Abstract
Background
Reductions in glial density and enlargement of glial nuclei have been reported in the dorsolateral prefrontal cortex (dlPFC) in mood disorders. In alcohol dependence, often comorbid with depression, it is unclear whether there are changes in the density and size of glial cells in the dlPFC.
Methods
The packing density and size of Nissl-stained glial cell nuclei were analyzed postmortem in the cortical layers of the dlPFC from 21 control and 17 alcohol-dependent (Alc) subjects without Wernicke or Korsakoff syndromes. Eight Alc subjects had depressive symptoms. The density of glial cells was measured with a three-dimensional cell counting method, and the areal fraction of glial fibrillary acidic protein immunoreactivity (GFAP) was also determined.
Results
Glial density was reduced by 11–14% in layers V and VI and in all layers combined in the Alc group. The size of glial nuclei was decreased by 3.2% in Alc subjects. The Alc subjects with depressive symptoms showed the lowest values of density and size. There was no difference in GFAP immunoreactivity, although the lowest values were in the Alc group.
Conclusions
Alcohol dependence is characterized by decreases in both density and size of glia in the dlPFC. Glial pathology may be more severe in Alc subjects with depressive symptoms.
Keywords: Alcoholism, neuropathology, postmortem, comorbidity, morphometry, immunohistochemistry
Introduction
There is abundant evidence for a preferential involvement of the prefrontal cortex in the brain neuropatho-logic changes associated with alcohol abuse (for a review, see Harper 1998). Characteristic behavioral abnormalities and reduced regional glucose metabolism or blood flow in medial, dorsolateral, and orbitofrontal regions of the pre-frontal cortex in chronic alcoholics indicate an abnormal functioning of these cortical regions (George et al 1999; Hoaken et al 1998; Pihl et al 1980; Sullivan et al 2000; Volkow et al 1992; Weingartner et al 1994). Moreover, a significant correlation between the behavioral and cognitive abnormalities and the haemodynamic and metabolic alterations have been demonstrated in the temporal and prefrontal cortical areas of alcoholics (Adams et al 1993, 1995; Dally et al 1988; Nicolas et al 1993). Neuroanatomic studies have shown that the shrinkage of the forebrain observed in alcoholics (Kril and Halliday 1999) is more prominent in the frontal and temporal lobes than in other cortical areas (Harper and Kril 1990; Kril et al 1997; Pfefferbaum et al 1997). These volumetric and functional changes in alcohol disorders suggest that the morphology and function of cortical neurons and their glial companions are altered in particular regions of the prefrontal cortex. Accordingly, two studies of alcoholic subjects have reported loss or reduced density of neurons in the cortex of the superior frontal gyrus (part of the dorsolateral prefrontal cortex [dlPFC]; Kril et al 1997; Kril and Harper 1989). It seems, however, that in the brain of alcoholics without Wernicke's encephalopathy or Korsakoff psychosis—so-called uncomplicated alcoholics—neuronal loss is not a generalized phenomenon (Harper 1998; Jensen and Pakkenberg 1993; Kril and Halliday 1999). Pathologic changes in the brain in alcoholism may be related to neuronal alterations not directly leading to cell death. Neuronal alterations in alcoholism may also involve the more abundant glial cells.
Despite the growing evidence for crucial roles of glial cells in regulating activity in the CNS (Laming et al 2000), and their recent implication in psychiatric diseases (reviewed by Cotter et al 2001b; Rajkowska 2000), only a few studies have addressed the putative involvement of glial cells in the histopathology of alcoholism in the human cerebral cortex. In a group of alcoholics, including individuals with Wernicke's encephalopathy, the density of glial cells was increased in the superior frontal gyrus (Harper et al 1987; Kril and Harper 1989), although these studies did not distinguish cortical layers and used two-dimensional cell counting techniques. In contrast, a recent study in the hippocampus of alcoholics with neither Wernicke's encephalopathy nor Korsakoff psychosis (Korbo 1999) reported dramatic reductions (37%) in the number of glial cells and a preferential reduction in the number of astrocytes. One study in severe alcoholism reports a patchy loss of glial fibrillary acidic protein (GFAP) immunoreactive structures, a beading of astrocytic fibers, and soma swelling in individual astrocytes in some regions of the brain (Cullen and Halliday 1994). Thus, it is still unclear whether the putative glial pathology in alcoholism encompasses reductions or increases in the density of glial cells and their subcellular components, and whether there is a differential involvement of particular cortical layers.
The regionally restricted prefrontal pathology and reduced metabolism in the dlPFC of chronic alcoholics bears some resemblance to cortical prefrontal pathology that has been recently described in major depressive disorder (MDD) and bipolar disorder (BPD; Drevets 1995, 1999; Ketter et al 1996; Öngür et al 1998; Rajkowska et al 1999, 2001). This pathology involves reductions in the density of glial cells and neurons and, at least in young adults, may involve reduced GFAP content in astrocytes of young adult MDD subjects in the dlPFC (Miguel-Hidalgo et al 2000). In fact, prolonged alcohol exposure in some alcohol-dependent subjects appears to facilitate episodes of MDD, dysthymia, or depressed mood. These changes are considered to be secondary to alcohol dependence or alcohol abuse in a majority of cases, because the depressive symptoms disappear after a period of abstinence (Davidson 1995; Davidson and Blackburn 1998; Nakamura et al 1983). Nonetheless, there have been no studies approaching glial pathology in alcohol dependence with comorbid depressive symptoms. Accordingly, the aim of this study was to use a direct three-dimensional (3-D) cell counting method to determine whether the density of glial cells is changed in the dlPFC of neurologically “uncomplicated” alcohol-dependent subjects compared with normal control subjects. In addition, we compared the size of glial nuclei and the areal fraction covered by immuno-staining for the astrocytic marker GFAP. We also address the possibility that different glial neuropathology might distinguish in a group of alcohol-dependent subjects those with depressive symptoms or a mood disorder and those without depressive symptoms.
Methods and Materials
Human Subjects
Human postmortem brain tissues were obtained at autopsies performed at the Cuyahoga County Coroner's Office in Cleveland, Ohio. Retrospective psychiatric assessments for all subjects were performed in accordance with institutional review board policies, and written consent was obtained from the next-of-kin. A retrospective psychiatric assessment was used for establishing psychiatric histories of control and alcoholic subjects based on information obtained from significant others or first-degree family members and medical records. Information about psychiatric symptoms was gathered using the Structured Clinical Interview for DSM-IV Psychiatric Disorders (First 1997), and a diagnosis for alcohol-dependence was based on the DSM-IV (American Psychiatric Association 1995). Kelly and Mann (1996) have shown that there is good agreement between informant-based retrospective psychiatric assessments of deceased subjects and diagnoses of living subjects by clinicians. Information was obtained about previous hospital medical records, prior medical or substance abuse problems, and toxicology reports. Subjects were excluded from the study if there was any evidence of head trauma or neurologic disease. Only one of the alcoholic subjects was shown to have cirrhosis of the liver at the time of death. Tremors and blackouts were described for several subjects in the family interviews, but they were all reported to occur during the periods of alcohol intoxication. The records available and the family reports did not reveal that any of the alcoholic subjects in our study suffered Korsakoff's psychosis or Wernicke's encephalopathy. In the alcohol-dependent group (subjects 22 to 38), five subjects were found to have suffered episodes of MDD (cases 22, 24, 27, 37, and 38), one subject was considered to have had mild BPD (case 25), one was diagnosed with alcohol-related mood disorder (case 31), and one was described as presenting repeated episodes of depressed mood during the last year with duration of 1 week each (case 26, Table 1). The mood disorders and disturbances detected in this group were reported as starting following alcohol dependence. Subjects with a history of MDD, BPD, or depressed mood (depression that did not reach DMS-IV criteria for MDD or BPD) beginning before the alcohol dependence were excluded from the study. These eight cases with depressive symptoms were considered a subgroup and termed Alc-D. The remaining nine subjects in the alcohol-dependent group were not described to have suffered depressive symptoms and were included in the Alc-ND subgroup (cases 23, 28–30, 32–36). The goal was to ascertain whether the two subgroups of alcohol-dependent subjects could be differentiated according to the pattern of changes in glial cell densities, glial nuclear sizes, and in the distribution of GFAP immunoreactivity. Brains were used from subjects who were younger than 73 years of age, had a postmortem delay equal to or shorter than 30 hours (only one subject had 36 hours), and had a fixation time in formalin that did not exceed four years. Fixation times for each block of tissue are listed in Table 1. A pH value for each brain was determined in frozen cerebellar tissue.
Table 1.
Characteristics of Subjects
| Age/gender/race | PMI | TF | pH | Cause of death | Medicationa | Toxicology | History of depression | Family history | |
|---|---|---|---|---|---|---|---|---|---|
| Control | |||||||||
| 1 | 71/M/C | 24.0 | 5 | 6.82 | N | None known | NDD | None | Alcohol abuse |
| 2 | 58/M/C | 21.5 | 5 | 6.78 | N | digoxin | NDD | None | Alcohol and drug abuse |
| 3 | 24/M/AAm | 15.0 | 24 | 6.84 | H | None known | NDD | None | None |
| 4 | 30/F/C | 9.0 | 29 | 6.75 | N | None known | NDD | None | None |
| 5 | 47/M/C | 17.0 | 7 | 6.89 | N | famotidine | NDD | None | Alcohol abuse |
| 6 | 23/F/C | 11.0 | 23 | 6.85 | A | levonorgestrel implant | NDD | None | None |
| 7 | 46/F/C | 24.0 | 25.5 | 6.32 | H | maxitrol | NDD | None | None |
| 8 | 57/M/C | 10.0 | 12 | 6.65 | N | naprosyn | NDD | None | None |
| 9 | 33/M/C | 27.0 | 15 | 6.70 | N | None known | cannabinoids | None | None |
| 10 | 27/F/C | 15.0 | 21 | 7.01 | N | captopril, ENDURONYL, ENALAPRIL, TOPRAL, lopressor | NDD | None | None |
| 11 | 60/F/AAm | 19.0 | 11 | 6.74 | N | None known | NDD | None | None |
| 12 | 69/M/C | 18.0 | 49 | 6.70 | N | None known | NDD | None | None |
| 13 | 60/M/AAm | 27.0 | 45.7 | 6.75 | H | None known | NDD | None | None |
| 14 | 51/M/C | 28.0 | 24.5 | 6.64 | N | None known | NDD | None | None |
| 15 | 52/M/C | 17.0 | 45.9 | 6.28 | N | None known | NDD | None | None |
| 16 | 53/M/AAm | 23.0 | 45.2 | 6.77 | E | None known | NDD | None | None |
| 17 | 18/F/C | 27.0 | 43.4 | 6.78 | A | None known | NDD | None | None |
| 18 | 39/M/C | 21.0 | 13.9 | 6.74 | N | None known | lidocaine | None | None |
| 19 | 59/M/C | 6.0 | 42.5 | 6.79 | N | None known | lidocaine | None | None |
| 20 | 38/M/C | 30.0 | 42.3 | 6.74 | N | None known | NDD | None | None |
| 21 | 42/M/C | 20.0 | 11.9 | 6.82 | N | None known | NDD | None | None |
| Average | 45.6 | 20.0 | 25.8 | 6.73 |
| Age/gender/race | PMI | TF | pH | Cause of death | Medicationa | Toxicology | History of depression | Family history | Duration of alcoholismb | Age of onset | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohol | |||||||||||
| 22 | 50/M/C | 17.0 | 34 | 6.67 | S | TRAZODONE-NON COMPLIANT | EtOH 0.12 | MDD | Father: Alcohol abuse | 2 | 48 |
| 23 | 61/M/AAm | 27.0 | 30 | 6.68 | N | NITROGLYCERIN | NDD | No | Father and brothers: Alcohol abuse | 46 | 15 |
| 24 | 36/M/C | 15.0 | 13 | 6.72 | S | AMITRIP, chlordiazepoxide, metronidazole, buspirone | EtOH 0.19, amitrip, diazepam, cocaine metabolite | MDD | Alcohol abuse, suicide attempts, affective disorders | 21 | 15 |
| 25 | 48/F/C | 30.0 | 18 | 6.23 | A | IMIPRAMINE, PAROXETINE, VALPROATE, CLONAZEPAM, LUVOX? | imipramine, acetone, isopropanol, lidocaine | Moderate BPD, recently depressed | Father: Alcohol abuse, Maternal grandmother: bipolar depression | 33 | 15 |
| 26 | 29/M/C | 27.0 | 24 | 6.75 | N | None known | NDD | Depressed mood, several episodes in last year | Father: attempted suicide, Alcoholic uncle | 14 | 15 |
| 27 | 37/M/C | 19.0 | 16 | 6.89 | S | SERTRALINE 1DAY, Hx: paroxetine | EtOH 0.13 | moderate MDD | Both parents alcoholic | 23 | 14 |
| 28 | 30/M/C | 12.0 | 18 | 6.49 | A | None known | EtOH 0.17, CO 49% | No | Father and brother: Alcohol abuse | 15 | 14 |
| 29 | 54/M/C | 24.0 | 12 | 6.42 | N | None known | lidocaine | No | Sister: heavy drinker | 39 | 15 |
| 30 | 41/F/C | 24.0 | 11 | 6.74 | N | None known | diazepam, chlorpheniramine, lidocaine | No | Alcohol abuse | 20 | 21 |
| 31 | 51/M/C | 22.0 | 9 | 7.07 | S | SERTRALINE | EtOH 0.16 | Depressed mood, long periods | Alcohol abuse | 33 | 18 |
| 32 | 59/M/C | 36.0 | 37.5 | 6.74 | S | None known | EtOH 0.08 | No | None | 39 | 20 |
| 33 | 46/M/C | 26.0 | 35.1 | 6.84 | N | None known | NDD | No | Father, grandfather: Alcoholic | 34 | 12 |
| 34 | 50/M/C | 14.0 | 30.1 | 6.66 | A | GLUXOTROL, ISOSORBIDE, BUMETANIDE, DILTIAZEM, FOSINOPRIL | EtOH 0.27 | No | Aunt: depression, suicide attempt. Brothers (3): emotional, alcohol, drug problems | 20 | 30 |
| 35 | 44/M/C | 17.0 | 28.8 | 6.87 | N | None | EtOH 0.12 | No | Not reported | 26 | 18 |
| 36 | 40/F/C | 27.5 | 15.7 | 6.67 | N | ALBUTEROL | NDD | No | Sister: emotional problems | 21 | 19 |
| 37 | 44/M/C | 20.0 | 13.7 | 6.77 | S | FLUOXETINE, LORAZEPAM, diclofenac | fluoxetine | MDD, mild | Father: alcoholic, Aunt: depression | 10 | 34 |
| 38 | 30/F/C | 17.0 | 13 | 6.77 | S | fluoxetine, sertraline, alprazolam, paroxetine, BUPROPRION | EtOH 0.26 | MDD, single moderate | Aunts and uncles with alcohol problems | 9 | 21 |
| Average | 44.2 | 21.9 | 21.11 | 6.70 |
AAm, African American; A, accident; BPD, bipolar disorder; C, caucasian; E, electrocution; F, female; H, homicide; M, male; MDD, major depressive disorder; N, natural; NDD, no drugs detected; PMI, postmortem interval (hours), defined as the time between death and the beginning of the formalin-fixation; S, suicide, TF, time in formalin (months); EtOH, ethanol blood level; Amitrip, Amitriptyline.
Capitalized drugs were prescribed in last month of life.
The duration (years) of alcohol dependence covers the time between the first display (and not necessarily diagnosis) of signs of dependence and the date of death.
In the Results section, the groups described here are compared with a group of MDD subjects who were included in a previous study on glia in the dlPFC (Rajkowska et al 1999). The average postmortem delay and time in fixative in that study were not significantly different from the control and alcohol-dependent groups in this report. The average age of the MDD group was older but not significantly different, and the matching for gender and race was within the guidelines for the other groups presented in this article.
Tissue Sampling
Brain tissue was collected postmortem from the dlPFC (Brodmann's area 9) in 21 psychiatrically normal control subjects and 17 subjects who were retrospectively diagnosed with alcohol dependence. These two groups were closely matched by age, gender, race, postmortem delay, and fixation time (see Table 1). There were no significant differences between groups for these variables. The precise location and extent of dlPFC (area 9) has been described previously based on quantitative cytoarchitectonic (Rajkowska and Goldman-Rakic 1995a, 1995b). Blocks of tissue (3 × 3 cm) from area 9 were embedded in celloidin, cut into 40-μm-thick sections, and stained for Nissl substance.
3-D Cell Counting and Measurements of Nuclear Size
Computer-assisted image analysis with 3-D cell counting capabilities based on the optical disector method (Gundersen 1986; Williams 1989; Williams and Rakic 1988) was used to estimate cortical thickness and glial cell morphometric parameters (glial cell density, size of glial cell nuclei). Glial cell densities are expressed as number of cells × 103 × mm−3; sizes of glial cell nuclei are calculated in μm as an equivalent diameter-circle, that is, (4 × area) / (circumference of the area of the nucleus); and cortical thickness is measured as the distance between the overlying pia and underlying white matter (for further details, see Rajkowska et al 1999). The area of the projected nucleus in its equatorial plane was considered the area of the nucleus. The morphometric parameters were measured in three cortical probes evenly spaced at 400-μm intervals along rostro-caudal levels of area 9, with the first section chosen at random within area 9. The quantification was carried out with a 100× magnification objective with a numerical aperture of 1.3. Each probe consisted of an uninterrupted series of 3-D counting boxes (90 × 60 × 25 μm), spanning the entire depth of the cortex (for further details on the method, see Rajkowska et al 1998; Selemon et al 1995). Alternating brain sections were coded and counted by two researchers (JW and JJMH) who were unaware of the diagnoses of the subjects while counting.
Two size analyses were performed to determine whether morphologic size changes had taken place in the glial cells of alcohol-dependent subjects. One of the analyses considered the average nuclear size in individual layers and in all layers combined. The other analysis studied the absolute density of different classes (size classes) of glial cells sorted according to small, medium-sized, large, and extra-large glial cell nuclei. The four size-classes were obtained using the mean (M) and the standard deviation (δ) of the control cases as follows: small (smaller than M − 1∂), medium-sized (M − 1∂ to M), large (M to M + 1∂), and extra large (larger than M + 1∂). Nuclei of glial cells were distinguished from small neurons in cresyl violet stained sections for lacking a Nissl-stained cytoplasm, the presence of several dark chromatin granules (with the exception of the nucleolus chromatin in neuronal nuclei, which is more uniform and with smooth appearance), and a thick condensation of stained material in the periphery of the nucleus that gives the appearance of a heavily stained membrane enclosing the nuclear content.
Thickness of the cortex was determined by the accumulated thickness of the six layers at the places where measurements of cell density and nuclear size were taken.
Areal Fraction of GFAP Immunoreactivity
The area occupied by GFAP immunoreactivity in layers III + IV and in layer V was examined. These layers were chosen for analysis because significant reductions in glial cell density and glial nuclear size in these same layers have been previously reported in MDD (Rajkowska et al 1999), for which glial reductions were also observed. The methods employed to detect GFAP immunoreactivity and measure the areal fraction of GFAP (area of GFAP staining in a region of interest divided by the total area of the region of interest and multiplied by 100) have been described in detail in previous publications (Miguel-Hidalgo et al 2000). The average of three GFAP areal fraction measurements per subject was considered as the individual value of areal fraction and used for statistical analysis.
Statistics
The mean values for all layers combined of the individual morphometric parameters obtained from the three cortical probes were compared between the alcohol-dependent and control groups using single factor (disease) analyses of variance (ANOVA, p < .05). We also compared the groups with a repeated measures analysis considering the values of density or nuclear size in each of the six cortical layers as repeated measures. For the analysis of glial size classes, each layer was studied separately, and diagnosis effects were determined with a repeated measures ANOVA with the four size classes as repeated measures. The repeated measures analysis of variance (ANOVA) for comparing groups was performed using both PROC GLM and PROC MIXED from the SAS system (Cary, NC). PROC GLM and PROC MIXED give identical results when compound symmetry is assumed for the covariance structure and where there are no missing data, as was the case in our study. Analysis of the data was also performed using an unstructured covariance pattern (using postmortem delay, fixation time, and pH as covariates). This resulted in slightly different values for testing the groups main effect but no general difference in the overall significance levels or conclusions. Because the assumption of compound symmetry is appropriate here and because results from using a mixed models approach with compound symmetry in the presence of complete data are identical to those from the linear models approach, all repeated measures tests reported here are from PROC GLM. Multiple correlation analysis was used to separately examine the influence of age, postmortem delay, storage time in formalin, and pH of the brain on cortical thickness, glial density, nuclear sizes, and GFAP areal fraction.
Significance levels in selected contrasts involving individual layers or individual size classes were established after Bonferroni adjustments for the number layers (six) or number of size classes (four), and only levels with p < .01 were considered significant.
Results
Packing Density of Glial Cells
The average density of glial cells in all layers combined was significantly reduced by 11% in the alcohol-dependent (Alc) compared with the control group [F(1,36) = 11.76; p < .003] (Figure 1). Analysis of the density of glial cells considering the values for individual layers in a repeated measures ANOVA also revealed a significant difference between Alc subjects and control subjects [F(1,36) = 11.58; p < .003]. Selected contrasts for individual layers revealed significantly lower density of glial cells in layers V (12%) and VI (14%) in the Alc group as compared with control subjects. There was a trend (.01 > p > .05) for lower glial density in layers I (12.2%) and II (13%; Table 2, Figure 2) in the Alc compared with the control group. No significant layer by diagnostic group interaction was found (F = 1.33, p = .254).
Figure 1.
Plots showing the distribution of individual values for packing density of glial cells in all layers combined in the control (n = 21) and alcohol-dependent (n = 17) groups. A horizontal bar indicates the median value.
Table 2.
Packing Density of Glial Cell Nucleia in All Layers Combined and in Each of the Six Layers of Cortical Area 9
| Control | ALC(ALL) | p | F(1,36) | ALC-ND | p | F(1,35) | ALC-D | p | F(1,35) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 101.0 ± 9.7 | 90.2 ± 9.6b | .002 | 11.8 | 92.1 ± 10.8b | .028 | 5.3 | 88.0 ± 8.2b | .003 | 10.4 |
| Layer I | 89.6 ± 11.7 | 78.7 ± 13.7c | .012 | 7.1 | 77.5 ± 11.6c | .023 | 5.7 | 80.0 ± 16.4 | .078 | 3.3 |
| Layer II | 81.6 ± 11.8 | 70.9 ± 14.8c | .018 | 6.1 | 75.5 ± 14.6 | .248 | 1.4 | 65.7 ± 14.1b | .006 | 8.6 |
| Layer III | 91.6 ± 10.9 | 85.0 ± 11.7 | .084 | 3.1 | 87.4 ± 13.9 | .359 | 0.9 | 82.4 ± 8.7 | .058 | 3.8 |
| Layer IV | 106.8 ± 17.1 | 100.6 ± 15.7 | .258 | 1.3 | 102.8 ± 18.9 | .554 | 0.4 | 98.1 ± 12.0 | .218 | 1.6 |
| Layer V | 100.8 ± 10.1 | 89.3 ± 13.3b | .005 | 9.2 | 93.9 ± 12.7 | .133 | 2.4 | 84.2 ± 12.9b | .001 | 12.5 |
| Layer VI | 129.0 ± 19.0 | 111.2 ± 15.2b | .003 | 9.8 | 109.3 ± 14.5b | .008 | 7.9 | 113.4 ± 16.7c | .04 | 4.5 |
Density data as mean ± standard deviaiton.
Glial Cells × 103/mm3.
Statistically significant compared with the control value only before Bonferroni adjustment.
Statistically significant compared with the control value after Bonferroni adjustment.
Figure 2.
Bar chart of the density of glial cells in individual cortical layers (I, II, III, IV, V, and VI) and in all layers combined (ALL) of the dorsolateral prefrontal cortex in control subjects (n = 21), the subgroup of alcohol-dependent subjects without depressive symptoms (ALC-ND; n = 9), and the subgroup of alcohol-dependent subjects with depressive symptoms (ALC-D; n = 8). Two asterisks indicate significant reduction in the disease groups (p < .01) compared with the control group; one asterisk indicates a nonsignificant trend to reduced density compared with the control group (.01 < p < .05). Error bars represent the standard error of the mean.
Further analyses were performed after dividing the group of Alc subjects into those with depression (Alc-D) and those without depression (Alc-ND). Although glial densities did not significantly differ between Alc-D and Alc-ND, in most measures the highest values of average glial packing density were found in the control group, the lowest values were observed in the Alc-D group, and intermediate values appeared in the Alc-ND group (Table 2). When considering the glial density in all layers combined (overall density) of the three groups (control, Alc-D, Alc-ND), one-way ANOVA showed significant effect of diagnostic group [F(2,35) = 6.22; p < .005]. In selected pairwise univariate, contrasts the glial density of all layers combined was reduced (8.8%) in the Alc-ND and in the Alc-D group (13%) compared with control subjects (Table 2). In a repeated measures ANOVA with layers as repeated measures, there was also a significant effect of the diagnostic group [F(1,35) = 6.06; p < .005]. Again there were no significant layer-by-diagnostic group interactions. Selected contrast analysis (Table 2) showed significantly lower glial densities in layers I (13.5%) and VI (15.3%) in the Alc-ND compared with the control group. In the Alc-D group, reductions in glial densities were found in layers II (19.4%), V (16.5%), and VI (15.3%) compared with control subjects, although only differences in layers II and V remained significant after statistically adjusting for the number of comparisons (Table 2).
We also divided the Alc group into those subjects with onset of alcohol dependence starting at or before 15 years of age and those starting at age 16 and later. No significant differences were found between these subgroups, either for individual layers or all layers combined. Likewise, no significant gender differences were found in either the controls or the Alc group.
Analysis of the Size of Glial Nuclei
ANALYSIS OF AVERAGE NUCLEAR SIZES
There was a significant difference in the average size of glial nuclei in all layers pooled together, with the average size in the alcohol-dependent subjects (4.995 ± 0.060 μm) being significantly smaller than in the control group by 3.2% (5.158 ± 0.054 μm, F = 4.108, p < .05, Figure 3). When separating the alcohol-dependent group into Alc-D and Alc-ND groups, no significant difference was detected between groups in the average size of glial cell nuclei (F = 1.994, p = .151). Nevertheless, the average values of glial size in the Alc-D group were still the lowest of the three groups (Figure 3). A tendency for a reduction of average glial size in layer III [4.4%, F(1,27) = 4.65; p < .04] was found in the Alc-D compared with the control group, but the difference was not significant after adjusting for multiple comparisons. No significant differences or a tendency for smaller glial sizes was found when comparing the Alc-ND group to the control group.
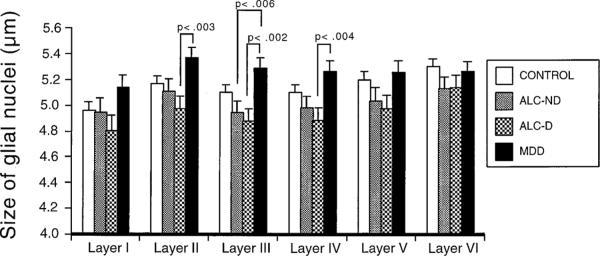
Figure 3.
Graph comparing the average size of glial cell nuclei in individual cortical layers in the group of control subjects, the subgroups of alcohol dependent subjects, and a group of subjects diagnosed with major depressive disorder (MDD). Only the level of significance (p) for differences between the MDD group and the subgroups of alcohol-dependent subjects is shown. Error bars represent the standard error of the mean.
ANALYSIS OF THE DENSITY OF GLIA SIZE-CLASSES
When considering all cortical layers combined, the density of large glial cell nuclei (diameter 5.1–5.8 μm) was significantly lower in the alcohol-dependent than in control subjects by 19% [F(1,36) = 5.28; p < .03]. When separating the groups into control, Alc-D, and Alc-ND, significant diagnosis effects were found for all cortical layers combined [F(2,35) = 7.19; p < .002] and individually for layers I [F(2,35) = 3.87; p < .03], II [F(2,35) = 4.64; p < .02], V [F(2,35) = 8.99; p < .001], and VI [F(2,35) = 5.26; p < .01]. In Alc-D (compared with control subjects), the density of large glia was found to be significantly reduced in all layers combined [23.7%, F(1,35) = 5.08; p < .04; Figure 3].
Comparison to a Group of MDD Subjects
We performed comparisons of the new data from this study with those of glial density and size from a group of 12 MDD subjects (non–alcohol dependent) who were included in our previous study (Rajkowska et al 1999). These data were obtained from the same cortical region, using the same methodology as in the report presented here. Data from this group of MDD patients (n = 12) was considered the fourth group in the ANOVA, the other three groups being the control subjects (n = 21), Alc-ND (n = 9), and Alc-D (n = 8) subjects. There were significant differences in overall glial density [F = 4.571, p < .006] as well as in nuclear glial size [F = 4.572, p < .02] between the four groups. Also significant was the difference between groups as analyzed by ANOVA when considering the data for the six cortical layers as repeated measures (for density p < .005, for size p < .05). Univariate contrasts confirmed the differences reported earlier between the control group and the Alc-ND and Alc-D groups. Only glial density in layer VI of the MDD subjects was significantly higher than in the Alc-D group (F = 6.017, p < .02). By contrast, in the MDD group the average nuclear size was increased in layers II, III, and IV compared with the Alc-D group and in layer III compared with the Alc-ND group.
Cortical Thickness
The overall thickness of the cortical gray matter in the sampled area 9 was not significantly different in subjects with alcohol dependence compared with control subjects [F(1,36) = 2.023, p = .164]; however, contrast analysis of the relative width of individual cortical layers showed that the thickness of layer V tended to be reduced by 17.3% in the subjects with alcohol dependence [F(1,36) = 4.423, p = .043]. No tendencies or significant differences in the thickness of the other layers were observed between the MDD group and each of the Alc subgroups.
Correlation with Age, Postmortem Delay, Time in Formalin, and pH
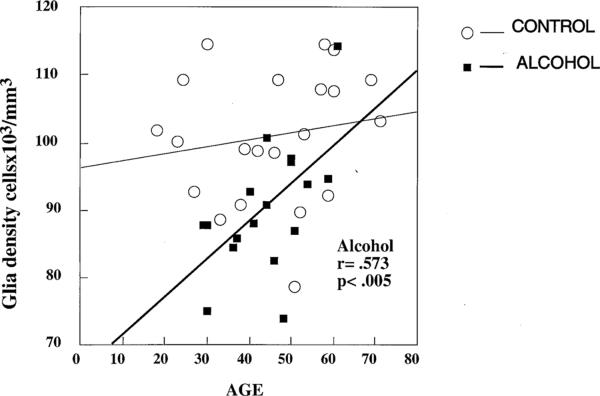
In the control group, there was a positive correlation between age and glial density only in layer II (r = .661, p < .001) but not when considering all layers combined. In the Alc group, there was a significant positive correlation between age and the glial density in all layers combined (r = .573, p < .02; Figure 4) and in layer V (r = .651, p < .005). Also in the Alc group, there was a trend for correlation between age and glial density in layers III (r = .525, p < .03) and IV (r = .512, p = .04). Unlike glial density, average glial size in the Alc group was not correlated with age for any layer, but in the control group it was positively correlated with age in each cortical layer and when all layers were combined (r = .576, p < .007). In both the control and Alc groups, however, the density of large (Alc, r = .546, p < .03; control, r = .533, p < .02), and extra-large (Alc, r = .528, p < .03; control, r = .546, p < .01) glial cells tended to increase with age. Pearson correlation analysis showed that postmortem interval, time in formalin, or tissue pH were not significantly correlated with either the size or the density of glial cells either in individual cortical layers or in the combination of all layers. The estimated age of onset and duration of alcohol abuse were not significantly correlated with glial density or size of glial nuclei, either for individual layers or all layers combined.
Figure 4.
Scatter plot of glial cell density against age in control and alcohol-dependent subjects with regression lines showing the significant correlation (r = .573, p < .005) between age and density of glial cells in the alcohol-dependent group.
GFAP Immunostaining
There was considerable variability in the distribution of GFAP from subject to subject in both control and Alc subjects; however, as described in our previous study (Miguel-Hidalgo et al 2000) layers I, II, and VI were generally richer in immunoreactive structures than layers III, IV, and V and the abundance of GFAP immunorective structures was higher in the oldest subjects. Visual inspection suggested that immunoreactivity in sections from Alc subjects was sparser than in control subjects (Figure 5), although the analysis of areal fraction did not confirm that impression. In many cases, whether control or Alc, GFAP immunostaining was intense in layers I, II, and VI and covered these layers, making it unreliable to measure the areal fraction of GFAP for subsequent comparisons. To establish meaningful comparisons between GFAP areal fraction in this study and GFAP areal fraction in our previous studies of MDD and control subjects (Miguel-Hidalgo et al 2000; Miguel-Hidalgo and Rajkowska 1999), we chose to measure the areal fraction of GFAP immunostaining in layers III + IV and in layer V.
Figure 5.
Microphotographs encompassing the six cortical layers in sections through cortical area 9. (A) Nissl-stained section showing the general cytoarchitecture of area 9 and the division into layers (I, II, III, IV, V, VI) in a control subject (male, 38 years of age). (B) Distribution of GFAP immunoreactivity in an adjacent section in the same subject as in A. (C) Distribution of GFAP immunoreactivity at a comparable level of area 9 in an alcohol-dependent subject (male, 37 years of age, with depressive symptoms). Calibration bar: 250 μm, same for A, B, and C.
LAYERS III + IV
The average areal fraction (%) of GFAP immunoreactivity in layers III + IV of the Alc subjects (40.06 ± 6.50) was not different from the average in the control group (45.94 ± 5.85); however, the values for the Alc subjects were more variable than values in the control group (coefficients of variation 0.74 and 0.53, respectively). In addition, in the Alc group there were seven subjects with GFAP areal fraction values under 20%, but only two such values in the control group. Among the seven Alc subjects with low values of GFAP areal fraction (less than 20%), five of these subjects were alcoholics with a history of depressive episodes. By contrast, only 3 of 10 Alc subjects with GFAP areal fraction values over 20% were alcohol-dependent subjects with a history of depressive episodes. The GFAP areal fraction was positively correlated with age in both the control (r = .605, p < .004) and the Alc (r = .533, p < .03) groups.
Because our recent study on MDD showed an influence of age on the measurements of GFAP areal fraction (Miguel-Hidalgo et al 2000), each of the alcohol-dependent and control groups were divided into two subgroups: subjects younger than 46 years of age and subjects 46 years of age and older. There were no significant differences in mean GFAP areal fraction when comparing the younger control subjects with the younger alcohol-dependent subjects or when comparing the older control subjects with the older Alc subjects.
LAYER V
The average GFAP areal fraction (%) in layer V of the alcohol-dependent subjects (63.01 ± 8.04) was not significantly different from the control subjects (71.60 ± 5.26). Pearson correlation analysis revealed a significant positive correlation between age and GFAP areal fraction in the Alc-ND subgroup (r = .712, p < .04) and in the control group (r = .448, p < .05), but not in the Alc-D group (r = .185, p = .66). In layers III + IV and layer V, GFAP areal fraction was not correlated with either postmortem delay, time in fixative, or tissue pH. In layer V, there was no significant difference between control and alcohol-dependent subjects in either the younger or the older subjects.
Discussion
In this study, the average density of glial cells in dIPFC (cortical area 9) of alcohol-dependent subjects was significantly reduced compared with psychiatrically normal control subjects. The absence of an interaction of layer and diagnostic group indicates that the reduction occurred across all cortical layers. In addition, there was a significant reduction in the average size of glial nuclei in the same area of the alcohol-dependent subjects. Although statistical adjustment for the number of layers analyzed does not allow for a firm conclusion regarding size changes in particular layers, a smaller average size (with p < .05) was observed in layers I, III, IV, and V of alcohol-dependent subjects. The lower glial density and smaller nuclear size found in dIPFC is consistent with the hypothesis that deficient glial function contributes to the functional and behavioral alterations in subjects with alcohol dependence. Systematic studies of glial and neuronal numbers in the prefrontal cortex of control animals and of animal models of alcohol dependence should help establish the time course and eventually the cellular and molecular pathology caused by alcohol in prefrontal cells.
Evidence for the involvement of glial cells in the alcohol-related neuropathology of the prefrontal cortex was reported by Kril and Harper (1989) in the superior frontal gyrus of alcoholics (which is mostly covered by cytoarchitectonic area 9; Rajkowska and Goldman-Rakic (1995a, 1995b). Unlike the results presented here, Kril and Harper (1989) described an increase in the density of glial cells. One reason for the discrepancy might be that the area sampled by Kril and Harper (1989) was not defined by quantitative cytoarchitectonic criteria as used in our study. This possible discrepancy is important because marked regional differences in glial and neuronal alterations are reported in the prefrontal pathology in other psychiatric disorders (MMD, BPD, schizophrenia) (Cotter et al 2001a, 2002; Önguür et al 1998; Rajkowska et al 1997, 1999, 2001). A further potential confound in the Kril and Harper (1989) report is that the authors differentiated neurons from glial cells solely based on their size (glia smaller than 40 μm2). However, Kril and Harper (1989) also reported a significant reduction by 18% in the average neuronal size. Consequently, many of the smaller neurons in the alcoholic group might have been inadvertently classified as glia. In contrast, in our study glial cells were distinguished from neurons not only according to size but also by shape and the morphology of chromatin in glial nuclei. These criteria allow a more reliable distinction of glial cells from small neurons. It is also important to stress that the average age of the individuals in the study of Kril and Harper (1989) was about 10 years older (57 for control and 58 for alcoholic subjects) than in our study. The age distinction is relevant because there is a positive correlation between age and glial density that appears to be more marked in older alcoholics. Additionally, an effect of age might have been further complicated by the presence of Wernicke's encephalopathy or cirrhosis in several alcoholic subjects included in the study of Kril and Harper (1989). In these advanced cases, ongoing neuronal degeneration and age may have produced a reactive secondary increase in glial cells that would not be detectable in our subjects. In agreement with the reductions in glial density observed in our study, Korbo (1999) reported a marked (37%) decrease in the number of glial cells in the hippocampus of alcoholics without Wernicke's encephalopathy that were closer in age (average 44 years) to the sample in our study.
Age appears to influence the effects of alcohol on glial cells of the dIPFC. In the control group, there was no correlation between age and the overall density of glial cells, but in the alcoholic group, a positive correlation was found between these two parameters. Because no correlation was detected between those same parameters and the estimated duration of alcohol dependence, it appears that in aged subjects, alcohol dependence would cause changes consistent with a degenerative pattern of increased glia proliferation that is not present in younger subjects. A degenerative process would be consistent with the MRI findings of Pfefferbaum et al (1992) that aging in alcoholics accelerates the loss in volume of brain gray and white matter. Although the expectation would be that the density of glial cells increases with age also in the control subjects, our data do not rule out that an increase may occur in aged subjects over 65 years old, because our sample only included three such subjects.
Our study of glial packing density in Nissl-stained sections does not inform whether all or only some types of glia are affected by alcoholism. Nissl-stained material does not allow unequivocal identification of each of the three major types of glial cells—microglia, astroglia, and oligodendroglia; however, there is abundant evidence on the effects of ethanol on astrocyte proliferation, differentiation, and synthesis of the cytoskeletal astrocytic marker GFAP (Guerri and Renau-Piqueras 1997; Guerri et al 1993; Lokhorst and Druse 1993). Therefore, the participation of astrocytes in the glial neuropathology of alcoholism was assessed by measuring the areal fraction covered by GFAP immunostaining in tissue sections of the dIPFC. Although there was no significant difference in the average GFAP areal fraction between the alcohol-dependent and control groups, a closer examination of the data revealed that the lower values of GFAP were more frequent in the alcohol-dependent group. Additionally, the lower values in alcohol-dependent subjects were specially concentrated in those subjects with depressive symptoms. Unlike younger subjects with MDD, in whom significantly lower values of GFAP areal fraction were measured compared with younger control subjects (Miguel-Hidalgo et al 2000), GFAP areal fraction in younger alcoholics was not different from that in younger control subjects in either layers III + IV or layer V.
The high variability of GFAP staining in both groups, but especially in the alcohol-dependent subjects, may be related to the apparently dual actions of ethanol on GFAP expression in vivo. Exposure to high doses of ethanol that produce neuronal damage also induce gliosis and an increase in GFAP expression. The synthesis of GFAP, however, is down-regulated after prolonged exposure to ethanol (Franke 1995; Franke et al 1997). It is possible that an increase in GFAP staining as a part of the glial reaction to neuronal or synaptic injury is paralleled by astrocytes experiencing a toxic decrease in their numbers as exposure to alcohol lags. There is in vitro evidence that ethanol inhibits the proliferation of astrocytes by reducing the synthesis of mRNA and proteins (Vallés et al 1996, 1997). In primary cultures of astrocytes from the adult human brain, ethanol directly inhibits cell proliferation (Kane et al 1996). In our study, however, analysis of the areal fraction of GFAP immunoreactivity did not support the sole involvement of astroglia in the reduction of glial packing density. The results rather point to additional types of glia being affected in alcohol-dependent subjects and contributing to the average reduction in glial density in these individuals. Indeed, there is experimental evidence in vitro and in vivo for alcohol-induced alterations in the activity, proliferation, and survival of oligodendroglia and microglia (Aroor and Baker 1998; Colton et al 1998; Davies and Ross 1991; Korbo 1999; Snyder 1996). Chronic exposure to ethanol alters the lipid composition of myelin in rats (Sun et al 1979); reduces 2′,3′-cyclic nucleotide 3′-phosphohydrolase activity (Sedmak et al 1978); and also decreases myelin proteins during myelination (Sedmak et al 1978). These changes in myelin components may be one of the main causes of white matter shrinkage and enlarged ventricles observed in the brains of alcoholics. Recently, it has been shown that phagocytosis and superoxide anion production by microglia are reduced by ethanol in vitro (Aroor and Baker 1998). Consequently, pathology affecting not only astrocytes but also oligodendrocytes and microglia in the gray matter may explain the reduced numbers and nuclear sizes of glial cells observed in our study. Further studies evaluating these cell types in alcohol dependence are clearly needed.
A reduction in the density of glial cells in specific cortical layers of area 9 has been found in the postmortem brain of subjects with MDD and BPD (Rajkowska et al 1999, 2001). In these disorders, the decrease in glial density is observed mainly in layers III and V, but not when all six cortical layers are combined. By contrast, in alcoholic subjects there was a significant decrease in glial density in layers II, V, and VI and also in the cortex as a whole, indicating that prefrontal glial pathology in alcoholism is more widely distributed across layers than in mood disorders. Other features of glial pathology also appear to distinguish alcoholism from MDD and BPD. In MDD, the average glial nuclear size was shown to be increased in sublayer IIIb and the density of extra-large glial cell nuclei to be significantly greater in layer III. In BPD, the average size of glial nuclei was increased in sublayer IIIc, as was the density of extra-large glial nuclei in the same layer (Rajkowska et al 2001). In alcoholic subjects, however, the mean glial nuclear size was found to be significantly decreased in layers III, IV, and in sublayer Va compared with control subjects. Moreover, the average glial size in layers II, III, and IV of the MDD subjects was significantly larger than in the group of alcoholics with depressive symptoms even after the Bonferroni adjustment. In summary, in the dIPFC, the density of glial cells is lower in both MDD and Alc subjects compared with control subjects; however, the changes in average glial size are divergent in these disorders compared with control subjects. Glia nuclei are smaller in Alc and larger in MDD. It could be argued that one defining factor of glial involvement in the manifestation of the depressive pathology is a reduction in the glial distribution in the dIPFC that is reflected in a reduced glial density. Consequently, reduced glial nuclear size in alcoholism, together with reduced packing density, might be related to the cytotoxic effects of prolonged alcohol exposure. It cannot be ruled out that subjects who are prone to alcohol dependence do share some glial alterations with MDD and BPD subjects before onset of protracted alcohol abuse. Long term exposure to alcohol may just complicate a predisposition to glial alterations. Alternatively, alcohol may directly and independently affect those glial cells in the prefrontal cortex that are involved in the pathology of depression. In either case, it should be expected that the glial alterations in alcohol-dependent subjects with depressive pathology are more marked than in uncomplicated alcoholics. In fact, the subgroup of alcoholics with depression did display a larger reduction in overall glial density and a highly significant reduction in the density of glial cells in layer V. It cannot be ruled out that antidepressant medication also contributes to the differences between Alc-ND and Alc-D because all but one of our subjects with depressive symptoms had taken antidepressant medication at some point of their lives. In summary, whether by itself or in combination with other constitutive or induced brain differences, the glial pathology of prolonged alcohol dependence appears to differ from the alterations found in other psychiatric disorders.
The reduction of both glial density and size described here, and the absence of a generalized reduction in neuronal numbers in most cortical areas (Harper 1998; Jensen and Pakkenberg 1993; Kril and Halliday 1999) and in the hippocampus (Korbo 1999), are consistent with the reversal of cognitive symptoms and gross anatomic alterations observed after prolonged abstinence from alcohol (Johnson-Greene et al 1997; Pfefferbaum et al 1995; Zipursky et al 1989). Although the loss of many neurons (as seen in neurodegenerative diseases) is likely to produce some degree of permanent deficits, the preferential loss and structural alterations in glial cells allows recovery, because glial cells retain the ability to proliferate in the central nervous system (Giulian et al 1986, 1991; Wood and Bunge 1991). If this explanation is correct, future studies should show a normalization of the density and size of glial cells after a sufficiently long period of abstinence from alcohol.
Acknowledgments
Supported by a grant from the Alcoholic Beverage Medical Research Foundation (JJM-H), a grant from the Foundation for Clinical Neuroscience Research and Education (CAS), and NIH Grant Nos. MH45488 (CAS), MH55872 (GR), and MH61578 (GR).
We acknowledge the excellent assistance of the Cuyahoga County Coroner's Office in Cleveland, Ohio. The authors thank Lisa Konick and Ginny Dilley (Case Western Reserve University, Cleveland, Ohio, USA) for assistance in collecting information on many of the subjects under study and Drs. Herbert Y. Meltzer and Bryan Roth for their assistance in the psychiatric diagnoses.
References
- Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, Dede D, et al. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcohol Clin Exp Res. 1993;17:205–210. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Adams KM, Gilman S, Koeppe B, Kluin K, Junck L, Lohman M, et al. Correlation of neuropsychological function with cerebral metabolic rate in subdivisions of the frontal cortex of older alcoholics patients measured with [18F]fluorodeoxyglucose and position emision tomography. Neuropsychology. 1995;9:275–280. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, DC: 1995. [Google Scholar]
- Aroor AR, Baker RC. Ethanol inhibition of phagocytosis and superoxide anion production by microglia. Alcohol. 1998;15:277–280. doi: 10.1016/s0741-8329(97)00129-8. [DOI] [PubMed] [Google Scholar]
- Colton CA, Snell-Callanan J, Chernyshev ON. Ethanol induced changes in superoxide anion and nitric oxide in cultured microglia. Alcohol Clin Exp Res. 1998;22:710–716. [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001a;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: The evidence and implications. Brain Res Bull. 2001b;55:585–595. doi: 10.1016/s0361-9230(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Cullen KM, Halliday GM. Chronic alcoholics have substantial glial pathology in the forebrain and diencephalon. Alcohol Alcohol Suppl. 1994;2:253–257. [PubMed] [Google Scholar]
- Dally S, Luft A, Ponsin JC, Girre C, Mamo H, Fournier E. Abnormal pattern of cerebral blood flow distribution in young alcohol addicts. Br J Addict. 1988;83:105–109. doi: 10.1111/j.1360-0443.1988.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Davidson KM. Diagnosis of depression in alcohol dependence: Changes in prevalence with drinking status. Br J Psychiatry. 1995;166:199–204. doi: 10.1192/bjp.166.2.199. [DOI] [PubMed] [Google Scholar]
- Davidson KM, Blackburn IM. Co-morbid depression and drinking outcome in those with alcohol dependence. Alcohol Alcohol. 1998;33:482–487. doi: 10.1093/alcalc/33.5.482. [DOI] [PubMed] [Google Scholar]
- Davies DL, Ross TM. Long-term ethanol-exposure markedly changes the cellular composition of cerebral glial cultures. Brain Res Dev Brain Res. 1991;62:151–158. doi: 10.1016/0165-3806(91)90162-c. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Pet and the functional anatomy of major depression. In: Nakajima T, Ono T, editors. Emotion, Memory and Behavior: Studies on Human and Nonhuman Primates. Japan Scientific Societies Press; Tokyo: 1995. pp. 43–62. [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spi RL. Structured Clinical Interview for DSM-IV Axis I Disorders—Clinician V. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Franke H. Influence of chronic alcohol treatment on the GFAP-immunoreactivity in astrocytes of the hippocampus in rats. Acta Histochem. 1995;97:263–271. doi: 10.1016/S0065-1281(11)80187-X. [DOI] [PubMed] [Google Scholar]
- Franke H, Kittner H, Berger P, Wirkner K, Schramek J. The reaction of astrocytes and neurons in the hippocampus of adult rats during chronic ethanol treatment and correlations to behavioral impairments. Alcohol. 1997;14:445–454. doi: 10.1016/s0741-8329(96)00209-1. [DOI] [PubMed] [Google Scholar]
- George MS, Teneback CC, Bloomer CW, Horner MD, Anton RF. Using neuroimaging to understand the alcohol's brain effects. CNS Spectrums. 1999;4:88–92. [Google Scholar]
- Giulian D, Allen RL, Baker TJ, Tomozawa Y. Brain peptides and glial growth. I. Glia-promoting factors as regulators of gliogenesis in the developing and injured central nervous system. J Cell Biol. 1986;102:803–811. doi: 10.1083/jcb.102.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Johnson B, Krebs JF, George JK, Tapscott M. Microglial mitogens are produced in the developing and injured mammalian brain. J Cell Biol. 1991;112:323–333. doi: 10.1083/jcb.112.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C, Renau-Piqueras J. Alcohol, astroglia, and brain development. Mol Neurobiol. 1997;15:65–81. doi: 10.1007/BF02740616. [DOI] [PubMed] [Google Scholar]
- Guerri C, Saez R, Portoles M, Renau-Piqueras J. Derangement of astrogliogenesis as a possible mechanism involved in alcohol-induced alterations of central nervous system development. Alcohol Alcohol Suppl. 1993;2:203–208. [PubMed] [Google Scholar]
- Gundersen HJG. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol. 1998;57:101–110. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Harper C, Kril J, Daly J. Are we drinking our neurons away? Br Med J (Clin Res Ed) 1987;294:534–536. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Kril J. Neuropathology of alcoholism. Alcohol Alcohol. 1990;25:207–216. doi: 10.1093/oxfordjournals.alcalc.a044994. [DOI] [PubMed] [Google Scholar]
- Hoaken PN, Giancola PR, Pihl RO. Executive cognitive functions as mediators of alcohol-related aggression. Alcohol Alcohol. 1998;33:47–54. doi: 10.1093/oxfordjournals.alcalc.a008347. [DOI] [PubMed] [Google Scholar]
- Jensen GB, Pakkenberg B. Do alcoholics drink their neurons away? Lancet. 1993;342:1201–1204. doi: 10.1016/0140-6736(93)92185-v. [DOI] [PubMed] [Google Scholar]
- Johnson-Greene D, Adams KM, Gilman S, Koeppe RA, Junck L, Kluin KJ, et al. Effects of abstinence and relapse upon neuropsychological function and cerebral glucose metabolism in severe chronic alcoholism. J Clin Exp Neuropsychol. 1997;19:378–385. doi: 10.1080/01688639708403866. [DOI] [PubMed] [Google Scholar]
- Kane CJ, Berry A, Boop FA, Davies DL. Proliferation of astroglia from the adult human cerebrum is inhibited by ethanol in vitro. Brain Res. 1996;731:39–44. doi: 10.1016/0006-8993(96)00456-8. [DOI] [PubMed] [Google Scholar]
- Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: A comparison with clinician antemortem diagnosis. Acta Psychiatr Scand. 1996;94:337–343. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- Ketter TA, George MS, Kimbrell TA, Benson BE, Post RM. Functional brain imaging, limbic function, and affective disorders. Neuroscientist. 1996;2:55–65. [Google Scholar]
- Korbo L. Glial cell loss in the hippocampus of alcoholics. Alcohol Clin Exp Res. 1999;23:164–168. [PubMed] [Google Scholar]
- Kril JJ, Halliday GM. Brain shrinkage in alcoholics: A decade on and what have we learned? Prog Neurobiol. 1999;58:381–387. doi: 10.1016/s0301-0082(98)00091-4. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Harper CG. Neuronal counts from four cortical regions of alcoholic brains. Acta Neuropathol. 1989;79:200–204. doi: 10.1007/BF00294379. [DOI] [PubMed] [Google Scholar]
- Laming PR, Kimelberg H, Robinson S, Salm A, Hawrylak N, Muller C, et al. Neuronal-glial interactions and behaviour. Neurosci Biobehav Rev. 2000;24:295–340. doi: 10.1016/s0149-7634(99)00080-9. [DOI] [PubMed] [Google Scholar]
- Lokhorst DK, Druse MJ. Effects of ethanol on cultured fetal astroglia. Alcohol Clin Exp Res. 1993;17:810–815. doi: 10.1111/j.1530-0277.1993.tb00846.x. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, et al. GFAP-immunoreactivity in the prefrontal cortex distinguishes young from old adults in major depressive disorder. Biol Psychiatry. 2000;48:860–872. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Rajkowska G. Immunohistochemistry of neural markers for the study of the laminar cytoarchitecture in celloidin sections from the human cerebral cortex. J Neurosci Meth. 1999;93:69–79. doi: 10.1016/s0165-0270(99)00114-4. [DOI] [PubMed] [Google Scholar]
- Nakamura MM, Overall JE, Hollister LE, Radcliffe E. Factors affecting outcome of depressive symptoms in alcoholics. Alcohol Clin Exper Res. 1983;7:188–193. doi: 10.1111/j.1530-0277.1983.tb05437.x. [DOI] [PubMed] [Google Scholar]
- Nicolas JM, Catafau AM, Estruch R, Lomena FJ, Salamero M, Herranz R, et al. Regional cerebral blood flow-SPECT in chronic alcoholism: relation to neuropsychological testing. J Nucl Med. 1993;34:1452–1459. [PubMed] [Google Scholar]
- Öngür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Segal Z, Yankofsky L. The effect of alcohol and placebo on affective reactions of social drinkers to a procedure designed to induce depressive affect anxiety and hostility. J Clin Psychol. 1980;36:337–342. doi: 10.1002/1097-4679(198001)36:1<337::aid-jclp2270360148>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Quantitative criteria for distinguishing areas 9 and 46. Cereb Cortex. 1995a;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46. Cereb Cortex. 1995b;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon L. Reductions in neuronal and glial density characterize the dorsolateral pre-frontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Marked glial neuropathology in prefrontal cortex distinguishes bipolar disorder from schizophrenia. Schizophr Res. 1997;24:41. [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: A postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Sedmak PA, Sedmak D, Fritz HI, Peterson GR. Myelination in chronically-alcoholic mice. Experientia. 1978;34:1059–1060. doi: 10.1007/BF01915346. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex: A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Snyder A. Responses of glia to alcohol. In: Aschner N, Kimelberg H, editors. The Role of Glia in Neurotoxicity. CRC Press; Boca Raton, FL: 1996. pp. 111–135. [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24:611–621. [PubMed] [Google Scholar]
- Sun GY, Danopoulos V, Sun AY. The effect of chronic ethanol administration on myelin lipids. Curr Alcohol. 1979;7:83–91. [PubMed] [Google Scholar]
- Vallés S, Pitarch J, Renau-Piqueras J, Guerri C. Ethanol exposure affects glial fibrillary acidic protein gene expression and transcription during rat brain development. J Neurochem. 1997;69:2484–2493. doi: 10.1046/j.1471-4159.1997.69062484.x. [DOI] [PubMed] [Google Scholar]
- Vallés S, Sancho-Tello M, Miñana R, Climent E, Renau-Piqueras J, Guerri C. Glial fibrillary acidic protein expression in rat brain and in radial glia culture is displayed by prenatal ethanol exposure. J Neurochem. 1996;67:2425–2433. doi: 10.1046/j.1471-4159.1996.67062425.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Burr G, Pascani K, et al. Decreased brain metabolism in neurologically intact healthy alcoholics. Am J Psychiatry. 1992;149:1016–1022. doi: 10.1176/ajp.149.8.1016. [DOI] [PubMed] [Google Scholar]
- Weingartner HJ, Eckardt MJ, Hommer D, Johnson DN. Impairments in reflective cognitive functions in alcoholics: A neuropharmacological model. Alcohol Alcohol Suppl. 1994;2:291–298. [PubMed] [Google Scholar]
- Williams RW. Three-dimensional counting: An accurate and direct method to estimate numbers of cells in sectioned material (Erratum) J Comp Neurol. 1989;281:335. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Three-dimensional counting: An accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol. 1988;278:344–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- Wood PM, Bunge RP. The origin of remyelinating cells in the adult central nervous system: the role of the mature oligodendrocyte. Glia. 1991;4:225–232. doi: 10.1002/glia.440040214. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Lim KC, Pfefferbaum A. MRI study of brain changes with short-term abstinence from alcohol. Alcohol Clin Exp Res. 1989;13:664–666. doi: 10.1111/j.1530-0277.1989.tb00401.x. [DOI] [PubMed] [Google Scholar]