Abstract
The multi-kinase inhibitor sunitinib malate (SUT) has been reported to reduce levels of myeloid suppressor cells and Treg cells in cancer patients, hypothetically diminishing intrinsic impediments for active immunization against tumor-associated antigens in such individuals. The goal of this study was to identify longitudinal immune molecular and cellular changes associated with tumor regression and disease-free status after the treatment of established day 7 s.c. MO5 (B16.OVA) melanomas with SUT alone (1 mg/day via oral gavage for 7 days), vaccination using OVA peptide-pulsed DC (VAC) alone, or the combination of SUT and VAC (SUT/VAC). We observed superior anti-tumor efficacy for SUT/VAC combination approaches, particularly when SUT was applied at the time of the initial vaccination or the vaccine boost. Treatment effectiveness was associated with the acute loss of (and/or failure to recruit) cells bearing myeloid-derived suppressor cells (MDSC) or Treg phenotypes within the tumor microenvironment (TME) and the corollary, prolonged enhancement of Type-1 anti-OVA CD8+ T cell responses in the tumor-draining lymph node (TDLN) and the TME. Enhanced Type-1 T cell infiltration of tumors was associated with treatment-induced expression of VCAM-1 and CXCR3 ligand chemokines in vascular/peri-vascular cells within the TME, with SUT/VAC therapy benefits conditionally negated upon adminsitration of CXCR3 or VCAM-1 blocking antibodies. These data support the ability of a short 7 day course of SUT to (re)condition the TME to become more receptive to the recruitment and prolonged therapeutic action of (VAC-induced) anti-tumor Tc1 cells.
Keywords: Sunitinib, Vaccine, Dendritic Cell, Melanoma, Tumor Microenvironment
INTRODUCTION
Tumor progression has been linked with enhancement in lesional angiogenesis and both locoregional, as well as, systemic immune dysfunction (1–3), processes that may be supported by the coercion of receptor tyrosine kinase (RTK) signaling pathways (4, 5). In this light, the development of tyrosine kinase inhibitors (TKI) for the treatment of cancer has gained increasing inertia, with nearly a dozen FDA-approved agents now being investigated in clinical trials (6, 7). Despite an apparent lack of refined specificity among many kinase inhibitors, such agents have proven surprisingly safe (6). In particular, sunitinib (SUT), a VEGFR/PDGFR inhibitor (which interacts with more than 50 tyrosine kinases at nanomolar concentrations, ref. 6), has demonstrated clinical efficacy as a first-line therapeutic agent in the setting of renal cell carcinoma (RCC), via mechanisms that include the suppression of angiogenesis and inhibition of MDSC and Treg function in vivo (8–13). It has recently been suggested that these latter effects may be related to the inhibitory effects of SUT on c-Kit- and/or STAT3-mediated signaling (14–16).
Since cancer patients treated with SUT (or other anti-angiogenic agents such as bevacizumab) ultimately develop progressive disease that is resistant to re-treatment (9–13, 17, 18), the evolution of combinational therapies integrating such drugs is warranted. In this regard, “adjuvant-like” qualities have been suggested for SUT based on studies performed by Ozao-Choy et al. (14). In their transplantable murine MCA26 (H-2d) colon carcinoma model, SUT injections improved the anti-tumor effectiveness of co-treatment with IL-12 gene therapy along with a 4-1BB agonist antibody (14). The interruption of MDSC/Treg activity in cancer patients would be predicted to provide a window of opportunity for improving immune response to specific (active) vaccination against tumor-associated antigens; i.e. in tumor models in which Treg cells have been suppressed or deleted, enhanced immunoreactivity against coordinately applied VAC has been observed (19–23). Hence, barring any untoward effects of kinase inhibitors on anti-tumor T effector cell survival and function in vivo, one would expect that these inhibitors might serve as useful conditioning agents allowing for the more effective activation of specific immunity in tumor-bearing hosts. Furthermore, therapeutic normalization of the tumor vasculature has been associated with enhanced infiltration of lymphocytes into the TME (22, 23), which could allow for improved anti-tumor efficacy mediated by vaccine-induced T cells (11, 13, 19, 20, 24, 25).
Using a murine MO5 (B16.OVA) melanoma model, we now demonstrate that the combination of orally-administered SUT along with specific VAC results in superior therapeutic efficacy (when compared with either single modality) and the improved activation and recruitment of Type-1 anti-tumor T cells into the TME. The enhanced presence of vaccine-induced Type-1 immunity within tumors after combinational therapy coincided with a pronounced and sustained reduction in cells bearing regulatory (MDSC, Treg) phenotypes, as well as, an increased activation of tumor vascular endothelial cells (based on VCAM-1 and CXCR3 ligand expression) in the TME, which was shown to be requisite for the recruitment of anti-OVA CD8+ tumor-infiltrating lymphocytes (TIL) and treatment efficacy.
MATERIALS AND METHODS
Mice
Female 6–8 week old C57BL/6 (B6; H-2b) mice were purchased from the Jackson laboratory (Bar Harbor, ME) and maintained in micro-isolator cages. Animals were handled under aseptic conditions, per an Institutional Animal Care and Use Committee (IACUC)-approved protocol.
Cell lines and Media
The MO5 (B16.OVA; H-2b) melanoma has been described previously (24). This cell line was free of mycoplasma contamination and was maintained in complete media [CM: RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 mM L-glutamine (all reagents from Life Technologies, Inc., Grand Island, NY)] in a humidified incubator at 5% CO2 and 37°C.
Viral vector
The Ad.mIL12 recombinant adenoviral vector (25) encoding the p35 and p40 subunits of murine IL-12, and the control (empty) Ad.ψ5 virus were produced and provided by the University of Pittsburgh Cancer Institute Vector Core Facility (a Shared Resource).
Peptides
Peptides OVA257–264 (SIINFEKL) and OVA323–339 (ISQAVHAAHAEINEAGR) were synthesized by 9-fluorenylmethoxycarbonyl (Fmoc) chemistry by the University of Pittsburgh Cancer Institute's Peptide Synthesis Facility (a Shared Resource). Peptides were >96% pure based on high performance liquid chromatography profile and mass spectrometric analysis performed by the University of Pittsburgh Cancer Institute's Protein Sequencing Facility (a Shared Resource).
DC.IL12 Vaccines
DC were generated from BM precursors isolated from the tibias/femurs of C57BL/6 mice and then infected on day 5 of culture with recombinant adenovirus encoding IL-12p70 (Ad.mIL-12) as previously described (25). On day 7, IL-12 gene modified DC (DC.IL12) were loaded with a mixture of 10 μM of each of the OVA257–264 and OVA323–339 synthetic peptides (for 4h at 37°C and 5% CO2), which serve as CD8+ and CD4+ T cell epitopes, respectively, in C57BL/6 mice (26, 27).
Animal Experiments
C57BL/6 mice received s.c. injections with 2 × 105 MO5 (B16.OVA) tumor cells in the right flank on day 0. On day 7, the animals were randomized into cohorts of 5 mice each exhibiting average tumor sizes of approximately 30–50 mm3. Tumor-bearing mice were either left untreated or treated with s. c. injection of 1×106 DC.IL12/OVA in 50 μl PBS in the left flank on day 7, days 7 and 14, or days 14 and 21 post-tumor inoculation as indicated in text. SUT (SUTENT™, Pfizer, New York, NY) was dissolved in Labrasol (Gattefossé Canada Inc., Toronto, Canada) and administered daily (at doses of 0.1 – 1.0 mg in 50 μl of Labrasol), via oral gavage for 7–14 consecutive days, beginning on day 7, 14 or 21 post-tumor inoculation, as indicated in text. In some experiments, 200 μg of blocking antibodies against murine CXCR3 (CXCR3-173: hamster IgG; non-T cell depleting; the kind gift of Dr. Robert D. Schreiber, Washington University School of Medicine; ref. 28) or murine VCAM-1 (R & D Systems, Minneapolis, MN; rat IgG) or species/isotype-matched control antibodies (Santa Cruz Biotech., San Diego, CA) were injected i.p. at the time of initiating treatment and every 2 days thereafter through day 15 (i.e. days 7, 9, 11, 13 and 15 post-tumor inoculation) to determine impact on therapeutic outcome. In all cases, individual tumor sizes were then assessed every 3–4 days and recorded in mm3 as determined by the formula: tumor volume = 0.5 × a2 × b, in which a is the smallest and b, the largest (orthogonal) superficial diameter. All measurements were taken using vernier calipers, with data reported as mean tumor volume ± SD.
Antibodies, MHC/peptide tetramer and flow cytometry analysis
Phycoerythrin (PE)-conjugated H-2Kb/SIINFEKL tetramers were purchased from Beckman Coulter (Brea, CA) and used to stain cells per the manufacturer's protocol. Single cell suspensions derived from tumors, tumor-draining lymph nodes (TDLN), vaccine-draining lymph nodes (VDLN) and splenocytes were also stained using directly-conjugated Abs (all from BD Biosciences, San Jose, CA): FITC-anti-CD8, PE-anti-CD8, FITC-anti-CD4, PE-anti-Gr1, FITC-anti-CD11b, PE-anti-CD86, PE-anti-Foxp3, PE-anti-CTLA-4 and FITC-anti-PD-1. Intracellular staining for Foxp3 performed after first staining the cell surface with FITC-antiCD4 mAb. Briefly, cells were incubated in permeabilization/fixation buffer (eBioscience, San Diego, CA) for a minimum of 30 min. After washing with permeabilization buffer, cells were blocked with the anti-FcR mAb and then stained with PE-anti-Foxp3 or isotype control mAb for 30 min at 4°C. Cells were washed in permeabilization buffer and resuspended in PBS. Flow cytometry was performed using Cell Quest software and a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Data analysis was performed using FlowJo software (Tree Star Inc., Ashland, OR).
Evaluation of anti-OVA T Cell Responses
CD3+ T cells were isolated using specific MACS™ beads (Miltenyi Biotec) from single cell suspensions of (mechanically-disrupted) TDLN or (enzymatically-digested; ref. 25) TIL prepared 28 days after tumor inoculation. For all analyses, T cells were pooled from 3 animals per treatment cohort. After their isolation, T cells were restimulated in vitro for 5 days with irradiated (100 Gy) MO5 tumor cells at a T cell-to-tumor ratio of 10:1. Recovered T cells were then co-cultured alone or with syngenic DC pulsed with no peptide or the SIINFEKL peptide (10 μM for 4h at 37°C and 5% CO2) for 48h. Cell-free supernatants were then harvested and assessed for levels of mIFN-γ using a specific ELISA (BD Biosciences) with a lower limit of detection of 32.5 pg/ml. Data are reported as the mean ± SD of triplicate determinations.
RT-PCR
Total RNA was extracted from single cell suspensions obtained via the enzymatic digestion of resected day 7, 10, 14, 21 and 28 tumors using Trizol (Invitrogen, Carlsbad, CA) per the manufacturer's protocol. Reverse transcription-PCR (RT-PCR) was performed using the primer pairs listed in Table S1. Cycling times and temperatures were as follows: initial denaturation at 94°C for 2 min (1 cycle), denaturation at 94°C for 30 s, annealing at 60°C for 30 s and elongation at 72°C for 1 min (35–40 cycles), final extension at 72°C for 5 min (1 cycle). PCR products were identified by image analysis software for gel documentation (LabWorks Software; UVP) following electrophoresis on 1.5% agarose gels and staining with ethidium bromide.
Western Blot
Western blots were performed on lysates generated from single cell suspensions of day 28 tumors as previously described (25). Primary blotting Abs were directed against STAT-1, STAT-3, pSTAT-1 and pSTAT-3 (all from Santa Cruz Biotechnology, San Jose, CA) and (control) β-actin (Cambridge, MA). After washed blots were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (Santa Cruz Biotechnology), blots were visualized by Western Lighting chemiluminescence detection kit (Perkin-Elmer, Waltham, MA) and exposed to X-Omat film (Eastman Kodak, New Haven, CT) for 30 sec to 2 min. Densitometry analysis was performed using a GDS 8000 bioimaging system and LabWorks4.6 software (UVP, LLC, Upland, CA).
Fluorescence Imaging of Tumor Sections
Tumor tissue samples were prepared and sectioned as previously reported (25). All washing steps were performed using wash buffer (WB; 0.5% BSA in PBS, Sigma-Aldrich). All blocking steps were performed using 2% BSA. For analysis of T cell subsets, sections were incubated with rat-anti-mouse-CD4 (BD Biosciences) followed by Goat-anti-Rat CY3 conjugated Fab 1 Fragments (Jackson ImmunoResearch, West Grove, PA). Sections were then incubated with Alexa 488-conjugated rat-anti-mouse-Foxp3 (eBioscience). MDSC were defined using FITC-conjugated rat anti-mouse-CD11b and PE-conjugated rat-anti-mouse-Gr1 mAbs (BD Biosciences). For analysis of the CD31 versus VCAM-1 or CXCL9/Mig rat-anti-mouse-CD31 (BD Biosciences) was combined with either goat-anti-mouse-VCAM-1 or goat-anti-mouse-CXCL9 (both from R&D Systems Inc., Minneapolis, MN). Each combination was then treated with a mixture of Alexa 488-conjugated donkey-anti-rat (Invitrogen) and CY3-conjugated donkey-anti-goat CY3 (Jackson ImmunoResearch). All sections were treated with DAPI (Sigma-Aldrich) and then mounted. Images were acquired using either an Olympus 1000 Scanning Confocal Microscope or an Olympus BX 51 Fluorescent Microscope (both from Olympus America, Melville, NY). Metamorph (Molecular Devices, Sunnyvale, CA) software was used for cell quantification.
Statistical analysis
Student t-test and one-way ANOVA were used to test for overall differences among the groups (StatMate III, ATMS Co., Tokyo, Japan), with a p value < 0.05 considered significant.
RESULTS
Combination therapy using SUT and specific DC-based vaccination provides superior anti-tumor efficacy against s.c. MO5 melanomas
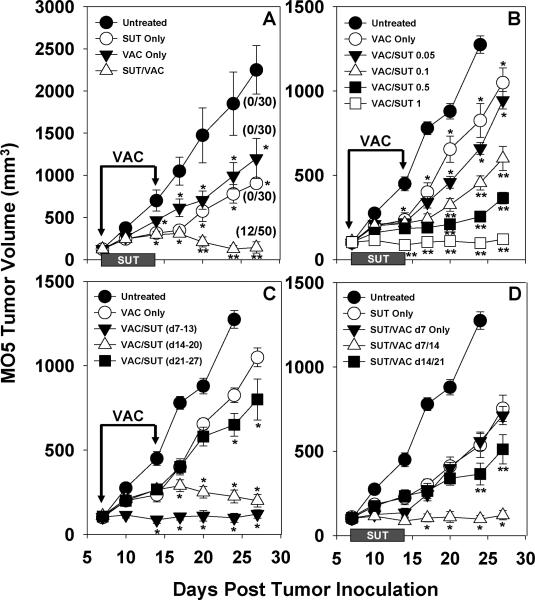
C57BL/6 mice harboring established day 7 s.c. MO5 (B16.OVA) melanomas were left untreated or they were administered SUT (1 mg/day via oral gavage for 7 consecutive days) and/or a syngenic cellular vaccine (DC.IL12 pulsed with the OVA257–264 and OVA323–339 peptide epitopes) on days 7 and 14 post-tumor inoculation. As shown in Fig. 1A, a seven day course of SUT alone or a single course of DC-based VAC monotherapy yielded partial protection against melanoma progression (p < 0.05 versus the untreated control for all time points after day 10). We also observed that each combination therapy provided superior protection vs. either of the single modality treatments (p < 0.05 versus single modality therapy for time points after day 17). Notably, only the combination treatment (SUT/VAC) cohort contained regressor animals, with 24% (i.e. 12/50) of mice rendered tumor-free through day 60 post-tumor inoculation (Fig. 1A).
Figure 1. SUT enhances the therapeutic efficacy of DC/OVA peptide-based vaccines in the MO5 (B16.OVA) melanoma model.
A. C57BL/6 mice (5 mice/cohort) bearing day 7 s.c. MO5 melanomas in their right flanks were untreated or treated with SUT (1 mg/day for 7 days via oral gavage) and/or DC/peptide vaccines (VAC; provided s.c. in the right flank on days 7 and 14 post-tumor inoculation) as described in Materials and Methods. The number of tumor-free mice on day 28 post-tumor inoculation is indicated in parentheses for 4 independent experiments performed. *p < 0.05 versus untreated control; **p < 0.05 versus either SUT or VAC monotherapy, and untreated control. B. C57BL/6 mice bearing established, day 7 MO5 melanomas were untreated or treated with VAC only on days 7 and 14 or VAC + SUT (at varying doses of sunitinib from 0.05 – 1 mg delivered daily on days 7–13). *p < 0.05 versus untreated controls; **p < 0.05 versus VAC monotherapy and untreated control. C. C57BL/6 mice bearing established, day 7 MO5 melanomas were untreated or treated with VAC only on days 7 and 14 or VAC + SUT (with sunitinib applied at a dose of 1 mg/day for 7 days beginning on day 7, day 14 or day 21 post-tumor inoculation). *p < 0.05 versus VAC only. D. C57BL/6 mice bearing established, day 7 MO5 melanomas were untreated or treated with SUT (1 mg/day on days 7–13) +/− VAC (provided on day 7 only, days 7 and 14, or days 14 and 21 post-tumor inoculation. *p < 0.05 versus SUT/VAC day 7 Only and untreated controls; **p < 0.05 versus either SUT monotherapy. In all cases, tumor size was monitored every 3–4 days and is reported as mean volume + SD. Data are representative of 4 independent experiments performed.
The impact of altering the dose of co-applied SUT or the schedule of SUT or VAC delivery on the anti-tumor efficacy of the combination therapy was next determined. As shown in Fig. 1B, all doses of SUT in excess of 0.05 mg/day resulted in an improvement in the anti-tumor effectiveness of DC-based vaccination (p < 0.05 versus VAC only for all time points after day 10). The timing of SUT administration in the context of vaccination appeared important, with superior efficacy demonstrated for combinational regimens in which SUT administration was initiated at the time of primary (day 7; p < 0.05 versus VAC only after day 10) or booster (day 14; p < 0.05 versus VAC only after day 14) vaccination (Fig. 1C). In contrast, if the application of SUT was delayed until day 21 (i.e. 7 days after the booster vaccination), only a modest deflection in tumor growth was observed (p < 0.05 versus VAC only on days 24 and 28). Data provided in Fig. 1D, suggest a major anti-tumor benefit for repeat vaccination vs. single vaccine administration in the context of SUT co-therapy (p < 0.05 for SUT/VAC day 7/14 versus SUT/VAC day 7 only for all time points after day 14). Furthermore, while a delay in the initiation of vaccination until day 14 (a day after SUT discontinuation) still resulted in a small degree of added protection to treated animals (p < 0.05 vs. SUT only on days 24 and 28), optimal co-therapy benefit was associated with the temporal overlap in administration of SUT and VAC (Fig. 1D; p < 0.05 for SUT/VAC d7/14 versus SUT/VAC d14/21 after day 10).
Combination SUT/VAC therapy elicits superior antigen-specific Type-1 T cell responses and losses in cells bearing a regulatory phenotype in the TME and TDLN in vivo
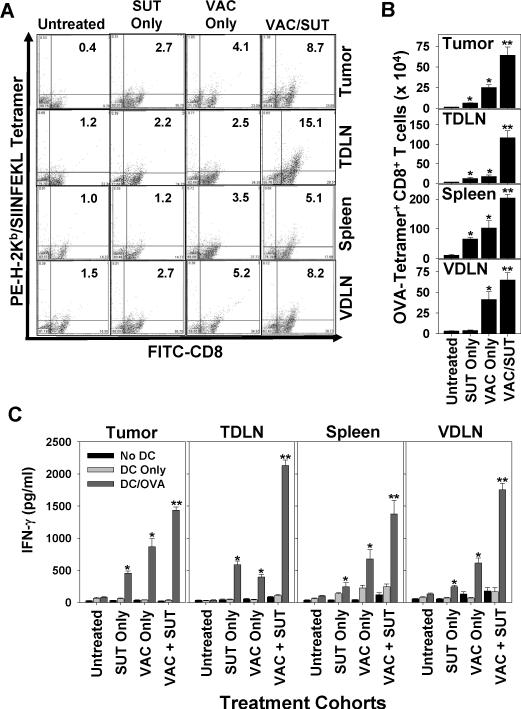
MO5-bearing mice were treated as outlined in Fig. 1A, with TIL, vaccine-draining lymph node (VDLN), tumor-draining lymph node (TDLN) and spleen cells harvested on day 28 post-tumor inoculation. MHC/peptide tetramer-based analyses of day 28 CD8+ T cells monitored by flow cytometry (Fig. 2A, 2B) revealed that treatment with SUT alone resulted in modest increases in the frequency and absolute numbers of OVA-specific CD8+ T cells in spleen, TDLN and tumor (p < 0.05 versus untreated). Treatment with VAC alone improved the prevalence of anti-OVA CD8+ T cells systemically (p < 0.05 versus untreated in all tissues analyzed). Combination SUT/VAC treatment yielded the highest frequencies of tetramer+ CD8+ T cells in all tissue compartments analyzed (p < 0.05 versus untreated or single modality therapy).
Figure 2. Combinational SUT + VAC therapy promotes superior anti-OVA Tc1 responses in treated mice.
C57BL/6 mice bearing established day 7 s.c. MO5 melanomas were treated as described in Fig. 1A. A. On day 28, post-tumor inoculation, spleens, VDLN, TDLN and TIL cells were harvested and analyzed by flow cytometry as indicated in Materials and Methods for the frequency of CD8+ T cells reacting with the PE-H-2Kb/SIINFEKL tetramer. Inset numbers reflect the % of tetramer+ events among all CD8+ events. In B, the absolute number of tetramer+CD8+T cells (mean +/− SD) based on multiplying the frequency of tetramer+CD8+ T cells among all flow-gated, viable cells by the total viable single-cell yield obtained from each tissue specimen. In C., TIL, TDLN, spleen and VDLN CD8+ T cells were isolated from day 28 untreated or treated animals, then stimulated for 5 days with irradiated MO5 tumor cells, prior to the co-culture of responder T cells with syngenic DC pulsed with no peptide or the OVA257–264 peptide. After 48h, cell-free supernatants were isolated and analyzed for levels of mIFN-γ by specific ELISA. Data are reported as the mean + SD of triplicate determinations. Similar data were obtained in 3 independent experiments performed. *p < 0.05 versus untreated control; **p < 0.05 versus untreated control and SUT or VAC monotherapy.
We next evaluated the ability of day 28 spleen, VDLN, TDLN and TIL CD8+ T cells to secrete IFN-γ in response to in vitro stimulation with the H-2Kb-presented OVA257–264 peptide (Fig. 2C). Despite the potential for cross-priming CD8+ T cells reactive with OVA in MO5 (B16.OVA) tumor-bearing mice, anti-OVA Tc1 responses were negligible in all tissues analyzed from untreated animals. SUT or VAC monotherapy improved anti-OVA Tc1 responses systemically (p < 0.05 versus untreated controls in all tissue compartments evaluated), with superior antigen-specific responses observed in animals treated with combination SUT/VAC therapy (p < 0.05 versus SUT only, VAC only or untreated controls in all tissue compartments analyzed).
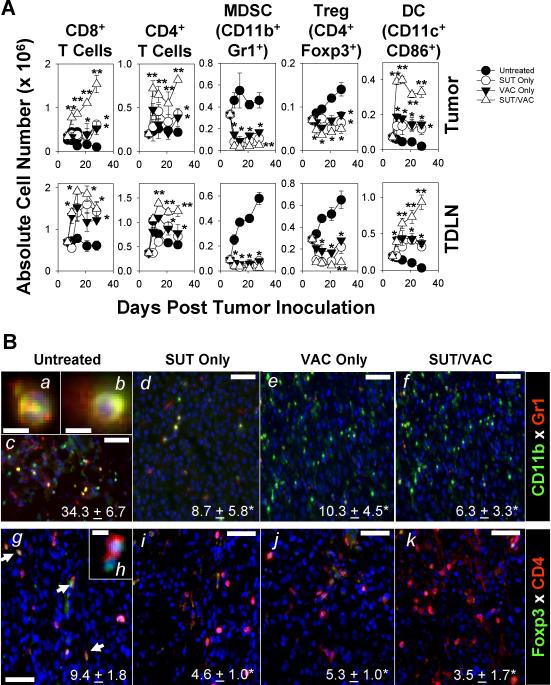
Previous reports have described the ability of SUT to reduce numbers of MDSC and Treg in the peripheral blood and TME of tumor-bearing hosts (12–14). Hence, we next asked whether single or combination modality treatment of MO5-bearing animals would alter the presence of cells bearing an MDSC-associated phenotype (i..e CD11b+Gr1+), a Treg-associated phenotype (i.e. CD4+Foxp3+), a mature DC-associated phenotype (i.e. CD11c+CD86+), as well as, total CD8+ and CD4+ T cells in the TME and TDLN through day 28 post-tumor inoculation. As shown in Fig. 3, while treatment of mice with SUT (or VAC) alone resulted in the partial, sustained reduction in the number of cells bearing MDSC (Fig. 3A, 3Ba–Bf) and Treg (Fig. 3A, 3Bg–Bk) phenotypes in the TME and TDLN, with a somewhat greater and more durable loss of these cell populations occurring in mice treated with the combination therapy. CD11b+Gr1+ cells in the TME were heterogeneous in their nuclear morphology, with some cells exhibiting multi- (i.e. PMN-like; Fig. 3Ba) and other cells displaying single- (i.e. monocyte-like; Fig. 3Bb) lobed nuclei. Combination SUT/VAC therapy was also superior in its capacity to acutely (within 3–7 days after initiating treatment) increase and sustain (through day 28; p < 0.05 versus single modality therapy after day 7) CD8+ T cell, CD4+ T cell and mature DC levels within the TME and TDLN (Fig. 3A).
Figure 3. Combination therapy acutely promotes a reduction in cells bearing CD11b+Gr1+ MDSC and CD4+Foxp3+ Treg phenotype, while improving T cell and “mature” DC numbers in the TME and TDLN.
A. TIL and TDLN were prepared as described in Fig. 1A on days 7, 10, 14, 21 and 28 post-tumor inoculation from the various treatment cohorts and analyzed by flow cytometry for the presence of T cells (CD8+ or CD4+), CD11b+Gr1+ cells (i.e. an MDSC phenotype), CD4+Foxp3+ cells (i.e. a Treg phenotype) and CD11c+CD86+ cells (i.e. a mature DC phenotype). Cumulative data from 3 independent experiments are reported as the absolute number (mean +/− SD) of cells in tumor and TDLN based on multiplying the frequency of cells bearing a given phenotype among all flow-gated, viable cells by the total viable single-cell yield obtained from each tissue specimen. In B., day 28 resected tumors were evaluated for the presence of cells bearing MDSC (panels Ba–Bf) and Treg (panels Bg–Bk) phenotypes by immunohistochemistry as outlined in the Materials and Methods. Arrows in panel Bg indicate CD4+Foxp3+ cells in situ. The average number (+/− SD) of cells bearing a given phenotype per high-power field (HPF) over 10 HPF/slide is indicated in panel inserts. *p < 0.05 versus untreated control. Bars = 50 microns with the exception of panels Ba, Bb and Bh (10 microns). Similar data were obtained in 3 independent experiments performed.
Combination SUT/VAC therapy results in a switch from a non-Type-1- to a Type-1-biased immune profile in the TME
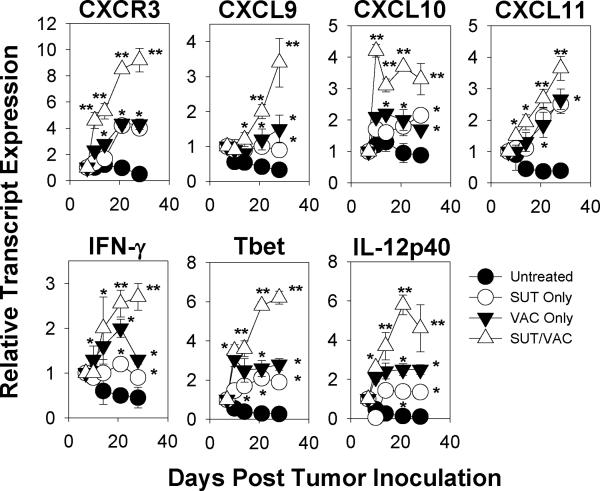
Given the suggestion of durable anti-tumor effects associated with day 7–14 treatment with SUT +/− VAC, we performed a more comprehensive analysis of immune parameters in the day 28 TME. Using RT-PCR and western blotting, we determined that SUT or VAC monotherapy, but even more so, SUT/VAC combination therapy resulted in a TME receptive towards Type-1 T cell recruitment (i.e. increased transcript expression for CXCR3 and the CXCR3 ligands CXCL9/Mig, CXCL10/IP-10, CXCL11/I-TAC; Fig. S1A) and functionality (i.e. increased transcripts for IFN-γ, IL-12p40 and T-bet and increased pSTAT1 protein expression; Fig. S1B, S1C, S1E). The TME post-treatment with SUT/VAC also could be characterized as being more refractory towards non-Type-1/regulatory cell recruitment (i.e. reduced transcript expression for known chemo-attractants for MDSC and Treg such as CXCL12/SDF-1α, CCL2/MCP-1, CXCL5/ENA-78, S100A8 and S100A9; Fig. S1A; refs. 29–36) and function (i.e. decreased expression of transcripts for IL-10, GATA-3 and Foxp3, as well as, the MDSC-associated gene products arginase-1, IDO-1 and iNOS/NOS2 (Fig. S1B and S1D) and reduced pSTAT3 protein expression; Fig. S1C, S1E). In contrast, expression of ROR-γt (a Th17-associated gene product) transcript levels appeared unaltered in the TME as a consequence of therapy (Fig. S1B). Additonal experiments support the TKI dose-dependency (for SUT administered at > 0.1 mg/day × 7 days; Fig. S2) of these molecular alterations in immune parameters. A confirmatory longitudinal evaluation of transcript changes in the TME suggests that single, and even more so, combination modality treatment results in the acute and sustained inhibition of many non-Type-1 immune indices (Fig. S3; p < 0.05 for SUT/VAC versus single modality therapy on day 28 for CXCR4, CXCL12, IL-10, S100A8, S100A9, Foxp3, Arg-1, IDO-1 and iNOS). A somewhat delayed kinetic (maximal by day 14–21 post-therapy initiation) was observed for improving many of the Type-1 immune indices in the TME (Fig. 4; p < 0.05 for SUT/VAC versus single modality therapy on day 28 for CXCR3, CXCL9, CXCL10, CXCL11, IFN-γ, Tbet and IL-12p40).
Figure 4. Longitudinal effects of mono- and combination therapy on Type-1-associated gene transcripts in the TME.
RT-PCR analyses were performed as outlined in Fig. S1 for the indicated gene products isolated from the untreated or treated TME on days 7, 10, 14, 21 and 28 post-tumor inoculation. The data depicted are the mean +/− SD values obtained from 3 independent experiments performed. *p < 0.05 versus untreated; **p < 0.05 for SUT/VAC versus SUT only or VAC only on day 28 post-tumor inoculation.
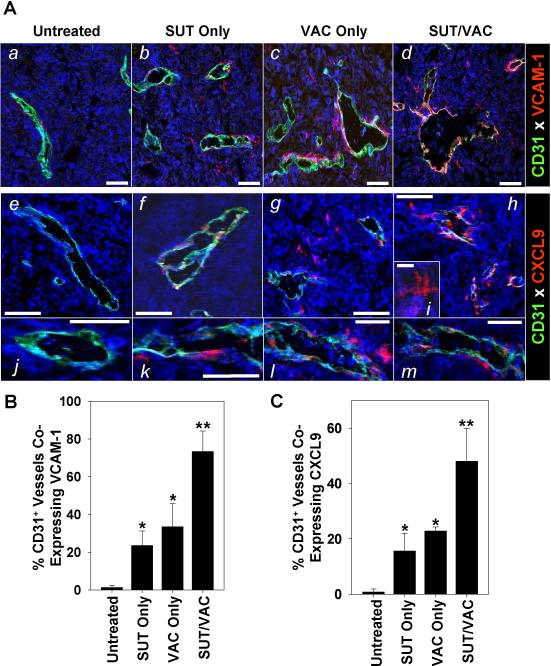
Previous work by our group and others provided support for the critical roles of VLA-4 (which binds to VCAM-1 expressed by activated endothelial cells) and CXCR3 (expressed by Type-1 CD8+ T cells) in the ability of protective anti-tumor T cells to effectively traffic into the TME (37–39). Consistent with the enhanced licensing of the day 28 TME for preferential recruitment of Type-1 T cells after SUT/VAC treatment, we observed remarkably enhanced expression of VCAM-1 (Fig. 5Aa–Ad; 5B, p < 0.05 for SUT/VAC versus all other cohorts, p < 0.05 for SUT or VAC versus untreated) and CXCL9 (a CXCR3 ligand; Fig. 5Ae–Am; 5C, p < 0.05 for SUT/VAC versus all other cohorts, p < 0.05 for SUT or VAC versus untreated) by tumor-associated CD31+ vascular endothelial cells in our fluorescence microscopy analyses. In the treated TME, we also observed CXCL9 to be expressed in situ by a population of “dendritic” cells (Fig. 5Ai) that are loosely-associated with the abluminal surface of tumor blood vessels (Fig. 5Ae–Am). Some, but not all, of these CD31negCXCL9/Mig+ cells appear to represent NG2+SMA+ vascular pericytes (Fig. S4).
Figure 5. Mono- and combinational therapies promote the activation of tumor vascular endothelial cells, resulting in the expression of VCAM-1 and CXCL9/Mig in the TME.
Day 28 MO5 lesions harvested from untreated or treated mice as outlined in Fig. 1A were fixed and tissue sections analyzed for expression of CD31 and VCAM-1 (panels Aa–Ad) or CD31 and CXCL9/Mig (panels Ae–Am) by fluorescence microscopy as described in Materials and Methods. Bars = 50 microns with the exception of panel Ai (10 microns). In B and C, cellular co-expression of VCAM-1 or CXCL9 with CD31, respectively, was quantitated over 10 high-power fluorescence microscopic fields and the data reported as the mean +/− SD per 1 high-power field. *p < 0.05 versus untreated; **p < 0.05 versus untreated, SUT only or VAC only. Similar data were obtained in 3 independent experiments performed.
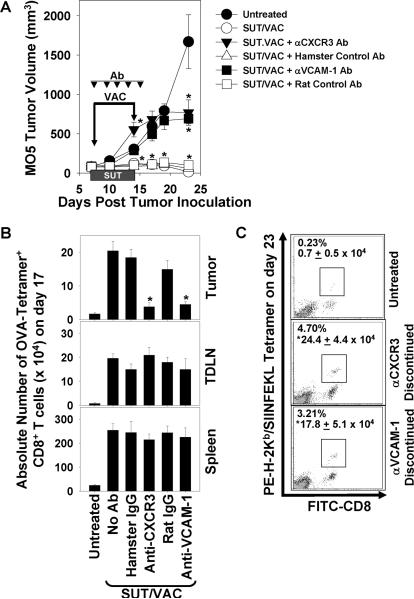
The anti-tumor efficacy of combination SUT/VAC therapy is negated upon administration of blocking antibodies against CXCR3 or VCAM-1
To address the biologic relevance of recruiting CXCR3+ (VLA-4+) anti-OVA Tc1 cells into the therapeutic TME, we injected MO5-bearing mice i.p. with blocking (non-depleting) antibodies against CXCR3 or VCAM-1, or the corresponding species/isotype control antibodies beginning on the day of initiating SUT/VAC treatment (i.e. 7 post-tumor inoculation). Antibodies were then re-injected every 2 days thereafter through day 15 (i.e. days 9, 11, 13 and 15) to sustain specific blockade. As shown in Fig. 6A, blockade of either CXCR3 or VCAM-1 completely abrogated protection associated with SUT/VAC treatment (p < 0.05 versus SUT/VAC and not significant versus untreated), while injection of control Abs had no discernable impact on therapy outcome. Flow cytometry-based OVA tetramer analyses performed on day 17 post-tumor inoculation revealed that CXCR3 or VCAM-1 blockade did not affect the ability of SUT/VAC combination therapy to expand the frequency of OVA-specific CD8+ T cells in the spleens or TDLN of treated animals (Fig. 6B). However, provision of the anti-CXCR3 or anti-VCAM-1 Abs, but not control Abs, prevented therapy-induced augmentation in the number of tetramer+CD8+ T cells in the day 17 TME (Fig. 6B). Interestingly, within 8 days of discontinuing the administration of anti-CXCR3 or anti-VCAM-1 Abs, these cohorts of animals began to display evidence for the recovery of SUT/VAC anti-tumor therapy benefit (Fig. 6A, day 23; *p < 0.05 versus untreated animals), which was associated with the renewed infiltration of tumor lesions by anti-OVA CD8+ T cells (Fig. 6C).
Figure 6. The anti-tumor efficacy of combined SUT/VAC therapy is CXCR3- and VCAM-1-dependent.
A. Mice bearing established day 7 M05 tumors were untreated or treated with SUT/VAC combined therapy as described in Fig. 1A. Beginning on day 7 and every 2 days thereafter through day 15, treated mice were injected i.p. with 200 μg of blocking antibodies against mCXCR3 or mVCAM-1, or species/isotype-matched antibodies. Tumor sizes were monitored every 3–4 days and are reported as mean volume ± SD. *p < 0.05 versus untreated control. On day 17 (B) and 23 (C) post-tumor inoculation, flow cytometry-based PE-H-2Kb/SIINFEKL tetramer analyses were performed on spleen, TDLN and/or TIL CD8+ T cells as described in Fig. 2A. In B, data are reported as the absolute number of tetramer+CD8+ T cells (mean +/− SD) based on multiplying the frequency of tetramer+CD8+ T cells among all flow-gated, viable cells by the total viable single-cell yield obtained from each tissue specimen. In C, flow dot-plots are provided with panel insets reporting the percentage of tetramer+CD8+ events in TIL and the absolute number of such events per tumor lesion. *p < 0.05 versus untreated control. All data are representative of 2 independent experiments performed.
DISCUSSION
Tumor-induced immune deviation in cancer patients is believed to represent a major impediment to the effectiveness of immunotherapies (including vaccines; 2–5, 12–16, 20–23, 29, 40). In such a paradigm, both local and systemic immune defects limit anti-tumor T cell responsiveness to treatment-induced activation, and they may support inappropriate T cell functionality and/or limit the durability of anti-tumor T effector cells within the TME (40–42). In this regard, both tumor cells themselves and tumor-associated stromal cells (including infiltrating MDSC and Treg) are believed to mitigate the efficacy of anti-tumor immunity in progressor lesions under constitutive, as well as, (immuno)therapeutic conditions.
Consistent with previous reports (12–14), we observed that all cohorts of animals receiving SUT exhibited reductions in levels of cells bearing the CD11b+Gr1+ MDSC or CD4+Foxp3+ (as well as CD4+CTLA-4+; Fig. S5) Treg phenotypes in both the TME and TDLN. These changes occurred acutely within 3–7 days of initiating SUT treatment and they remained in force even 14 days after discontinuation of SUT delivery (i.e. day 28 post-tumor inoculation), suggesting a degree of treatment durability may be associated with this drug. However, the natural cross-priming of Type-1 anti-tumor (anti-OVA) CD8+ T cell responses in MO5-bearing mice was only modestly accentuated by SUT monotherapy in spleen, TDLN and the TME. Hence, despite the capacity of SUT to foster the receptivity of the TME to Type-1 T cell recruitment (via upregulated expression of CXCR3 ligands and VCAM-1 by tumor-associated VEC), there were few systemic OVA-specific Tc1 cells to recruit, and all animals treated with SUT monotherapy ultimately exhibited terminal progressive disease.
Treatment of melanoma-bearing animals with VAC alone resulted in slowed tumor growth and the improved systemic activation of OVA-specific Tc1 cells in vivo. More anti-OVA CD8+ T cells were detected in the TME, presumably based on the ability of vaccine components to affect many of the same endpoints noted for SUT monotherapy (i.e. reduced numbers of cells bearing MDSC or Treg phenotype, enhanced CXCR3 ligand and VCAM-1 expression). In this setting, additional experiments evaluating therapeutic vaccines based on i) wild-type DC or control, Adψ5-infected DC pulsed with OVA peptides or ii) DC.IL12 devoid of OVA peptides, revealed that protection afforded by VAC is strictly dependent upon both IL-12p70 transgene expression in DC and OVA peptide presentation by these APC (data not shown). Nevertheless, despite the ability of DC.IL12 + OVA peptide vaccines alone to result in more anti-OVA Tc1 in MO5 tumors, none of the animals treated with VAC only were cured of their disease. While one could argue that the quality and durability of vaccine-induced T cells in the TME might remain sub-optimal, such TIL were strong secretors of IFN-γ in response to OVA peptide stimulation (in vitro) and they expressed reduced levels of pro-apoptotic molecules such as PD-1 throughout the progressive tumor growth phase when compared to analogous T cells isolated from untreated, control mice (Fig. S5).
Interestingly, we observed similarities for most readouts applied to cohorts of animals treated with either SUT (or VAC) alone, suggesting potential commonality in the underlying mechanism(s) of action for these modalities. Both single modalities were competent to activate tumor-associated vascular endothelial cells (to express VCAM-1 and CXCR3 ligands) and to switch the chemokine balance away from one previously reported to be linked to the recruitment/function of CXCR4+ MDSC and Treg (2, 30–36, 43–45), towards one associated with the infiltration/function of CXCR3+ Type-1 T cells (28, 37–39). Alternatively, as suggested by Vianello et al. (45), production of the CXCR4 ligand CXCL12 within the TME may serve as a “chemorepellant” for anti-tumor CD8+ T cells, with SUT +/− VAC therapies mediating the removal of such a barrier to effective treatment. Since therapy-induced CXCR3 ligand chemokines also serve as natural antagonists for Type-2 T cell expressed CCR3 (46), this could further bolster the dominance of Type-1 (Tbet+, IFN-γ+) over non-Type-1 (Th2 [GATA3+] or Treg [Foxp3+, IL-10+, TGF-β+, CTLA-4+]) TIL as a consequence of treatment with SUT +/− VAC. Both single modality therapies were also capable of replacing the prevalence of pSTAT3 in the untreated TME with pSTAT1 in the therapeutic TME, with the STAT3 change being consistent with a recent report by Xin et al. (16). Furthermore, mRNA transcripts of MDSC-associated “effector molecules” (i.e. arginase-1, IDO-1 and iNOS) in the TME were reduced in their expression after single modality treatment. This can be most simplistically explained by treatment-induced inhibition of signals that recruit cells bearing the CD11b+Gr1+ MDSC phenotype into the progressor TME (i.e. consistent with the observed reductions in transcript levels for chemoattractants such as CCL2, CXCL5, CXCL12, S100A8 and S100A9; refs. 33–36). Many of these immunologic changes in the TME were further augmented in a statistically-significant manner by combination SUT/VAC approaches that provided superior anti-tumor efficacy when compared to either monotherapy. Of these changes, we determined that the anti-tumor impact of SUT/VAC combination therapy was strongly dependent upon chemotactic signals mediated through the CXCR3 receptor and adhesion mediated through VCAM-1 that impact OVA-specific CD8+ T cell recruitment into the TME based on the results of our antibody blocking studies. Notably, these blocking antibodies did not inhibit peripheral induction of anti-OVA CD8+ T cells (in TDLN and spleen) as a result of SUT/VAC combined therapy, and upon the discontinuation of CXCR3 or VCAM-1 blockade, the TME again became permissive for the recruitment of OVA-tetramer+CD8+ T cells, resulting in disease stabilization.
While the relevant, functionally-dominant TK affected by sunitinib in our model remains to be elucidated, one can begin to rationale these results by considering simple conceptual models. If one focuses on c-kit in the TME for instance, sunitinib may block c-kit signaling (14), whereas, VAC-induced Type-1 TIL production of IFN-γ and/or TNF-α may reduce c-kit transcription and expression (47–49), yielding a similar net result in treated animals. Alternatively or additionally, STAT1 activation by either sunitinib or IFN-γ (elaborated by Type-1 TIL) may antagonize the immunosuppressive influence of activated STAT3 in the TME (50). Such possibilities warrant prospective study in order to further refine the utility and efficacy of both SUT- and VAC-based therapeutic approaches.
Of perhaps greatest importance, we observed that in contrast to SUT- or VAC-based monotherapies which failed to cure any tumor-bearing mice in our model, roughly one-quarter of mice could be rendered tumor-free after a single cycle of co-treatment with SUT/VAC. Cured animals subsequently rejected a challenge with either the MO5 (B16.OVA) or B16 parental (OVAneg) cell lines, supporting therapy-induced development of a protective T cell repertoire recognizing B16 tumor-associated antigens that are unrelated to OVA (data not shown), as has been suggested in alternate vaccination models using B16.OVA tumors (24). The most effective combination therapies involved the provision of SUT either at the time of the primary or the booster vaccination. If the application of SUT or VAC were separated from each other temporally by one week or more, combination treatment efficacy was sub-optimal. Systemic levels of anti-OVA Tc1 cells were optimized by combined therapy (in spleen, TDLN, VDLN and the TME). Our aggregate analysis of more than two dozen molecular indices in this study suggest that combined SUT/VAC therapy was superior to either SUT or VAC monotherapy in reducing non-Type-1 immune-associated parameters, while coordinately increasing Type-1 immune-associated parameters, in the TME.
Based on the standard clinical provision of SUT to RCC patients in 6 week cycles that include 28 consecutive days of administering this drug (followed by 2 weeks off drug; ref. 14, 15), one could argue that a more prolonged administration of SUT might yield even greater SUT/VAC co-therapy benefits. Our preliminary data evaluating this contention (Fig. S6), however, suggest that: i.) the growth of MO5 melanomas in vivo begins to become refractory to the suppressive effects of SUT monotherapy after 7–10 days of administration, and ii.) the anti-tumor efficacy of SUT/VAC co-therapy is fairly equitable when comparing a 7- versus 14-day course of orally-administered SUT in our protocol schema. However, there may be a modest benefit for the extended delivery of low-dose SUT (p < 0.05 for treatment with 0.1 mg/day × 14 days versus 0.1 mg/day × 7 days) in the SUT/VAC regimen at late time points (i.e. ≥ day 28). While we believe that the vast weight of our data supports the sufficiency of a one week course of SUT in order to maximize the effectiveness of the SUT/VAC treatment protocol, additional experiments are warranted before reaching unequivocal conclusions.
In summary, our data supports substantial and sustained treatment benefit(s) resulting from a short (7 day) course of SUT, particularly when applied in concert with specific DC/peptide-based vaccination. This combination therapy effectively and durably reduces immunologic indices associated with immune suppression, while promoting/recruiting Type-1 anti-tumor T cells into the TME. As a consequence, approximately one-quarter of animals receiving co-therapy may be rendered disease-free. Our data support the clinical translation of SUT/VAC combination modalities, which may be particularly cogent for cancer histologies that have previously demonstrated some degree of responsiveness to SUT or VAC monotherapies (i.e. such as melanoma or renal cell carcinoma among others), where additive/synergistic benefits might be anticipated. This may be most salient in preventing, or serving as a second line treatment option for patients that develop, progressive disease that is refractory to SUT monotherapy.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank Drs. Devin Lowe, Amy Wesa, Pawel Kalinski, Alicia Mathers and Kyle McKenna for their careful review and/or helpful discussions provided during the generation of this manuscript. This work was supported by NIH P01 grant CA109688 (to J.H.F. and W.J.S.) and P50 CA121973 (to W.J.S.) and the University of Pittsburgh Cancer Center Support Grant (CCSG) P30 CA047904. Author J.A.G. has a sponsored research agreement with, and B.I.R. is a paid consultant for, Pfizer.
Abbreviations Used
- BM
bone marrow
- DC
dendritic cell
- MDSC
myeloid-derived suppressor cell
- OVA
ovalbumin
- RTK
receptor tyrosine kinase
- SUT
sunitinib malate
- TDLN
tumor-draining lymph node
- TIL
tumor infiltrating lymphocytes
- TK
tyrosine kinase
- TKI
tyrosine kinase inhibitor
- TME
tumor microenvironment
- VAC
vaccine
- VCAM-1
vascular cell adhesion molecule-1
- VDLN
vaccine-draining lymph node
REFERENCES CITED
- 1.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 2.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68:2561–3. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 3.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–18. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 4.Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. BLOOD. 2008;111:219–28. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 6.Ghoreschi K, Laurence A, O'Shea JJ. Selectivity and therapeutic inhibition of kinases: to be or not to be? Nat Immunol. 2009;10:356–60. doi: 10.1038/ni.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arteaga CL, Khuri F, Krystal G, Sebti S. Overview of rationale and clinical trials with signal transduction inhibitors in lung cancer. Semin Oncol. 2002;29:15–26. doi: 10.1053/sonc.2002.31524. [DOI] [PubMed] [Google Scholar]
- 8.Egberts F, Kahler KC, Livingstone E, Hauschild A. Metastatic melanoma: scientific rationale for sorafenib treatment and clinical results. Onkologie. 2008;31:398–403. doi: 10.1159/000137714. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, Ginsberg MS, Bacik J, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–24. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006;24:5601–8. doi: 10.1200/JCO.2006.08.5415. [DOI] [PubMed] [Google Scholar]
- 12.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 13.Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, Wood L, Elson P, Garcia J, Dreicer R, Bukowski R. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–82. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 14.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–22. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Cruijsen H, Hoekman K, Stam AG, van den Eertwegh AJ, Kuenen BC, Scheper RJ, Giaccone G, de Gruijl TD. Defective differentiation of myeloid and plasmacytoid dendritic cells in advanced cancer patients is not normalized by tyrosine kinase inhibition of the vascular endothelial growth factor receptor. Clin Dev Immunol. 2007;2007:17315. doi: 10.1155/2007/17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–13. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helfrich I, Scheffrahn I, Bartling S, Weis J, von Felbert V, Middleton M, Kato M, Ergün S, Schadendorf D. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J Exp Med. 2010;207:491–503. doi: 10.1084/jem.20091846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian CN, Kahnoski R, Futreal PA, Furge KA, Teh BT. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70:1063–71. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregor PD, Wolchok JD, Ferrone CR, Buchinshky H, Guevara-Patiño JA, Perales MA, Mortazavi F, Bacich D, Heston W, Latouche JB, Sadelain M, Allison JP, et al. CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self antigens in animal and cellular model systems. Vaccine. 2004;22:1700–8. doi: 10.1016/j.vaccine.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 20.Cohen AD, Diab A, Perales MA, Wolchok JD, Rizzuto G, Merghoub T, Huggins D, Liu C, Turk MJ, Restifo NP, Sakaguchi S, Houghton AN. Agonist anti-GITR antibody enhances vaccine-induced CD8+ T-cell responses and tumor immunity. Cancer Res. 2006;66:4904–12. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruter J, Barnett BG, Kryczek I, Brumlik MJ, Daniel BJ, Coukos G, Zou W, Curiel TJ. Altering regulatory T cell function in cancer immunotherapy: a novel means to boost the efficacy of cancer vaccines. Front Biosci. 2009;14:1761–70. doi: 10.2741/3338. [DOI] [PubMed] [Google Scholar]
- 22.Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, Clay TM. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. BLOOD. 2008;112:610–8. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, Buchlis G, Fridlender ZG, Sun J, Kapoor V, Cheng G, Haas A, Cheung HK, Zhang X, Corbley M, Kaiser LR, Ling L, et al. Systemic blockade of transforming growth factor-beta signaling augments the efficacy of immunogene therapy. Cancer Res. 2008;68:10247–56. doi: 10.1158/0008-5472.CAN-08-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celluzzi CM, Falo LD., Jr. Epidermal dendritic cells induce potent antigen-specific CTL-mediated immunity. J Invest Dermatol. 1997;108:716–20. doi: 10.1111/1523-1747.ep12292095. [DOI] [PubMed] [Google Scholar]
- 25.Komita H, Zhao X, Taylor JL, Sparvero LJ, Amoscato AA, Alber S, Watkins SC, Pardee AD, Wesa AK, Storkus WJ. CD8+ T-cell responses against hemoglobin-beta prevent solid tumor growth. Cancer Res. 2008;68:8076–84. doi: 10.1158/0008-5472.CAN-08-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipford GB, Hoffman M, Wagner H, Heeg K. Primary in vivo responses to ovalbumin. Probing the predictive value of the Kb binding motif. J Immunol. 1993;150:1212–22. [PubMed] [Google Scholar]
- 27.Robertson JM, Jensen PE, Evavold BD. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin323-339 epitope. J Immunol. 2000;164:4706–12. doi: 10.4049/jimmunol.164.9.4706. [DOI] [PubMed] [Google Scholar]
- 28.Uppaluri R, Sheehan KC, Wang L, Bui JD, Brotman JJ, Lu B, Gerard C, Hancock WW, Schreiber RD. Prolongation of cardiac and islet allograft survival by a blocking hamster anti-mouse CXCR3 monoclonal antibody. Transplantation. 2008;86:137–47. doi: 10.1097/TP.0b013e31817b8e4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berhanu A, Huang J, Alber SM, Watkins SC, Storkus WJ. Combinational FLT3 ligand and granulocyte macrophage colony-stimulating factor treatment promotes enhanced tumor infiltration by dendritic cells and anti-tumor CD8+ T cell cross-priming but is ineffective as a therapy. Cancer Res. 2006;66:4895–903. doi: 10.1158/0008-5472.CAN-05-2384. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y, Xu L, Jin H, Zhong Y, Di J, Lin QD. CXCL12 enhances exogenous CD4+CD25+ T cell migration and prevents embryo loss in non-obese diabetic mice. Fertil Sterl. 2009;91:2687–96. doi: 10.1016/j.fertnstert.2008.01.109. [DOI] [PubMed] [Google Scholar]
- 31.Grauer OM, Nierkens S, Bennink E, Toonen LW, Boon L, Wesseling P, Sutmuller RP, Adema GJ. CD4+Foxp3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007;121:95–105. doi: 10.1002/ijc.22607. [DOI] [PubMed] [Google Scholar]
- 32.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–5. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 33.Huang B, Lei Z, Zhao J, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, Zou W. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–75. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasaki K, Zhao X, Pardee AD, Ueda R, Fujita M, Sehra S, Kaplan MH, Kane LP, Okada H, Storkus WJ. Stat6 signaling suppresses VLA-4 expression by CD8+ T cells and limits their ability to infiltrate tumor lesions in vivo. J Immunol. 2008;181:104–8. doi: 10.4049/jimmunol.181.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobrzanski MJ, Reome JB, Hylind JC, Rewers-Felkins KA, Abulsamad K, Adams SL. Ag-specific type 1 CD8 effector cells enhance methotrexate-mediated antitumor responses by modulating differentiated T cell localization, activation and chemokine production in established breast cancer. Clin Immunol. 2008;128:205–18. doi: 10.1016/j.clim.2008.03.518. [DOI] [PubMed] [Google Scholar]
- 39.Nishimura F, Dusak JE, Eguchi J, Zhu X, Gambotto A, Storkus WJ, Okada H. Adoptive transfer of type 1 CTL mediates effective anti-central nervous system tumor response: critical roles of IFN-inducible protein-10. Cancer Res. 2006;66:4478–87. doi: 10.1158/0008-5472.CAN-05-3825. [DOI] [PubMed] [Google Scholar]
- 40.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–12. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–61. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 42.Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother. 2006;29:233–40. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- 43.Kryczek I, Wei S, Keller E, Liu R, Zou W. Stroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesis. Am J Physiol Cell Physiol. 2007;292:C987–C995. doi: 10.1152/ajpcell.00406.2006. [DOI] [PubMed] [Google Scholar]
- 44.Schmid MC, Varner JA. Myeloid Cells in the Tumor Microenvironment: Modulation of Tumor Angiogenesis and Tumor Inflammation. J Oncol. 2010;2010:201026. doi: 10.1155/2010/201026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vianello F, Papeta N, Chen T, Kraft P, White N, Hart WK, Kircher MF, Swart E, Rhee S, Palù G, Irimia D, Toner M, Weissleder R, Poznansky MC. Murine B16 melanomas expressing high levels of the chemokine stromal-derived factor-1/CXCL12 induce tumor-specific T cell chemorepulsion and escape from immune control. J Immunol. 2006;176:2902–14. doi: 10.4049/jimmunol.176.5.2902. [DOI] [PubMed] [Google Scholar]
- 46.Loetscher P, Pellegrino A, Gong JH, Mattioli I, Loetscher M, Bardi G, Baggiolini M, Clark-Lewis I. The ligands of CXC chemokine receptor 3, I-TAC, Mig, and IP10, are natural antagonists for CCR3. J Biol Chem. 2001;276:2986–91. doi: 10.1074/jbc.M005652200. [DOI] [PubMed] [Google Scholar]
- 47.Khoury E, Andre C, Pontvert-Delucq S, Drenou B, Baillou C, Guigon M, Najman A, Lemoine FM. Tumor necrosis factor alpha (TNF alpha) downregulates c-kit protooncogene product expression in normal and acute myeloid leukemia CD34+ cells via p55 TNF alpha receptors. BLOOD. 1994;84:2506–14. [PubMed] [Google Scholar]
- 48.Buzby JS, Knoppel EM, Cairo MS. Coordinate regulation of Steel factor, its receptor (Kit), and cytoadhesion molecule (ICAM-1 and ELAM-1) mRNA expression in human vascular endothelial cells of differing origins. Exp Hematol. 1994;22:122–9. [PubMed] [Google Scholar]
- 49.Rusten LS, Smeland EB, Jacobsen FW, Lien E, Lesslauer W, Loetscher H, Dubois CM, Jacobsen SE. Tumor necrosis factor-α inhibits stem cell factor-induced proliferation of human bone marrow progenitor cells in vitro. Role of p55 and p75 tumor necrosis factor receptors. J Clin Invest. 1994;94:165–72. doi: 10.1172/JCI117303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka K, Ichiyama K, Hashimoto M, Yoshida H, Takimoto T, Takaesu G, Torisu T, Hanada T, Yasukawa H, Fukuyama S, Inoue H, Nakanishi Y, et al. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-γ on STAT3 and Smads. J Immunol. 2008;180:3746–56. doi: 10.4049/jimmunol.180.6.3746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.