Abstract
The purpose of this study was to investigate roles for Toll-like receptor 4 (TLR4) in host responses to sterile tissue injury. Hydrochloric acid was instilled into the left mainstem bronchus of TLR4-defective (both C3H/HeJ and congenic C.C3-Tlr4Lps-d/J) and control mice to initiate mild, self-limited acute lung injury (ALI). Outcome measures included respiratory mechanics, barrier integrity, leukocyte accumulation, and levels of select soluble mediators. TLR4-defective mice were more resistant to ALI, with significantly decreased perturbations in lung elastance and resistance, resulting in faster resolution of these parameters [resolution interval (Ri); ∼6 vs. 12 h]. Vascular permeability changes and oxidative stress were also decreased in injured HeJ mice. These TLR4-defective mice paradoxically displayed increased lung neutrophils [(HeJ) 24×103 vs. (control) 13×103 cells/bronchoalveolar lavage]. Proresolving mechanisms for TLR4-defective animals included decreased eicosanoid biosynthesis, including cysteinyl leukotrienes (80% mean decrease) that mediated CysLT1 receptor-dependent vascular permeability changes; and induction of lung suppressor of cytokine signaling 3 (SOCS3) expression that decreased TLR4-driven oxidative stress. Together, these findings indicate pivotal roles for TLR4 in promoting sterile ALI and suggest downstream provocative roles for cysteinyl leukotrienes and protective roles for SOCS3 in the intensity and duration of host responses to ALI.—Hilberath, J N., Carlo, T., Pfeffer, M. A., Croze, R. H., Hastrup, F., Levy, B. D. Resolution of Toll-like receptor 4-mediated acute lung injury is linked to eicosanoids and suppressor of cytokine signaling 3.
Keywords: inflammation, lung elastance, vascular permeability, lipid mediators, macrophages
Acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) are life-threatening disorders that contribute significantly to critical illness (1, 2). Up to 75,000 patients die from ARDS each year in the United States (3). In response to lung injury, permeability of the alveolar-capillary barrier is increased (4), and there is an exuberant acute inflammatory response. These changes result in influx of protein-rich edema fluid and accumulation of activated leukocytes, in particular, polymorphonuclear leukocytes (PMNs) that fill alveoli and impair gas exchange (1, 5, 6). Host protective responses to restrain acute inflammation in ALI/ARDS and to promote its resolution are currently not well understood. Thus, treatment options for ALI/ARDS are still limited to supportive measures and represent a major unmet clinical need (1).
Toll-like receptors (TLRs) are critical effector mechanisms in innate immunity that recognize specific microbial components and initiate acute inflammatory responses (7). Recently, TLR4 signaling was linked to the pathogenesis of sterile tissue injury in acid-induced ALI (8). Nonenzymatic oxidation of phospholipids triggered lung injury via local cytokine production in a TLR4-dependent manner. Suppressor of cytokine signaling (SOCS) proteins are intracellular immune regulators that modulate TLR signaling cascades by altering the quality and quantity of signal transducer and activator of transcription (STAT) signals from cytokine receptors (9). SOCS proteins are induced in alveolar macrophages and other immune cells by several soluble stimuli, including cytokines, and act in classical negative-feedback loops to inhibit cytokine signal transduction (9, 10). In particular, SOCS3 has been linked to the pathogenesis of sepsis (11) and immune complex-mediated ALI (12). Macrophages are activated during ALI (13) and contribute to both the initiation and resolution of inflammatory responses (14, 15). Activation of pulmonary leukocytes leads to increased generation of eicosanoids that can serve as important mediators of inflammation, vascular leak, and tissue catabasis (16, 17).
Here, we used a self-limited and nonlethal model of acid-induced ALI to determine the effect of TLR4 and its downstream effectors on ALI and its resolution.
MATERIALS AND METHODS
Animals
All studies were reviewed and approved by the Harvard Medical Area standing committee on animals. Male 8- to 12-week-old C3H/HeJ (HeJ) and wild-type matched C3H/HeOuJ (OuJ) mice (Jackson Laboratories, Bar Harbor, ME, USA) were maintained in a barrier facility under specific pathogen-free conditions. HeJ mice exhibit resistance to lipopolysaccharide (LPS) due to a spontaneous mutation at the LPS response locus in the intracellular domain of TLR4 (Tlr4LPS-d), which is not shared by control OuJ mice that express the wild-type TLR4 receptor (18). Given the presence of additional genetic differences between HeJ and OuJ mice, select experiments were performed with congenic C.C3-Tlr4Lps-d/J mice (C.C3) that have the C3H/HeJ point mutation in Tlr4 in the BALB/cByJ (BALB) background (Jackson Laboratories; ref. 19). In view of the TLR4 defect in HeJ and C.C3 mice, the microbiome was determined in stool samples diluted in PBS (n=3) from all strains using CHROMagar Orientation plates (Becton Dickinson, Franklin Lakes, NJ, USA). Enterococci predominated, with minor variation in less abundant bacteria (Supplemental Fig. S1). In addition, there was no detectable endotoxin in samples of bronchoalveolar lavage fluid (BALF) or serum as measured by the Limulus amebocyte lysate test (Lonza, Walkersville, MD, USA; limit of detection 0.25 endotoxin units/ml).
Acid-initiated ALI
Mice were anesthetized with i.p. injections of ketamine (80 mg/kg body wt; Phoenix Scientific, St. Joseph, MO, USA) and xylazine (10 mg/kg body wt; Phoenix Scientific). Hydrochloric acid (0.1 N HCl, pH 1.5, 50 μl, endotoxin free; Sigma-Aldrich, St. Louis, MO, USA) was instilled selectively into the left lung, followed by a bolus of air (150 μl), as described previously (20). At timed intervals (2, 12, 24, 48 h), anesthetized mice (pentobarbital sodium, 70 mg/kg body wt i.p.; Abbott Laboratories, North Chicago, IL, USA) were mechanically ventilated with a flexiVent small animal ventilator (Scireq, Montreal, QC, Canada) to determine airway mechanics. Whole-lung BALF was obtained at the same time points (1 ml PBS with 0.6 mM EDTA×2). In some animals, lung tissue was snap-frozen for RNA or protein analysis. Total cells in BALFs were counted using a hemocytometer, and differential cell counts were determined after cytospin using Wright-Giemsa staining. Some animals received recombinant murine interleukin-6 (rmIL-6; 2 μg; Biolegend, San Diego, CA, USA) in 1% BSA or vehicle intranasally 2 h after initiation of ALI. Cell-free BALFs were portioned into aliquots and kept at −80°C.
Endothelial permeability
Evans Blue dye was utilized as a marker for endothelial barrier function (21). At 30 min prior to termination of the experiments, Evans Blue dye (40 mg/kg) was injected via tail vein, and extravasation of dye into BALFs was quantified by spectrophotometry (absorbance at 650 nm). In some experiments, HeJ mice were given montelukast (10 μg) or vehicle (0.5% ethanol) in 0.1 ml sterile 0.9% saline (i.v.) 30 min before initiation of ALI. Montelukast sodium was isolated from Singulair tablets by extraction and chromatography at Berlex Biosciences (Richmond, CA, USA). Chemical and NMR analytical data were consistent with the desired structure (as in ref. 22).
Measurement of mediators in BALFs
The amounts of lipoxin A4 (LXA4; Neogen, Lexington, KY, USA), cysteinyl leukotrienes (CysLTs) and 8-isoprostane (Cayman Chemicals, Ann Arbor, MI, USA) were measured in BALFs using specific ELISA kits, according to the manufacturer's instructions. Select cytokines and chemokines were measured in BALFs by protein bead array (Aushon Biosystems, Billerica, MA, USA).
Quantitative PCR
Total RNA was extracted from snap-frozen left lung using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). Total RNA (2 μg) was reverse transcribed using TaqMan qPCR gene expression assays (Applied Biosystems, Foster City, CA, USA), according to the manufacturer's instructions. TaqMan probes specific for murine SOCS1 and SOCS3 were used as target genes and murine cyclophilin A served as the reference gene. Cycle threshold (CT) values were determined in triplicate using an Applied Biosystems 7300 real-time PCR system, and relative expression was analyzed using the comparative 2−ΔΔCT method.
Western blot analysis
Analysis of SOCS1, SOCS3, STAT3, and phosphoSTAT3 protein was performed by Western blot analysis. β-Actin was used as a control. Antibodies were used in the manufacturer's recommended concentrations (1 μg/ml; Abcam, Cambridge, MA, USA and Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Lung histopathology
After dissection, lungs were perfusion fixed at 20 cmH2O in IHC zinc fixative buffer (BD Pharmingen, San Diego, CA, USA). Tissue blocks were obtained from midsagittal slices of lungs embedded in paraffin. After deparaffinization, tissue sections were incubated for 1 h at room temperature with LY-6G (1:100 dilution, BD Pharmingen), or SOCS3 (1:200 dilution, Abcam), followed by horseradish peroxidase-conjugated secondary antibody. H&E stains were also obtained.
Macrophage transfection
The murine alveolar cell line AMJ2-C8 was obtained from American Type Culture Collection (ATCC; Manassas, VA, USA). Macrophages were plated 24 h (37°C, 5% CO2) at 1 × 106 cells/ml in culture plates containing serum-free modified Eagle's medium (MEM) supplemented with 10% FBS (ATCC) and penicillin-streptomycin. Chariot (Active Motif, Carlsbad, CA, USA), a noncytotoxic protein transfection reagent (23–25), was used to transfer SOCS3 antibody and rabbit IgG into the cells, according to manufacturer's directions. This approach was chosen because of the potential for posttranscriptional regulation of SOCS3. Macrophages were transfected with anti-SOCS3 or polyclonal IgG antibody (1 μg/105 cells) for 2 h. After transfection, cells were exposed to 1 μg/ml LPS (Escherichia coli 055:B5; Sigma-Aldrich) or vehicle (H2O) for 4–24 h (37°C, 5% CO2).
Statistical analysis
Numerical results are expressed as means ± se. ANOVA was used to determine significance for differences between >2 groups. For analyses between 2 groups, cohorts were compared by Student's t test. Values of P < 0.05 were considered significant. Statistics were performed using GraphPad Prism 5 for Windows (GraphPad, San Diego, CA, USA).
RESULTS
TLR4 influences the time course for acid-initiated ALI and its resolution
To determine the time course for host responses to ALI, a self-limited experimental model of gastric acid aspiration was used. Sterile hydrochloric acid (0.1 N HCl, pH 1.5, 50 μl) was instilled selectively into the left main stem bronchus. This nonlethal model of ALI did not require mechanical ventilation for survival and provided an experimental window into cellular and molecular mechanisms of resolution. After acid instillation, lung elastance (E) and dynamic resistance (R) were measured (flexiVent) at baseline and 2, 12, 24, and 48 h after initiation of ALI (Fig. 1A, B). E and R values significantly increased and peaked 24 h (Φmax) after ALI in wild-type OuJ mice, returning to near-baseline levels at 48 h. HeJ mice have a TLR4-receptor defect and were protected after ALI with significant relative decrements in both peak E and R at 24 h. The time to maximal response Φmax was 12 h for both E and R in HeJ mice, and the resolution interval (Ri, defined as the time from Φmax to half-maximal response) for these parameters was ∼6 h. These measures of organ injury, Φmax, and resolution, Ri, in the TLR4-receptor defective HeJ mice were decreased by ∼50% compared to wild-type OuJ mice, indicating that TLR4 signaling both increases the extent of organ injury and delays its resolution.
Figure 1.
Effect of TLR4 on the kinetics of acid-initiated ALI and resolution. A, B) Lung elastance (A) and resistance (B) were determined at the indicated time points after acid instillation of HeJ mice that have TLR4 receptor mutation and wild-type OuJ mice. C) Evans Blue dye extravasation into BALF was determined 24 h after ALI. Values represent means ± se for n ≥ 3 at each time point. *P < 0.05 vs. 0 h; #P < 0.01 vs. OuJ; +P < 0.05 vs. OuJ.
Given these transient changes in lung mechanics, the effect of acid injury on airway barrier integrity was next determined. Leakage permeability changes were measured by extravasation of intravenous Evans Blue dye into BALFs. Wild-type OuJ mice had a significant increase in BALF Evans Blue dye 24 h after ALI (Fig. 1C). In contrast, HeJ mice did not display significant changes in endothelial permeability in response to lung injury at this time point, and the amounts of BALF Evans Blue dye were significantly less than in the OuJ mice (Fig. 1C), suggesting a more rapid restitution of barrier integrity in the HeJ mice.
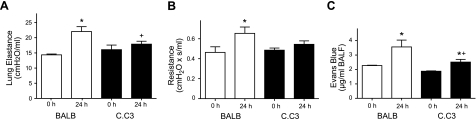
In addition to TLR4, HeJ and OuJ mice display other genetic differences that are not well characterized. To provide further evidence for TLR4 as a critical locus for regulation of host responses to sterile ALI, select experiments were performed with congenic C.C3-Tlr4Lps-d/J and control BALB/cByJ mice. Similar to the HeJ mice, the C.C3 mice were protected from acid-initiated ALI (at 24 h), with E and R significantly increased in only wild-type BALB mice (Fig. 2A, B). The effect of acid injury on endothelial permeability was also determined in both strains. While both wild-type BALB and TLR4-defective C.C3 mice showed a significant increase in BALF Evans Blue dye 24 h after ALI, the changes in permeability in the C.C3 strain were significantly less than in wild-type BALB mice (Fig. 2C).
Figure 2.
Effect of TLR4 on lung mechanics and endothelial leak in C.C3-Tlr4Lps-d/J congenic mice. A, B) Lung elastance (A) and resistance (B) were determined at 0 and 24 h in congenic C.C3-Tlr4Lps-d/J (solid bars) and BALB/cByJ mice (open bars). C) Evans Blue dye extravasation into BALF was also determined 24 h after ALI (see Materials and Methods). Values represent means ± se for n ≥ 3 at each time point. *P < 0.05 vs. 0 h; +P < 0.05 vs. BALB.
CysLTs lead to endothelial leak after ALI
CysLTs are potent vasoactive mediators (26), so their levels were determined during this self-limited model of ALI. Compared to HeJ mice, BALF levels of CysLTs were significantly increased in OuJ mice 24 h after acid instillation (OuJ vs. HeJ: 1008±321.6 vs. 206.8±253.7 pg/ml; P<0.05, n=4; Fig. 3A). To examine the connection between CysLTs and pulmonary endothelial permeability, the CysLT1 receptor antagonist montelukast was administered intravenously (10 μg in 100 μl) to OuJ mice 30 min prior to initiation of lung injury. There was significantly less extravasation of Evans Blue dye into BALFs in OuJ mice treated with montelukast relative to control animals (vehicle, 0.5% ethanol in 0.9% NaCl, 100 μl; Fig. 3B). Because LXA4 is an endogenous counterregulatory eicosanoid that blocks cysLT-mediated actions in the lung (22) and vasculature (27), BALF LXA4 levels were determined after ALI. Similar to CysLTs, LXA4 was significantly increased in OuJ mice compared to HeJ mice 24 h after ALI (OuJ vs. HeJ: 227.6±131.2 vs. 62±78.3 pg/ml; P<0.05, n=4; Fig. 3C). Of interest, the relative conversion of arachidonic acid to CysLTs rather than LXA4 was increased by ∼30% in the OuJ mice (mean CysLT/LXA4 ratio was 4.4 in OuJ vs. 3.3 in HeJ), favoring increased vascular permeability changes with intact TLR4 signaling.
Figure 3.
TLR4 signaling induces cysteinyl leukotriene-mediated vascular leak after ALI. A) CysLT levels were determined by ELISA in BALF 24 h after ALI. B) Vascular leak was determined by Evans Blue dye extravasation after administration of the CysLT1-receptor antagonist montelukast (10 μg) or vehicle (EtOH) 30 min prior to ALI. C) LXA4 levels were determined in BALF 24 h after ALI by ELISA. Values represent means ± se for n = 4. *P < 0.05 vs. HeJ, #P < 0.05 vs. vehicle.
TLR4 influences the time course for acid-initiated lung inflammation and its resolution
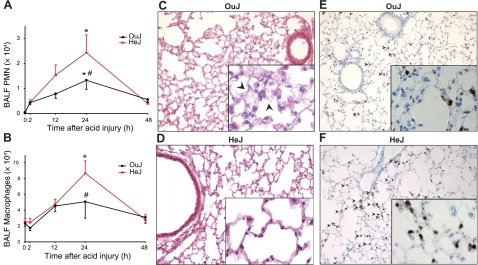
In contrast to the decreased perturbations in lung mechanics and permeability in the HeJ mice, acid-initiated ALI in these animals was not similarly associated with decreased inflammation (Fig. 4). After ALI, leukocyte counts transiently increased with Φmax for BALF PMNs and macrophages at 24 h (Fig. 4A, B), which was concomitant with peak increases in E and R (Fig. 1A, B). Of interest, the modest increases in BALF PMNs and macrophages in HeJ mice 24 h after ALI were ∼2-fold greater than in wild-type OuJ mice 24 h after ALI (Fig. 4A, B). While Φmax for BALF PMNs and macrophages was increased in the HeJ mice, Ri was not significantly changed for these parameters of lung inflammation, indicating that TLR4 signaling in ALI leads to both proinflammatory and anti-inflammatory responses without significantly influencing the pace of leukocyte resolution.
Figure 4.
TLR4 influences the time course for acid-initiated lung inflammation. A, B) Leukocyte trafficking into the lung was measured in BALF by enumeration of PMNs (A) and macrophages (B). Values represent means ± se for n ≥ 3 at each time point. *P < 0.05 vs. 0 h; #P < 0.01 vs. OuJ. C–F) Representative (n=3) lung tissue sections from ALI injured OuJ (C, E) and HeJ (D, F) mice were obtained from fixed, paraffin-embedded lung tissue and stained with either hematoxylin and eosin (C, D) or Ly-6G (1:100 dilution; E, F). Arrowheads indicate thickening of alveolar septa and interstitium with a modest increase in leukocyte trafficking in OuJ mice in H&E stains; accumulation of PMNs in Ly-6G stains. Original view: ×200 (main panels); ×400 (insets).
Lung histopathology 24 h after ALI revealed pulmonary edema as the predominant consequence of acid injury, including thickening of the alveolar septa and interstitium that was concomitant with peak changes in airway mechanics (Fig. 4C). The alveolar and interstitial changes were less marked in HeJ mice (Fig. 4D). Relative to uninjured lung (data not shown), Ly-6G immunostaining identified an increase in leukocyte trafficking in OuJ mice 24 h after ALI, and, consistent with the BALF analyses (Fig. 4), HeJ mice had modestly increased lung PMNs at this time point (Fig. 4E, F).
TLR4 signaling in ALI regulates cytokine and SOCS3 expression
BALF levels of interleukin-6 (IL-6) were significantly increased in OuJ mice at 2 and 12 h after ALI compared to baseline and HeJ mice (Supplemental Fig. S2A). To determine whether this cytokine would increase lung injury (i.e., lung elastance), recombinant murine IL-6 (rmIL-6, 2 μg, i.n.) was administered to HeJ mice 2 h after ALI. Animals receiving rmIL-6 displayed significantly increased airway PMNs 24 h after ALI, but no significant changes in lung elastance were elicited (Supplemental Fig. S2B, C). BALF levels of additional mediators linked to ALI were also determined by protein bead array, and strain-specific differences were present for keratinocyte chemoattractant (KC) and monocyte chemotactic protein-1 (MCP-1) (Supplemental Fig. S3). Because the levels of KC were discordant with BALF PMN numbers, the kinetics for KC production in this model were not further examined. BALF LTB4 was also detected 24 h after ALI, but only in low picogram amounts in both cohorts (OuJ vs. HeJ: 5.7±2.7 vs. 1.8±0.2 pg/ml; P<0.05, n=4).
Without significant differences in BALF cytokine levels, the SOCS family proteins, which regulate cytokine signaling, were next examined. Acid-initiated ALI induced a marked increase in lung SOCS3 expression in both mouse strains as early as 2 h after injury (Fig. 5A). Of interest, SOCS3 expression in HeJ mice remained markedly increased 12 h after ALI, while SOCS3 expression in the OuJ mice decreased substantially at this time point (ΔCT, OuJ vs. HeJ: 6.6±0.26 vs. 11.03±1.96; P<0.05). In addition, 24 h after acid-initiated ALI, SOCS3 expression was still 4-fold increased over baseline in HeJ mice. For purposes of comparison, expression of the related gene SOCS1 was also determined and found to be increased early (2 h) after ALI and decreased with time. Relative to SOCS3, these changes in SOCS1 expression were of much smaller amplitude, and no significant differences between genotypes were present (Fig. 5B). Western blot analyses confirmed the increased SOCS3 expression in HeJ mice 12 h after ALI (Fig. 5C). SOCS1, as well as STAT3 protein, a pivotal intracellular signaling checkpoint for SOCS proteins (reviewed in ref. 28), was not significantly increased in HeJ mouse lung compared to OuJ mice (Fig. 5C). Immunohistochemistry 24 h after ALI revealed increased lung SOCS3 expression in HeJ mice in the cytoplasm of both airway lining epithelial cells and alveolar macrophages (Fig. 5D, E).
Figure 5.
Effect of TLR4 on SOCS3 expression in ALI. A, B) SOCS3 (A) and SOCS1 (B) expression in whole-lung homogenates from acid-injured mice was determined by real-time PCR at indicated time points after ALI. Data are expressed as fold change in transcript over 0 h baseline expression (defined as 1 and marked by horizontal line) and represent means for n ≥ 3 at each time point. C) Western blot at 12 h after ALI for n = 3 OuJ (left) and HeJ (right) mice, with densitometric analysis of protein levels relative to β-Actin (bottom panel). Values represent means ± sd. *P < 0.05 vs. HeJ. D, E) Representative lung tissue sections were obtained 24 h after ALI and stained for SOCS3 (1:200 dilution) in OuJ (D) and HeJ (E) mice. Arrowheads indicate staining of alveolar macrophages and airway lining cells. Original view: ×200 (main panels); ×400 (insets).
SOCS3 regulates alveolar macrophage responses to soluble stimuli
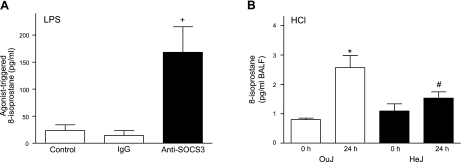
Because of the reported relationship between TLR4 signaling in ALI and oxidative stress (8) and now in view of the change in SOCS3 expression in alveolar macrophages (Fig. 5), we investigated a potential link between TLR4, SOCS3, and oxidative stress by introducing an anti-SOCS3 antibody into murine alveolar macrophages (AMJ2-C8 cell line) to regulate SOCS3 function in these cells. First, to determine the effect of the introduction of this anti-SOCS3 antibody on cell signaling, cells were exposed to the TLR4-agonist LPS (E. coli 055:B5, 1 μg/ml) for 4 h (37°C, 5% CO2) and phosphorylation of STAT3 on tyrosine 705 (pYSTAT3) was quantitated by densitometry on immunoblot. Cell activation by LPS increased the ratio of pYSTAT3 to STAT3, and introduction of anti-SOCS3 led to further increases in pYSTAT3 that were significantly greater than the changes in cells receiving control IgG (0.35 ± 0.08 vs. 0.14 ± 0.05 densitometric units, respectively; P<0.03, n=6), indicating that this anti-SOCS3 antibody displayed blocking properties for SOCS3 signaling. Next, in the presence of anti-SOCS3 or control antibody, the AMJ2-C8 cells were exposed to LPS (E. coli 055:B5, 1 μg/ml) for 24 h (37°C, 5% CO2), and levels of 8-isoprostane, a sensitive marker of oxidative stress (29), were determined in cell supernatants. After transfection of anti-SOCS3 antibody, LPS initiated a significant increase in 8-isoprostane relative to cells transfected with polyclonal IgG or unstimulated control cells (Fig. 6A). In addition, 8-isoprostane levels were significantly increased in BALFs 24 h after ALI in the OuJ mice compared to baseline and relative to HeJ mice (Fig. 6B).
Figure 6.
SOCS3 regulates oxidative stress in LPS-activated murine alveolar macrophages. A) Anti-SOCS3 or control IgG was introduced into AMJ2-C8 cells (1×105), and cells were exposed to LPS (1 μg/ml) for 24 h (37°C, 5% CO2). 8-Isoprostane formation was determined in cell-free supernatants by ELISA and compared to control cells unexposed to protein transfection reagents. +P < 0.05 vs. other groups. B) 8-Isoprostane levels in BALF obtained 24 h after ALI in OuJ (open bars) and HeJ (solid bars) mice were determined by ELISA. Values represent means ± se for n ≥ 3. *P < 0.05 vs. 0 h; #P < 0.05 vs. OuJ.
DISCUSSION
TLR4 is well appreciated for its role in microbial host defense, but there is growing evidence for a broader role in regulating innate immune responses (7). Here, TLR4 played a pivotal role in regulating not only the development of sterile acid-induced ALI, but also its resolution. In particular, TLR4-intact mice had more marked increases in lung elastance, lung resistance, and vascular permeability after ALI. TLR4-defective mice had significantly decreased Φmax and Ri for lung elastance, indicating that injury was dampened and ALI resolution was facilitated. Acid-initiated ALI triggered the enzymatic conversion of arachidonic acid to CysLTs, and the transient ALI-induced changes in vascular leak were CysLT1 receptor dependent. In the HeJ mice, there was a modest, yet paradoxical, increase in lung peak inflammation Φmax for both PMNs and macrophages, but no significant changes were evident in Ri for those parameters compared to the wild-type OuJ mice. Select BALF cytokines and SOCS expression in the lung after ALI differed between the genotypes. Significant increases in lung SOCS3 expression 2 h after ALI were present in both OuJ and HeJ mice, but persisted only in the HeJ mice. Delivery of an anti-SOCS3 antibody into alveolar macrophages linked SOCS3 to regulation of TLR4-driven oxidative stress. Together, these findings indicate a pivotal role for TLR4 in mediating lung response to sterile injury and suggest roles for eicosanoids in provoking inflammation and vascular leak during ALI and SOCS3 in the regulation of oxidative stress to promote resolution.
In addition to prominent roles in innate immunity, TLRs also participate in host responses to noninfectious stimuli, including tissue injury (30, 31). In ARDS, TLRs are selectively regulated. Decreased expression of TLR4 in the early phase of ARDS is more common in disease survivors (32) and in the self-limited ALI model used here, a defect in TLR4 signaling lessened ALI severity and accelerated ALI resolution. Of interest, discordant measures of inflammation and disease severity have also been observed in TLR3-deficient mice (33). These findings point to a direct role TLR signaling in lung resident cells in responses to injury that is distinct from the regulation of subsequent leukocyte trafficking. Macrophages are vital to clearance of inflammatory cells and debris from injured tissue (15, 34), and alveolar macrophages express TLR4 (35). BALF macrophages were significantly increased after ALI in TLR4-receptor defective HeJ mice, highlighting the tissue protective roles for these cells during resolution of ALI.
SOCS3 is an important intracellular regulator of TLR4 signaling (36) and has been linked to the genesis of ALI (37). SOCS3-deficient mice do not survive past day 13 of gestation due to placental insufficiency (38), so were not available to us for study. SOCS3 is one of the most abundantly induced proteins in macrophages following exposure to LPS (39) and can negatively regulate inflammatory cytokine production, NF-кB-dependent transcription, and apoptosis (39, 40). Here, ALI induced SOCS3 and regulation of cytokine levels. Oxidized phospholipids induce cytokine production in ALI via TLR4 activation and oxidative stress can exacerbate ALI (8). 8-Isoprostane is a nonenzymatic product of arachidonate metabolism that serves as a sensitive marker of oxidative stress and has been detected in exhaled breath condensates from patients with ALI and ARDS (41). Mice with a TLR4 defect displayed lower BALF levels of 8-isoprostane, and introduction of a blocking anti-SOCS3 antibody markedly increased 8-isoprostane generation by TLR4-activated macrophages, indicating that SOCS3 can protect from the propagation of ALI by regulating oxidative stress. Thus, the discordance between improved lung mechanics and increased BALF leukocytes after ALI in HeJ mice could be explained by increased macrophage recruitment and SOCS3 expression, leading to blunting of oxidative stress. TLR4-independent mechanisms for regulating alveolar fluid clearance and PMN and macrophage trafficking in ALI were recently assigned to adenosine and Netrin-1-mediated regulation of A2B adenosine receptors (4, 46, 47). Together, these findings are consistent with a growing recognition of resolution macrophages that have distinct patterns of receptor expression and function (14).
In addition to serving as a biomarker for oxidative stress, 8-isoprostane indicates that arachidonic acid was released during ALI and LPS activation of murine alveolar macrophages. The CysLTs (LTC4, D4, and E4) are products of arachidonic acid metabolism that can potently regulate cell function (as reviewed in ref. 26) and were generated in substantial amounts after ALI by TLR4 intact mice. Inhibition of LT generation protects mice from ALI (42), and here, defective TLR4 signaling in ALI markedly decreased CysLT generation. Montelukast is a CysLT1 receptor antagonist that protected OuJ animals from ALI-induced endothelial permeability. The anti-inflammatory and proresolving eicosanoid LXA4 can serve as an endogenous counterregulatory signal for CysLT-mediated host responses and as a CysLT1 receptor antagonist to block CysLT-mediated vascular events (27). While TLR4-defective animals had decreased BALF levels of LXA4, the conversion of arachidonic acid to provocative CysLTs relative to organ protective LXA4 was also decreased in these mice, which would serve to lessen the LT-mediated vascular permeability changes. LXs also regulate differentiated bronchial epithelial cell responses to acid injury via cognate LXA4 receptors (43), and transgenic mice expressing the human LXA4 receptor display a more rapid resolution of ALI (20). Statins can decrease mucosal inflammation in ALI via the generation of 15-epi-lipoxin A4 (44), which can also serve as a receptor antagonist for CysLT1 and ligand for LXA4 receptors (27, 45). Together, these findings point to eicosanoid generation as an important regulatory program for vascular permeability after TLR4 activation in ALI.
In summary, our results indicate that TLR4 signaling is integral to innate responses to sterile tissue injury in ALI. Mice defective in TLR4 function responded to ALI with decreased perturbations in tissue elastance and resistance that resolved more rapidly. These changes were related, in part, to decreased vascular permeability changes and oxidative stress, despite modest increases in lung leukocytes. Induction of vascular permeability in ALI was linked to CysLT-CysLT1 receptor signaling, and ALI resolution was associated with increased expression of SOCS3 that can regulate oxidative stress. Together, these findings highlight important sentinel roles for TLRs in tissue injury, as well as potential proresolving roles for SOCS3 and macrophages in ALI.
Supplementary Material
Acknowledgments
The authors acknowledge Bonna Ith for expert help with histology, and Anton Serhan for assistance with the transfection experiments.
This study was supported by departmental funds (J.N.H.) and National Institutes of Health grants HL068669 and HL090927 (B.D.L.). B.D.L. is a coinventor on patents on the use of lipoxins in lung disease that are assigned to Brigham and Women's Hospital, which have been licensed for clinical development.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Ware L. B., Matthay M. A. (2000) The acute respiratory distress syndrome. N. Engl. J. Med. 342, 1334–1349 [DOI] [PubMed] [Google Scholar]
- 2. Marik P. E. (2001) Aspiration pneumonitis and aspiration pneumonia. N. Engl. J. Med. 344, 665–671 [DOI] [PubMed] [Google Scholar]
- 3. Rubenfeld G. D., Caldwell E., Peabody E., Weaver J., Martin D. P., Neff M., Stern E. J., Hudson L. D. (2005) Incidence and outcomes of acute lung injury. N. Engl. J. Med. 353, 1685–1693 [DOI] [PubMed] [Google Scholar]
- 4. Eckle T., Grenz A., Laucher S., Eltzschig H. K. (2008) A2b adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J. Clin. Invest. 118, 3301–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reutershan J., Basit A., Galkina E. V., Ley K. (2005) Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L807–L815 [DOI] [PubMed] [Google Scholar]
- 6. Zarbock A., Singbartl K., Ley K. (2006) Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J. Clin. Invest. 116, 3211–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akira S., Takeda K. (2004) Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 [DOI] [PubMed] [Google Scholar]
- 8. Imai Y., Kuba K., Neely G. G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y. H., Wang H., Liu H., Sun Y., Pasparakis M., Kopf M., Mech C., Bavari S., Peiris J. S., Slutsky A. S., Akira S., Hultqvist M., Holmdahl R., Nicholls J, Jiang C., Binder C. J., Penninger J. M. (2008) Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133, 235–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshimura A., Naka T., Kubo M. (2007) Socs proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 7, 454–465 [DOI] [PubMed] [Google Scholar]
- 10. Starr R., Willson T. A., Viney E. M., Murray L. J., Rayner J. R., Jenkins B. J., Gonda T. J., Alexander W. S., Metcalf D., Nicola N. A., Hilton D. J. (1997) A family of cytokine-inducible inhibitors of signalling. Nature 387, 917–921 [DOI] [PubMed] [Google Scholar]
- 11. Fang M., Dai H., Yu G., Gong F. (2005) Gene delivery of socs3 protects mice from lethal endotoxic shock. Cell. Mol. Immunol. 2, 373–377 [PubMed] [Google Scholar]
- 12. Gao H., Hoesel L. M., Guo R. F., Rancilio N. J., Sarma J. V., Ward P. A. (2006) Adenoviral-mediated overexpression of SOCS3 enhances IgG immune complex-induced acute lung injury. J. Immunol. 177, 612–620 [DOI] [PubMed] [Google Scholar]
- 13. Frank J. A., Wray C. M., McAuley D. F., Schwendener R., Matthay M. A. (2006) Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L1191–L1198 [DOI] [PubMed] [Google Scholar]
- 14. Bystrom J., Evans I., Newson J., Stables M., Toor I., van Rooijen N., Crawford M., Colville-Nash P., Farrow S., Gilroy D. W. (2008) Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by camp. Blood 112, 4117–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freire-de-Lima C. G., Xiao Y. Q., Gardai S. J., Bratton D. L., Schiemann W. P., Henson P. M. (2006) Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and no synthesis in murine macrophages. J. Biol. Chem. 281, 38376–38384 [DOI] [PubMed] [Google Scholar]
- 16. Serhan C. N. (2000) Preventing injury from within, using selective cpla2 inhibitors. Nat. Immunol. 1, 13–15 [DOI] [PubMed] [Google Scholar]
- 17. Serhan C. N. (2007) Resolution phase of inflammation: Novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 25, 101–137 [DOI] [PubMed] [Google Scholar]
- 18. Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 [DOI] [PubMed] [Google Scholar]
- 19. Vogel S. N., Wax J. S., Perera P. Y., Padlan C., Potter M., Mock B. A. (1994) Construction of a BALB/c congenic mouse, C.C3H-Lpsd, that expresses the Lpsd allele: analysis of chromosome 4 markers surrounding the Lps gene. Infect. Immun. 62, 4454–4459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukunaga K., Kohli P., Bonnans C., Fredenburgh L. E., Levy B. D. (2005) Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J. Immunol. 174, 5033–5039 [DOI] [PubMed] [Google Scholar]
- 21. Udaka K., Takeuchi Y., Movat H. Z. (1970) Simple method for quantitation of enhanced vascular permeability. Proc. Soc. Exp. Biol. Med. 133, 1384–1387 [DOI] [PubMed] [Google Scholar]
- 22. Levy B. D., Lukacs N. W., Berlin A. A., Schmidt B., Guilford W. J., Serhan C. N., Parkinson J. F. (2007) Lipoxin a4 stable analogs reduce allergic airway responses via mechanisms distinct from cyslt1 receptor antagonism. FASEB J. 21, 3877–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morris M. C., Depollier J., Mery J., Heitz F., Divita G. (2001) A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 19, 1173–1176 [DOI] [PubMed] [Google Scholar]
- 24. Peddibhotla S., Lam M. H., Gonzalez-Rimbau M., Rosen J. M. (2009) The DNA-damage effector checkpoint kinase 1 is essential for chromosome segregation and cytokinesis. Proc. Natl. Acad. Sci. U. S. A. 106, 5159–5164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keller M., Lidington D., Vogel L., Peter B. F., Sohn H. Y., Pagano P. J., Pitson S., Spiegel S., Pohl U., Bolz S. S. (2006) Sphingosine kinase functionally links elevated transmural pressure and increased reactive oxygen species formation in resistance arteries. FASEB J. 20, 702–704 [DOI] [PubMed] [Google Scholar]
- 26. Rovati G. E., Capra V. (2007) Cysteinyl-leukotriene receptors and cellular signals. Sci. World J. 7, 1375–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gronert K., Martinsson-Niskanen T., Ravasi S., Chiang N., Serhan C. N. (2001) Selectivity of recombinant human leukotriene d(4), leukotriene b(4), and lipoxin a(4) receptors with aspirin-triggered 15-epi-lxa(4) and regulation of vascular and inflammatory responses. Am. J. Pathol. 158, 3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murray P. J. (2007) The JAK-STAT signaling pathway: Input and output integration. J. Immunol. 178, 2623–2629 [DOI] [PubMed] [Google Scholar]
- 29. Morrow J. D, Frei B., Longmire A. W., Gaziano J. M., Lynch S. M., Shyr Y., Strauss W. E., Oates J. A., Roberts L. J., 2nd. (1995) Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N. Engl. J. Med. 332, 1198–1203 [DOI] [PubMed] [Google Scholar]
- 30. Marshak-Rothstein A. (2006) Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6, 823–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trompette A., Divanovic S., Visintin A., Blanchard C., Hegde R. S., Madan R., Thorne P. S., Wills-Karp M., Gioannini T. L., Weiss J. P., Karp C. L. (2009) Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457, 585–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramirez Cruz N. E., Maldonado Bernal C., Cuevas Uriostegui M. L., Castanon J., Lopez Macias C., Isibasi A. (2004) Toll-like receptors: dysregulation in vivo in patients with acute respiratory distress syndrome. Rev. Alerg. Mex. 51, 210–217 [PubMed] [Google Scholar]
- 33. Cavassani K. A., Ishii M., Wen H., Schaller M. A., Lincoln P. M., Lukacs N. W., Hogaboam C. M., Kunkel S. L. (2008) Tlr3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J. Exp. Med. 205, 2609–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gordon S. (2003) Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35 [DOI] [PubMed] [Google Scholar]
- 35. Garantziotis S., Li Z., Potts E. N., Lindsey J. Y., Stober V. P., Polosukhin V. V., Blackwell T. S., Schwartz D. A., Foster W. M., Hollingsworth J. W. (2009) Tlr4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am. J. Respir. Crit. Care Med. 181, 666–675 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 36. Liu X., Zhang Y., Yu Y., Yang X., Cao X. (2008) SOCS3 promotes TLR4 response in macrophages by feedback inhibiting TGF-beta1/Smad3 signaling. Mol. Immunol. 45, 1405–1413 [DOI] [PubMed] [Google Scholar]
- 37. Ma S. F., Grigoryev D. N., Taylor A. D., Nonas S., Sammani S., Ye S. Q., Garcia J. G. (2005) Bioinformatic identification of novel early stress response genes in rodent models of lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L468–L477 [DOI] [PubMed] [Google Scholar]
- 38. Roberts A. W., Robb L., Rakar S., Hartley L., Cluse L., Nicola N. A., Metcalf D., Hilton D. J., Alexander W. S. (2001) Placental defects and embryonic lethality in mice lacking suppressor of cytokine signaling 3. Proc. Natl. Acad. Sci. U. S. A. 98, 9324–9329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoshimura A. (2009) Regulation of cytokine signaling by the SOCS and Spred family proteins. Keio J. Med. 58, 73–83 [DOI] [PubMed] [Google Scholar]
- 40. Jo D., Liu D., Yao S., Collins R. D., Hawiger J. (2005) Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat. Med. 11, 892–898 [DOI] [PubMed] [Google Scholar]
- 41. Carpenter C. T., Price P. V., Christman B. W. (1998) Exhaled breath condensate isoprostanes are elevated in patients with acute lung injury or ARDS. Chest 114, 1653–1659 [DOI] [PubMed] [Google Scholar]
- 42. Nagase T., Uozumi N., Ishii S., Kume K., Izumi T., Ouchi Y., Shimizu T. (2000) Acute lung injury by sepsis and acid aspiration: A key role for cytosolic phospholipase A2. Nat. Immunol. 1, 42–46 [DOI] [PubMed] [Google Scholar]
- 43. Bonnans C., Fukunaga K., Levy M. A., Levy B. D. (2006) Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am. J. Pathol. 168, 1064–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Planaguma A, Pfeffer MA, Rubin G, Croze R, Uddin M, Serhan CN, Levy B. D. Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A(4). Mucosal Immunol. 3, 270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chiang N., Serhan C. N., Dahlen S. E., Drazen J. M., Hay D. W., Rovati G. E., Shimizu T., Yokomizo T., Brink C. (2006) The lipoxin receptor alx: potent ligand-specific and stereoselective actions in vivo. Pharmacol. Rev. 58, 463–487 [DOI] [PubMed] [Google Scholar]
- 46. Reutershan J., Vollmer I., Stark S., Wagner R., Ngamsri K. C., Eltzschig H. K. (2009) Adenosine and inflammation: Cd39 and Cd73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 23, 473–482 [DOI] [PubMed] [Google Scholar]
- 47. Rosenberger P., Schwab J. M., Mirakaj V., Masekowsky E., Mager A., Morote-Garcia J. C., Unertl K., Eltzschig H. K. (2009) Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat. Immunol. 10, 195–202 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.