGE2 (Cayman), 2.0 and 5.0 μg (dose selection based on Ref. 17) 30 min before lights out, and food (standard chow) was available immediately after injections. For this experiment, injections were preceded by 16 h food deprivation (12 h of which was during the light cycle) to ensure a higher baseline level of food intake against which to judge any PGE2-induced anorexic effect (17). Food intake was recorded at 1, 2, 3, 6, and 24 h after injections.
Experiment 2: evaluation of PBN, NTS, and PVH administration of PGE2 on TC, TIBAT, HR, SPA, and food intake
To determine energetic/sympathetic effects of direct parenchymal PGE2 administration into PBN, NTS, or PVH sites, rats received vehicle injections (0.1 μl aCSF) counterbalanced with several doses (that were subthreshold when administered to the ventricle) of PGE2: 0.1 or 0.2 μg. TC, HR, and SPA were recorded telemetrically for 1 h before injections and 4 h after injections every 5 min (TC and SPA) or 60 sec (HR). Food was removed at the time of injections (early in the light cycle) and returned 4 h later. Food intake and body weight measurements were made 24 h after the injection of drug. Given this design, all noted differences in food intake reflect longer latency effects of PGE2 (i.e. intake from 4–24 h after injection). For ad libitum feeding of rats, food was always available during the dark cycle, and a minimum of 48 h was allotted between experimental testing for all animals.
Statistical analysis
All parameters were analyzed by ANOVA followed by post hoc Tukey test, as appropriate. All statistical analysis was conducted using STATISTICA software (StatSoft, Tulsa, OK). Differences were considered significant at P < 0.05.
Results
Experiment 1: evaluation of hindbrain (fourth v) and forebrain (third v) PGE2 administration on TC, TIBAT, HR, SPA and food intake
Core temperature
Both forebrain (third v) and hindbrain (fourth v) PGE2 injections produced rapid, dose-related hyperthermic responses in TC with a 90-min average effect size of 0.05 C (P = 0.99), 0.63 C (P = 0.28), 1.20 C (P < 0.005), and 1.55 C (P < 0.0005) for third v and 0.29 C (P = 0.75), 0.45 C (P = 0.42), 0.87 C (P < 0.01), and 1.05C (P < 0.005) for fourth v for the 0.01-, 0.1-, 1.0-, and 5.0-μg doses, respectively (Figs. 1A and 2A).
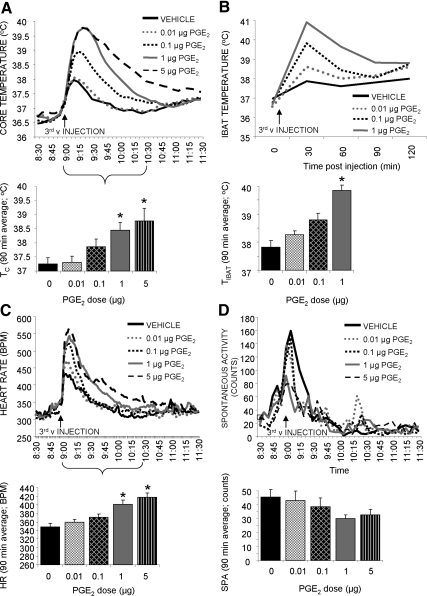
Fig. 1.
Effect of forebrain ventricle PGE2 administration (1 μl, third v delivery) on TC (A), TIBAT (B), HR (C), and SPA (D). Line graphs represent across-rat average parameter measurements through the 3-h recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms. The histograms provide 1.5-h postinjection averages + sem for each parameter at each dose. *, P < 0.05. BPM, Beats per minute.
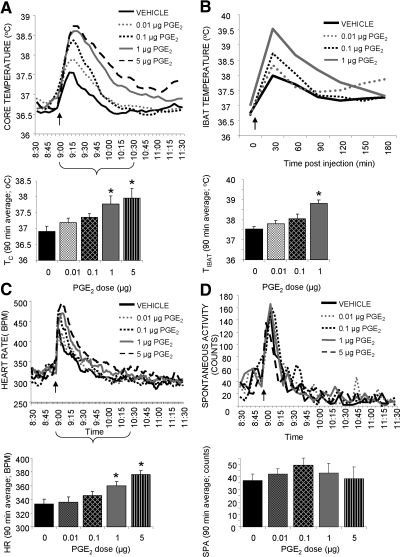
Fig. 2.
Effect of hindbrain ventricle PGE2 administration (1 μl, fourth v delivery) on TC (A), TIBAT (B), HR (C), and SPA (D). Line graphs represent across-rat average parameter measurements through the 3-h recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms. The histograms provide 1.5-h postinjection averages + sem for each parameter at each dose. *, P < 0.05.
IBAT temperature
Both forebrain (third v) and hindbrain (fourth v) PGE2 injections produced rapid, dose-related hyperthermic responses in TIBAT with a 90-min average effect size of 0.45 C (P = 0.09), 0.99 C (P < 0.0005), and 2.02 C (P < 0.0005) for third v and 0.27 C (P = 0.95), 0.51 C (P = 0.71), and 1.28 C (P < 0.05) for fourth v for the 0.01-, 0.1-, and 1.0-μg doses, respectively (Figs. 1B and 2B).
Heart rate
Both forebrain (third v) and hindbrain (fourth v) PGE2 injections produced short-latency, dose-related tachycardic responses with a 90-min average effect size of 10 (P = 0.79), 22 (P = 0.15), 52 (P < 0.0005), and 69 (P < 0.0005) beats/min for third v and 2 (P = 0.99), 12 (P = 0.61), 26 (P < 0.05), and 42 (P < 0.0005) beats/min for fourth v for the 0.01-, 0.1-, 1.0-, and 5.0-μg doses, respectively (Figs. 1C and 2C).
Spontaneous activity
Neither forebrain (third v) nor hindbrain (fourth v) PGE2 injections produced any significant changes in spontaneous activity (Fig. 1d and 2d).
There were no significant differences between the TC, HR, and SPA responses of rats treated with PGE2 in the third v, and rats receiving fourth v injections as two-way ANOVA (ventricle and drug) did not indicate a significant interaction. Although there was no significant interaction between site of administration and drug for TIBAT parameter, there was a trend (P = 0.08), which is likely due to the fact that third v administration of PGE2 produced a significant BAT hyperthermia at both 0.1 and 1.0 μg, when only the 1.0-μg dose was effective in the fourth v.
Food intake
Third v PGE2 administration induced a significant but small anorexic effect only at the 24 h measurement point (5.0 μg PGE2; P < 0.01). Hindbrain (fourth v) PGE2 stimulation did not alter food intake (Fig. 3).
Fig. 3.
Effect of third v and fourth v PGE2 administration on food intake. The histograms provide average values ± sem. *, P < 0.05.
Experiment 2: evaluation of PBN, NTS, and PVH PGE2 delivery on TC, TIBAT, HR, SPA, and food intake
PBN
Figure 4 shows the physiological response of rats with confirmed PBN cannula placements and indicates that PGE2 injection into PBN significantly increased TC (P < 0.0005, 0.1 μg; and P < 0.0005, 0.2 μg) and HR (P = 0.33, 0.1 μg; and P < 0.05, 0.2 μg). Spontaneous activity and 24-h food intake did not change significantly.
NTS
Figure 5 displays the physiological response of rats with confirmed NTS cannula placements and shows that PGE2 injection in this site did not significantly change any of the measured parameters.
PVH
Figure 6 shows the physiological response of rats with anatomically confirmed PVH cannula placements and indicates that PGE2 injection significantly increased TC (P < 0.001, 0.1 μg; and P < 0.0005, 0.2 μg) and HR (P < 0.005, 0.1 μg; and P < 0.005, 0.2 μg). Spontaneous activity did not change significantly. The 24-h food intake was significantly reduced (P = 0.78, 0.1 μg; and P < 0.01, 0.2 μg). The 24-h measurement point was chosen based on the results of our third v study, where a significant PGE2 anorexia was observed only at this time point. The inclusion of this point also had design advantages because it allowed for the short-latency measurement of energetic cardiovascular parameters in the absence of food, the return of food, and the subsequent measurement of intake effects for the period that food was available until the 24-h point. We note that it is possible that if the rats received access to food immediately after the injection and earlier time points where included in the analysis, the size of the effect may have differed.
Discussion
This study provides evidence to support the hypothesis that pharmacological stimulation of EP-R located outside of the POA contributes to the pyrogenic and cardiovascular effects induced by PGE2 delivery to the brain. Here we show that hindbrain-directed (fourth v) PGE2 induced short-latency fever, increased TIBAT, and tachycardia, similar in magnitude and duration to that obtained after forebrain (third v) injection. Furthermore, data obtained with parenchymal application of doses of PGE2 that were subthreshold for effect when delivered into the ventricles define, at least in part, the neuronal populations mediating the responses observed in the ventricular studies. Parenchymal PGE2 application triggered a short-latency fever and tachycardia when applied to the PVH in the basal forebrain and to the PBN in the caudal brainstem. Together these data suggest that PGE2 thermogenic and cardiovascular control is distributed anatomically rather than localized exclusively in the POA.
Our data uncover a direct contribution of PBN neurons to PGE2-induced fever and tachycardia. A role for PBN neurons in autonomic processes is well established. There are clear neuroanatomical connections to sympathetic circuitry controlling BAT (43) and the heart, and electrical stimulation of the PBN has been shown to induce changes in temperature and HR (44, 45). Recent data indicate a role for PBN neurons in response to immune system challenge (30–32). Parabrachial neurons are essential for IL-1β-induced activation of the amygdala, the bed nucleus of the stria terminalis, and the ventrolateral medulla (46). This critical role of PBN neuronal processing has been suggested to result from activation of upstream projections from other sites that respond directly to PGE2 (e.g. NTS) (46). Here, however, we show that direct PGE2 stimulation in the PBN elicits fever and elevates HR (whereas NTS stimulation does not). These data are consistent with previous molecular data pointing to a direct role of PGE2 in this site; e.g. PBN neurons that densely express EP3/4 receptors are activated by immune challenge with lipopolysaccharide (LPS) (32). Little is known about the neurochemical phenotype of these EP3/4-expressing neurons; however, coexpression of EP3 or EP4 with several peptides such as dynorphin, enkephalin, calcitonin gene-related peptide, and cholecystokinin neurons in various subnuclei of the PBN was recently described (30). Intracellular effects of PGE2 stimulation of these neurons and their downstream projections require further investigation to better define the circuitry underlying the PGE2-driven responses. Nonetheless, projections to both forebrain and hindbrain are likely involved, because PBN lesions clearly attenuated systemic infection activation of several forebrain structures and also some hindbrain autonomic centers (e.g. ventrolateral medulla) (46).
The lack of effect for direct PGE2 stimulation in the NTS is interesting in light of the clear expression of EP-R, projection of NTS neurons to sympathetic outflow to BAT (47), and neuronal activation during an immune challenge of this area. Another structure that clearly expresses EP-R and has well-established connections to the control of BAT thermogenesis is the raphe pallidus (RPa). Despite that, and similar to our NTS data, direct injection of PGE2 to this area does not produce a fever (48). These findings directly address a suggestion by Oka (28) that extra-POA EP3, including those expressed on NTS and RPa neurons, were possible contributors to PGE2-elicited fever because his paper reported that injection of EP3-specific agonist into the POA of rats failed to trigger hyperthermia, whereas knockout of EP3 in mice eliminated the fever triggered by LPS treatment. Although no direct involvement of RPa EP-R was found, RPa neurons are an essential downstream mediator of the pyrogenic PGE2 effect in POA (49). Therefore, it is still possible that the NTS neurons, like those in the RPa, do participate in immune challenge, either indirectly (i.e. by mediating signals downstream of direct targets of PGE2) in the responses tested here or by playing a role in other acute-phase responses not tested here. We also cannot exclude the possibility that parts of the nucleus more caudal to the NTS region examined here might play a role in PGE2-mediated energetic responses.
These data are the first to show that direct stimulation of PVH EP-R produces a clear pyrogenic, tachycardic, and anorexic (discussed below) response. PVH neurons express EP4 and are involved in a variety of regulatory functions, at least in part, via its role in the control of sympathetic outflow (21, 34, 35). Capillary endothelial cells might be the source of endogenous PGE2, but also, interestingly, PVH neurons contain cyclooxygenase 2, a PGE2-synthesizing enzyme that provides an alternate source of PGE2 production (50). A wealth of molecular and electrophysiological data indicates a role of PVH neural processing in PGE2-induced responses. That chronic lesions of PVH block LPS-induced fever shows a critical role for this nucleus in the systemic immune pyrogenic response (35). A potential direct role of PGE2 in PVH was indicated by data showing that IL-1β systemic injection increases PGE2 release in PVH (39), which in turn results in depolarized PVH neurons (37). Interestingly, PVH EP4 are up-regulated after LPS injection (21, 34, 35), but the underlying mechanism of this change is not clear; however, this could potentially result in a higher sensitivity of PVH neurons to PGE2. It is therefore possible that the direct responses investigated here could have been greater if these endogenous conditions of increased EP-R in the PVH were mimicked. Downstream neurochemical mediators and pathways from the PVH are not clear; however, direct projections to spinal cord autonomic neurons might be involved, because LPS-induced Fos-positive neurons in dorsal PVH project to the intermediolateral cell column (51). In general, PVH neurons are well documented to contribute to thermoregulatory control (through BAT activation but also via vasoconstriction) either through direct projections to the spinal cord intermediolateral cell column or through projections to other autonomic relays (e.g. rostroventral medulla) (52, 53).
Microbial infections induce anorexia; this response is in part mediated by PGE2 (54). Central (third v) PGE2 application decreases food intake in food-deprived rats and mice (17, 55); however, the site of action of PGE2 for this response is unclear. Our data indicate that food intake was not significantly altered after any of the hindbrain-targeted PGE2 treatments (fourth v, NTS, and PBN). By contrast, a forebrain site of anorexic PGE2 action was supported by our third v studies, indicating that PGE2 anorexia is induced by stimulation of an EP-R-expressing site that is accessed by third but not fourth v drug application. Consistent with this anatomical distinction is the anorexic effect we report with the PVH-applied PGE2 on 24-h food intake. A role for the PVH in control of feeding behavior is well established, and our study shows that PGE2 stimulation of the PVH induces small but significant anorexia. Oxytocin and corticotropin-releasing factor are just a few of the many neuropeptides expressed in the PVH neurons that are implicated in food intake and released during a microbial infection (54). It will be important to neurochemically phenotype EP-R-expressing PVN neurons to better understand or define downstream pathways mediating the anorexic and immune response effects of PGE2 in the PVH.
Taken together, the data described in this report are consistent with the view that PGE2 action is anatomically distributed, meaning that the induction of fever, elevation in TIBAT, and tachycardia arises from PGE2 action at sites in addition to the POA that include the PVH and PBN. Future work is required to determine the downstream circuits that mediate the PGE2-driven responses from PVN and PBN. Such details have been described for the POA because it has been under investigation in connection with PGE and more generally with thermoregulatory function for some time (22–24). Evidence from this study suggests a novel perspective on the neural mediation of PGE2-induced immune responses, one that involves action of the endogenous ligand at one of several individual sites and possibly at several sites simultaneously. This perspective is similar to that previously indicated for functional effects of other neuromodulators, e.g. melanocortin and leptin (40, 41, 56). Further work will be needed to examine whether the sites mediating a specific response can vary as a function of type of immune challenge stimulus and the amount of infectious agent available. We note here that evidence exists for species differences in the contribution of EP3 in neural control of PGE2-induced fever. For the mouse, POA EP3 play a critical role in PGE- and LPS-induced fever (57). For the rat, local injection and lesion studies of POA fail to support the view that POA is the exclusive site mediating PGE-induced fever (28, 29). Our results are consistent with a role for extra-POA mediation of PGE2-induced fever but in no way should be interpreted to detract from the perspective that POA contributes importantly to the thermoregulatory neural control circuit. The current data set reveals that although EP-R stimulation of some nuclei engage the neural pathways mediating both anorexic and pyrogenic/sympathetic responses for others, e.g. PBN, only the pyrogenic/sympathetic responses are triggered.
Acknowledgments
This work was supported by the National Institutes of Health Research Grant DK-21397 (to H.J.G.) and NRSA NS-059254 (to K.P.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- Artificial cerebral spinal fluid
- EP3
- PGE2 receptor 3
- EP-R
- PGE receptor
- HR
- heart rate
- IBAT
- interscapular brown adipose tissue
- LPS
- lipopolysaccharide
- NTS
- nucleus of the solitary tract
- PBN
- parabrachial nucleus
- PGE2
- prostaglandin 2
- POA
- preoptic area
- PVH
- paraventricular nucleus of the hypothalamus
- RPa
- raphe pallidus
- SPA
- spontaneous physical activity
- TC
- core temperature
- v
- ventricle.
References
- 1. Hopkins SJ, Rothwell NJ. 1995. Cytokines and the nervous system. I. Expression and recognition. Trends Neurosci 18:83–88 [PubMed] [Google Scholar]
- 2. Rothwell NJ, Hopkins SJ. 1995. Cytokines and the nervous system. II. Actions and mechanisms of action. Trends Neurosci 18:130–136 [DOI] [PubMed] [Google Scholar]
- 3. Murray MJ, Murray AB. 1979. Anorexia of infection as a mechanism of host defense. Am J Clin Nutr 32:593–596 [DOI] [PubMed] [Google Scholar]
- 4. Vaughn LK, Veale WL, Cooper KE. 1980. Antipyresis: its effect on mortality rate of bacterially infected rabbits. Brain Res Bull 5:69–73 [DOI] [PubMed] [Google Scholar]
- 5. Vaughn LK, Veale WL, Cooper KE. 1981. Effects of antipyresis on bacterial numbers in infected rabbits. Brain Res Bull 7:175–180 [DOI] [PubMed] [Google Scholar]
- 6. Plata-Salamán CR. 2000. Central nervous system mechanisms contributing to the cachexia-anorexia syndrome. Nutrition 16:1009–1012 [DOI] [PubMed] [Google Scholar]
- 7. Schiltz JC, Sawchenko PE. 2003. Signaling the brain in systemic inflammation: the role of perivascular cells. Front Biosci 8:s1321–s1329 [DOI] [PubMed] [Google Scholar]
- 8. Elmquist JK, Breder CD, Sherin JE, Scammell TE, Hickey WF, Dewitt D, Saper CB. 1997. Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. J Comp Neurol 381:119–129 [DOI] [PubMed] [Google Scholar]
- 9. Miettinen S, Fusco FR, Yrjänheikki J, Keinänen R, Hirvonen T, Roivainen R, Närhi M, Hökfelt T, Koistinaho J. 1997. Spreading depression and focal brain ischemia induce cyclooxygenase-2 in cortical neurons through N-methyl-d-aspartic acid-receptors and phospholipase A2. Proc Natl Acad Sci USA 94:6500–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Engblom D, Ek M, Saha S, Ericsson-Dahlstrand A, Jakobsson PJ, Blomqvist A. 2002. Prostaglandins as inflammatory messengers across the blood-brain barrier. J Mol Med 80:5–15 [DOI] [PubMed] [Google Scholar]
- 11. Pecchi E, Dallaporta M, Jean A, Thirion S, Troadec JD. 2009. Prostaglandins and sickness behavior: old story, new insights. Physiol Behav 97:279–292 [DOI] [PubMed] [Google Scholar]
- 12. Engblom D, Saha S, Engström L, Westman M, Audoly LP, Jakobsson PJ, Blomqvist A. 2003. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat Neurosci 6:1137–1138 [DOI] [PubMed] [Google Scholar]
- 13. Vane JR. 1971. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 231:232–235 [DOI] [PubMed] [Google Scholar]
- 14. Lugarini F, Hrupka BJ, Schwartz GJ, Plata-Salaman CR, Langhans W. 2002. A role for cyclooxygenase-2 in lipopolysaccharide-induced anorexia in rats. Am J Physiol Regul Integr Comp Physiol 283:R862–R868 [DOI] [PubMed] [Google Scholar]
- 15. Pecchi E, Dallaporta M, Thirion S, Salvat C, Berenbaum F, Jean A, Troadec JD. 2006. Involvement of central microsomal prostaglandin E synthase-1 in IL-1β-induced anorexia. Physiol Genomics 25:485–492 [DOI] [PubMed] [Google Scholar]
- 16. Feldberg W, Gupta KP. 1973. Pyrogen fever and prostaglandin-like activity in cerebrospinal fluid. J Physiol 228:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levine AS, Morley JE. 1981. The effect of prostaglandins (PGE2 and PGF2α) on food intake in rats. Pharmacol Biochem Behav 15:735–738 [DOI] [PubMed] [Google Scholar]
- 18. Feldberg W, Saxena PN. 1971. Fever produced in rabbits and cats by prostaglandin E1 injected into the cerebral ventricles. J Physiol 215:23P–24P [PubMed] [Google Scholar]
- 19. Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. 2001. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 41:661–690 [DOI] [PubMed] [Google Scholar]
- 20. Ek M, Arias C, Sawchenko P, Ericsson-Dahlstrand A. 2000. Distribution of the EP3 prostaglandin E2 receptor subtype in the rat brain: relationship to sites of interleukin-1-induced cellular responsiveness. J Comp Neurol 428:5–20 [DOI] [PubMed] [Google Scholar]
- 21. Oka T, Oka K, Scammell TE, Lee C, Kelly JF, Nantel F, Elmquist JK, Saper CB. 2000. Relationship of EP(1–4) prostaglandin receptors with rat hypothalamic cell groups involved in lipopolysaccharide fever responses. J Comp Neurol 428:20–32 [DOI] [PubMed] [Google Scholar]
- 22. Amir S, Schiavetto A. 1990. Injection of prostaglandin E2 into the anterior hypothalamic preoptic area activates brown adipose tissue thermogenesis in the rat. Brain Res 528:138–142 [DOI] [PubMed] [Google Scholar]
- 23. Zaretsky DV, Hunt JL, Zaretskaia MV, DiMicco JA. 2006. Microinjection of prostaglandin E2 and muscimol into the preoptic area in conscious rats: comparison of effects on plasma adrenocorticotrophic hormone (ACTH), body temperature, locomotor activity, and cardiovascular function. Neurosci Lett 397:291–296 [DOI] [PubMed] [Google Scholar]
- 24. Katsuura G, Arimura A, Koves K, Gottschall PE. 1990. Involvement of organum vasculosum of lamina terminalis and preoptic area in interleukin 1β-induced ACTH release. Am J Physiol 258:E163–E171 [DOI] [PubMed] [Google Scholar]
- 25. Tanaka M, McKinley MJ, McAllen RM. 2009. Roles of two preoptic cell groups in tonic and febrile control of rat tail sympathetic fibers. Am J Physiol Regul Integr Comp Physiol 296:R1248–R1257 [DOI] [PubMed] [Google Scholar]
- 26. Yoshida K, Nakamura K, Matsumura K, Kanosue K, König M, Thiel HJ, Boldogköi Z, Toth I, Roth J, Gerstberger R, Hübschle T. 2003. Neurons of the rat preoptic area and the raphe pallidus nucleus innervating the brown adipose tissue express the prostaglandin E receptor subtype EP3. Eur J Neurosci 18:1848–1860 [DOI] [PubMed] [Google Scholar]
- 27. Elmquist JK, Scammell TE, Jacobson CD, Saper CB. 1996. Distribution of Fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J Comp Neurol 371:85–103 [DOI] [PubMed] [Google Scholar]
- 28. Oka T. 2004. Prostaglandin E2 as a mediator of fever: the role of prostaglandin E (EP) receptors. Front Biosci 9:3046–3057 [DOI] [PubMed] [Google Scholar]
- 29. Matsumura K, Cao C, Watanabe Y, Watanabe Y. 1998. Prostaglandin system in the brain: sites of biosynthesis and sites of action under normal and hyperthermic states. Prog Brain Res 115:275–295 [DOI] [PubMed] [Google Scholar]
- 30. Engblom D, Ek M, Ericsson-Dahlstrand A, Blomqvist A. 2004. EP3 and EP4 receptor mRNA expression in peptidergic cell groups of the rat parabrachial nucleus. Neuroscience 126:989–999 [DOI] [PubMed] [Google Scholar]
- 31. Engblom D, Ek M, Hallbeck M, Ericsson-Dahlstrand A, Blomqvist A. 2000. Distribution of prostaglandin EP3 and EP4 receptor mRNA in the rat parabrachial nucleus. Neurosci Lett 281:163–166 [DOI] [PubMed] [Google Scholar]
- 32. Engblom D, Ek M, Ericsson-Dahlstrand A, Blomqvist A. 2001. Activation of prostanoid EP3 and EP4 receptor mRNA-expressing neurons in the rat parabrachial nucleus by intravenous injection of bacterial wall lipopolysaccharide. J Comp Neurol 440:378–386 [DOI] [PubMed] [Google Scholar]
- 33. Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Negishi M. 2000. Immunohistochemical localization of prostaglandin EP3 receptor in the rat nervous system. J Comp Neurol 421:543–569 [DOI] [PubMed] [Google Scholar]
- 34. Freeman PH, Wellman PJ. 1987. Brown adipose tissue thermogenesis induced by low level electrical stimulation of hypothalamus in rats. Brain Res Bull 18:7–11 [DOI] [PubMed] [Google Scholar]
- 35. Horn T, Wilkinson MF, Landgraf R, Pittman QJ. 1994. Reduced febrile responses to pyrogens after lesions of the hypothalamic paraventricular nucleus. Am J Physiol 267:R323–R328 [DOI] [PubMed] [Google Scholar]
- 36. Scammell TE, Elmquist JK, Griffin JD, Saper CB. 1996. Ventromedial preoptic prostaglandin E2 activates fever-producing autonomic pathways. J Neurosci 16:6246–6254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferri CC, Ferguson AV. 2005. Prostaglandin E2 mediates cellular effects of interleukin-1β on parvocellular neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol 17:498–508 [DOI] [PubMed] [Google Scholar]
- 38. Ferri CC, Yuill EA, Ferguson AV. 2005. Interleukin-1β depolarizes magnocellular neurons in the paraventricular nucleus of the hypothalamus through prostaglandin-mediated activation of a non selective cationic conductance. Regul Pept 129:63–71 [DOI] [PubMed] [Google Scholar]
- 39. Watanobe H, Takebe K. 1994. Effects of intravenous administration of interleukin-1-β on the release of prostaglandin E2, corticotropin-releasing factor, and arginine vasopressin in several hypothalamic areas of freely moving rats: estimation by push-pull perfusion. Neuroendocrinology 60:8–15 [DOI] [PubMed] [Google Scholar]
- 40. Skibicka KP, Grill HJ. 2009. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology 150:5351–5361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Skibicka KP, Grill HJ. 2008. Energetic responses are triggered by caudal brainstem melanocortin receptor stimulation and mediated by local sympathetic effector circuits. Endocrinology 149:3605–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ritter RC, Slusser PG, Stone S. 1981. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213:451–452 [DOI] [PubMed] [Google Scholar]
- 43. Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. 2003. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 460:303–326 [DOI] [PubMed] [Google Scholar]
- 44. Mraovitch S, Kumada M, Reis DJ. 1982. Role of the nucleus parabrachialis in cardiovascular regulation in cat. Brain Res 232:57–75 [DOI] [PubMed] [Google Scholar]
- 45. Kobayashi A, Osaka T. 2003. Involvement of the parabrachial nucleus in thermogenesis induced by environmental cooling in the rat. Pflugers Arch 446:760–765 [DOI] [PubMed] [Google Scholar]
- 46. Buller KM, Allen T, Wilson LD, Munro F, Day TA. 2004. A critical role for the parabrachial nucleus in generating central nervous system responses elicited by a systemic immune challenge. J Neuroimmunol 152:20–32 [DOI] [PubMed] [Google Scholar]
- 47. Bamshad M, Song CK, Bartness TJ. 1999. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol 276:R1569–R1578 [DOI] [PubMed] [Google Scholar]
- 48. Tanaka M, McAllen RM. 2005. A subsidiary fever center in the medullary raphe? Am J Physiol Regul Integr Comp Physiol 289:R1592–R1598 [DOI] [PubMed] [Google Scholar]
- 49. Madden CJ, Morrison SF. 2003. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience 122:5–15 [DOI] [PubMed] [Google Scholar]
- 50. Breder CD, Dewitt D, Kraig RP. 1995. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol 355:296–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang YH, Lu J, Elmquist JK, Saper CB. 2000. Lipopolysaccharide activates specific populations of hypothalamic and brainstem neurons that project to the spinal cord. J Neurosci 20:6578–6586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen QH, Toney GM. 2010. In vivo discharge properties of hypothalamic paraventricular nucleus neurons with axonal projections to the rostral ventrolateral medulla. J Neurophysiol 103:4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen QH, Toney GM. 2003. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience 118:797–807 [DOI] [PubMed] [Google Scholar]
- 54. Asarian L, Langhans W. 2010. A new look on brain mechanisms of acute illness anorexia. Physiol Behav 100:464–471 [DOI] [PubMed] [Google Scholar]
- 55. Ohinata K, Suetsugu K, Fujiwara Y, Yoshikawa M. 2006. Activation of prostaglandin E receptor EP4 subtype suppresses food intake in mice. Prostaglandins Other Lipid Mediat 81:31–36 [DOI] [PubMed] [Google Scholar]
- 56. Skibicka KP, Grill HJ. 2009. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology 150:1705–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. 2007. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci 10:1131–1133 [DOI] [PubMed] [Google Scholar]